Phenolic Compound Profiles, Cytotoxic, Antioxidant, Antimicrobial Potentials and Molecular Docking Studies of Astragalus gymnolobus Methanolic Extracts
Abstract
:1. Introduction
2. Results
2.1. Extraction of Plants
2.2. Phytochemical Analysis of Extracts
2.3. Cytotoxic Effects of A. gymnolobus Extracts
2.4. Colorimetric Protease (Caspase-2, -3, -6, -8, -9) Assay
2.5. Cellular DNA Fragmentation
2.6. Antimicrobial Activity
2.7. Determination of Antioxidant Activities of Extracts
2.8. Pearson Correlation Analysis
2.9. Docking Studies
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction
4.3. Identification of Phenolic Compounds by Liquid Chromatography–Electrospray Tandem Mass Spectrometry (LC–ESI–MS/MS)
4.4. Cell Culture
4.5. Cell Proliferation (WST-1) Assay
4.6. DNA Fragmentation
4.7. Measurement of Caspases Activities
4.8. Antimicrobial Activity
4.8.1. Bacteria
4.8.2. Disc Diffusion Method
4.8.3. Broth Microdilution Method
4.9. Antioxidant Activity
4.9.1. Total Phenolic Contents of the Extracts
4.9.2. Determination of Total Flavonoid Contents
4.9.3. DPPH Radical Scavenging Assay
4.9.4. Superoxide Radical (•O2−) Scavenging Assay
4.9.5. Determination of Copper Ion Reducing Effect (CUPRAC)
4.9.6. Determination of Fe2+ Chelating Effect
4.9.7. Determination of Lipid Peroxidation Inhibitory Effect in β-Carotene/Linoleic Acid System
4.9.8. Determination of Total Antioxidant Capacity
4.9.9. Determination of Antioxidant Capacity Equivalent to Trolox
4.10. Pearson Correlation Analysis
4.11. Docking Studies
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwell, M.; Rahman, P.K.S.M. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- Podlech, D.; Zarre, S.H. Taxonomic Revision of the Genus Astragalus L. (Leguminosae) in the Old World; Vienna Natural History Museum: Vienna, Austria, 2013; Volume 2, ISBN 9783902421760. [Google Scholar]
- Aytac, Z.; Ekici, M. Astragalus L. Türkiye Bitkileri Listesi (Damarlı Bitkiler), 1st ed.; Güner, A., Aslan, S., Ekim, T., Vural, M., Babaç, M., Eds.; Nezahat Gökyiğit Botanik Bahçesi ve Flora Araştırmaları Derneği Yayınları: Istanbul, Turkey, 2012; pp. 427–456. ISBN 978-605-60425-7-7. [Google Scholar]
- Oltulu, B. Regeneration of Astragalus trojanus (Geven) in Different In Vitro Nutrient Media. Master’s Thesis, Ege University, Bornova, Turkey, 2008. [Google Scholar]
- Calis, I.; Sticher, O. Triterpene Saponins from Plants of the Flora of Turkey. In Saponins Used in Traditional and Modern Medicine; Waller, G., Yamasaki, K., Eds.; Springer: New York, NY, USA, 1996; Volume 404, pp. 485–500. ISBN 978-1-4899-1367-8. [Google Scholar]
- Calis, I.; Yürüker, A.; Taşdemir, D.; Wright, A.; Sticher, O.; Luo, Y.-D.; Pezzuto, J. Cycloartane Triterpene Glycosides from the Roots of Astragalus melanophrurius. Planta Med. 1997, 63, 183–186. [Google Scholar] [CrossRef]
- Bedir, E.; Calis, I.; Khan, I.A.; Macrophyllosaponin, E. A Novel Compound from the Roots of Astragalus olelfolius. Chem. Pharm. Bull. 2000, 48, 1081–1083. [Google Scholar] [CrossRef]
- Li, X.; Qu, L.; Dong, Y.; Han, L.; Liu, E.; Fang, S.; Zhang, Y.; Wang, T. A Review of Recent Research Progress on the Astragalus Genus. Molecules 2014, 19, 18850–18880. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Sahinler, S.S.; Tepe, B. Astragalus gymnolobus, A. leporinus Var. Hirsutus, and A. onobrychis: Phytochemical Analysis and Biological Activity. Ind. Crops Prod. 2020, 150, 112366. [Google Scholar] [CrossRef]
- Kondeva-Burdina, M.; Shkondrov, A.; Simeonova, R.; Vitcheva, V.; Krasteva, I.; Ionkova, I. In Vitro/In Vivo Antioxidant and Hepatoprotective Potential of Defatted Extract and Flavonoids Isolated from Astragalus spruneri Boiss. (Fabaceae). Food Chem. Toxicol. 2018, 111, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Noh, H.-J.; Choi, J.; Lee, K.-H.; Lee, M.-H.; Lee, J.-H.; Hong, Y.; Lee, S.-E.; Kim, S.-Y.; Kim, G.-S. Anti-Inflammatory Cycloartane-Type Saponins of Astragalus membranaceus. Molecules 2013, 18, 3725–3732. [Google Scholar] [CrossRef] [PubMed]
- Maamria, L.; Long, C.; Haba, H.; Lavaud, C.; Cannac, A.; Benkhaled, M. Cycloartane Glycosides from Astragalus gombo. Phytochem. Lett. 2015, 11, 286–291. [Google Scholar] [CrossRef]
- Yesilada, E.; Bedir, E.; Çalış, İ.; Takaishi, Y.; Ohmoto, Y. Effects of Triterpene Saponins from Astragalus Species on In Vitro Cytokine Release. J. Ethnopharmacol. 2005, 96, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Özipek, M.; Dönmez, A.A.; Çalış, İ.; Brun, R.; Rüedi, P.; Tasdemir, D. Leishmanicidal Cycloartane-Type Triterpene Glycosides from Astragalus oleifolius. Phytochemistry 2005, 66, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Gariboldi, P. Cycloartane Triterpene Glycosides from Astragalus trigonus. Phytochemistry 1995, 40, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Sevimli-Gür, C.; Onbaşılar, İ.; Atilla, P.; Çakar, N.; Deliloğlu-Gürhan, İ.; Bedir, E. Wound Healing Effects of Cycloartane-Type Triterpenes Isolated from Astragalus Species. Planta Med. 2009, 75, PA57. [Google Scholar] [CrossRef]
- Bisignano, G.; Iauk, L.; Kirjavainen, S.; Galati, E.M. Anti-Inflammatory, Analgesic, Antipyretic and Antibacterial Activity of Astragalus siculus Biv. Int. J. Pharmacogn. 1994, 32, 400–405. [Google Scholar] [CrossRef]
- Rios, J.L.; Waterman, P.G. A Review of the Pharmacology and Toxicology of Astragalus. Phytother. Res. 1997, 11, 411–418. [Google Scholar] [CrossRef]
- Radwan, M.M.; El-Sebakhy, N.A.; Asaad, A.M.; Toaima, S.M.; Kingston, D.G.I. Kahiricosides II–V, Cycloartane Glycosides from an Egyptian Collection of Astragalus kahiricus. Phytochemistry 2004, 65, 2909–2913. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Uyar, E.; Turker, A. In Vitro Culture of Endemic Astragalus gymnolobus Fischer and Comparison of Its Antibacterial, Antioxidant, and Phenolic Profiles with Field Grown Plants. J. Agr. Sci. Tech. 2020, 22, 815–828. [Google Scholar]
- Turker, A.U.; Koyluoglu, H. Biological Activities of Some Endemic Plants in Turkey. Rom. Biotechnol. Lett. 2012, 17, 6949–6961. [Google Scholar]
- Carvalho, A.J.S.; Ishikawa, T.; Gouvêa, C.M.C.P. Aqueous Extract of Plinia edulis Leaves: Antioxidant Activity and Cytotoxicity to Human Breast Cancer MCF-7 Cell Line. S. Afr. J. Bot. 2012, 81, 1–7. [Google Scholar] [CrossRef]
- Engel, N.; Oppermann, C.; Falodun, A.; Kragl, U. Proliferative Effects of Five Traditional Nigerian Medicinal Plant Extracts on Human Breast and Bone Cancer Cell Lines. J. Ethnopharmacol. 2011, 137, 1003–1010. [Google Scholar] [CrossRef]
- WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. Available online: https://www.who.int/publications/i/item/9789240001299 (accessed on 12 February 2024).
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Last 25 Years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Ghaffari, M.; Chaudhry, B.; Uzair, M.; Imran, M.; Haneef, M.; Ashfaq, K. Biological and Phytochemical Investigations of Crude Extracts of Astragalus creticus. Pak. J. Pharm. Sci. 2021, 64, 403–409. [Google Scholar] [CrossRef]
- Lekmine, S.; Boussekine, S.; Kadi, K.; Martín-García, A.I.; Kheddouma, A.; Nagaz, K.; Bensouici, C. A Comparative Study on Chemical Profile and Biological Activities of Aerial Parts (Stems, Flowers, Leaves, Pods and Seeds) of Astragalus gombiformis. Biocatal. Agric. Biotechnol. 2020, 27, 101668. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I. The Role of Phenolic Compounds in the Fight Against Cancer—A Review. Anticancer Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant Activity of Phenolic Compounds: From In Vitro Results to In Vivo Evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Cooray, H.C.; Janvilisri, T.; van Veen, H.W.; Hladky, S.B.; Barrand, M.A. Interaction of the Breast Cancer Resistance Protein with Plant Polyphenols. Biochem. Biophys. Res. Commun. 2004, 317, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and Cancer Chemoprevention. Evid. Based Complement. Altern. Med. 2011, 2011, 591356. [Google Scholar] [CrossRef]
- Kızılkeçili, Ö. Determining Antifungal, Antibacterial and Antituberculosis Activity of Methanol, Ethanol Extracts and Volatile Oils of Salvia crypthanta Montbret & Auchr Ex Bentham and Salvia pomifera L. Species. Master’s Thesis, Balikesir University, Balıkesir, Turkey, 2007. [Google Scholar]
- Isıksolugu, M. Association of Flavonoids and Tea Intake between Cardiovascular Disease. Turk. Hij. Den. Biyol. Derg. 2000, 57, 181–188. [Google Scholar]
- Choi, E.J.; Ahn, W.S.; Bae, S.M. Equol Induces Apoptosis through Cytochrome C-Mediated Caspases Cascade in Human Breast Cancer MDA-MB-453 Cells. Chem. Biol. Interact. 2009, 177, 7–11. [Google Scholar] [CrossRef]
- Sghaier, M.B.; Skandrani, I.; Nasr, N.; Franca, M.-G.D.; Chekir-Ghedira, L.; Ghedira, K. Flavonoids and Sesquiterpenes from Tecurium Ramosissimum Promote Antiproliferation of Human Cancer Cells and Enhance Antioxidant Activity: A Structure–Activity Relationship Study. Environ. Toxicol. Pharmacol. 2011, 32, 336–348. [Google Scholar] [CrossRef]
- Hasimi, N.; Ertas, A.; Yılmaz, M.A.; Boğa, M.; Temel, H.; Demirci, S.; Yılmaz-Özden, T.; Yener, I.; Kolak, U. LC-MS/MS and GC-MS Analyses of Three Endemic Astragalus Species from Anatolia towards Their Total Phenolic Flavonoid Contents and Biological Activities. Biol. Divers. Conserv. 2017, 10, 18–30. [Google Scholar]
- Sarikurkcu, C.; Zengin, G. Polyphenol Profile and Biological Activity Comparisons of Different Parts of Astragalus macrocephalus subsp. finitimus from Turkey. Biology 2020, 9, 231. [Google Scholar] [CrossRef]
- Arumugam, R.; Kirkan, B.; Sarikurkcu, C. Phenolic Profile, Antioxidant and Enzyme Inhibitory Potential of Methanolic Extracts from Different Parts of Astragalus ponticus Pall. S. Afr. J. Bot. 2019, 120, 268–273. [Google Scholar] [CrossRef]
- Al-Harbi, D.; Awad, N.; Alsberi, H.; Abdein, M. Apoptosis Induction, Cell Cycle Arrest and In Vitro Anticancer Potentiality of Convolvulus spicatus and Astragalus vogelii. World J. Environ. Biosci. 2020, 8, 69–75. [Google Scholar]
- Dhanya, R.; Jayamurthy, P. In Vitro Evaluation of Antidiabetic Potential of Hesperidin and Its Aglycone Hesperetin under Oxidative Stress in Skeletal Muscle Cell Line. Cell Biochem. Funct. 2020, 38, 419–427. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, L.; Wang, X.; Lan, R.; Wang, M.; Du, G.; Guan, W.; Liu, J.; Brennan, M.; Guo, H.; et al. Antioxidant Activity Evaluation of Dietary Flavonoid Hyperoside Using Saccharomyces cerevisiae as a Model. Molecules 2019, 24, 788. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Shen, C.-Y.; Jiang, J.-G. Antidepressant Active Ingredients from Herbs and Nutraceuticals Used in TCM: Pharmacological Mechanisms and Prospects for Drug Discovery. Pharmacol. Res. 2019, 150, 104520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ying, M.-D.; Wu, Y.-P.; Zhou, Z.-H.; Ye, Z.-M.; Li, H.; Lin, D.-S. Hyperoside, a Flavonoid Compound, Inhibits Proliferation and Stimulates Osteogenic Differentiation of Human Osteosarcoma Cells. PLoS ONE 2014, 9, e98973. [Google Scholar] [CrossRef]
- Qi, X.-C.; Li, B.; Wu, W.-L.; Liu, H.-C.; Jiang, Y.-P. Protective Effect of Hyperoside against Hydrogen Peroxide-Induced Dysfunction and Oxidative Stress in Osteoblastic MC3T3-E1 Cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 377–383. [Google Scholar] [CrossRef]
- Kim, D.K.; Jeon, H.; Cha, D.S. p-Hydroxybenzoic Acid-Mediated Lifespan Extension in Caenorhabditis elegans. J. Funct. Foods 2014, 7, 630–640. [Google Scholar] [CrossRef]
- Wang, L.; Lee, I.-M.; Zhang, S.M.; Blumberg, J.B.; Buring, J.E.; Sesso, H.D. Dietary Intake of Selected Flavonols, Flavones, and Flavonoid-Rich Foods and Risk of Cancer in Middle-Aged and Older Women. Am. J. Clin. Nutr. 2009, 89, 905–912. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant Properties of Catechins: Comparison with Other Antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric Acid and Its Conjugates: Dietary Sources, Pharmacokinetic Properties and Biological Activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Zhang, J.; Gao, J.; Ge, X.; Lou, G. Hesperidin Inhibits HeLa Cell Proliferation through Apoptosis Mediated by Endoplasmic Reticulum Stress Pathways and Cell Cycle Arrest. BMC Cancer 2015, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Shen, J.; Chen, Z.; Lin, Z.; Long, L.; Wu, J.; Long, C.; Huang, S.; Lian, P.; Luo, G. Antioxidant and Antitumor Activities of Newly Synthesized Hesperetin Derivatives. Molecules 2022, 27, 879. [Google Scholar] [CrossRef]
- Kumar, S. Caspase Function in Programmed Cell Death. Cell Death Differ. 2007, 14, 32–43. [Google Scholar] [CrossRef]
- Matkowski, A. Plant In Vitro Culture for the Production of Antioxidants—A Review. Biotechnol. Adv. 2008, 26, 548–560. [Google Scholar] [CrossRef]
- Verma, A.K.; Ahmed, S.F.; Hossain, M.S.; Bhojiya, A.A.; Mathur, A.; Upadhyay, S.K.; Srivastava, A.K.; Vishvakarma, N.K.; Barik, M.; Rahaman, M.M.; et al. Molecular Docking and Simulation Studies of Flavonoid Compounds against PBP-2a of Methicillin-resistant Staphylococcus aureus. J. Biomol. Struct. Dyn. 2022, 40, 10561–10577. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Reddy, B.D.; Padukudru, M.A.; Kumari Chitturi, C.H.M.; Vimalambike, M.G.; Madhunapantula, S.V. Naturally Occurring Benzoic Acid Derivatives Retard Cancer Cell Growth by Inhibiting Histone Deacetylases (HDAC). Cancer Biol. Ther. 2017, 18, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Alsahli, M.A.; Almatroudi, A.; Almogbel, M.A.; Khan, A.A.; Anwar, S.; Almatroodi, S.A. The Potential Role of Apigenin in Cancer Prevention and Treatment. Molecules 2022, 27, 6051. [Google Scholar] [CrossRef]
- Taghizadeh, M.S.; Niazi, A.; Moghadam, A.; Afsharifar, A. Experimental, Molecular Docking and Molecular Dynamic Studies of Natural Products Targeting Overexpressed Receptors in Breast Cancer. PLoS ONE 2022, 17, e0267961. [Google Scholar] [CrossRef]
- Singh Tuli, H.; Kumar, A.; Ramniwas, S.; Coudhary, R.; Aggarwal, D.; Kumar, M.; Sharma, U.; Chaturvedi Parashar, N.; Haque, S.; Sak, K. Ferulic Acid: A Natural Phenol That Inhibits Neoplastic Events through Modulation of Oncogenic Signaling. Molecules 2022, 27, 7653. [Google Scholar] [CrossRef]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A Review of Its Structure, Synthesis, Pharmacology, Pharmacokinetics and Toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef] [PubMed]
- Boyenle, I.D.; Adelusi, T.I.; Ogunlana, A.T.; Oluwabusola, R.A.; Ibrahim, N.O.; Tolulope, A.; Okikiola, O.S.; Adetunji, B.L.; Abioye, I.O.; Kehinde Oyedele, A.-Q. Consensus Scoring-Based Virtual Screening and Molecular Dynamics Simulation of Some TNF-Alpha Inhibitors. Inform. Med. Unlocked 2022, 28, 100833. [Google Scholar] [CrossRef]
- Turker, A.; Yıldırım, A. Evaluation of Antibacterial and Antitumor Activities of Some Turkish Endemic Plants. Trop. J. Pharm. Res. 2013, 12, 1003. [Google Scholar] [CrossRef]
- Keskin, C.; Ozen, H.Ç.; Toker, Z.; Kizil, G.; Kizil, M. Determination of In Vitro Antioxidant and Antimicrobial Properties of Shoot and Root Extracts of Astragalus diphtherites FENZL var. diphtherites and Astragalus gymnalopecias RECH. FIL. Obtained by Different Solvents. KSU J. Agric. Nat. 2018, 21, 157–166. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Selim, S.; Albaqawi, A. Antimicrobial Activity of Methanol Extract of Anziroat (Astragalus Sp.). In Proceedings of the 2nd Int’l Conference on Advances in Environment, Agriculture & Medical Sciences (ICAEAM’15), Antalya, Turkey, 11–12 June 2015; pp. 69–71. [Google Scholar]
- Jaradat, N.; AL-Masri, M.; Zaid, A.N.; Hussien, A. Preliminary Phytochemical Screening and In-Vitro Evaluation of Antioxidant and Antimicrobial Activities for Astragalus pelecinus from Palestine. J. Mater. Environ. Sci. 2017, 8, 1492–1497. [Google Scholar]
- Albayrak, S.; Kaya, O. Antioxidant, Antimicrobial and Cytotoxic Activities of Endemic Astragalus argaeus Boiss. from Turkey. Hacettepe J. Biol. Chem. 2019, 47, 87–97. [Google Scholar]
- Teyeb, H.; Zanina, N.; Neffati, M.; Douki, W.; Najjar, M.F. Cytotoxic and Antibacterial Activities of Leaf Extracts of Astragalus gombiformis Pomel (Fabaceae) Growing Wild in Tunisia. Turk. J. Biol. 2012, 36, 53–58. [Google Scholar] [CrossRef]
- Güllüce, M.; Sökmen, M.; Agar, G.; Adiguzel, A.; Bariş, Ö.; Sahin, F. Antimicrobial and Antioxidant Activities of Methanol and Hexane Extract of Some Endemic Astragalus Species. Asian J. Chem. 2008, 20, 2125–2132. [Google Scholar]
- Adigüzel, A.; Sökmen, M.; Ozkan, H.; Agar, G.; Güllüce, M.; Sahin, F. In Vitro Antimicrobial and Antioxidant Activities of Methanol and Hexane Extract of Astragalus Species Growing in the Eastern Anatolia Region of Turkey. Turk. J. Biol. 2009, 33, 65–71. [Google Scholar] [CrossRef]
- Cittan, M.; Çelik, A. Development and Validation of an Analytical Methodology Based on Liquid Chromatography–Electrospray Tandem Mass Spectrometry for the Simultaneous Determination of Phenolic Compounds in Olive Leaf Extract. J. Chromatogr. Sci. 2018, 56, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-N.; Wu, R.-C.; Cheng, C.-T.; Tsai, C.-Y.; Chang, Y.-R.; Yeh, T.-S.; Wu, T.-H.; Lee, W.-C.; Chiang, K.-C. HO-1 Is a Favorable Prognostic Factor for HBV-HCC Patients Who Underwent Hepatectomy. Cancer Manag. Res. 2018, 10, 6049–6059. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, R.P.; Kim, S.; Cheresh, P.; Williams, D.B.; Morales-Nebreda, L.; Cheng, Y.; Yeldandi, A.; Bhorade, S.; Pardo, A.; Selman, M.; et al. SIRT3 Deficiency Promotes Lung Fibrosis by Augmenting Alveolar Epithelial Cell Mitochondrial DNA Damage and Apoptosis. FASEB J. 2017, 31, 2520–2532. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayal, K.; Alafeefy, A.; Vaali-Mohammed, M.-A.; Mahmood, A.; Zubaidi, A.; Al-Obeed, O.; Khan, Z.; Abdulla, M.; Ahmad, R. Novel Derivative of Aminobenzenesulfonamide (3c) Induces Apoptosis in Colorectal Cancer Cells through ROS Generation and Inhibits Cell Migration. BMC Cancer 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Twelfth Edition. CLSI document M02-A12; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of Propolis: Some Parameters and Procedures for Chemical Quality Control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Akter, S.; Ahmed, M.; Eun, J.-B. Solvent Effects on Antioxidant Properties of Persimmon (Diospyros kaki L. cv. Daebong) Seeds. Int. J. Food Sci. Technol. 2010, 45, 2258–2264. [Google Scholar] [CrossRef]
- Aydın, A.; Orhan, H.; Sayal, A.; Özata, M.; Şahin, G.; Işımer, A. Oxidative Stress and Nitric Oxide Related Parameters in Type II Diabetes Mellitus: Effects of Glycemic Control. Clin. Biochem. 2001, 34, 65–70. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Guo, T.; Wei, L.; Sun, J.; Hou, C.; Fan, L. Antioxidant Activities of Extract and Fractions from Tuber. indicum Cooke & Massee. Food Chem. 2011, 127, 1634–1640. [Google Scholar] [CrossRef]
- Miller, H.E. A Simplified Method for the Evaluation of Antioxidants. J. Am. Oil Chem. Soc. 1971, 48, 91. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Akhtar, W.; Ali, G.; Ashraf, N.; Fatima, I.; Kayani, W.K.; Shaheen, H.; Ghoneim, M.M.; Abdelgawad, M.A.; Khames, A. Efficiency of Multiple Extraction Solvents on Antioxidant, Cytotoxic, and Phytotoxic Potential of Taraxacum officinale (L.) Weber Ex F.H. Wigg. from Poonch Valley, Azad Kashmir, Pakistan. Evid. Based Complement. Altern. Med. 2022, 2022, 5118553. [Google Scholar] [CrossRef]
- Das, P.R.; Darwish, A.G.; Ismail, A.; Haikal, A.M.; Gajjar, P.; Balasubramani, S.P.; Sheikh, M.B.; Tsolova, V.; Soliman, K.F.A.; Sherif, S.M.; et al. Diversity in Blueberry Genotypes and Developmental Stages Enables Discrepancy in the Bioactive Compounds, Metabolites, and Cytotoxicity. Food Chem. 2022, 374, 131632. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Chacko, S.; Bose, P.; Lapenna, A.; Pattanayak, S.P. Structure Based Multitargeted Molecular Docking Analysis of Selected Furanocoumarins against Breast Cancer. Sci. Rep. 2019, 9, 15743. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.W.; Zhang, Y. DockRMSD: An Open-Source Tool for Atom Mapping and RMSD Calculation of Symmetric Molecules through Graph Isomorphism. J. Cheminform. 2019, 11, 40. [Google Scholar] [CrossRef] [PubMed]

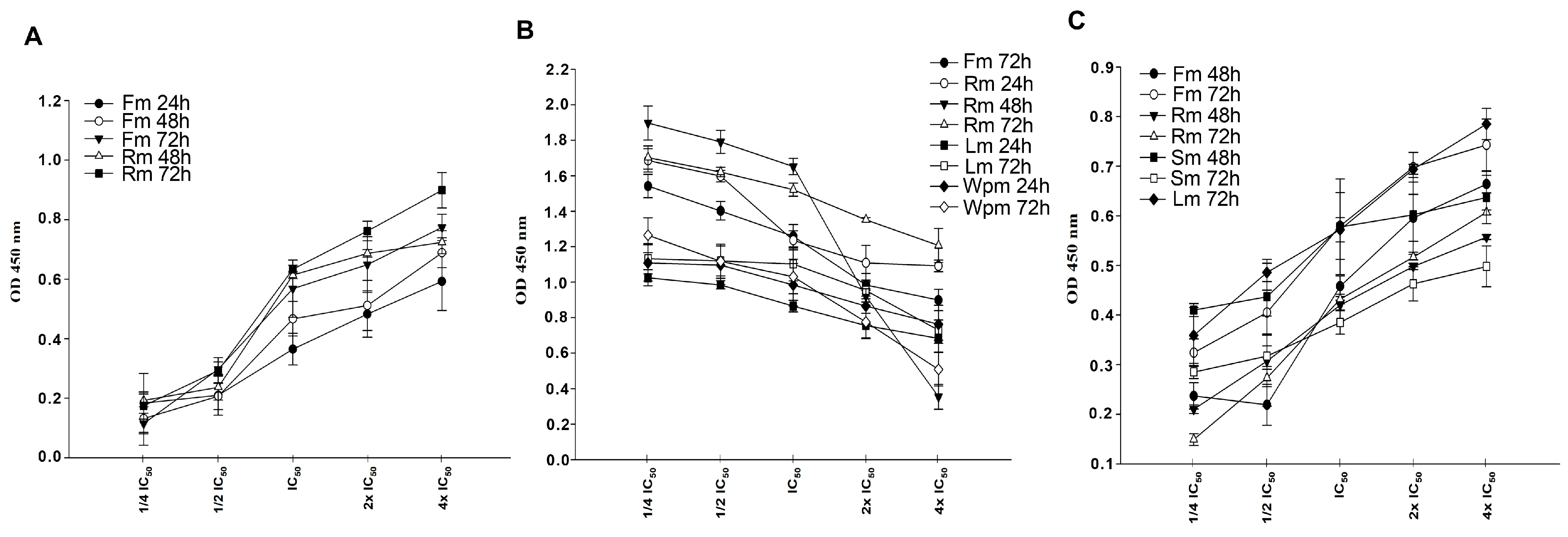
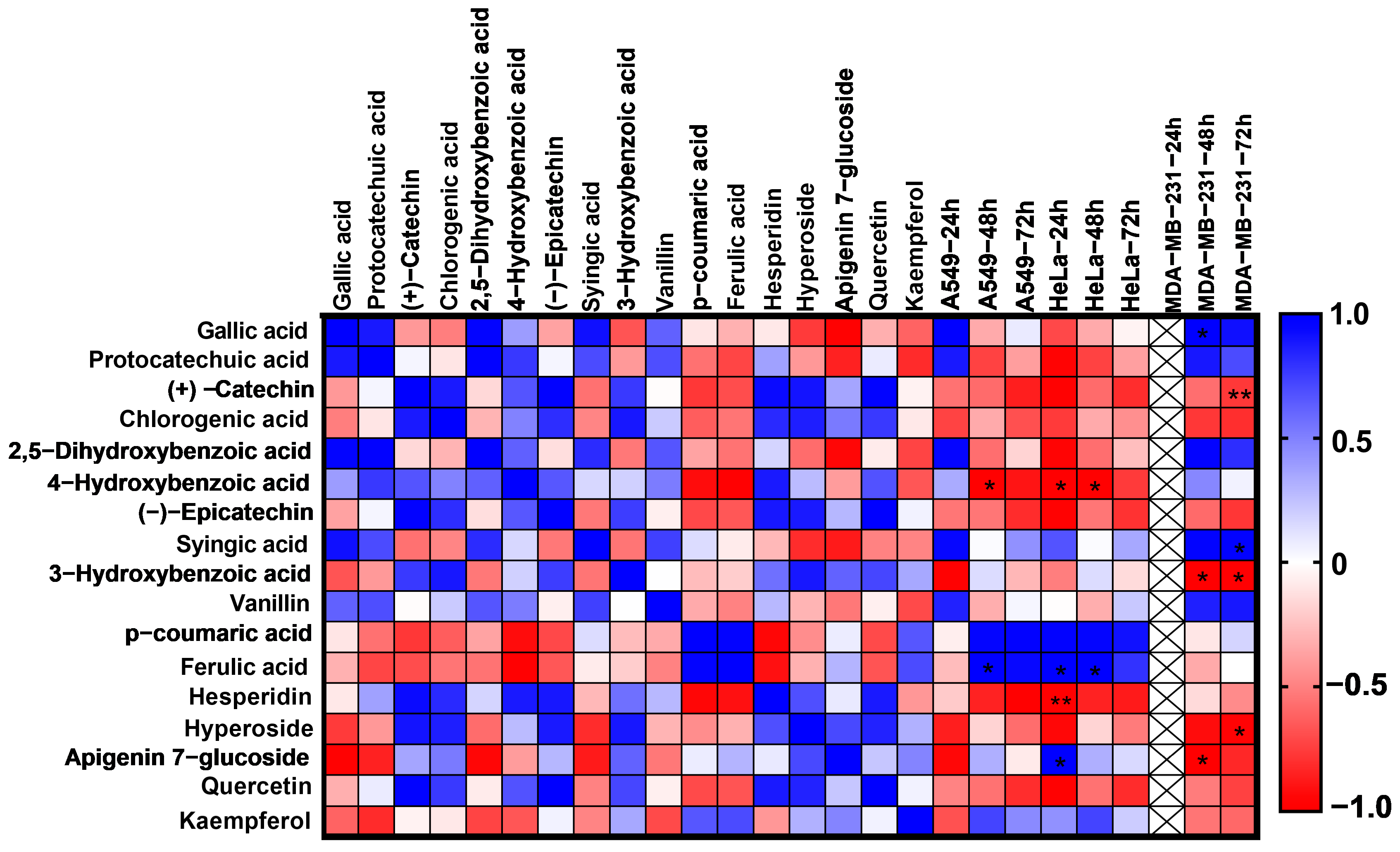
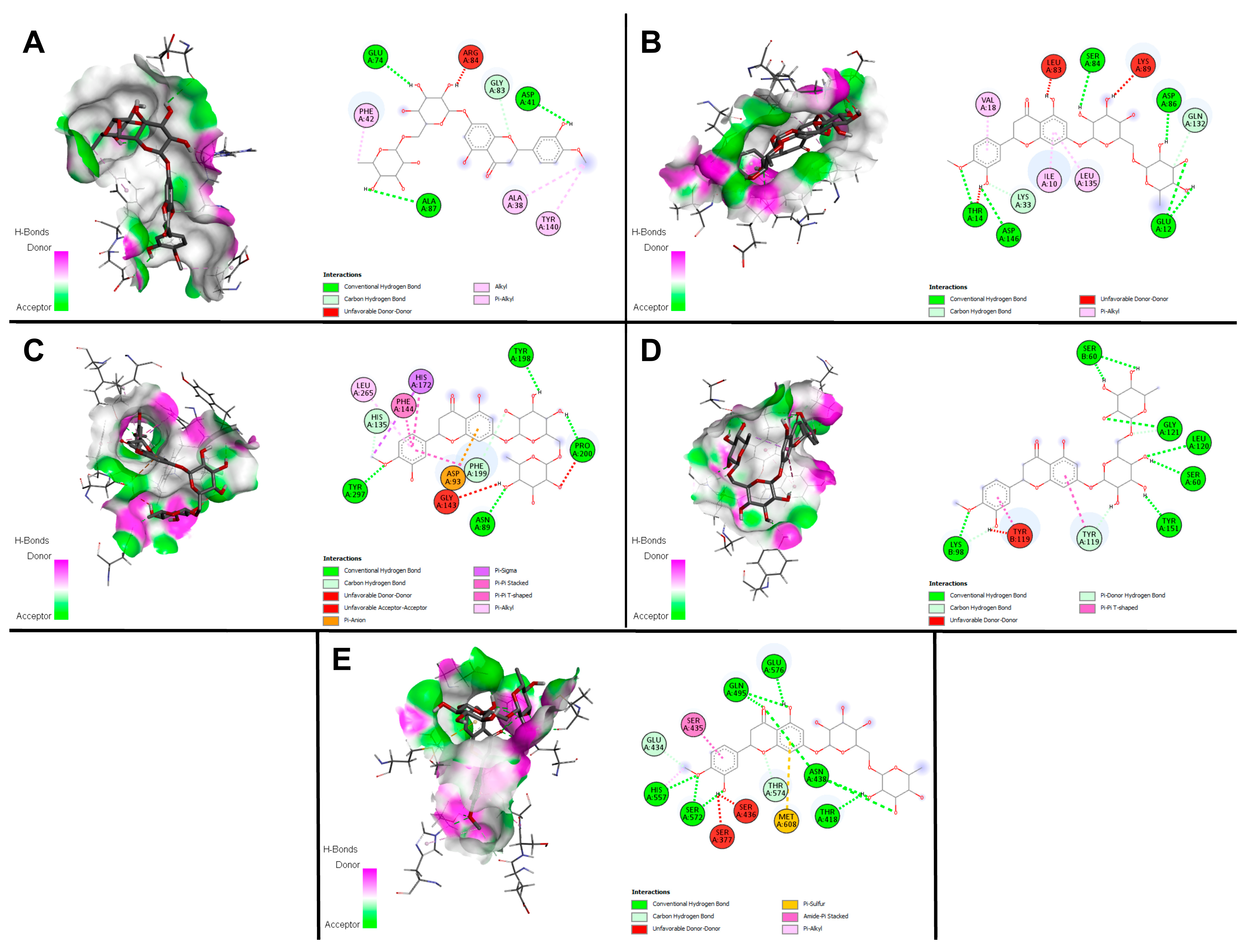
| Compounds | Fm | WPm | Rm | Sm | Lm |
|---|---|---|---|---|---|
| Gallic acid | 39.5 ± 0.6 a | 11.4 ± 0.4 c | 9.20 ± 0.37 d | 14.2 ± 0.1 b | 14.0 ± 0.5 b |
| Protocatechuic acid | 348 ± 2 a | 20.7 ± 1.1 e | 144 ± 1 c | 167 ± 3 b | 85.2 ± 0.8 d |
| (+)-Catechin | 8.49 ± 0.62 c | nd | 365 ± 3 b | 454 ± 1 a | 12.8 ± 0.6 c |
| Chlorogenic acid | 4.87 ± 0.03 d | 8.52 ± 0.53 c | 18.8 ± 0.6 a | 14.5 ± 0.9 b | 3.08 ± 0.15 d |
| 2,5-Dihydroxybenzoic acid | 57.5 ± 1.7 a | 2.36 ± 0.07 d | 11.8 ± 1.2 c | 20.4 ± 0.5 b | 10.8 ± 0.9 c |
| p-Hydroxybenzoic acid | 609 ± 3b | 66.0 ± 0.8 e | 565 ± 3 c | 648 ± 1 a | 183 ± 1 d |
| (-)-Epicatechin | nd | nd | 9.13 ± 0.46 b | 15.5 ± 0.7 a | nd |
| Syringic acid | 16.0 ± 0.8 a | 5.52 ± 0.58 b | nd | nd | nd |
| 3-Hydroxybenzoic acid | 1.92 ± 0.31 a | 2.20 ± 0.10 a | 2.26 ± 0.05 a | 2.28 ± 0.19 a | 1.93 ± 0.08 a |
| Vanillin | 16.0 ± 0.2 a | 13.4 ± 1.3 ab | 14.5 ± 0.2 ab | 12.6 ± 1.5 ab | 10.8 ± 0.5 b |
| p-Coumaric acid | 57.6 ± 0.6 c | 93.1 ± 1.8 a | 41.1 ± 0.8 e | 48.5 ± 1.1 d | 74.3 ± 0.6 b |
| Ferulic acid | 24.9 ± 0.1 c | 161 ± 1 a | 13.7 ± 0.9 d | 15.4 ± 0.2 d | 121 ± 2 b |
| Hesperidin | 18581 ± 103 b | 147 ± 6 d | 40174 ± 136 a | 40122 ± 27 a | 4854 ± 168 c |
| Hyperoside | 363 ± 4 e | 1455 ± 3 d | 2444 ± 13 b | 2677 ± 13 a | 1237 ± 15 c |
| Apigenin 7-glucoside | 1.53 ± 0.18 c | 11.1 ± 1.4 ab | 13.2 ± 0.3 a | 8.99 ± 0.56 b | 10.5 ± 0.8 ab |
| Quercetin | 6.07 ± 0.67 c | 3.24 ± 0.29 d | 34.2 ± 0.4 b | 61.8 ± 0.4 a | 4.18 ± 0.25 d |
| Kaempferol | nd | 25.5 ± 0.8 a | 4.35 ± 0.23 c | 19.5 ± 0.1 b | 17.1 ± 2.0 b |
| IC50 Values (µg/mL) | |||||
|---|---|---|---|---|---|
| Extracts | Hours | A549 | HeLa | MDA-MB-231 | 293T |
| Fm | 24 h | 2479.518 | na | na | na |
| 48 h | 328.782 | na | 100.284 | na | |
| 72 h | 409.253 | 2.950 | 23.013 | na | |
| Rm | 24 h | na | 1.175 | na | na |
| 48 h | 1407.938 | 2.907 | 17.742 | na | |
| 72 h | 966.808 | 5.031 | 12.465 | na | |
| Sm | 24 h | na | na | na | na |
| 48 h | na | na | 7.121 | na | |
| 72 h | na | na | 10.102 | na | |
| Lm | 24 h | na | 0.069 | na | na |
| 48 h | na | na | na | na | |
| 72 h | na | 0.751 | 7.121 | na | |
| WPm | 24 h | na | 0.940 | na | na |
| 48 h | na | na | na | na | |
| 72 h | na | 2.637 | na | na | |
| Bacteria | Disc Diffusion Results | ||||||
|---|---|---|---|---|---|---|---|
| Fm (mm) | Lm (mm) | Sm (mm) | Rm (mm) | WPm (mm) | A (mm) | N (mm) | |
| Staphylococcus aureus ATCC 25923 | 9 | - | 8 | - | 9 | 32 (P) | - |
| Staphylococcus aureus ATCC 29213 | 8 | - | 8 | - | 9 | 20 (P) | - |
| Staphylococcus aureus ATCC 43300 | 8 | - | 8 | - | 9 | 15 (FOX) | - |
| Staphylococcus epidermidis ATCC 12228 | - | - | - | - | - | 20 (VA) | - |
| Enterococcus faecalis ATCC 51299 | - | - | - | - | - | 15 (VA) | - |
| Enterococcus faecalis ATCC 29212 | - | - | - | - | - | 20 (VA) | - |
| Streptococcus pyogenes ATCC 19615 | 10 | 9 | 10 | 9 | 11 | 42 (P) | - |
| Escherichia coli ATCC 25922 | - | - | - | - | - | 21 (AMC) | - |
| Escherichia coli ATCC 35218 | - | - | - | - | - | 18 (AMC) | - |
| Klebsiella pneumoniae ATCC 13883 | - | - | - | - | - | 25 (CAZ) | - |
| Klebsiella pneumoniae ATCC 700603 | - | - | - | - | - | 14 (CAZ) | - |
| Enterobacter cloacae ATCC 23355 | - | - | - | - | - | 33 (MEM) | - |
| Serratia marcescens ATCC 8100 | - | - | - | - | - | 30 (MEM) | - |
| Proteus vulgaris ATCC 13315 | - | - | - | - | - | 35 (FEP) | - |
| Salmonella typhimurium ATCC 14028 | - | - | - | - | - | 20 (AMP) | - |
| Pseudomonas aeruginosa ATCC 27853 | - | - | - | - | - | 32 (MEM) | - |
| Extract | TP (mg GAE/g) | TF (mg QE/g) | TOAC (mg AAE/g) | TEAC (µM TE/g) |
|---|---|---|---|---|
| Rm | 200.53 ± 13.62 | 34.43 ± 4.97 | 103 ± 1.99 | 231 ± 13.48 |
| Sm | 173.58 ± 5.21 | 26.57 ± 6.73 | 86 ± 6.49 | 187 ± 7.29 |
| Fm | 130.27 ± 7.39 | 9.73 ± 1.44 | 56 ± 4.37 | 153 ± 6.38 |
| Lm | 374.41 ± 14.37 | 66.85 ± 3.49 | 304 ± 7.31 | 794 ± 8.37 |
| WPm | 367.10 ± 15.29 | 44.78 ± 4.28 | 258 ± 4.84 | 678 ± 14.31 |
| Extract | DPPH (EC 50 µg/mL) | FRAP (mM FeSO4/g) | CUPRAC (mg AAE/g) | β-Caroten (% Activity of 1 mg/mL) |
|---|---|---|---|---|
| Rm | 465 ± 16.34 | 1.62 ± 0.05 | 47 ± 2.79 | 68.41 ± 4.17 |
| Sm | 676 ± 12.39 | 0.91 ± 0.09 | 31 ± 5.48 | 59.52 ± 1.34 |
| Fm | 759 ± 9.37 | 0.65 ± 0.03 | 19 ± 1.65 | 41.94 ± 2.25 |
| Lm | 152 ± 6.13 | 3.72 ± 0.09 | 148 ± 4.63 | 90.37 ± 1.84 |
| WPm | 143 ± 7.42 | 3.15 ± 0.08 | 132 ± 8.7 | 83.02 ± 1.94 |
| Compound | Binding Affinity | ||||
|---|---|---|---|---|---|
| BCL-2 | CDK1 | HDAC2 | TNFα | PBP2a | |
| Hesperidin | −9.3 | −8.7 | −7.7 | −9.0 | −9.4 |
| Apigenin 7-glucoside | −7.7 | −7.8 | −7.6 | −7.9 | −9.2 |
| Hyperoside | −7.2 | −7.8 | −6.5 | −8.0 | −7.7 |
| Catechin | −6.8 | −8.0 | −7.5 | −7.1 | −8.2 |
| Ferulic acid | −5.6 | −6.7 | −7.6 | −5.9 | −6.6 |
| 3-Hydroxybenzoic acid | −5.6 | −4.1 | −6.5 | −5.4 | −5.8 |
| p-Hydroxybenzoic acid | −5.2 | −5.7 | −6.4 | −5.2 | −5.7 |
| Gallic acid | −5.2 | −5.9 | −5.9 | −5.6 | −6.3 |
| Syringic acid | −5.3 | −6.2 | −5.5 | −5.8 | −6.0 |
| Co-crystallized ligands | −11.6 a | −9.1 b | −7.0 c | −8.9 d | −7.5 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydemir, E.; Odabaş Köse, E.; Yavuz, M.; Kilit, A.C.; Korkut, A.; Özkaya Gül, S.; Sarikurkcu, C.; Celep, M.E.; Göktürk, R.S. Phenolic Compound Profiles, Cytotoxic, Antioxidant, Antimicrobial Potentials and Molecular Docking Studies of Astragalus gymnolobus Methanolic Extracts. Plants 2024, 13, 658. https://doi.org/10.3390/plants13050658
Aydemir E, Odabaş Köse E, Yavuz M, Kilit AC, Korkut A, Özkaya Gül S, Sarikurkcu C, Celep ME, Göktürk RS. Phenolic Compound Profiles, Cytotoxic, Antioxidant, Antimicrobial Potentials and Molecular Docking Studies of Astragalus gymnolobus Methanolic Extracts. Plants. 2024; 13(5):658. https://doi.org/10.3390/plants13050658
Chicago/Turabian StyleAydemir, Esra, Elif Odabaş Köse, Mustafa Yavuz, A. Cansu Kilit, Alaaddin Korkut, Serap Özkaya Gül, Cengiz Sarikurkcu, Mehmet Engin Celep, and R. Süleyman Göktürk. 2024. "Phenolic Compound Profiles, Cytotoxic, Antioxidant, Antimicrobial Potentials and Molecular Docking Studies of Astragalus gymnolobus Methanolic Extracts" Plants 13, no. 5: 658. https://doi.org/10.3390/plants13050658
APA StyleAydemir, E., Odabaş Köse, E., Yavuz, M., Kilit, A. C., Korkut, A., Özkaya Gül, S., Sarikurkcu, C., Celep, M. E., & Göktürk, R. S. (2024). Phenolic Compound Profiles, Cytotoxic, Antioxidant, Antimicrobial Potentials and Molecular Docking Studies of Astragalus gymnolobus Methanolic Extracts. Plants, 13(5), 658. https://doi.org/10.3390/plants13050658






