Melatonin Mitigates Water Deficit Stress in Cenchrus alopecuroides (L.) Thunb through Up-Regulating Gene Expression Related to the Photosynthetic Rate, Flavonoid Synthesis, and the Assimilatory Sulfate Reduction Pathway
Abstract
:1. Introduction
2. Results and Discussion
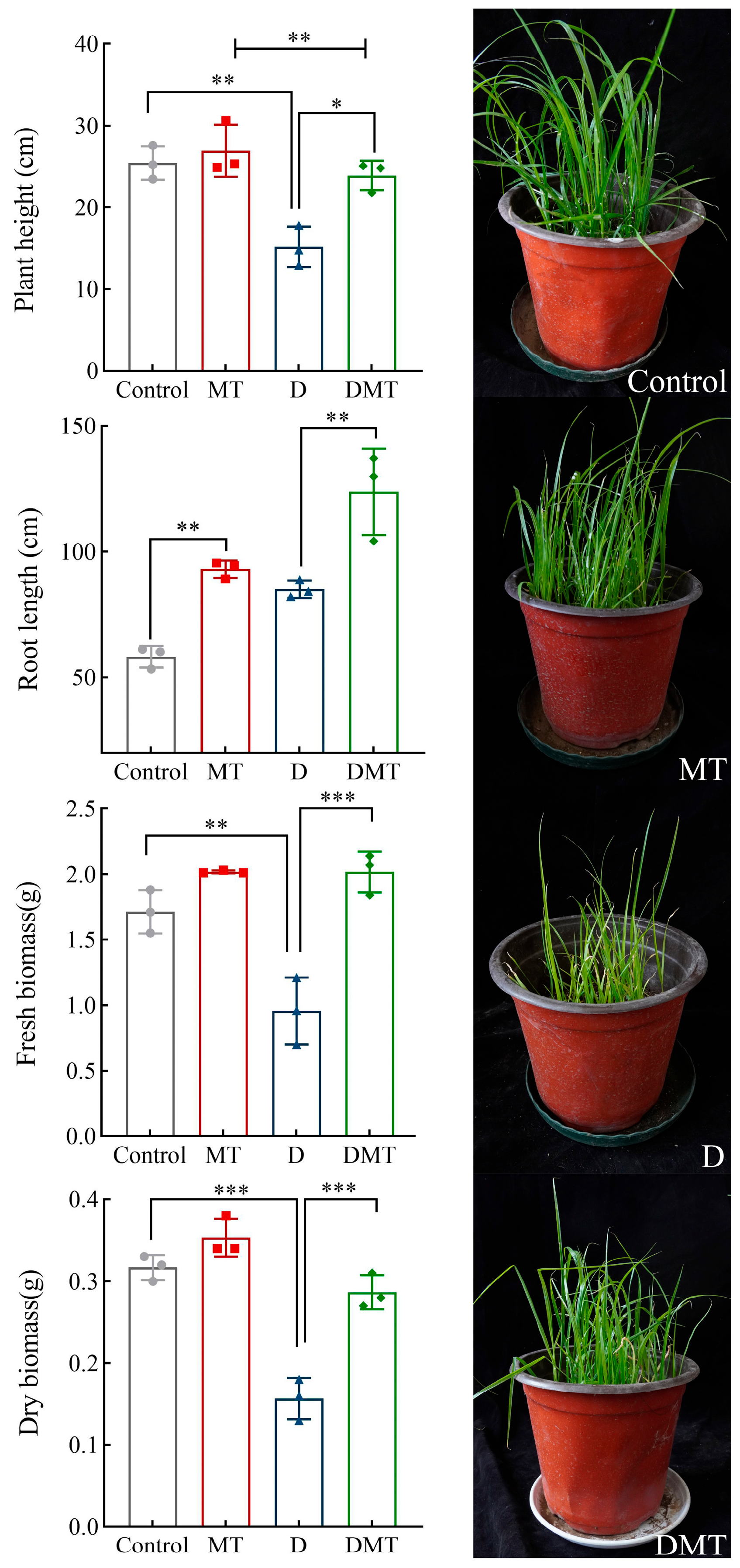
2.1. Plant Responses to Water Stress Induced by Melatonin
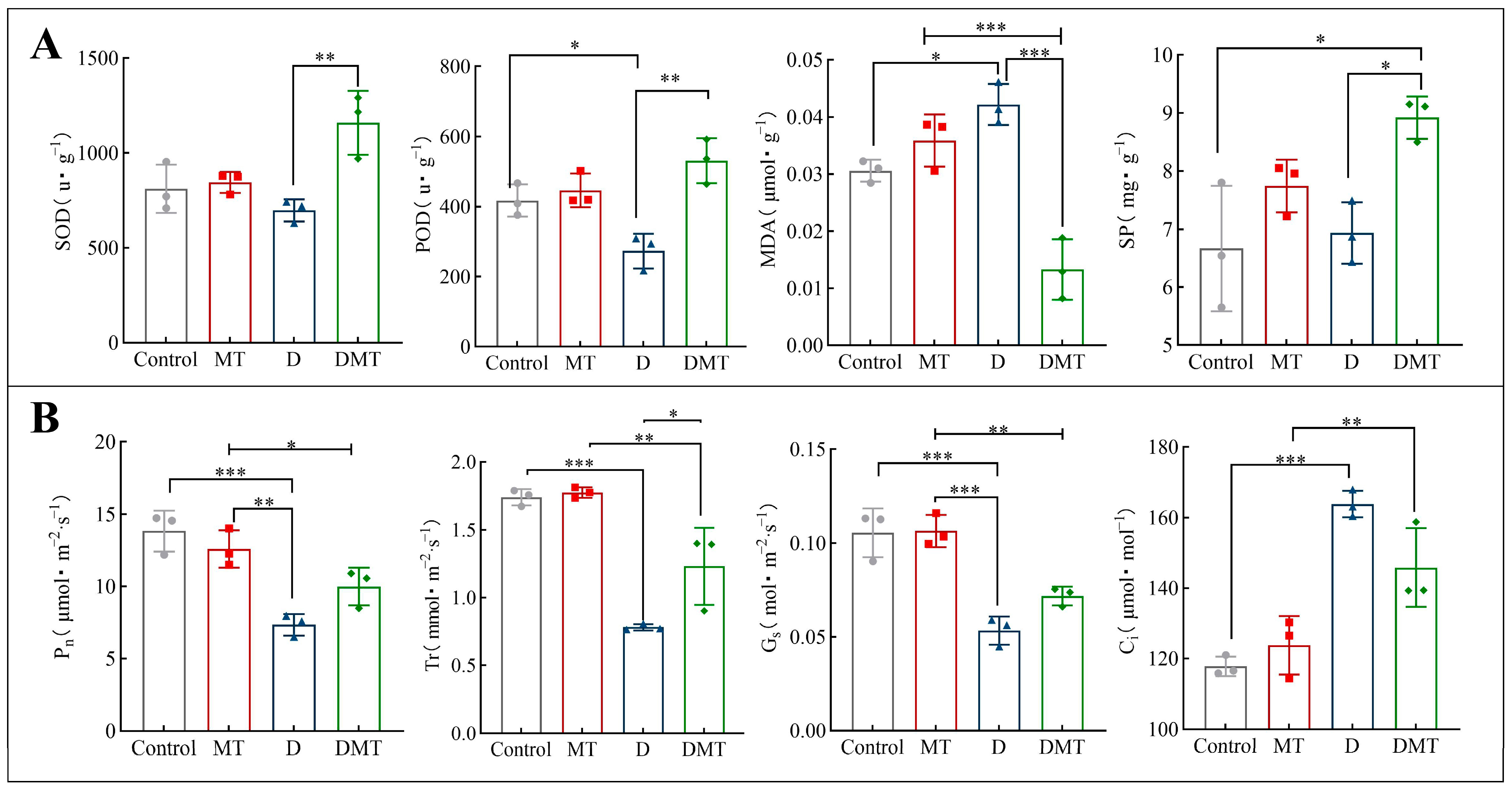
2.2. Melatonin Improves Gas Exchange Parameters under Water Stress
2.3. Melatonin Modulates Transcriptomic Responses to Water Stress
2.3.1. Transcriptome Sequencing Data and Quality Assessment
2.3.2. Analysis of the Number of Differentially Expressed Genes
2.3.3. GO and KEGG Enrichment Analyses
2.4. Melatonin Regulates Gene Transcription Levels in Secondary Metabolic Pathways
2.4.1. Melatonin Regulates the Phenylpropanoid and Flavonoid Synthesis Pathways
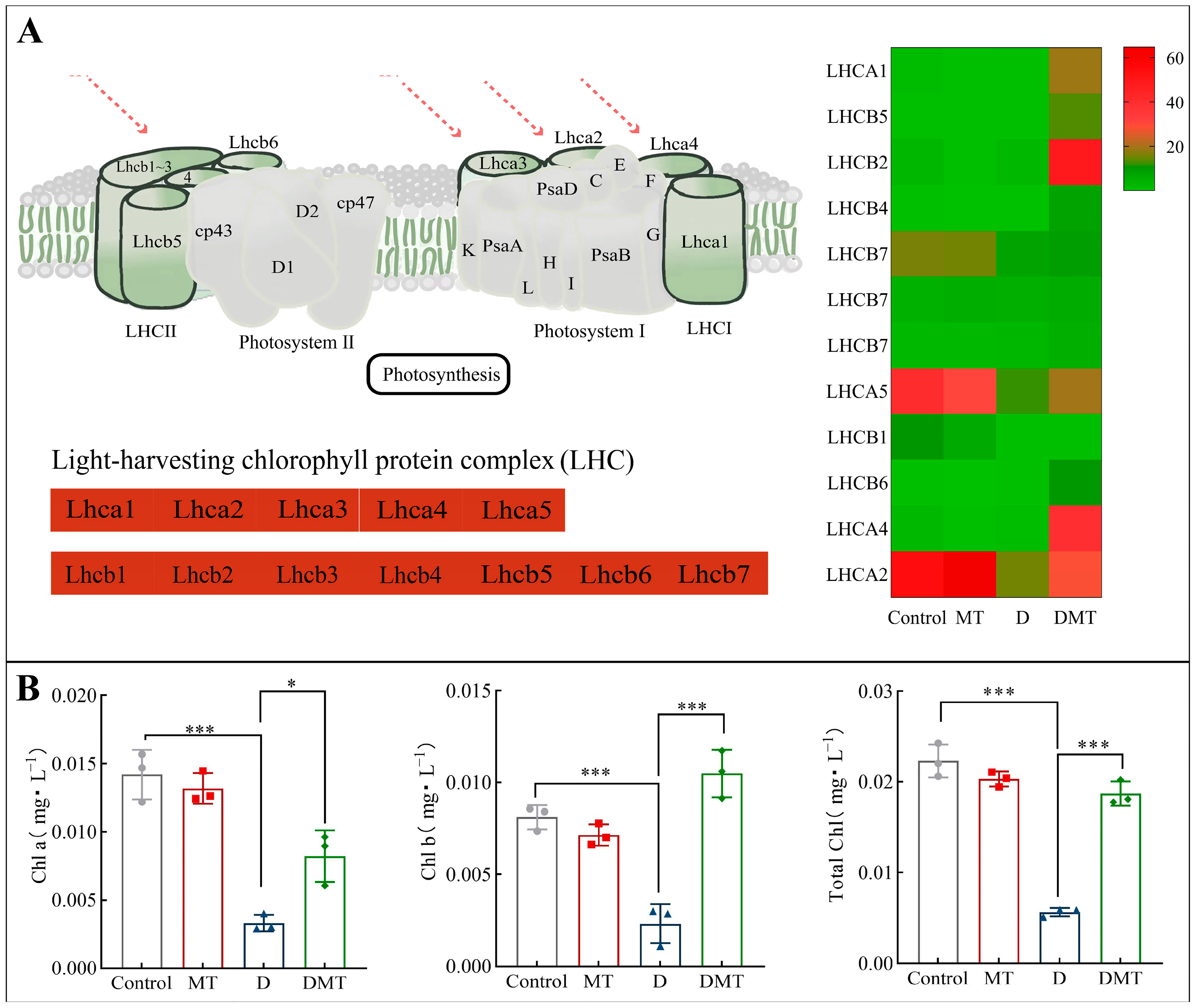
2.4.2. Melatonin Regulates Photosynthesis-Antenna Proteins
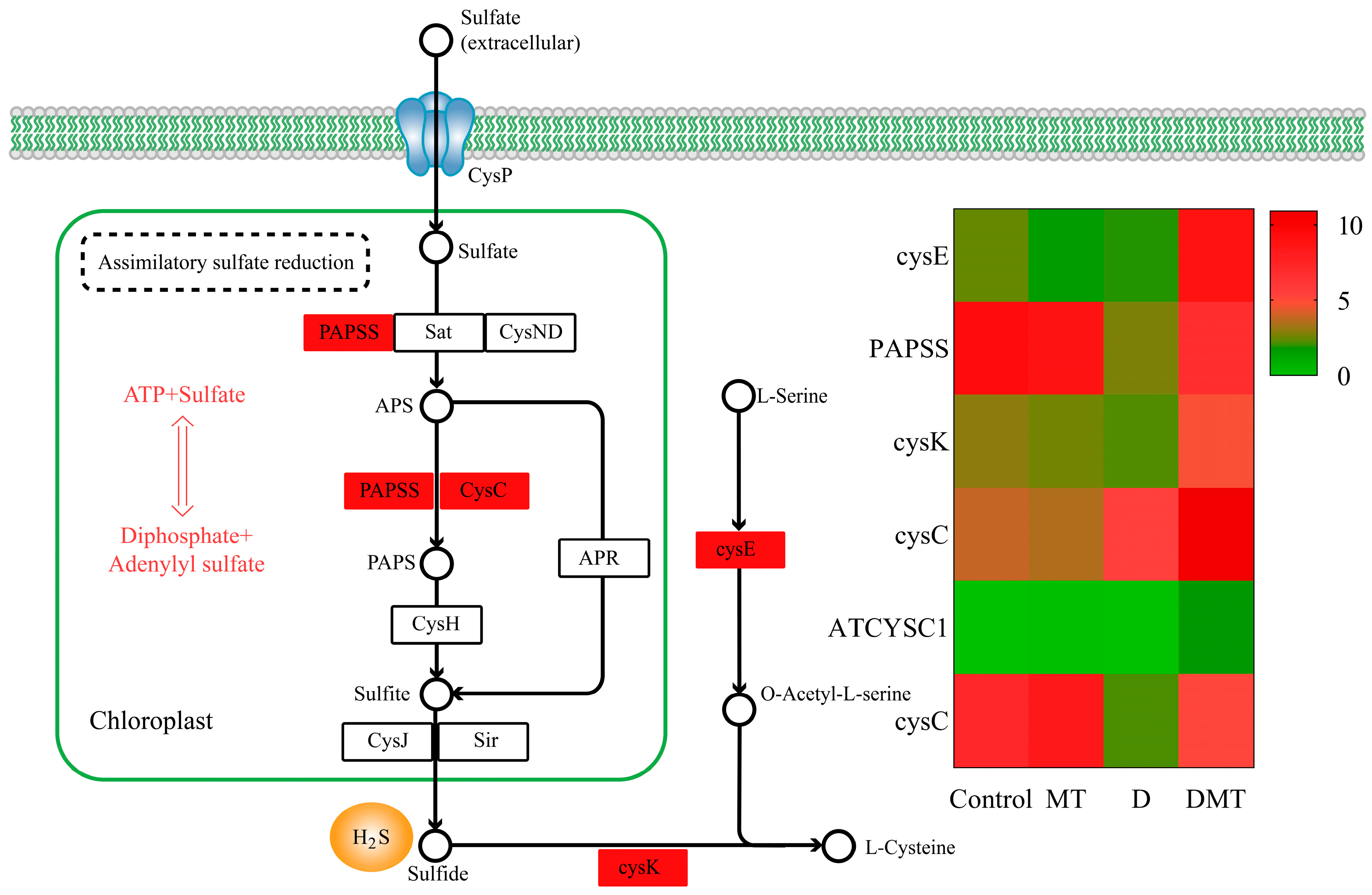
2.4.3. Melatonin Regulates the Assimilatory Sulfate Reduction (ASR) Pathway of Sulfur Metabolism
2.5. RT-qPCR Validation of Transcriptome Data
2.6. C. alopecuroides Transcription Factors Are Affected by Exogenous Melatonin
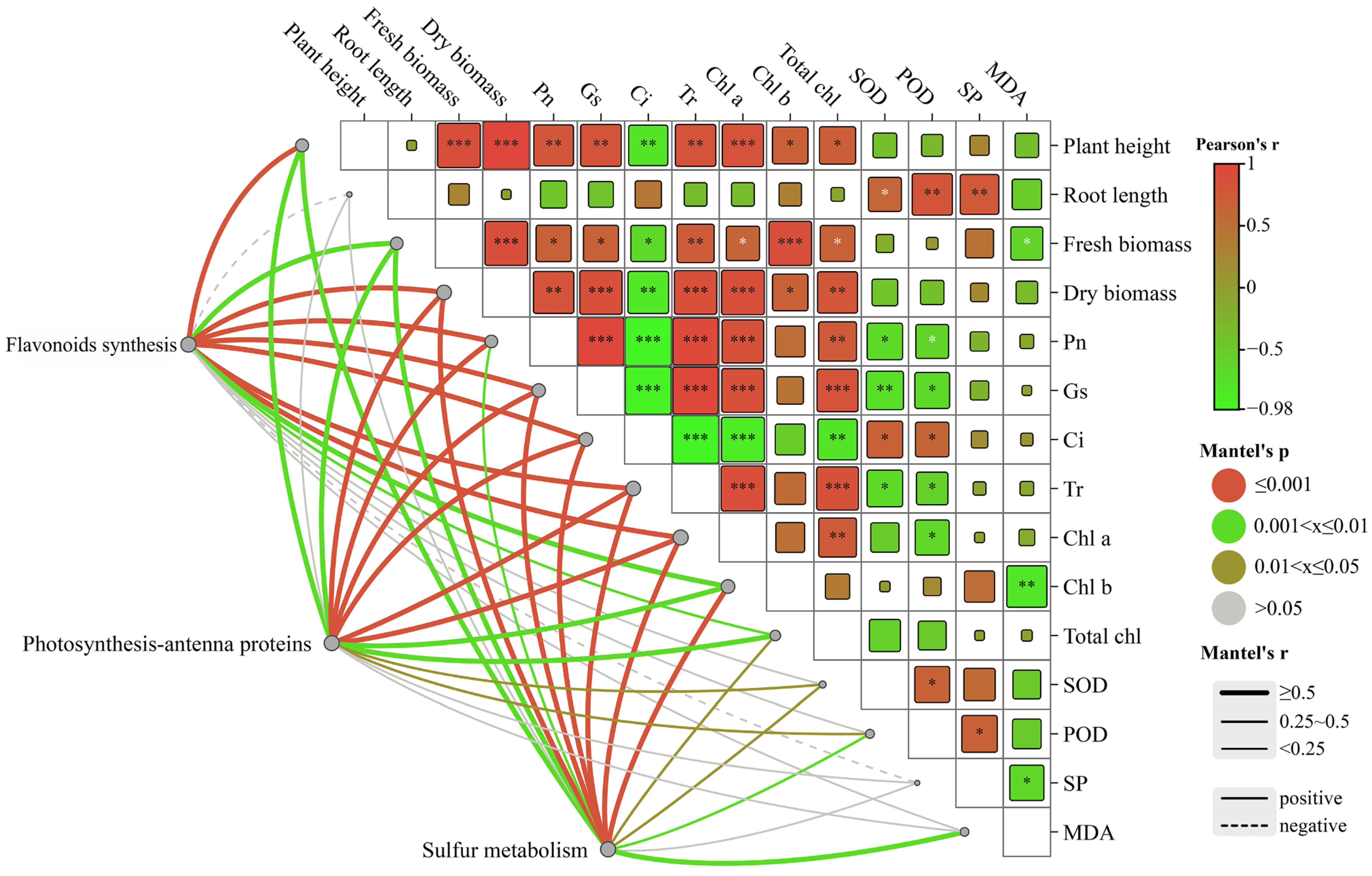
2.7. Correlations between Environmental Factors and Related Genes in the Growth of C. alopecuroides under Different Treatments
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Experimental Designs
3.3. Growth of Morphological and Physiological Indicators
3.3.1. Growth of Morphological Indicators
3.3.2. Measurement of Physiological Indicators
3.4. RNA Sequencing Experimental Method
3.4.1. RNA Isolation and Library Preparation
3.4.2. RNA Sequencing Analysis Process
3.5. Real-Time Fluorescence Quantitative PCR (RT-qPCR)
3.6. Statistical Analysis of Data
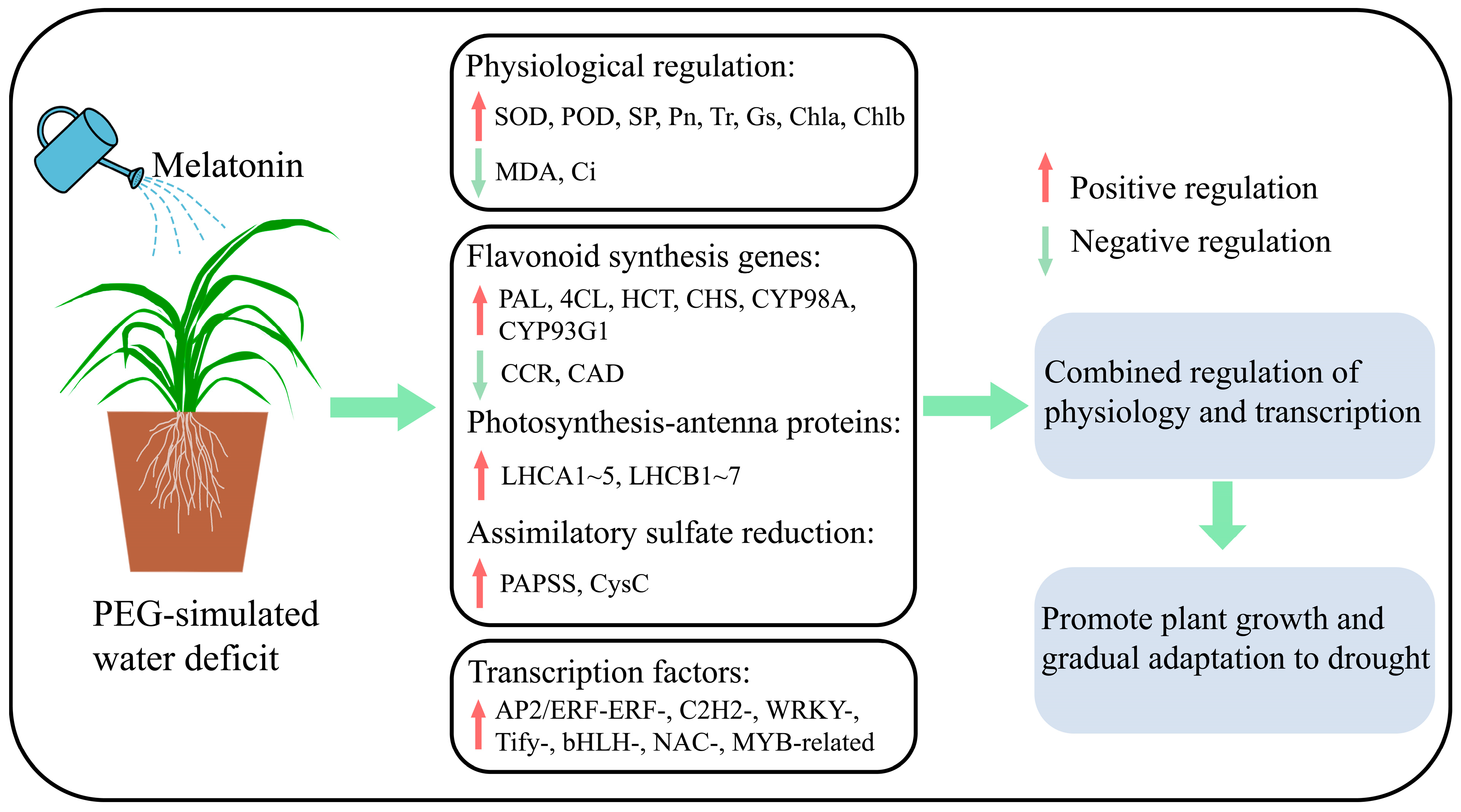
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, Y.; Fu, J.; Lu, Y.; Liu, B. Three-dimensional-based global drought projection under global warming tendency. Atmos. Res. 2023, 291, 106812. [Google Scholar] [CrossRef]
- Kulundžić, A.M.; Josipović, A.; Kočar, M.M.; Vuletić, M.V.; Dunić, J.A.; Varga, I.; Cesar, V.; Sudarić, A.; Lepeduš, H. Physiological insights on soybean response to drought. Agric. Water Manag. 2022, 268, 107620. [Google Scholar] [CrossRef]
- Pandey, K.; Kumar, R.S.; Prasad, P.; Pande, V.; Trivedi, P.K.; Shirke, P.A. Coordinated regulation of photosynthesis and sugar metabolism in guar increases tolerance to drought. Environ. Exp. Bot. 2022, 194, 104701. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, X.; Wang, Y.; Zhang, J. Response of three dominant forages to drought stress at different growth stages. Pratacultural Sci. 2021, 38, 2210–2220. [Google Scholar]
- Mehralian, M.; Bidabadi, S.S.; Azad, M.; Ebrahimi, S.N.; Mirjalili, M.H. Melatonin-mediated alleviation of drought stress by modulation of physio-biochemical and metabolic status in Dracocephalum kotschyi Boiss. (Lamiaceae). Ind. Crops Prod. 2023, 204, 117321. [Google Scholar] [CrossRef]
- Hayat, K.; Zhou, Y.; Menhas, S.; Bundschuh, J.; Hayat, S.; Ullah, A.; Wang, J.; Chen, X.; Zhang, D.; Zhou, P. Pennisetum giganteum: An emerging salt accumulating/tolerant non-conventional crop for sustainable saline agriculture and simultaneous phytoremediation. Environ. Pollut. 2020, 265, 114876. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Feng, Z.; Xu, W.; Vetukuri, R.R.; Xu, X. Exogenous melatonin-stimulated transcriptomic alterations of Davidia involucrata seedlings under drought stress. Trees 2021, 35, 1025–1038. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.K.; Zinta, G. Mechanistic insights on melatonin-mediated drought stress mitigation in plants. Physiol. Plant. 2021, 172, 1212–1226. [Google Scholar] [CrossRef]
- Wang, X.; Cao, M.; Li, H.; Liu, Y.; Fan, S.; Zhang, N.; Guo, Y.-D. Strategies and prospects for melatonin to alleviate abiotic stress in horticultural plants. Hortic. Plant J. 2023; in press. [Google Scholar] [CrossRef]
- Supriya, L.; Durgeshwar, P.; Muthamilarasan, M.; Padmaja, G. Melatonin Mediated Differential Regulation of Drought Tolerance in Sensitive and Tolerant Varieties of Upland Cotton (Gossypium hirsutum L.). Front. Plant Sci. 2022, 13, 821353. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Zhu, Y.; Zheng, W.; Li, M.; Hu, J.; Fei, Y.; Zhu, S. Exogenous application of melatonin to mitigate drought stress-induced oxidative damage in Phoebe sheareri seedlings. PeerJ 2023, 11, e15159. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zou, J.; Qin, B.; Bei, S.; Ma, W.; Yan, B.; Jin, X.; Zhang, Y. Response of exogenous melatonin on transcription and metabolism of soybean under drought stress. Physiol. Plant. 2023, 175, e14038. [Google Scholar] [CrossRef] [PubMed]
- Shreya, S.; Supriya, L.; Padmaja, G. Melatonin induces drought tolerance by modulating lipoxygenase expression, redox homeostasis and photosynthetic efficiency in Arachis hypogaea L. Front. Plant Sci. 2022, 13, 1069143. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, N.; Iqbal, S.; Hayat, F.; Raziq, A.; Ayaz, A.; Zaman, W. Melatonin in Micro-Tom Tomato: Improved Drought Tolerance via the Regulation of the Photosynthetic Apparatus, Membrane Stability, Osmoprotectants, and Root System. Life 2022, 12, 1922. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Lv, Y.; Bai, J.; Li, T.; Yang, X.; Liu, L.; Zhou, H. Comparative transcriptomics reveals new insights into melatonin-enhanced drought tolerance in naked oat seedlings. PeerJ 2022, 10, e13669. [Google Scholar] [CrossRef]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Chen, L.; Xie, Z.M.; Peng, X. Combined transcriptome and metabolome analysis revealed pathways involved in improved salt tolerance of Gossypium hirsutum L. seedlings in response to exogenous melatonin application. BMC Plant Biol. 2022, 22, 552. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Guo, H.; Wu, X.; Hao, M.; Zhang, R. Integrative transcriptome and metabolome analysis reveals the mechanism of exogenous melatonin alleviating drought stress in maize roots. Plant Physiol. Biochem. 2023, 199, 107723. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant 2016, 38, 48. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, W.; Yang, Y.; Xie, W.; Zhao, L. Comprehensive Evaluation of Drought Resistance of Two Different Pennisetum Species during Seed Germination and Seedling Growth Stages. Chin. J. Grassl. 2023, 45, 50–59. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Jiang, H.; Wei, C.; Ji, C. Effect of Replacing Leymus chinensis with Pennisetum on Growth Performance and Blood Indexes of Fattening Dezhou Donkeys. Feed Ind. 2023, 1–6. Available online: https://link.cnki.net/urlid/21.1169.S.20230921.1918.009 (accessed on 21 January 2024).
- Lee, D.; Nam, H.; Seo, M.W.; Lee, S.H.; Tokmurzin, D.; Wang, S.; Park, Y.-K. Recent progress in the catalytic thermochemical conversion process of biomass for biofuels. Chem. Eng. J. 2022, 447, 137501. [Google Scholar] [CrossRef]
- Cui, T.; Yu, H.; Li, H.; Bian, X.; Wang, L. Effect of drought stress and rewatering on physiological characteristics of Pennisetum alopecuroides seedlings. Pratacultural Sci. 2017, 34, 788–793. [Google Scholar]
- Imran, M.; Khan, A.L.; Shahzad, R.; Khan, M.A.; Bilal, S.; Khan, A.; Kang, S.-M.; Lee, I.-J. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. Aob. Plants 2021, 13, plab26. [Google Scholar] [CrossRef] [PubMed]
- Michelena, V.A.; Boyer, J.S. Complete turgor maintenance at low water potentials in the elongating region of maize leaves. Plant Physiol. 1982, 69, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, L.; Proe, M.F.; Cameron, A.D. Interactive effects of nitrogen and water availabilities on gas exchange and whole-plant carbon allocation in poplar. Tree Physiol. 1998, 18, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zheng, B. Melatonin Mediated Regulation of Drought Stress: Physiological and Molecular Aspects. Plants 2019, 8, 190. [Google Scholar] [CrossRef]
- Ahmad, R.; Alsahli, A.A.; Alansi, S.; Altaf, M.A. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic efficiency and antioxidant defense system of pea (Pisum sativum L.). Sci. Hortic. 2023, 322, 112431. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, M.; Wu, X.; Wang, Y.; Zhang, R. Physiological and transcriptomic analyses of the effects of exogenous melatonin on drought tolerance in maize (Zea mays L.). Plant Physiol. Biochem. 2021, 168, 128–142. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, X.; Gu, L.; Liu, P.; Zhao, B.; Zhang, J.; Ren, B. The effects of high temperature, drought, and their combined stresses on the photosynthesis and senescence of summer maize. Agric. Water Manag. 2023, 289, 108525. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Ahmad, R.; Manzoor, M.; Muhammad, H.M.D.; Altaf, M.A.; Shakoor, A. Exogenous Melatonin Spray Enhances Salinity Tolerance in Zizyphus Germplasm: A Brief Theory. Life 2023, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Karaca, P.; Cekic, F.Ö. Exogenous melatonin-stimulated defense responses in tomato plants treated with polyethylene glycol. Int. J. Veg. Sci. 2019, 25, 601–609. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Burgess, P.; Rossi, S.; Huang, B. Interactive effects of melatonin and cytokinin on alleviating drought-induced leaf senescence in creeping bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2018, 145, 1–11. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, M.; Cai, H.; Chen, J.; Lin, Y.; Wang, F.; Wang, L.; Zhang, X.; Liu, J. The activity comparison of six dietary flavonoids identifies that luteolin inhibits 3T3-L1 adipocyte differentiation through reducing ROS generation. J. Nutr. Biochem. 2023, 112, 109208. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Q.; Gao, T.; Wang, H.; Zhang, Z.; Liang, B.; Wei, Z.; Liu, C.; Ma, F. The mitigation effects of exogenous melatonin on replant disease in apple. J. Pineal Res. 2018, 65, e12523. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, Z.; Liu, X.; Wu, Q.; Chen, Q.; Liu, Q.; van Nocker, S.; Ma, F.; Li, C. Physiological and transcriptome analyses of the effects of exogenous dopamine on drought tolerance in apple. Plant Physiol. Biochem. 2020, 148, 260–272. [Google Scholar] [CrossRef]
- Khadka, K.; Raizada, M.N.; Navabi, A. Recent Progress in Germplasm Evaluation and Gene Mapping to Enable Breeding of Drought-Tolerant Wheat. Front. Plant Sci. 2020, 11, 1149. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Dong, B.; Niu, L.; Song, Z.; Wang, L.; Amin, R.; Cao, H.; Li, H.; Yang, Q.; Fu, Y. The pigeon pea CcCIPK14-CcCBL1 pair positively modulates drought tolerance by enhancing flavonoid biosynthesis. Plant J. 2021, 106, 1278–1297. [Google Scholar] [CrossRef] [PubMed]
- Genzel, F.; Dicke, M.D.; Junker-Frohn, L.V.; Neuwohner, A.; Thiele, B.; Putz, A.; Usadel, B.; Wormit, A.; Wiese-Klinkenberg, A. Impact of Moderate Cold and Salt Stress on the Accumulation of Antioxidant Flavonoids in the Leaves of Two Capsicum Cultivars. J. Agric. Food Chem. 2021, 69, 6431–6443. [Google Scholar] [CrossRef] [PubMed]
- Gill, U.S.; Uppalapati, S.R.; Gallego-Giraldo, L.; Ishiga, Y.; Dixon, R.A.; Mysore, K.S. Metabolic flux towards the (iso)flavonoid pathway in lignin modified alfalfa lines induces resistance against Fusarium oxysporum f. sp. medicaginis. Plant Cell Environ. 2018, 41, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Hu, T.; Huo, Y.; Wang, L.; Zhang, L.; Yan, R. Effects of Exogenous Melatonin on Chrysanthemum Physiological Characteristics and Photosynthesis under Drought Stress. Horticulturae 2023, 9, 106. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Q.; Shen, W.; El Mohtar, C.A.; Zhao, X.; Gmitter, F.G. Functional study of CHS gene family members in citrus revealed a novel CHS gene affecting the production of flavonoids. BMC Plant Biol. 2018, 18, 189. [Google Scholar] [CrossRef] [PubMed]
- Velez-Ramirez, A.I.; van Ieperen, W.; Vreugdenhil, D.; van Poppel, P.M.J.A.; Heuvelink, E.; Millenaar, F.F. A single locus confers tolerance to continuous light and allows substantial yield increase in tomato. Nat. Commun. 2014, 5, 4549. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Liu, F.; Luo, P.; Wang, Y.; Liu, D.; Wu, X.; Zhang, Z.; Wu, J. Transcriptomic and physiological analysis revealed the ammonium tolerance mechanisms of Myriophyllum aquaticum. Environ. Exp. Bot. 2021, 187, 104462. [Google Scholar] [CrossRef]
- Levin, G.; Schuster, G. LHC-like Proteins: The Guardians of Photosynthesis. Int. J. Mol. Sci. 2023, 24, 2503. [Google Scholar] [CrossRef]
- Huang, D.; Huo, J.; Liao, W. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2021, 302, 110733. [Google Scholar] [CrossRef]
- Zhou, L.; Ou, P.; Zhao, B.; Zhang, W.; Yu, K.; Xie, K.; Zhuang, W.-Q. Assimilatory and dissimilatory sulfate reduction in the bacterial diversity of biofoulant from a full-scale biofilm-membrane bioreactor for textile wastewater treatment. Sci. Total Environ. 2021, 772, 145464. [Google Scholar] [CrossRef] [PubMed]
- Saito, K. Regulation of sulfate transport and synthesis of sulfur-containing amino acids. Curr. Opin. Plant Biol. 2000, 3, 188–195. [Google Scholar] [CrossRef]
- Thakur, M.; Anand, A. Hydrogen sulfide: An emerging signaling molecule regulating drought stress response in plants. Physiol. Plant. 2021, 172, 1227–1243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mu, Y.; Zhang, L.; Liu, Z.; Liu, D.; Jin, Z.; Pei, Y. Hydrogen sulfide mediated the melatonin induced stoma closure by regulating the K+ channel in Arabidopsis thaliana. Environ. Exp. Bot. 2023, 205, 105125. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Zhang, J.; Wang, Y.; Yang, Y.; Chen, X.; Wang, Y. How does Malus crabapple resist ozone? Transcriptomics and metabolomics analyses. Ecotoxicol. Environ. Saf. 2020, 201, 110832. [Google Scholar] [CrossRef] [PubMed]
- Zainy, Z.; Fayyaz, M.; Yasmin, T.; Hyder, M.Z.; Haider, W.; Farrakh, S. Antioxidant enzymes activity and gene expression in wheat-stripe rust interaction at seedling stage. Physiol. Mol. Plant Pathol. 2023, 124, 101960. [Google Scholar] [CrossRef]
- Gu, T.; Wang, Y.; Cao, J.; Zhang, Z.; Li, G.; Shen, W.; Lou, Y.; Wang, H. Hydrogen-Rich Water Pretreatment Alleviates the Phytotoxicity of Bispyribac-Sodium to Rice by Increasing the Activity of Antioxidant Enzymes and Enhancing Herbicide Degradation. Agronomy 2022, 12, 2821. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Zhang, Z. Experimental Guide to Plant Physiology; China Agricultural Science and Technology Press: Beijing, China, 2004; pp. 179–195. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Yun, M.; Ma, Y.; Qu, T. Melatonin Mitigates Water Deficit Stress in Cenchrus alopecuroides (L.) Thunb through Up-Regulating Gene Expression Related to the Photosynthetic Rate, Flavonoid Synthesis, and the Assimilatory Sulfate Reduction Pathway. Plants 2024, 13, 716. https://doi.org/10.3390/plants13050716
Jiang L, Yun M, Ma Y, Qu T. Melatonin Mitigates Water Deficit Stress in Cenchrus alopecuroides (L.) Thunb through Up-Regulating Gene Expression Related to the Photosynthetic Rate, Flavonoid Synthesis, and the Assimilatory Sulfate Reduction Pathway. Plants. 2024; 13(5):716. https://doi.org/10.3390/plants13050716
Chicago/Turabian StyleJiang, Li, Minqiang Yun, Yinxi Ma, and Tongbao Qu. 2024. "Melatonin Mitigates Water Deficit Stress in Cenchrus alopecuroides (L.) Thunb through Up-Regulating Gene Expression Related to the Photosynthetic Rate, Flavonoid Synthesis, and the Assimilatory Sulfate Reduction Pathway" Plants 13, no. 5: 716. https://doi.org/10.3390/plants13050716





