In Situ Accumulation of CaOx Crystals in C. quitensis Leaves and Its Relationship with Anatomy and Gas Exchange
Abstract
:1. Introduction
2. Results
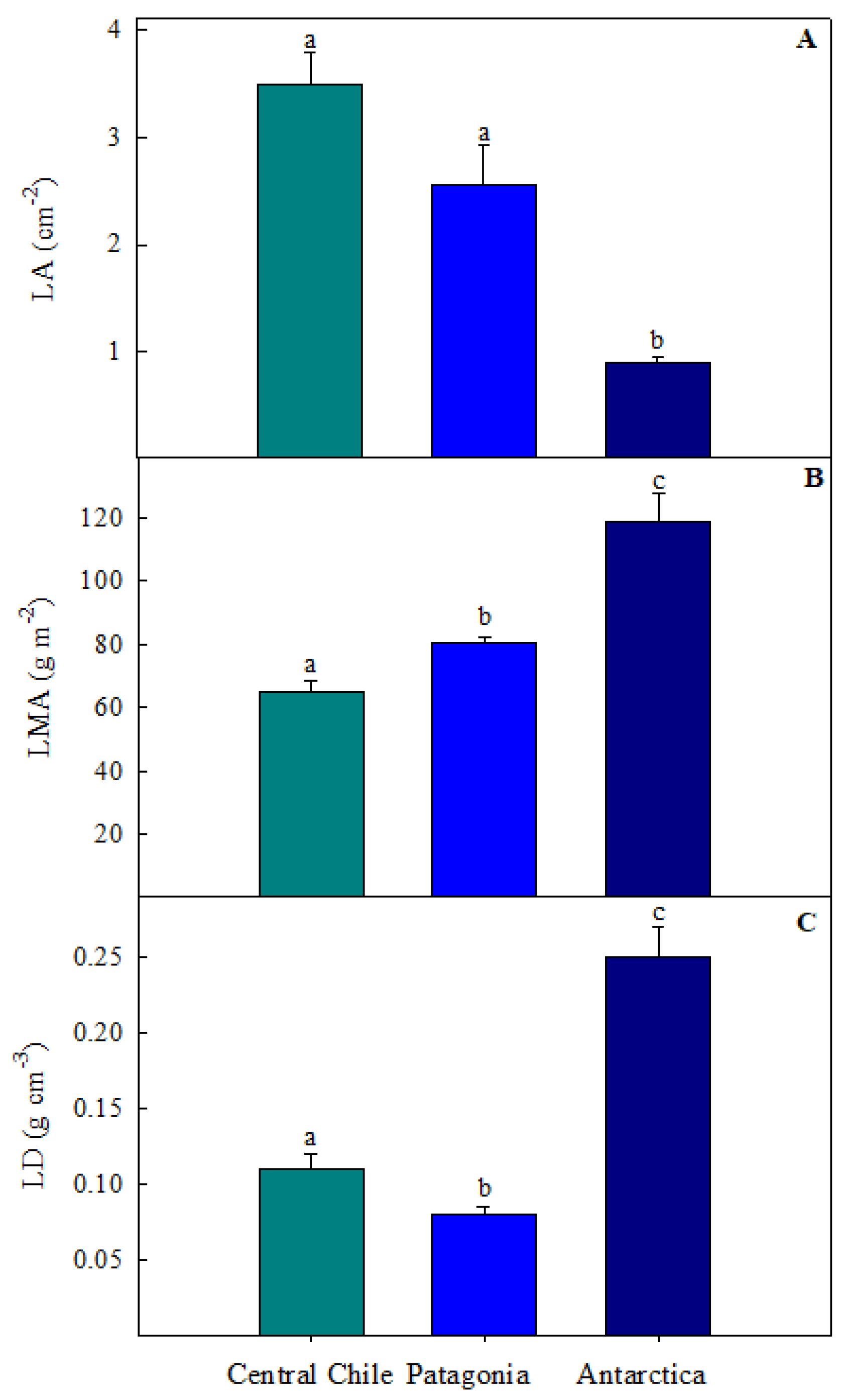
2.1. Morphological Leaf Traits
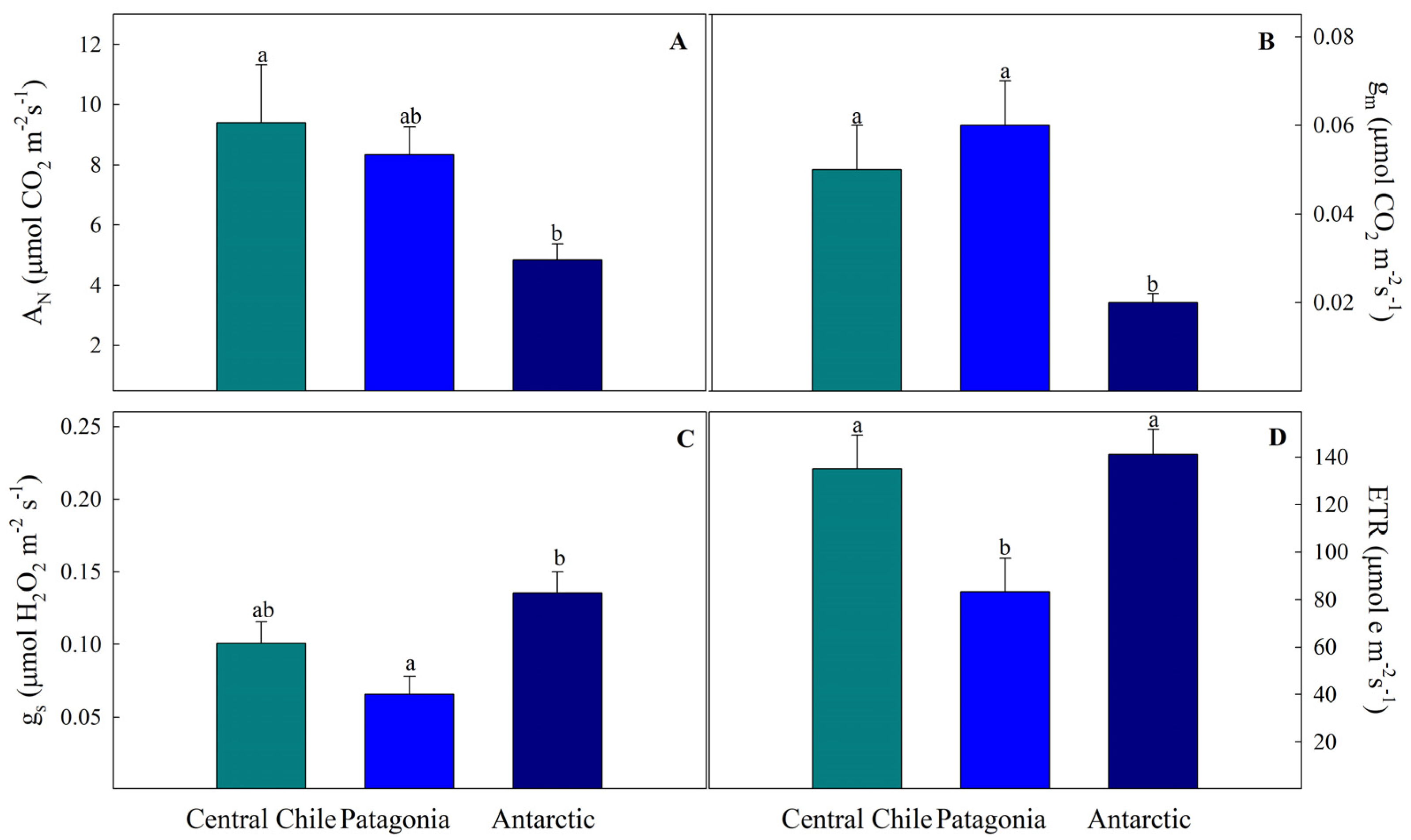
2.2. Leaf Gas Exchange
2.3. Leaf CaOx Crystal
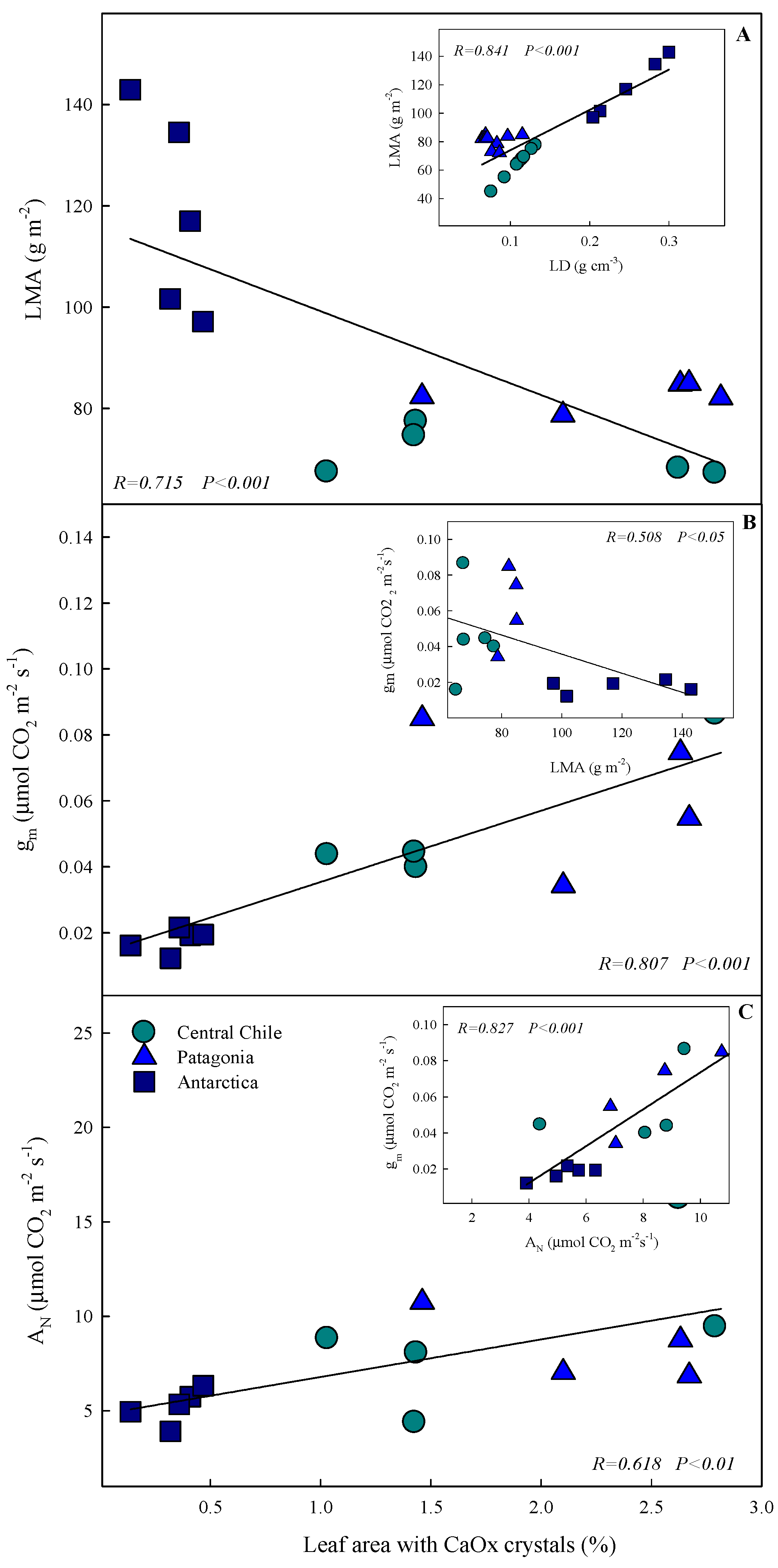
2.4. Relationship between CaOx Crystal and the Anatomical and Photosynthetic Traits
3. Discussion
4. Materials and Methods
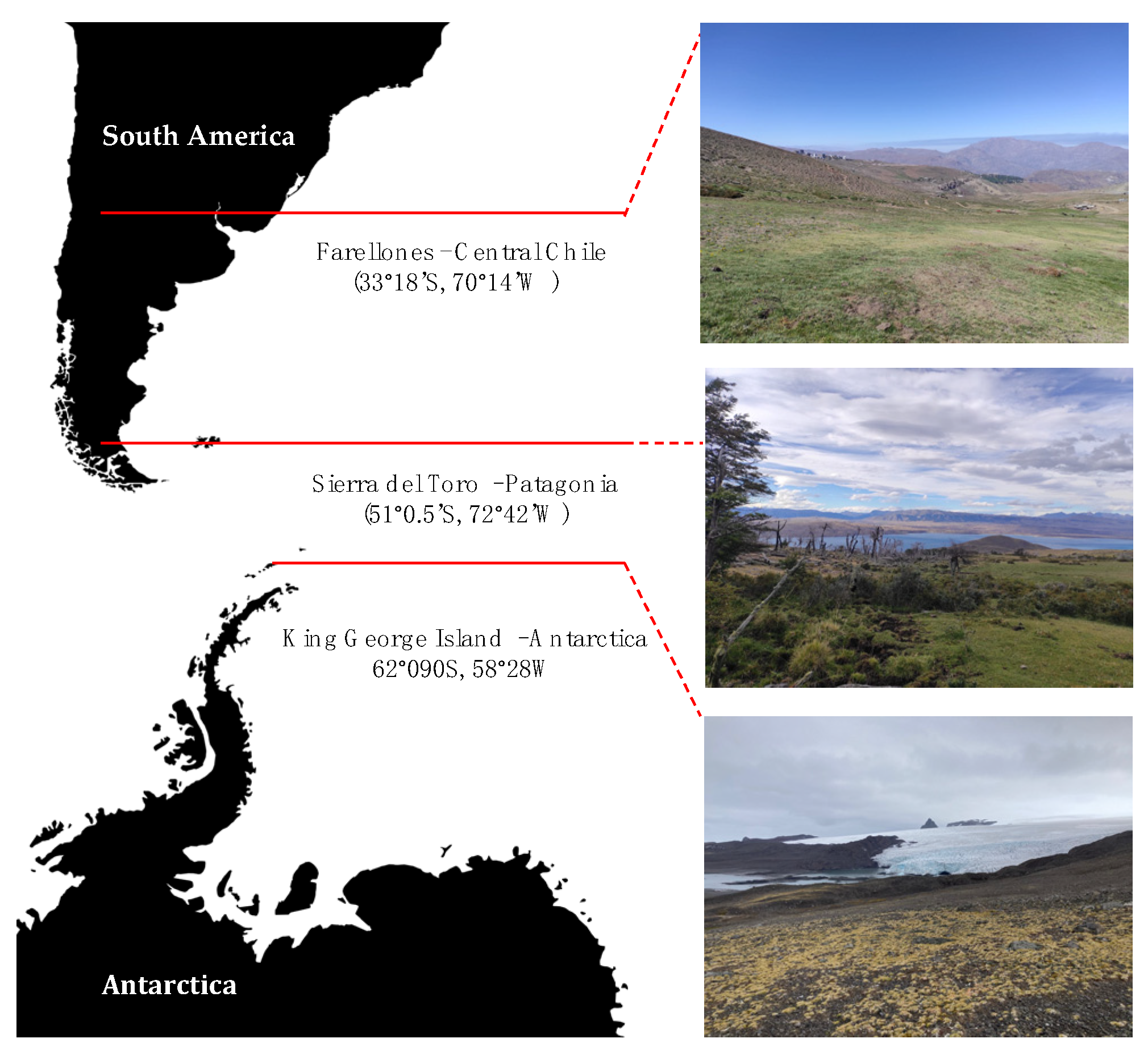
4.1. Study Sites and Plant Collection
4.2. Leaf Mass Area and Leaf Density
4.3. Leaf Gas Exchange and Chl Fluorescence
4.4. CaOx Crystal Measurements in the Leaves
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Frenne, P.; Graae, B.J.; Rodríguez-Sánchez, F.; Kolb, A.; Chabrerie, O.; Decocq, G.; De Kort, H.; De Schrijver, A.; Diekmann, M.; Eriksson, O.; et al. Latitudinal Gradients as Natural Laboratories to Infer Species’ Responses to Temperature. J. Ecol. 2013, 101, 784–795. [Google Scholar] [CrossRef]
- IPCC. Global Warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Pörtner, H.-O., Skea, J., Zhai, P., Roberts, D., Shukla, P.R., Pirani, A., Pidcock, R., Chen, Y., Lonnoy, E., et al., Eds.; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Koc, J.; Androsiuk, P.; Chwedorzewska, K.J.; Cuba-Díaz, M.; Górecki, R.; Giełwanowska, I. Range-Wide Pattern of Genetic Variation in Colobanthus quitensis. Polar Biol. 2018, 41, 2467–2479. [Google Scholar] [CrossRef]
- Gianoli, E.; Inostroza, P.; Zúñiga-Feest, A.; Reyes-Díaz, M.; Cavieres, L.A.; Bravo, L.A.; Corcuera, L.J. Ecotypic Differentiation in Morphology and Cold Resistance in Populations of Colobanthus quitensis (Caryophyllaceae) from the Andes of Central Chile and the Maritime Antarctic. Arct. Antarct. Alp. Res. 2004, 36, 484–489. [Google Scholar] [CrossRef]
- Acuña-Rodríguez, I.S.; Torres-Díaz, C.; Hereme, R.; Molina-Montenegro, M.A. Asymmetric Responses to Simulated Global Warming by Populations of Colobanthus quitensis along a Latitudinal Gradient. PeerJ 2017, 5, e3718. [Google Scholar] [CrossRef]
- Cuba-Díaz, M.; Klagges, M.; Fuentes-Lillo, E.; Cordero, C.; Acuña, D.; Opazo, G.; Troncoso-Castro, J.M. Phenotypic Variability and Genetic Differentiation in Continental and Island Populations of Colobanthus quitensis (Caryophyllaceae: Antarctic Pearlwort). Polar Biol. 2017, 41, 267–268. [Google Scholar] [CrossRef]
- Biersma, E.M.; Torres-Díaz, C.; Molina-Montenegro, M.A.; Newsham, K.K.; Vidal, M.A.; Collado, G.A.; Acuña-Rodríguez, I.S.; Ballesteros, G.I.; Figueroa, C.C.; Goodall-Copestake, W.P.; et al. Multiple Late-Pleistocene Colonisation Events of the Antarctic Pearlwort Colobanthus quitensis (Caryophyllaceae) Reveal the Recent Arrival of Native Antarctic Vascular Flora. J. Biogeogr. 2020, 47, 1663–1673. [Google Scholar] [CrossRef]
- Ramírez, C.F.; Cavieres, L.A.; Sanhueza, C.; Vallejos, V.; Gómez-Espinoza, O.; Bravo, L.A.; Sáez, P.L. Ecophysiology of Antarctic Vascular Plants: An Update on the Extreme Environment Resistance Mechanisms and Their Importance in Facing Climate Change. Plants 2024, 13, 449. [Google Scholar] [CrossRef]
- Gómez-Espinoza, O.; González-Ramírez, D.; Bresta, P.; Karabourniotis, G.; Bravo, L.A. Decomposition of Calcium Oxalate Crystals in Colobanthus quitensis under CO2 Limiting Conditions. Plants 2020, 9, 1307. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Horner, H.T.; Bresta, P.; Nikolopoulos, D.; Liakopoulos, G. New Insights into the Functions of Carbon–Calcium Inclusions in Plants. New Phytol. 2020, 228, 845–854. [Google Scholar] [CrossRef]
- Tooulakou, G.; Giannopoulos, A.; Nikolopoulos, D.; Bresta, P.; Dotsika, E.; Orkoula, M.G.; Kontoyannis, C.G.; Fasseas, C.; Liakopoulos, G.; Klapa, M.I.; et al. Alarm Photosynthesis: Calcium Oxalate Crystals as an Internal CO2 Source in Plants. Plant Physiol. 2016, 171, 2577–2585. [Google Scholar] [CrossRef]
- Sáez, P.L.; Galmés, J.; Ramírez, C.F.; Poblete, L.; Rivera, B.K.; Cavieres, L.A.; Clemente-Moreno, M.J.; Flexas, J.; Bravo, L.A. Mesophyll Conductance to CO2 Is the Most Significant Limitation to Photosynthesis at Different Temperatures and Water Availabilities in Antarctic Vascular Species. Environ. Exp. Bot. 2018, 156, 279–287. [Google Scholar] [CrossRef]
- Sáez, P.L.; Bravo, L.A.; Cavieres, L.A.; Vallejos, V.; Sanhueza, C.; Font-Carrascosa, M.; Gil-Pelegrín, E.; Javier Peguero-Pina, J.; Galmés, J. Photosynthetic Limitations in Two Antarctic Vascular Plants: Importance of Leaf Anatomical Traits and Rubisco Kinetic Parameters. J. Exp. Bot. 2017, 68, 2871–2883. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Espinoza, O.; González-Ramírez, D.; Méndez-Gómez, J.; Guillén-Watson, R.; Medaglia-Mata, A.; Bravo, L. Calcium Oxalate Crystals in Leaves of the Extremophile Plant Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae). Plants 2021, 10, 1787. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Forn, D.; Sancho-Knapik, D.; Ferrio, J.P.; Peguero-Pina, J.J.; Bueno, A.; Onoda, Y.; Cavender-Bares, J.; Niinemets, Ü.; Jansen, S.; Riederer, M.; et al. Revisiting the Functional Basis of Sclerophylly Within the Leaf Economics Spectrum of Oaks: Different Roads to Rome. Curr. For. Rep. 2020, 6, 260–281. [Google Scholar] [CrossRef]
- Onoda, Y.; Westoby, M.; Adler, P.B.; Choong, A.M.F.; Clissold, F.J.; Cornelissen, J.H.C.; Díaz, S.; Dominy, N.J.; Elgart, A.; Enrico, L.; et al. Global Patterns of Leaf Mechanical Properties. Ecol. Lett. 2011, 14, 301–312. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Wright, I.J.; Evans, J.R. Leaf Mesophyll Diffusion Conductance in 35 Australian Sclerophylls Covering a Broad Range of Foliage Structural and Physiological Variation. J. Exp. Bot. 2009, 60, 2433–2449. [Google Scholar] [CrossRef]
- Niinemets, Ü. Does the Touch of Cold Make Evergreen Leaves Tougher? Tree Physiol. 2016, 36, 267–272. [Google Scholar] [CrossRef]
- Li, B.; Suzuki, J.I.; Hara, T. Latitudinal Variation in Plant Size and Relative Growth Rate in Arabidopsis thaliana. Oecologia 1998, 115, 293–301. [Google Scholar] [CrossRef]
- Luo, Y.; Hu, H.; Zhao, M.; Li, H.; Liu, S.; Fang, J. Latitudinal Pattern and the Driving Factors of Leaf Functional Traits in 185 Shrub Species across Eastern China. J. Plant Ecol. 2019, 12, 67–77. [Google Scholar] [CrossRef]
- Smith, R.I. The Enigma of Colobanthus quitensis and Deschampsia antarctica in Antarctica. In Antarctic Biology in a Global Context; Huiskes, A., Gieskes, W., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2003; pp. 234–239. [Google Scholar]
- Elferjani, R.; DesRochers, A.; Tremblay, F. Plasticity of Bud Phenology and Photosynthetic Capacity in Hybrid Poplar Plantations along a Latitudinal Gradient in Northeastern Canada. Environ. Exp. Bot. 2016, 125, 67–76. [Google Scholar] [CrossRef]
- Figueroa, J.A.; Cabrera, H.M.; Queirolo, C.; Hinojosa, L.F. Variability of Water Relations and Photosynthesis in Eucryphiacordifolia Cav. (Cunoniaceae) over the Range of Its Latitudinal and Altitudinal Distribution in Chile. Tree Physiol. 2010, 30, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, G.; Cui, Q.; Huang, Z.; Ye, X.; Cornelissen, J.H.C. New Field Wind Manipulation Methodology Reveals Adaptive Responses of Steppe Plants to Increased and Reduced Wind Speed. Plant Methods 2021, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.J.; Retkute, R.; Preston, S.P.; Jensen, O.E.; Pound, M.P.; Pridmore, T.P.; Murchie, E.H. The 4-Dimensional Plant: Effects of Wind-Induced Canopy Movement on Light Fluctuations and Photosynthesis. Front. Plant Sci. 2016, 7, 217696. [Google Scholar] [CrossRef] [PubMed]
- Di Castri, F.; Hajek, E. Bioclimatología de Chile; Universidad Católica de Chile: Santiago, Chile, 1976. [Google Scholar]
- Gajardo Michell, R. La Vegetación Natural de Chile. Clasificación y Distribución Geográfica; Editorial Universitaria: Santiago, Chile, 1994. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- InfoStat; Version 2008; Manual Del Usuario; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina; Editorial Brujas: Córdoba, Argentina, 2008.

| El Colorado Station (Lo Barnechea)—Central Chile | Río Serrano Station (Torres del Paine)—Patagonia | C.M.A. Eduardo Frei Montalva Station—King George Island | |
|---|---|---|---|
| Average temperature (°C) | 10.5 | 8 | 0 |
| Max temperature (°C) | 25 | 20 | 2 |
| Min temperature (°C) | 5 | 3 | −4 |
| Total Precipitation (mm) | 0.2 | 5 | 45 |
| Solar radiation kwh/m² | 13,180.6 | 7467.5 | - |
| UV Index | 4 | - | 4 |
| Light Hours | 13.5 | 14.6 | 18.5 |
| Average wind speed (m/s) | 5.1 | 6.4 | 10.2 |
| Average relative humidity (%) | 51.5 | 75 | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Espinoza, O.; Fuentes, F.I.; Ramírez, C.F.; Bravo, L.A.; Sáez, P.L. In Situ Accumulation of CaOx Crystals in C. quitensis Leaves and Its Relationship with Anatomy and Gas Exchange. Plants 2024, 13, 769. https://doi.org/10.3390/plants13060769
Gómez-Espinoza O, Fuentes FI, Ramírez CF, Bravo LA, Sáez PL. In Situ Accumulation of CaOx Crystals in C. quitensis Leaves and Its Relationship with Anatomy and Gas Exchange. Plants. 2024; 13(6):769. https://doi.org/10.3390/plants13060769
Chicago/Turabian StyleGómez-Espinoza, Olman, Francisca I. Fuentes, Constanza F. Ramírez, León A. Bravo, and Patricia L. Sáez. 2024. "In Situ Accumulation of CaOx Crystals in C. quitensis Leaves and Its Relationship with Anatomy and Gas Exchange" Plants 13, no. 6: 769. https://doi.org/10.3390/plants13060769








