New Epigenetic Modifier Inhibitors Enhance Microspore Embryogenesis in Bread Wheat
Abstract
:1. Introduction
2. Results
2.1. Selection of Candidate Epigenetic Modifiers
2.2. Screening of Different Epigenetic Modifiers to Induce ME in Wheat
2.3. Effect of Aurora Kinase Inhibitor II on the Efficiency of Green DH Plant Production
2.3.1. Application of Aurora Kinase Inhibitor II during a 24 h Stress Treatment
2.3.2. Application of Aurora Kinase Inhibitor II during a 5-Day Stress Treatment
3. Discussion
3.1. Effect of Different Epigenetic Modifiers on the Early Stages of Wheat ME Induction
3.2. Effect of Aurora Kinase Inhibitor II on the Efficiency of Green DH Plant Production
4. Materials and Methods
4.1. Growing Conditions of Donor Plants and Harvesting of Spikes
4.2. Anther Stress Pretreatment
4.3. Preparation of Ovary Pre-Conditioned Medium
4.4. Anther Culture
4.5. Screening of Different Compounds to Induce ME in Wheat
4.6. Effect of Aurora Kinase Inhibitor II on the Efficiency of Green Plant Production
4.6.1. Application of Aurora Kinase Inhibitor II during a 24-h Stress Treatment
4.6.2. Application of Aurora Kinase Inhibitor II during a 5-Day Stress Treatment
4.7. Stereoscopic and Microscopic Observation
4.8. Ploidy Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 0.4AUKI-II | 0.4 µM AUKI-II treatment in SM solid medium for 5 days |
| 0.8AUKI-II | 0.8 µM AUKI-II treatment in SM solid medium for 5 days |
| 1.2AUKI-II | 1.2 µM AUKI-II treatment in SM solid medium for 5 days |
| ANOVA | analysis of variance |
| AUKI-II | aurora kinase inhibitor II |
| CM | control treatment in SM solid medium for 5 days |
| CM + DMSO | control DMSO in SM solid medium for 5 days |
| DH | doubled haploid |
| EMB | number of embryos/100 anthers |
| GP | number of green plants/100 anthers |
| GPDH | number of green DH plants/100 anthers |
| L-CM | control treatment in SM liquid medium for 24 h |
| L-CM + DMSO | control treatment in SM liquid medium with DMSO for 24 h |
| L-0.4AUKI-II | 0.4 µM AUKI-II treatment in SM liquid medium for 24 h |
| L-0.8AUKI-II | 0.8 µM AUKI-II treatment in SM liquid medium for 24 h |
| L-0.4CARMIN1I | 0.4 µM CARMIN1 inhibitor treatment in SM liquid medium for 24 h |
| L-0.4Chaetocin | 0.4 µM chaetocin treatment in SM liquid medium for 24 h |
| L-0.4Hesperadin | 0.4 µM hesperadin treatment in SM liquid medium for 24 h |
| L-0.4TSA | 0.4 µM TSA treatment in SM liquid medium for 24 h |
| ME | microspore embryogenesis |
| PDH | percentage of DH plants/100 plants |
| PEMB | number of pro-embryos/100 anthers |
| PGP | number of green plants/100 total plants |
| TSA | trichostatin A |
References
- Tadesse, W.; Sanchez-Garcia, M.; Tawkaz, S.; Baum, M. Doubled haploid production in wheat. In Advances in Crop Breeding Techniques for Cereal Crops; Burleigh Dodds Science Publishing: Cambridge, UK, 2019. [Google Scholar]
- Shen, K.; Qu, M.; Zhao, P. The Roads to Haploid Embryogenesis. Plants 2023, 12, 243. [Google Scholar] [CrossRef]
- Soriano, M.; Li, H.; Boutilier, K. Microspore embryogenesis: Establishment of embryo identity and pattern in culture. Plant Reprod. 2013, 26, 181–196. [Google Scholar] [CrossRef]
- Lantos, C.; Weyen, J.; Orsini, J.M.; Gnad, H.; Schlieter, B.; Lein, V.; Kontowski, S.; Jacobi, A.; MihÁly, R.; Broughton, S.; et al. Efficient application of in vitro anther culture for different European winter wheat (Triticum aestivum L.) breeding programmes. Plant Breed. 2013, 132, 149–154. [Google Scholar] [CrossRef]
- Weigt, D.; Kiel, A.; Siatkowski, I.; Zyprych-Walczak, J.; Tomkowiak, A.; Kwiatek, M. Comparison of the androgenic response of spring and winter wheat (Triticum aestivum L.). Plants 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Devaux, P.; Cistué Sola, L. Wheat doubled haploids: Production to sequencing. What makes them so appealing? In The World Wheat Book; Lavoisier: Paris, France, 2016; Volume 3, pp. 885–919, Part 2, Breeding Strategies. [Google Scholar]
- Muñoz-Amatriaín, M.; Svensson, J.T.; Castillo, A.M.; Cistué, L.; Close, T.J.; Vallés, M.P. Transcriptome analysis of barley anthers: Effect of mannitol treatment on microspore embryogenesis. Physiol. Plant. 2006, 127, 551–560. [Google Scholar] [CrossRef]
- Lee, K.; Seo, P.J. Dynamic epigenetic changes during plant regeneration. Trends Plant Sci. 2018, 23, 235–247. [Google Scholar] [CrossRef]
- Testillano, P.S. Microspore embryogenesis: Targeting the determinant factors of stress-induced cell reprogramming for crop improvement. J. Exp. Bot. 2019, 70, 2965–2978. [Google Scholar] [CrossRef]
- Li, H.; Soriano, M.; Cordewener, J.; Muiño, J.M.; Riksen, T.; Fukuoka, H.; Angenent, G.C.; Boutilier, K. The histone deacetylase inhibitor trichostatin a promotes totipotency in the male gametophyte. Plant Cell 2014, 26, 195–209. [Google Scholar] [CrossRef]
- Solís, M.T.; El-Tantawy, A.A.; Cano, V.; Risueño, M.C.; Testillano, P.S. 5-azacytidine promotes microspore embryogenesis initiation by decreasing global DNA methylation, but prevents subsequent embryo development in rapeseed and barley. Front. Plant Sci. 2015, 6, 472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Gao, Y.; Jiang, X.; Zhang, M.; Wu, H.; Liu, Z.; Feng, H. Effects of histone deacetylase inhibitors on microspore embryogenesis and plant regeneration in Pakchoi (Brassica rapa ssp. chinensis L.). Sci. Hortic. 2016, 209, 61–66. [Google Scholar] [CrossRef]
- Berenguer, E.; Bárány, I.; Solís, M.T.; Pérez-Pérez, Y.; Risueño, M.C.; Testillano, P.S. Inhibition of histone H3K9 methylation by BIX-01294 promotes stress-induced microspore totipotency and enhances embryogenesis initiation. Front. Plant Sci. 2017, 8, 1161. [Google Scholar] [CrossRef]
- Pandey, P.; Daghma, D.S.; Houben, A.; Kumlehn, J.; Melzer, M.; Rutten, T. Dynamics of post-translationally modified histones during barley pollen embryogenesis in the presence or absence of the epi-drug trichostatin A. Plant Reprod. 2017, 30, 95–105. [Google Scholar] [CrossRef]
- Nowicka, A.; Juzoń, K.; Krzewska, M.; Dziurka, M.; Dubas, E.; Kopeć, P.; Zieliński, K.; Żur, I. Chemically-induced DNA de-methylation alters the effectiveness of microspore embryogenesis in triticale. Plant Sci. 2019, 287, 110189. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, P.; Laurie, J.D. Triticale Isolated Microspore Culture for Doubled Haploid Production. In Doubled Haploid Technology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 1, pp. 295–312, General Topics, Alliaceae, Cereals. [Google Scholar]
- Liu, C.; Song, G.; Zhao, Y.; Fang, B.; Liu, Z.; Ren, J.; Feng, H. Trichostatin A induced microspore embryogenesis and promoted plantlet regeneration in ornamental kale (Brassica oleracea var. acephala). Horticulturae 2022, 8, 790. [Google Scholar] [CrossRef]
- Sai, C.B.; Chidambaranathan, P.; Samantaray, S. Role of histone deacetylase inhibitors in androgenic callus induction of Oryza sativa sub indica, in sight into evolution and mode of action of histone deacetylase genes. Mol. Biol. Rep. 2022, 49, 2169–2183. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Ryabova, D.; Diedhiou, J.; Hucl, P.; Randhawa, H.; Marillia, E.-F.; Foroud, N.A.; Eudes, F.; Kathiria, P. Trichostatin A increases embryo and green plant regeneration in wheat. Plant Cell Rep. 2017, 36, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Enns, J.L.; Nelson, K.L.; Brost, J.M.; Orr, T.D.; Ferrie, A.M.R. Improving the efficiency of wheat microspore culture methodology: Evaluation of pretreatments, gradients, and epigenetic chemicals. PCTOC 2019, 139, 589–599. [Google Scholar] [CrossRef]
- Castillo, A.M.; Valero-Rubira, I.; Burrell, M.Á.; Allué, S.; Costar, M.A.; Vallés, M.P. Trichostatin A affects developmental reprogramming of bread wheat microspores towards an embryogenic route. Plants 2020, 9, 1442. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, S. Degrees make all the difference: The multifunctionality of histone H4 lysine 20 methylation. Epigenetics 2009, 4, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, Y.; Liang, Y.; Zhou, D.; Li, S.; Lin, S.; Dong, H.; Huang, L. The function of histone lysine methylation related SET domain group proteins in plants. Protein Sci. 2020, 29, 1120–1137. [Google Scholar] [CrossRef]
- Suresh, S.; Huard, S.; Dubois, T. CARM1/PRMT4: Making its mark beyond its function as a transcriptional coactivator. Trends Cell Biol. 2021, 31, 402–417. [Google Scholar] [CrossRef]
- Greiner, D.; Bonaldi, T.; Eskeland, R.; Roemer, E.; Imhof, A. Identification of a specific inhibitor of the histone methyltransferase SU (VAR) 3-9. Nat. Chem. Biol. 2005, 1, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Weimer, A.K.; Demidov, D.; Lermontova, I.; Beeckman, T.; Van Damme, D. Aurora kinases throughout plant development. Trends Plant Sci. 2016, 21, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Demidov, D.; Van Damme, D.; Geelen, D.; Blattner, F.R.; Houben, A. Identification and dynamics of two classes of aurora-like kinases in Arabidopsis and other plants. Plant Cell 2005, 17, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, D.; De Rybel, B.; Gudesblat, G.; Demidov, D.; Grunewald, W.; De Smet, I.; Houben, A.; Beeckman, T.; Russinova, E. Arabidopsis α Aurora kinases function in formative cell division plane orientation. Plant Cell 2011, 23, 4013–4024. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, D.; Matsunaga, S.; Kawabe, A.; Fujimoto, S.; Noda, M.; Uchiyama, S.; Fukui, K. Aurora kinase is required for chromosome segregation in tobacco BY-2 cells. Plant J. 2006, 48, 572–580. [Google Scholar] [CrossRef]
- Kurihara, D.; Matsunaga, S.; Uchiyama, S.; Fukui, K. Live cell imaging reveals plant aurora kinase has dual roles during mitosis. Plant Cell Physiol. 2008, 49, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Echávarri, B.; Cistué, L. Enhancement in androgenesis efficiency in barley (Hordeum vulgare L.) and bread wheat (Triticum aestivum L.) by the addition of dimethyl sulfoxide to the mannitol pretreatment medium. Plant Cell Tissue Organ Cult. 2016, 125, 11–22. [Google Scholar] [CrossRef]
- Valero-Rubira, I.; Castillo, A.M.; Burrell, M.Á.; Valles, M.P. Microspore embryogenesis induction by mannitol and TSA results in a complex regulation of epigenetic dynamics and gene expression in bread wheat. Front. Plant Sci. 2023, 13, 1058421. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Özsoy, N.; Gürle, S.D.; Yaman, B.; Ari, Ş. Chaetocin enhances callus induction by decreasing the expression of major leaf polarity genes in Nicotiana tabacum. Turk. J. Bot. 2021, 45, 412–420. [Google Scholar] [CrossRef]
- Shishkova, E.; Zeng, H.; Liu, F.; Kwiecien, N.W.; Hebert, A.S.; Coon, J.J.; Xu, W. Global mapping of CARM1 substrates defines enzyme specificity and substrate recognition. Nat. Commun. 2017, 8, 15571. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Cao, X. Plant PRMTs broaden the scope of arginine methylation. J. Genet. Genom. 2012, 39, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Hernando, C.E.; Sanchez, S.E.; Mancini, E.; Yanovsky, M.J. Genome wide comparative analysis of the effects of PRMT5 and PRMT4/CARM1 arginine methyltransferases on the Arabidopsis thaliana transcriptome. BMC Genom. 2015, 16, 192. [Google Scholar] [CrossRef]
- Boruc, J.; Weimer, A.K.; Stoppin-Mellet, V.; Mylle, E.; Kosetsu, K.; Cedeño, C.; Jaquinod, M.; Njo, M.; De Milde, L.; Tompa, P.; et al. Phosphorylation of MAP65-1 by Arabidopsis Aurora kinases is required for efficient cell cycle progression. Plant Physiol. 2017, 173, 582–599. [Google Scholar] [CrossRef]
- Takagi, M.; Sakamoto, T.; Suzuki, R.; Nemoto, K.; Obayashi, T.; Hirakawa, T.; Matsunaga, T.M.; Kurihara, D.; Nariai, Y.; Urano, T.; et al. Plant Aurora kinases interact with and phosphorylate transcription factors. J. Plant Res. 2016, 129, 1165–1178. [Google Scholar] [CrossRef]
- Komaki, S.; Takeuchi, H.; Hamamura, Y.; Heese, M.; Hashimoto, T.; Schnittger, A. Functional analysis of the plant chromosomal passenger complex. Plant Physiol. 2020, 183, 1586–1599. [Google Scholar] [CrossRef]
- Demidov, D.; Lermontova, I.; Weiss, O.; Fuchs, J.; Rutten, T.; Kumke, K.; Sharbel, T.F.; Van Damme, D.; De Storme, N.; Geelen, D.; et al. Altered expression of Aurora kinases in Arabidopsis results in aneu-and polyploidization. Plant J. 2014, 80, 449–461. [Google Scholar] [CrossRef]
- Lee, K.H.; Utku, A.; Qi, L.; Wang, H. The α-Aurora kinases function in vascular development in Arabidopsis. Plant Cell Physiol. 2019, 60, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Boruc, J.; Deng, X.; Mylle, E.; Besbrugge, N.; Van Durme, M.; Demidov, D.; Tomaštíková, E.D.; Tan, T.-R.C.; Vandorpe, M.; Eeckhout, D.; et al. TPX2-LIKE PROTEIN3 is the primary activator of α-aurora kinases and is essential for embryogenesis. Plant Physiol. 2019, 180, 1389–1405. [Google Scholar] [CrossRef]
- Corral-Martínez, P.; Siemons, C.; Horstman, A.; Angenent, G.C.; de Ruijter, N.; Boutilier, K. Live Imaging of embryogenic structures in Brassica napus microspore embryo cultures highlights the developmental plasticity of induced totipotent cells. Plant Reprod. 2020, 33, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.M.; Cistué, L.; Vallés, M.P.; Soriano, M. Chromosome doubling in monocots. In Advances in Haploid Production in Higher Plant; Springer: Berlin/Heidelberg, Germany, 2009; pp. 329–338. [Google Scholar]
- Jakše, M.; Havey, M.J.; Bohanec, B. Chromosome doubling procedures of onion (Allium cepa L.) gynogenic embryos. Plant Cell Rep. 2003, 21, 905–910. [Google Scholar] [CrossRef]
- Hooghvorst, I.; Ribas, P.; Nogués, S. Chromosome doubling of androgenic haploid plantlets of rice (Oryza sativa) using antimitotic compounds. Plant Breed. 2020, 139, 754–761. [Google Scholar] [CrossRef]
- Soriano, M.; Cistué, L.; Vallés, M.P.; Castillo, A.M. Effects of colchicine on anther and microspore culture of bread wheat (Triticum aestivum L.). PCTOC 2007, 91, 225–234. [Google Scholar] [CrossRef]
- Soriano, M.; Cistué, L.; Castillo, A.M. Enhanced induction of microspore embryogenesis after n-butanol treatment in wheat (Triticum aestivum L.) anther culture. Plant Cell Rep. 2008, 27, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Dubas, E.; Castillo, A.M.; Żur, I.; Krzewska, M.; Vallés, M.P. Microtubule organization changes severely after mannitol and n-butanol treatments inducing microspore embryogenesis in bread wheat. BMC Plant Biol. 2021, 21, 586. [Google Scholar] [CrossRef]
- Petrovská, B.; Jeřábková, H.; Kohoutová, L.; Cenklová, V.; Pochylová, Ž.; Gelová, Z.; Kočárová, G.; Váchová, L.; Kurejová, M.; Tomaštíková, E.; et al. Overexpressed TPX2 causes ectopic formation of microtubular arrays in the nuclei of acentrosomal plant cells. J. Exp. Bot. 2013, 64, 4575–4587. [Google Scholar] [CrossRef] [PubMed]
- Panteris, E.; Adamakis, I.D.S. Aberrant microtubule organization in dividing root cells of p60-katanin mutants. Plant Signal. Behav. 2012, 7, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.M.; Valero-Rubira, I.; Allué, S.; Costar, M.A.; Vallés, M.P. Bread wheat doubled haploid production by anther culture. In Doubled Haploid Technology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 1, pp. 227–244, General Topics, Alliaceae, Cereals. [Google Scholar]
- Hunter, C.P. Plant Generation Method European Patent application No. 0245 892 A2, 17 February 1990.
- Hul, T.; Kasha, K.J. Improvement of isolated microspore culture of wheat (Triticum aestivum L.) through ovary co-culture. Plant Cell Rep. 1997, 16, 520–525. [Google Scholar] [CrossRef]
- Jensen, C.J. Monoploid production by chromosome elimination. In Applied and Fundamental Aspects of Plant Cell, Tissue, and Organ Culture; CABI: Wallingford, UK, 1977. [Google Scholar]
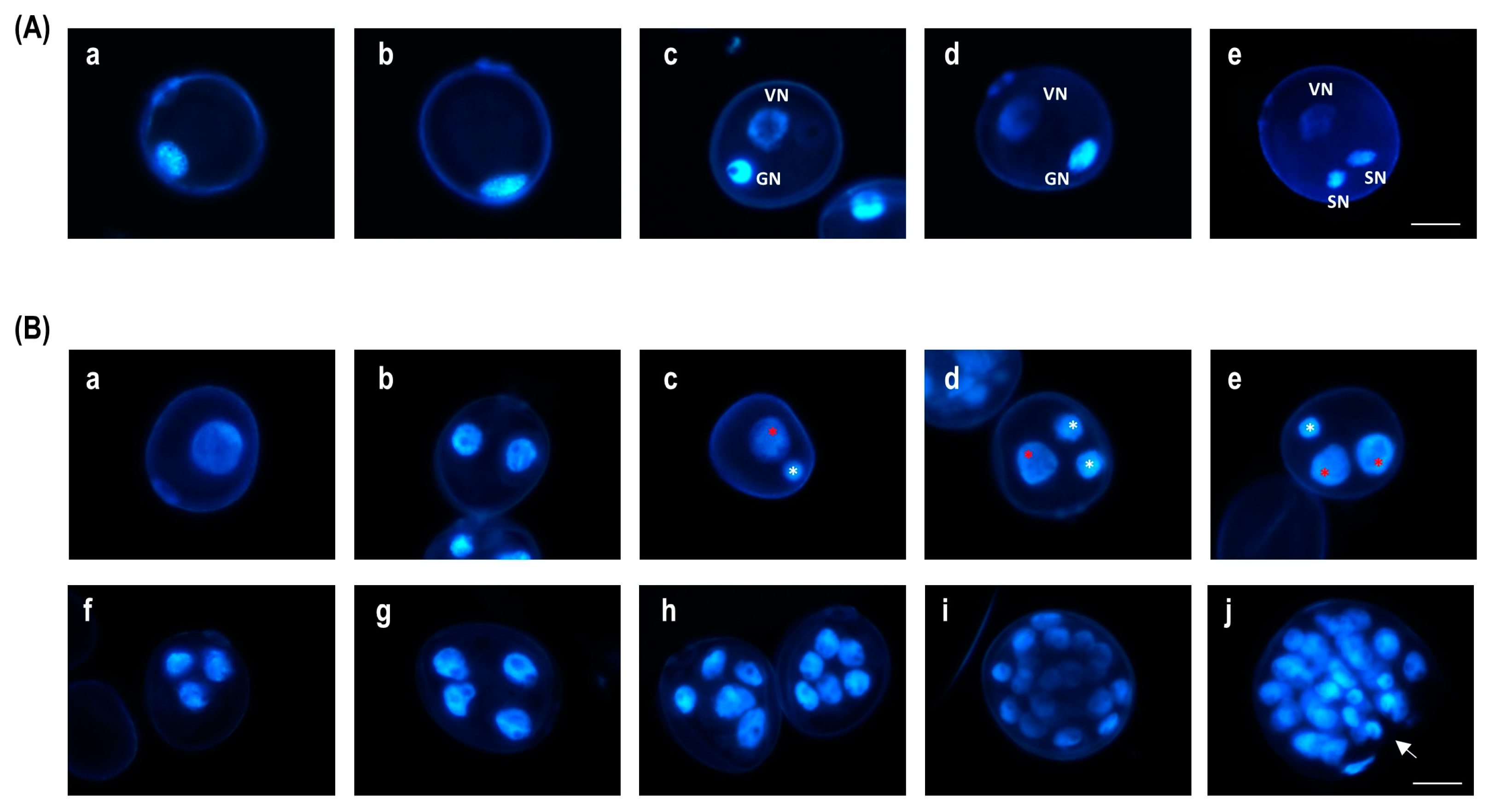
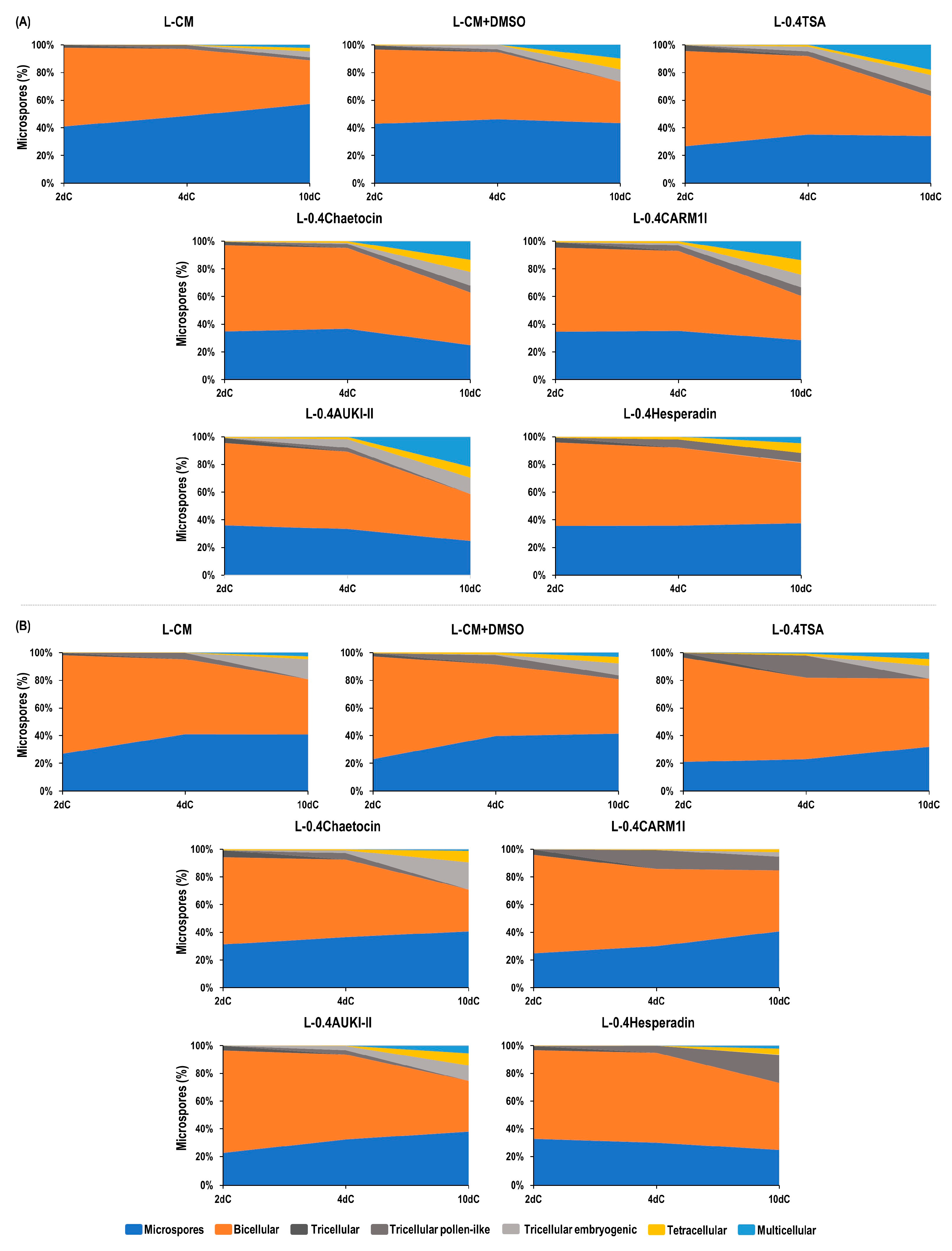
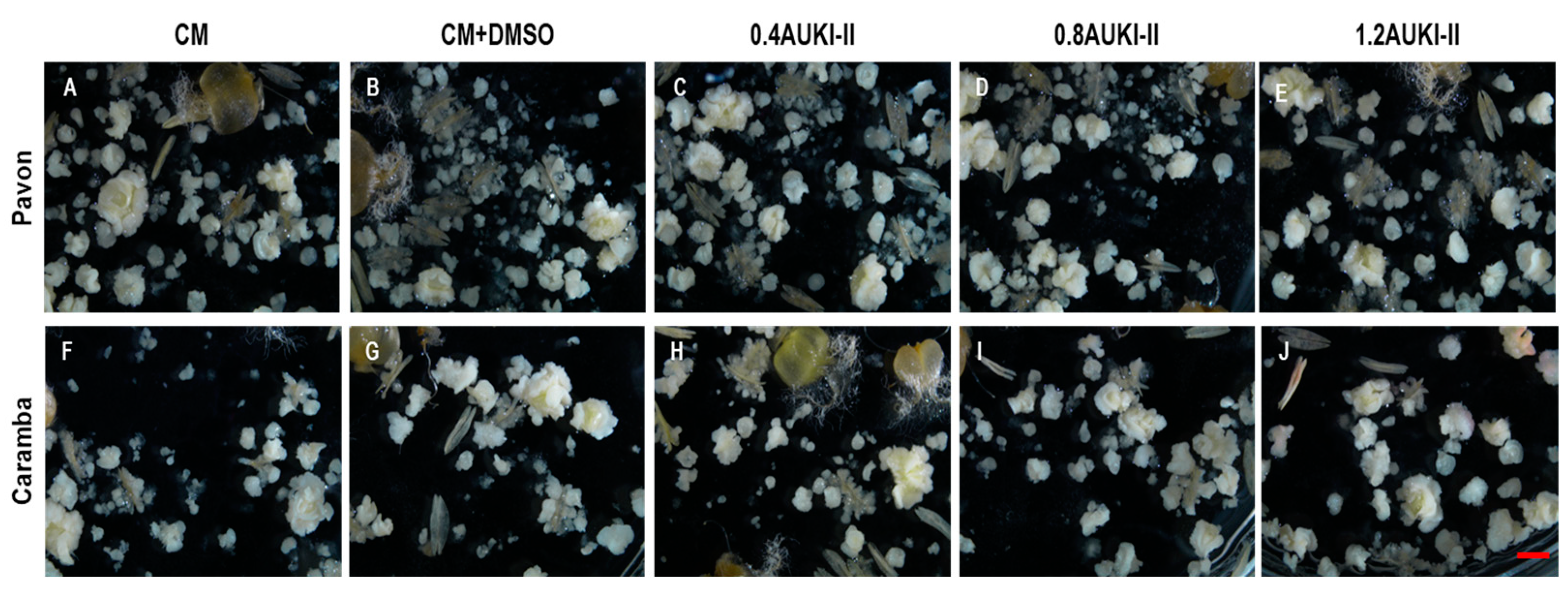

| Treatment | PEMB * | EMB * | GP * | AP * | PGP * | PREG * | PDH ** | GPDH * |
|---|---|---|---|---|---|---|---|---|
| L-CM | 482.9 a | 135.6 a | 17.0 a | 27.6 a | 36.8 a | 29.0 b | 65.8 a | 11.2 a |
| L-CM + DMSO | 434.4 a | 123.7 a | 26.2 a | 28.4 a | 44.3 a | 47.3 a | 46.4 b | 12.2 a |
| L-0.4AUKI-II | 376.7 a | 101.3 a | 15.6 a | 18.7 a | 47.3 a | 35.5 ab | 59.1 a | 9.3 a |
| L-0.8AUKI-II | 418.7 a | 123.1 a | 22.1 a | 26.9 a | 45.3 a | 38.8 ab | 68.5 a | 15.2 a |
| L-0.4TSA | 370.6 a | 112.4 a | 20.9 a | 22.2 a | 48.8 a | 28.2 ab | 45.4 b | 9.5 a |
| p-Value * | PEMB | EMB | GP | AP | PGP | PREG |
|---|---|---|---|---|---|---|
| Genotype (G) | ˂0.001 | ˂0.001 | ˂0.001 | 0.104 | ˂0.001 | 0.746 |
| Treatment (T) | 0.980 | 0.794 | 0.608 | 0.155 | 0.735 | 0.353 |
| G × T | 0.097 | 0.568 | 0.850 | 0.882 | 0.795 | 0.620 |
| Treatments | PEMB * | EMB * | GP * | AP * | PGP * | PREG * | PDH ** | GPDH * |
|---|---|---|---|---|---|---|---|---|
| Pavon | ||||||||
| CM | 767.0 a | 116.8 a | 40.2 a | 19.2 a | 64.4 a | 51.0 ab | 57.3 a | 23.0 a |
| CM + DMSO | 668.1 ab | 115.8 a | 36.4 a | 14.1 a | 72.4 a | 42.4 b | 56.0 a | 20.4 a |
| 0.4AUKI-II | 647.3 b | 110.0 a | 33.8 a | 13.0 a | 73.5 a | 41.7 b | 61.5 a | 20.8 a |
| 0.8AUKI-II | 663.8 ab | 113.2 a | 33.2 a | 13.7 a | 71.2 a | 43.0 b | 64.5 a | 21.4 a |
| 1.2AUKI-II | 603.0 b | 105.9 a | 37.8 a | 16.2 a | 71.0 a | 52.6 a | 59.8 a | 22.6 a |
| Caramba | ||||||||
| CM | 282.3 a | 54.3 a | 8.5 a | 14.3 a | 37.7 a | 54.2 a | 61.7 a | 4.6 ab |
| CM + DMSO | 352.8 a | 50.5 a | 7.8 a | 12.3 a | 43.4 a | 48.3 a | 44.3 bc | 3.1 b |
| 0.4AUKI-II | 373.2 a | 42.8 a | 7.3 a | 10.3 a | 34.9 a | 47.4 a | 39.0 c | 2.6 b |
| 0.8AUKI-II | 372.8 a | 57.7 a | 11.0 a | 13.2 a | 38.0 a | 47.1 a | 63.1 a | 6.1 a |
| 1.2AUKI-II | 393.0 a | 63.8 a | 10.2 a | 12.5 a | 41.9 a | 47.2 a | 58.0 ab | 5.2 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero-Rubira, I.; Vallés, M.P.; Echávarri, B.; Fustero, P.; Costar, M.A.; Castillo, A.M. New Epigenetic Modifier Inhibitors Enhance Microspore Embryogenesis in Bread Wheat. Plants 2024, 13, 772. https://doi.org/10.3390/plants13060772
Valero-Rubira I, Vallés MP, Echávarri B, Fustero P, Costar MA, Castillo AM. New Epigenetic Modifier Inhibitors Enhance Microspore Embryogenesis in Bread Wheat. Plants. 2024; 13(6):772. https://doi.org/10.3390/plants13060772
Chicago/Turabian StyleValero-Rubira, Isabel, María Pilar Vallés, Begoña Echávarri, Patricia Fustero, María Asunción Costar, and Ana María Castillo. 2024. "New Epigenetic Modifier Inhibitors Enhance Microspore Embryogenesis in Bread Wheat" Plants 13, no. 6: 772. https://doi.org/10.3390/plants13060772
APA StyleValero-Rubira, I., Vallés, M. P., Echávarri, B., Fustero, P., Costar, M. A., & Castillo, A. M. (2024). New Epigenetic Modifier Inhibitors Enhance Microspore Embryogenesis in Bread Wheat. Plants, 13(6), 772. https://doi.org/10.3390/plants13060772









