Deciphering the Enhancing Impact of Exogenous Brassinolide on Physiological Indices of Melon Plants under Downy Mildew-Induced Stress
Abstract
:1. Introduction
2. Results
2.1. Analysis of Phenotypic and Cytological Changes in Leaves
2.2. Analysis of Enzymatic Activity of Antioxidants and Permeability of Cell Membrane
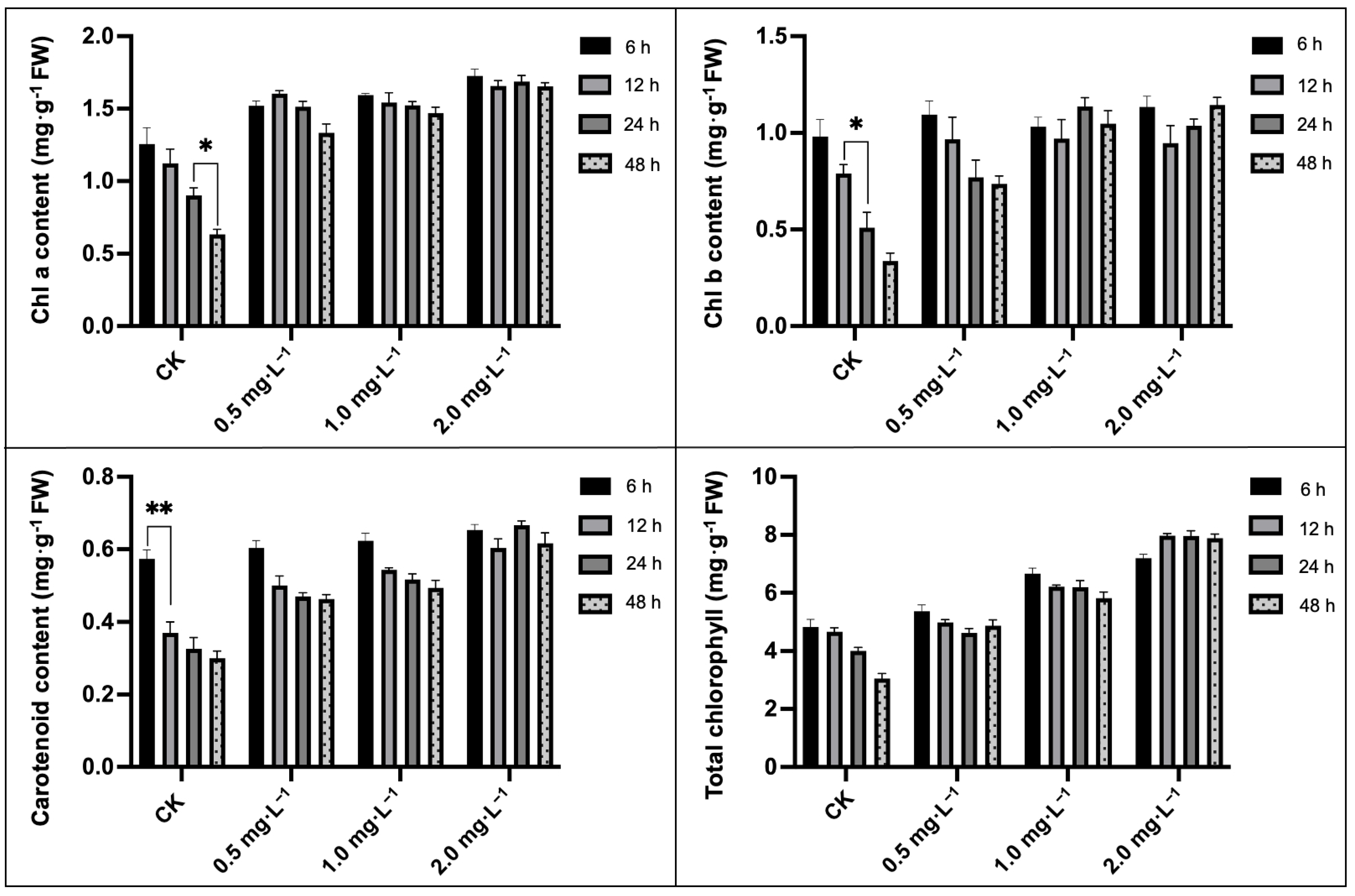
2.3. Analysis of Chlorophyll and Carotenoids Contents
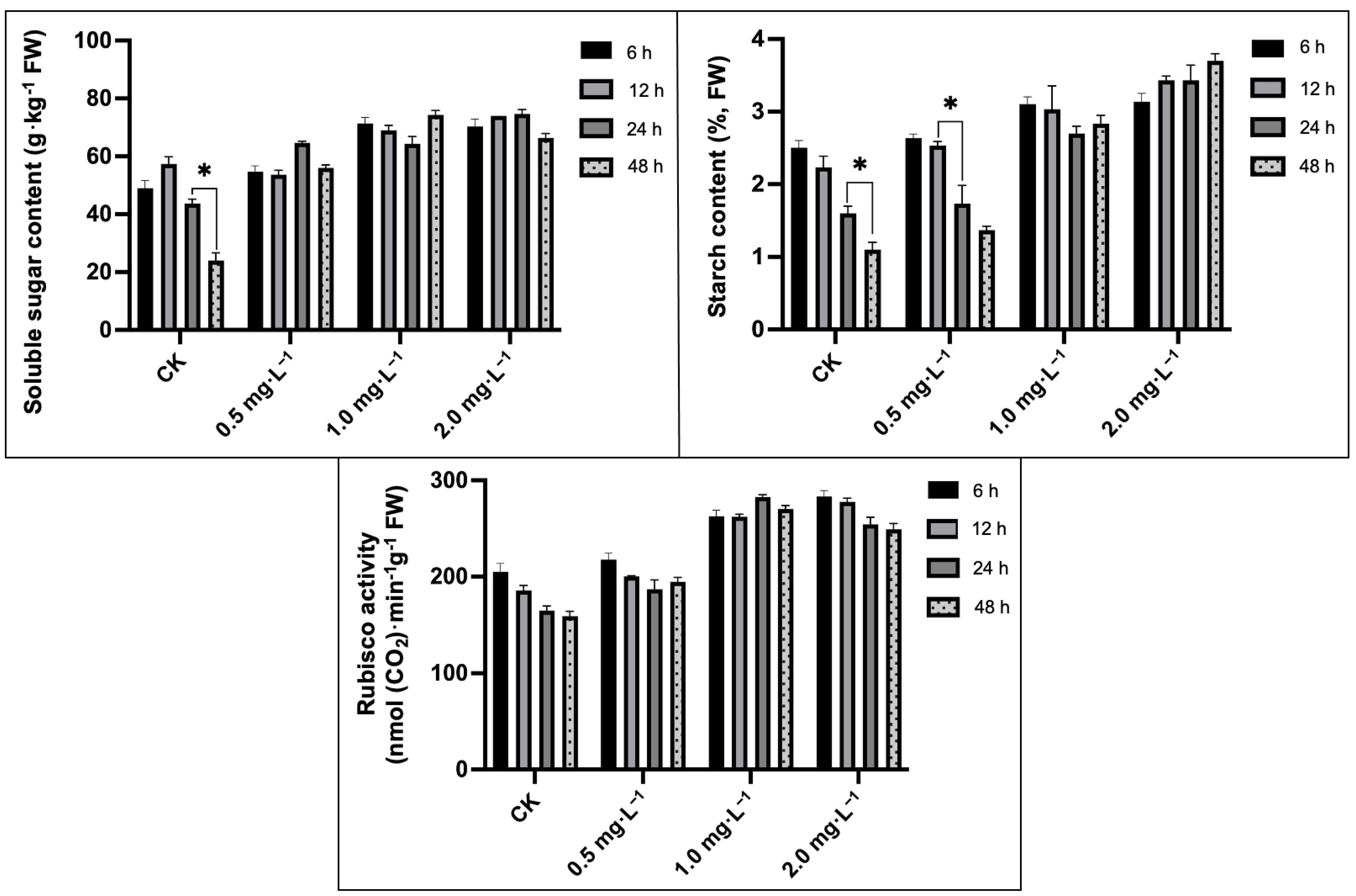
2.4. Analysis of Soluble Sugar, Starch Content, and Rubisco Activity
2.5. Analysis of Variations in Chlorophyll Fluorescence Parameters
2.6. Analysis of Relative Genes Expression Trends
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Downy-Mildew-Induced Stress
4.2. Observation of Disease Symptoms and Transmission Electron Microscopy
4.3. Evaluation of Antioxidant Enzymatic Activity and Cell Membrane Permeability
4.4. Estimation of Total Soluble Sugar, Starch Content and Rubisco Activity
4.5. Determination of Photosynthetic Pigment Content
4.6. Determination of Photosynthetic Fluorescence Parameters
4.7. Validation of Expression Trends of Putative Genes
4.8. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palti, J.; Cohen, Y. Downy mildew of Cucurbits (Pseudoperonospora Cubensis): The fungus and its hosts, distribution, epidemiology and control. Phytoparasitica 1980, 8, 109–147. [Google Scholar] [CrossRef]
- Thomas, C.E. Physiological specialization in Pseudoperonospora cubensis. Phytopathology 1987, 77, 1621–1624. [Google Scholar] [CrossRef]
- Savory, E.A.; Granke, L.L.; Quesada-Ocampo, L.M.; Varbanova, M.; Hausbeck, M.K.; Day, B. The cucurbit downy mildew pathogen Pseudoperonospora cubensis. Mol. Plant Pathol. 2011, 12, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.D.; Xiang, Y.J.; Han, S.; Zhang, H.Q.; Wu, J.; Li, H.B.; Liu, B.W.; Chen, G.H. Research progress on cucumber downy mildew and its resistance. J. South Agric. 2016, 10, 1709–1714. [Google Scholar]
- Bidaramali, V.; Bhutia, T.L.; Sureja, A.K.; Munshi, A.D.; Das, A.; Boopalakrishnan, G.; Gopalakrishnan, S.; Behera, T.K.; Dey, S.S. Genetics of downy mildew resistance in indigenous cucumber germplasm. Indian J. Hortic. 2023, 80, 50–56. [Google Scholar] [CrossRef]
- Xu, S.C.; Wang, H.B.; Feng, J.J.; Xiang, H.F.; Wu, M.D.; Wang, Z.M.; Wei, D.Y.; Zhang, H.C.; Tang, Q.L. Progress in cucumber downy mildew and mechanisms of host resistance. Chin. J. Biotechnol. 2021, 11, 1–14. [Google Scholar]
- Cui, L.; Siskos, L.; Wang, C.; Schouten, H.J.; Visser, R.; Bai, Y. Breeding melon (Cucumis melo) with resistance to powdery mildew and downy mildew. Hortic. Plant J. 2022, 8, 545–561. [Google Scholar] [CrossRef]
- Sedlárová, M.; Lebeda, A.; Pink, D.A.C. The early stages of interaction between effective and non-effective race-specific genes in Lactuca sativa, wild Lactuca spp. and Bremia lactucae (race NL 16). J. Plant Dis. Prot. 2001, 108, 477–489. [Google Scholar]
- Zhang, P.; Zhu, Y.Q.; Shen, C.J.; Zhou, S.J. Proteome analysis of cucumber responses to Pseudoperonospora cubensis infection. J. Plant Pathol. 2019, 4, 917–925. [Google Scholar] [CrossRef]
- Sedlárová, M.; Výtisková, M.; Dolezal, K.; Lebeda, A. Pre-incubation with cytokinins delays chlorophyll degradation in Lactuca spp. tissues and reduces Bremia lactucae sporulation. Adv. Downy Mildew Res. 2007, 3, 185–194. [Google Scholar]
- Chinnusamy, V.; Zhu, J.H.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef]
- Borrell, J.H.; Domènech, Ò.; Keough, K.M. Membrane Protein-Lipid Interactions: Physics and Chemistry in the Bilayer; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Reszczyńska, E.; Hanaka, A. Lipids composition in plant membranes. Cell Biochem. Biophys. 2020, 78, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, L.; Barkla, B.J. Membrane lipid remodeling in response to salinity. Int. J. Mol. Sci. 2019, 20, 4264. [Google Scholar] [CrossRef] [PubMed]
- Kalisch, B.; Dörmann, P.; Hölzl, G. DGDG and glycolipids in plants and algae. In Lipids in Plant and Algae Development; Nakamura, Y., Li-Beisson, Y., Eds.; Springer: Cham, Switzerland, 2016; Volume 86, pp. 51–83. [Google Scholar]
- Negi, J.; Munemasa, S.; Song, B.; Tadakuma, R.; Fujita, M.; Azoulay-Shemer, T.; Engineer, C.B.; Kusumi, K.; Nishida, I.; Schroeder, J.I.; et al. Eukaryotic lipid metabolic pathway is essential for functional chloroplasts and CO2 and light responses in Arabidopsis guard cells. Proc. Natl. Acad. Sci. USA 2018, 115, 9038–9043. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zheng, Q.; Shen, W.Y.; Cram, D.; Fowler, D.B.; Wei, Y.D.; Zou, J.T. Understanding the biochemical basis of temperature-induced lipid pathway adjustments in plants. Plant Cell 2015, 27, 86–103. [Google Scholar] [CrossRef]
- Campos, P.S.; Quartin, V.N.; Ramalho, J.C.; Nunes, M.A. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef]
- Moellering, E.R.; Muthan, B.; Benning, C. Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 2010, 330, 226–228. [Google Scholar] [CrossRef]
- Lamine, M.; Gargouri, M.; Mliki, A. Identifification of the NaCl-responsive metabolites in Citrus roots: A lipidomic and volatomic signature. Plant Signal. Behav. 2020, 15, 1777376. [Google Scholar] [CrossRef]
- Li, W.Q.; Wang, R.P.; Li, M.Y.; Li, L.X.; Wang, C.M.; Welti, R.; Wang, X.M. Differential degradation of extraplastidic and plastidic lipids during freezing and post-freezing recovery in Arabidopsis thaliana. J. Biol. Chem. 2008, 283, 461–468. [Google Scholar] [CrossRef]
- Jain, S.K.; Wettberg, E.J.v.; Punia, S.S.; Parihar, A.K.; Lamichaney, A.; Kumar, J.; Gupta, D.S.; Ahmad, S.; Pant, N.C.; Dixit, G.P.; et al. Genomic-Mediated Breeding Strategies for Global Warming in Chickpeas (Cicer arietinum L.). Agriculture 2023, 13, 1721. [Google Scholar] [CrossRef]
- Wang, Y.N.; Li, K.X.; Li, X. Auxin redistribution modulates plastic development of root system architecture under salt stress in Arabidopsis thaliana. J. Plant Physiol. 2009, 166, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z.L. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Tommasino, E.; Griffa, S.; Grunberg, K.; Ribotta, A.; Colomba, E.L.; Carloni, E.; Quiroga, M.; Luna, C.M. Malondialdehyde content as a potential biochemical indicator of tolerant Cenchrus ciliaris L. genotypes under heat stress treatment. Grass Forage Sci. 2012, 67, 456–459. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, G.Z.; Du, L.; Shang, X.G.; Cheng, C.Z.; Yang, B.; Hu, Y.; Cai, C.P.; Guo, W.Z. Genetic regulation of salt stress tolerance revealed by RNASeq in cotton diploid wild species, Gossypium davidsonii. Sci. Rep. 2016, 6, 20582. [Google Scholar] [CrossRef]
- Rose, J.K.C.; Braam, J.; Fry, S.C.; Nishitani, K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: Current perspectives and a new unifying nomenclature. Plant Cell Physiol. 2002, 43, 1421–1435. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef]
- Kaushik, N.; Bhatt, K.; Chakrabarti, S.K. Development of transgenic potato with increased starch production. Trends Carbohydr. Res. 2018, 10, 44–53. [Google Scholar]
- Zhang, L.; Zhu, L.; Xu, Y.; Lü, L.; Li, X.; Li, W.; Liu, W.; Ma, F.; Li, M.; Han, D. Genome-wide identification and function analysis of the sucrose phosphate synthase MdSPS gene family in apple. J. Integr. Agric. 2023, 22, 2080–2093. [Google Scholar] [CrossRef]
- Begum, H.H.; Osaki, M.; Shinano, T.; Miyatake, H.; Wasaki, J.; Yamamura, T.; Watanabe, T. The Function of a Maize-Derived Phosphoenolpyruvate Carboxylase (PEPC) in Phosphorus-Deficient Transgenic Rice. Soil Sci. Plant Nutr. 2005, 51, 497–506. [Google Scholar] [CrossRef]
- Liu, T.; Amanullah, S.; Xu, H.; Gao, P.; Du, Z.; Hu, X.; Han, M.; Che, Y.; Zhang, L.; Qi, G.; et al. RNA-Seq Identified Putative Genes Conferring Photosynthesis and Root Development of Melon under Salt Stress. Genes 2023, 14, 1728. [Google Scholar] [CrossRef]
- Zhang, J.F.; Li, J.; Xie, J.M.; Yu, J.H.; Dawuda, M.M.; Lyv, J.; Tang, Z.Q.; Zang, J.; Zhang, X.D.; Tang, C.N. Changes in photosynthesis and carotenoid composition of pepper (Capsicum annuum L.) in response to low-light stress and low temperature combined with low-light stress. Photosynthetica 2020, 58, 125–136. [Google Scholar] [CrossRef]
- Plücken, H.; Müller, B.; Grohmann, D.; Westhoff, P.; Eichacker, L.A. The HCF136 protein is essential for assembly of the photosystem II reaction center in Arabidopsis thaliana. FEBS Lett. 2002, 532, 85–90. [Google Scholar] [CrossRef]
- Lotta, V.S.; Serena, S.; Jörg, M.; Christiane, F.; Fikret, M.; Wolfgang, P.S. The PsbY protein of arabidopsis photosystem II is important for the redox control of cytochrome b559. Biochim. Biophys. Acta 2016, 1857, 1524–1533. [Google Scholar]
- Luan, S.; Bogorad, L. Nucleotide sequences of two genes encoding the light harvesting chlorophyll a/b binding protein of rice. Nucleic Acids Res. 1989, 17, 2357–2358. [Google Scholar] [CrossRef]
- Muzzalupo, I.; Stefanizzi, F.; Perri, E.; Chiappetta, A.A. Transcript levels of CHLP gene, antioxidants and chlorophylls contents in olive (Olea europaea L.) pericarps: A comparative study on eleven olive cultivars harvested in two ripening stages. Plant Foods Hum. Nutr. 2011, 66, 1–10. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, S.; Hu, Y.; Wen, Y. Biological Control of the Cucumber Downy Mildew Pathogen Pseudoperonospora cubensis. Horticulturae 2022, 8, 410. [Google Scholar] [CrossRef]
- Clouse, S.D.; Sasse, J.M. Brassinosteroids: Essential regulators of plant growth and development. Ann. Rev. Plant Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef]
- Creelman, R.A.; Mullet, J.E. Oligosaccharins, brassinolides, and jasmonates: Non-traditional regulators of plant growth development, and gene expression. Plant Cell 1997, 9, 1211–1223. [Google Scholar] [CrossRef]
- Li, B.Q.; Zhang, C.F.; Cao, B.H.; Qin, G.Z.; Wang, W.H.; Tian, S.P. Brassinolide enhances cold stress tolerance of fruit by regulating plasma membrane proteins and lipids. Amino Acids 2012, 43, 2469–2480. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.F.; Zhang, W.; Wang, W.W.; Meng, L.M.; Li, H.; Jin, Q.M.; Zhao, Z.W.; Dong, B.C. Analysis of test against chilling injury and phytotoxicity of seed coating agent to corn by additive brassinolide. J. Maize Sci. 2013, 21, 137–140. [Google Scholar]
- Behnamnia, M. Protective roles of brassinolide on tomato seedlings under drought stress. Int. J. Agric. Crop Sci. 2015, 8, 552–559. [Google Scholar]
- Anuradha, S.; Rao, S.S.R. Application of brassinosteroids to rice seeds (Oryza sativa L.) reduced the impact of salt stress on growth, prevented photosynthetic pigment loss and increased nitrate reductase activity. Plant Growth Regul. 2003, 40, 29–32. [Google Scholar] [CrossRef]
- Amanullah, S.; Gao, P.; Osae, B.A.; Saroj, A.; Yang, T.; Liu, S.; Weng, Y.; Luan, F. Genetic linkage mapping and QTLs identification for morphology and fruit quality related traits of melon by SNP based CAPS markers. Sci. Hortic. 2021, 278, 109849. [Google Scholar] [CrossRef]
- Sharma, S.K. Brassinosteroids application responses in fruit crops-a review. Int. J. Agric. Environ. Biotechnol. 2021, 14, 123–140. [Google Scholar] [CrossRef]
- Sun, Y.; He, Y.; Irfan, A.R.; Liu, X.; Yu, Q.; Zhang, Q.; Yang, D. Exogenous brassinolide enhances the growth and cold resistance of maize (Zea mays L.) seedlings under chilling stress. Agronomy 2020, 10, 488. [Google Scholar] [CrossRef]
- Ali, B.; Hayat, S.; Fariduddin, Q.; Ahmad, A. 24-Epibrassinolide protects against the stress generated by salinity and nickel in Brassica juncea. Chemosphere 2008, 72, 1387–1392. [Google Scholar] [CrossRef]
- Fazeli, F.; Ghorbanli, M.; Niknam, V. Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol. Plant. 2007, 51, 98–103. [Google Scholar] [CrossRef]
- Wang, D.; Li, W.; Li, D.; Li, L.; Luo, Z. Effect of high carbon dioxide treatment on reactive oxygen species accumulation and antioxidant capacity in fresh-cut pear fruit during storage. Sci. Hortic. 2021, 281, 109925. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Wang, Z.; Yang, Y.; Li, M.; Xu, B.; Xu, B. Abscisic acid and brassinolide combined application synergistically enhances drought tolerance and photosynthesis of tall fescue under water stress. Sci. Hortic. 2018, 228, 1–9. [Google Scholar] [CrossRef]
- Zhang, M.C.; Zhai, Z.X.; Tian, X.L.; Duan, L.S.; Li, Z.H. Brassinolide alleviated the adverse effect of water deficits on photosynthesis and the antioxidant of soybean (Glycine max L.). Plant Growth Regul. 2008, 56, 257–264. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Asghari, M.; Farmani, B.; Mohayeji, M.; Moradbeygi, H. Impact of postharvest brassinosteroids treatment on PAL activity in tomato fruit in response to chilling stress. Sci. Hortic. 2012, 144, 116–120. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Bhardwaj, R.; Gupta, B.D.; Dutt, P.; Gupta, R.K.; Kanwar, M.; Biondi, S. Enhancing effects of 24-epibrassinolide and putrescine on the antioxidant capacity and free radical scavenging activity of Raphanus sativus. Acta Physiol. Plant. 2011, 33, 1319–1333. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Xu, X.; Chen, Y.; Chu, J.; Yao, X. The combined treatments of brassinolide and zeaxanthin better alleviate oxidative damage and improve hypocotyl length, biomass, and the quality of radish sprouts stored at low temperature. Food Chem. X 2022, 15, 100394. [Google Scholar] [CrossRef]
- Cao, S.Q.; Xu, Q.T.; Cao, Y.J.; Qian, K.; An, K.; Zhu, Y.; Hu, B.Z.; Zhao, H.F.; Kuai, B.K. Loss-of-function mutations in DET2, gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol. Plant. 2005, 123, 57–66. [Google Scholar] [CrossRef]
- Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef]
- Tanaka, R.; Kobayashi, K.; Masuda, T. Tetrapyrrole metabolism in Arabidopsis thaliana. Arab. Book 2011, 9, e0145. [Google Scholar] [CrossRef]
- Polívka, T.S.; Frank, H.A. Molecular factors controlling photosynthetic light harvesting by carotenoids. Acc. Chem. Res. 2010, 43, 1125–1134. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Kang, C.; Zhai, H.; Xue, L.; Zhao, N.; He, S.; Liu, Q. A lycopene beta-cyclase gene, IbLCYB2, enhances carotenoid contents and abiotic stress tolerance in transgenic sweet potato. Plant Sci. 2018, 272, 243–254. [Google Scholar] [CrossRef]
- Tattini, M.; Loreto, F.; Fini, A.; Guidi, L.; Brunetti, C.; Velikova, V.; Gori, A.; Ferrini, F. Isoprenoids and phenylpropanoids are part of the antioxidant defense orchestrated daily by drought-stressed Platanus × acerifolia plants during Mediterranean summers. New Phytol. 2015, 3, 613–626. [Google Scholar] [CrossRef]
- Hussain, S.; Ulhassan, Z.; Brestic, M.; Zivcak, M.; Zhou, W.J.; Allakhverdiev, S.I.; Yang, X.H.; Safdar, M.E.; Yang, W.Y.; Liu, W.G. Photosynthesis research under climate change. Photosynth. Res. 2021, 150, 5–19. [Google Scholar] [CrossRef]
- Hashida, Y.; Hirose, T.; Okamura, M.; Hibara, K.; Ohsugi, R.; Aoki, N. A reduction of sucrose phosphate synthase (SPS) activity affects sucrose/starch ratio in leaves but does not inhibit normal plant growth in rice. Plant Sci. 2016, 253, 40–49. [Google Scholar] [CrossRef]
- O’Leary, B.; Park, J.; Plaxton, W.C. The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): Recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs. Biochem. J. 2011, 436, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.; Boscaiu, M.; Lull, C.; Ba, U.I.; Lid, N.A.; Vicente, O. Are soluble carbohydrates ecologically relevant for salt tolerance in halophytes? Funct. Plant Biol. 2013, 40, 805–818. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Gan, Y.; Yu, J.; Xie, J. Brassinosteroid alleviates chilling induced oxidative stress in pepper by enhancing antioxidation systems and maintenance of photosystem II. Acta Physiol. Plant. 2015, 37, 222. [Google Scholar] [CrossRef]
- Belkhodja, R.; Morales, F.; Abadia, A.; Medrano, H.; Abadia, J. Effects of salinity on chlorophyll fluorescence and photosynthesis of barley (Hordeum vulgare L.) grown under a triple-line-source sprinkler system in the field. Photosynthetica 1999, 36, 375–387. [Google Scholar] [CrossRef]
- Tsiupka, S.; Mesyats, N.; Smykov, A.; Tsiupka, V. Assessment of drought tolerance in some peach cultivars by chlorophyll fluorescence induction method. Acta Hortic. 2022, 1339, 353–362. [Google Scholar] [CrossRef]
- Han, J.; Gu, L.; Wen, J.; Sun, Y. Inference of photosynthetic capacity parameters from chlorophyll a fluorescence is affected by redox state of PSII reaction centers. Plant Cell Environ. 2022, 45, 1298–1314. [Google Scholar] [CrossRef] [PubMed]
- Pshybytko, N.L. Non-Photochemical Quenching of Chlorophyll a Fluorescence as an Indicator of the State of a Plant Photosynthetic Apparatus under Abiotic Stress. J. Appl. Spectrosc. 2023, 90, 60–65. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Tene, T.M.; Sari, H.; Canci, H.; Maaruf, A.; Eker, T.; Toker, C. Traits Related to Heat Stress in Phaseolus Species. Agriculture 2023, 13, 953. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Wijaya, L.; Ahmad, P. The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiol. Biochem. 2019, 143, 119–128. [Google Scholar] [CrossRef]
- Xue, J.; Guo, C.; Shen, Y.; Li, M.; Chu, J.; Yao, X. Brassinolide soaking and preharvest UV-B radiation influence the shelf life of small black bean sprouts. Food Chem. 2021, 352, 29322. [Google Scholar] [CrossRef]
- Farooq, S.; Chmeliov, J.; Wientjes, E.; Koehorst, R.; Bader, A.; Valkunas, L.; Trinkunas, G.; van Amerongen, H. Dynamic feedback of the photosystem II reaction centre on photoprotection in plants. Nat. Plants 2018, 4, 225–331. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.J. Effects of cesium accumulation on chlorophyll content and fluorescence of Brassica juncea L. J. Environ. Radioact. 2018, 195, 26–32. [Google Scholar] [CrossRef]
- Gao, X.N.; Xie, F.Q.; Wang, L.; Huang, L.L. Acibenzolar induced resistance to cucumber downy mildew. Agrochemicals 2004, 4, 190–191. [Google Scholar]
- Liu, J.J.; Wei, Z.; Li, J.H. Effects of copper on leaf membrane structure and root activity of maize seedling. Bot. Stud. 2014, 55, 47. [Google Scholar] [CrossRef]
- Cui, C.; Cai, J.; Zhang, S. Allelopathic effects of walnut (Juglans regia L.) rhizospheric soil extracts on germination and seedling growth of turnip (Brassica rapa L.). Allelopath. J. 2013, 32, 37–48. [Google Scholar]
- Narayanan, S.; Prasad, P.V.; Welti, R. Wheat leaf lipids during heat stress: II. Lipids experiencing coordinated metabolism are detected by analysis of lipid co-occurrence. Plant Cell Environ. 2016, 39, 608–617. [Google Scholar] [CrossRef]
- Wu, G.X.; Li, S.L.; Li, Y.M.; Bi, H.G.; Ai, X.Z. Effects of hydrogen sulphide, nitric oxide and their interaction on photosynthesis of cucumber seedlings under chilling stress. Plant Physiol. J. 2020, 56, 2221–2232. [Google Scholar]
- Mahadevan, A.; Sridhar, R. Methods in Physiological Plant Pathology, 2nd ed.; Sivakami Publications: Chennai, India, 1982. [Google Scholar]
- Li, W.; Yang, S.; Lu, Z.; He, Z.; Ye, Y.; Zhao, B.; Wang, L.; Jin, B. Cytological, physiological, and transcriptomic analyses of golden leaf coloration in Ginkgo biloba L. Hortic. Res. 2018, 5, 12. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Zhang, J.; Gong, X.; Zhang, Z.; Sun, J.; Chen, X.; Wang, Y. Exogenous melatonin improves physiological characteristics and promotes growth of strawberry seedlings under cadmium stress. Hortic. Plant J. 2021, 7, 13–22. [Google Scholar] [CrossRef]
- Dong, Z.; Men, Y.; Liu, Z.; Li, J.; Ji, J. Application of chlorophyll fluorescence imaging technique in analysis and detection of chilling injury of tomato seedlings. Comput. Electron. Agric. 2020, 168, 105109. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ihemere, U.; Arias-Garzon, D.; Lawrence, S.; Sayre, R. Genetic modification of cassava for enhanced starch production. Plant Biotechnol. J. 2006, 4, 453–465. [Google Scholar] [CrossRef]
- Sengupta, S.; Majumder, A.L. Insight into the salt tolerance factors of a wild halophytic rice, Porteresia coarctata: A physiological and proteomic approach. Planta 2009, 229, 911–929. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Wang, Y.; Huang, R.; Sun, C.; Xu, Z.; Zhu, J.; Gao, X.; Deng, X.; Wang, P. Identification of a Geranylgeranyl reductase gene for chlorophyll synthesis in rice. Springer Plus 2014, 3, 201. [Google Scholar] [CrossRef]

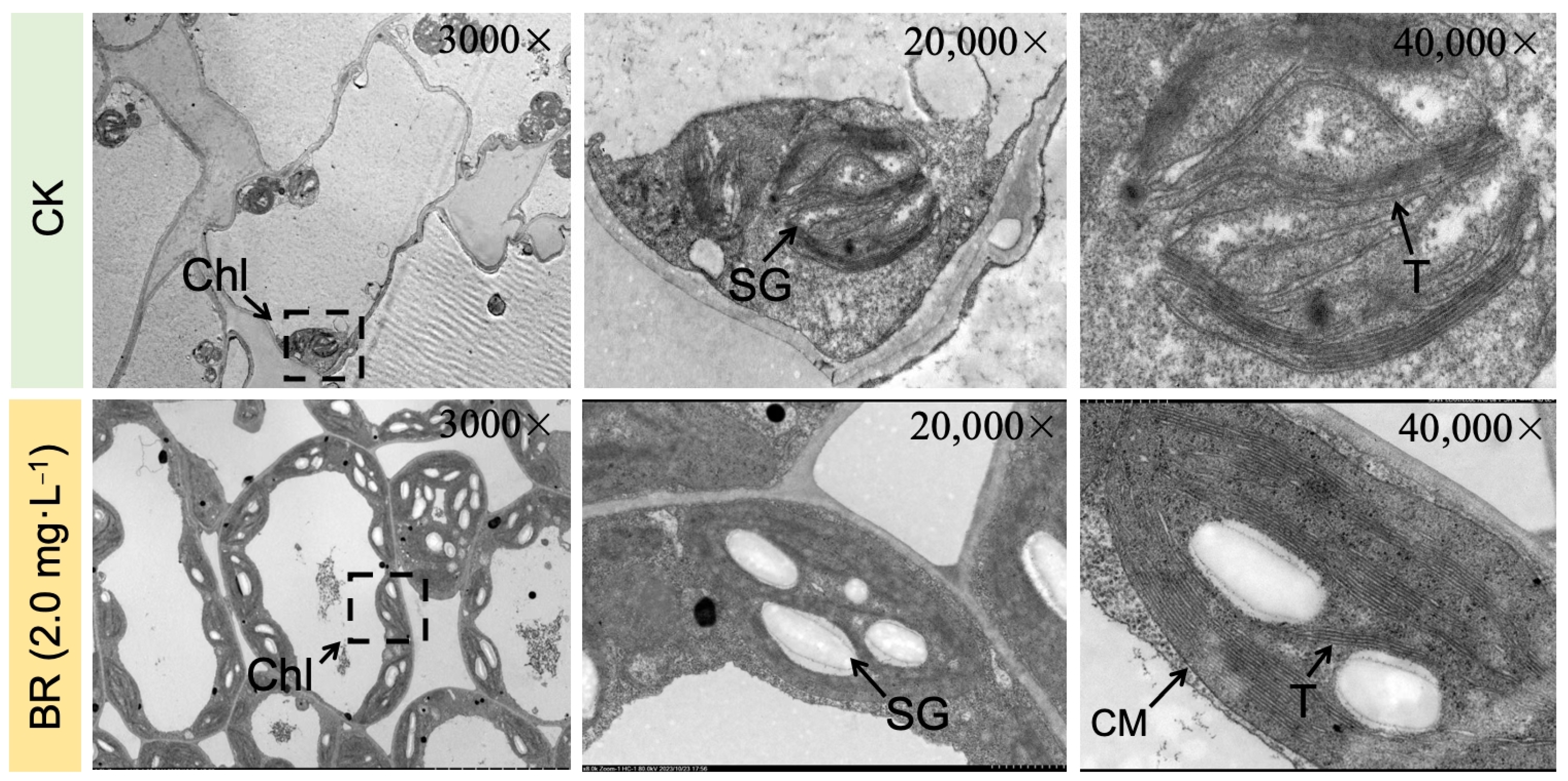


| Molar (%) | CK | BR (2.0 mg·L−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| 6 h | 12 h | 24 h | 48 h | 6 h | 12 h | 24 h | 48 h | |
| DGDG (%) | 26.73 ± 0.89 a | 22.80 ± 1.04 a | 17.65 ± 2.04 a | 13.84 ± 3.02 ab | 25.40 ± 1.12 a | 26.15 ± 0.75 a | 25.31 ± 0.80 a | 26.03 ± 0.85 a |
| MGDG (%) | 38.68 ± 1.67 a | 34.21 ± 2.23 b | 31.07 ± 0.77 a | 26.85 ± 2.80 b | 40.69 ± 1.03 a | 38.76 ± 1.18 a | 41.28 ± 1.57 a | 40.03 ± 0.41 a |
| Treatments | Duration | NPQ | Y (NPQ) | qP | F0 | Fv/Fm | Y (II) |
|---|---|---|---|---|---|---|---|
| CK | 6 h | 0.48 ± 0.09 a | 0.45 ± 0.09 a | 0.50 ± 0.04 a | 75.33 ± 0.07 a | 0.80 ± 0.09 a | 0.36 ± 0.04 a |
| 12 h | 0.59 ± 0.04 a | 0.53 ± 0.04 a | 0.48 ± 0.07 a | 78.26 ± 0.12 a | 0.76 ± 0.11 a | 0.38 ± 0.07 a | |
| 24 h | 0.32 ± 0.12 a | 0.34 ± 0.12 a | 0.40 ± 0.06 a | 70.87 ± 0.09 ab | 0.65 ± 0.08 a | 0.41 ± 0.06 a | |
| 48 h | 0.29 ± 0.06 a | 0.31 ± 0.06 a | 0.49 ± 0.03 a | 63.68 ± 0.10 a | 0.57 ± 0.10 a | 0.38 ± 0.12 b | |
| 0.5 mg·L−1 BR | 6 h | 0.53 ± 0.10 a | 0.50 ± 0.10 a | 0.40 ± 0.10 a | 80.69 ± 0.02 a | 0.78 ± 0.05 a | 0.40 ± 0.10 a |
| 12 h | 0.79 ± 0.04 a | 0.74 ± 0.04 a | 0.48 ± 0.07 a | 79.44 ± 0.13 a | 0.83 ± 0.16 ab | 0.37 ± 0.07 a | |
| 24 h | 0.83 ± 0.06 a | 0.80 ± 0.06 a | 0.50 ± 0.15 b | 82.37 ± 0.28 c | 0.85 ± 0.10 a | 0.45 ± 0.15 b | |
| 6 h | 1.14 ± 0.02 a | 1.02 ± 0.16 c | 0.46 ± 0.01 a | 85.20 ± 0.07 a | 0.86 ± 0.07 a | 0.38 ± 0.01 a | |
| 1.0 mg·L−1 BR | 6 h | 0.47 ± 0.07 a | 0.50 ± 0.07 a | 0.48 ± 0.04 a | 76.70 ± 0.15 a | 0.83 ± 0.15 a | 0.43 ± 0.07 a |
| 12 h | 0.66 ± 0.10 c | 0.59 ± 0.10 c | 0.38 ± 0.10 b | 80.50 ± 0.07 b | 0.88 ± 0.07 b | 0.40 ± 0.13 c | |
| 24 h | 0.89 ± 0.07 b | 0.85 ± 0.07 b | 0.36 ± 0.12 b | 90.45 ± 0.18 b | 0.92 ± 0.08 a | 0.36 ± 0.11 a | |
| 6 h | 1.25 ± 0.08 b | 1.14 ± 0.08 b | 0.41 ± 0.03 a | 91.66 ± 0.03 a | 0.90 ± 0.03 a | 0.42 ± 0.08 a | |
| 2.0 mg·L−1 BR | 6 h | 0.52 ± 0.10 b | 0.57 ± 0.10 b | 0.33 ± 0.09 a | 79.65 ± 0.13 a | 0.91 ± 0.13 a | 0.38 ± 0.07 a |
| 12 h | 0.94 ± 0.27 c | 0.90 ± 0.27 c | 0.38 ± 0.05 a | 88.41 ± 0.57 c | 0.88 ± 0.04 a | 0.33 ± 0.10 b | |
| 24 h | 0.87 ± 0.14 a | 0.91 ± 0.14 a | 0.40 ± 0.05 a | 85.40 ± 0.41 c | 0.90 ± 0.11 a | 0.42 ± 0.11 b | |
| 6 h | 1.38 ± 0.15 b | 1.21 ± 0.15 b | 0.42 ± 0.02 a | 90.57 ± 0.13 a | 0.95 ± 0.08 ab | 0.40 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Xu, H.; Amanullah, S.; Du, Z.; Hu, X.; Che, Y.; Zhang, L.; Jiang, Z.; Zhu, L.; Wang, D. Deciphering the Enhancing Impact of Exogenous Brassinolide on Physiological Indices of Melon Plants under Downy Mildew-Induced Stress. Plants 2024, 13, 779. https://doi.org/10.3390/plants13060779
Liu T, Xu H, Amanullah S, Du Z, Hu X, Che Y, Zhang L, Jiang Z, Zhu L, Wang D. Deciphering the Enhancing Impact of Exogenous Brassinolide on Physiological Indices of Melon Plants under Downy Mildew-Induced Stress. Plants. 2024; 13(6):779. https://doi.org/10.3390/plants13060779
Chicago/Turabian StyleLiu, Tai, Huichun Xu, Sikandar Amanullah, Zhiqiang Du, Xixi Hu, Ye Che, Ling Zhang, Zeyu Jiang, Lei Zhu, and Di Wang. 2024. "Deciphering the Enhancing Impact of Exogenous Brassinolide on Physiological Indices of Melon Plants under Downy Mildew-Induced Stress" Plants 13, no. 6: 779. https://doi.org/10.3390/plants13060779






