The Role of Snow-Related Environmental Variables in Plant Conservation Plans in the Mediterranean Mountains
Abstract
1. Introduction
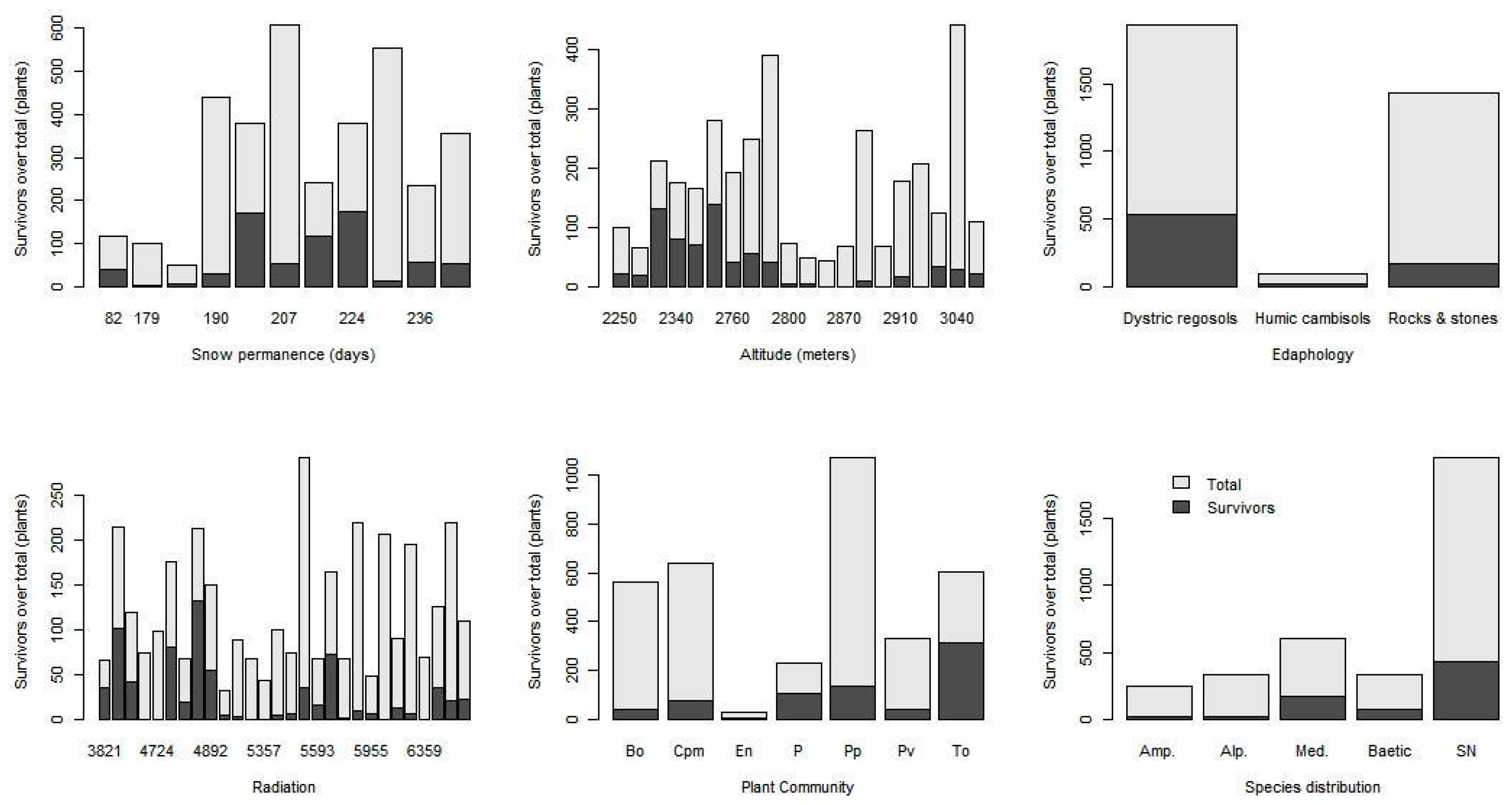
2. Results
3. Discussion
4. Materials and Methods
4.1. Selection of Species
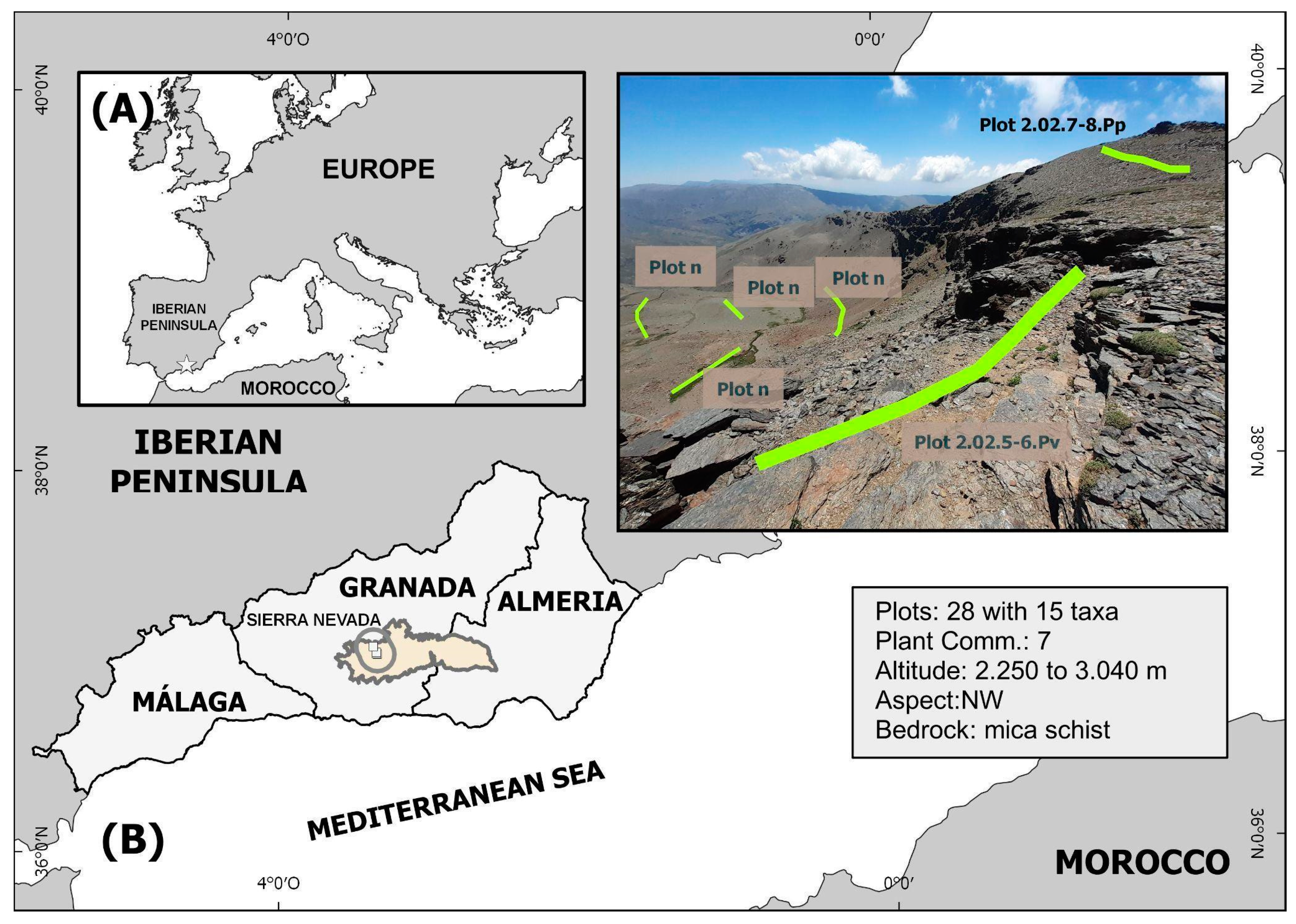
4.2. Selection of Experimental Sites and Variables
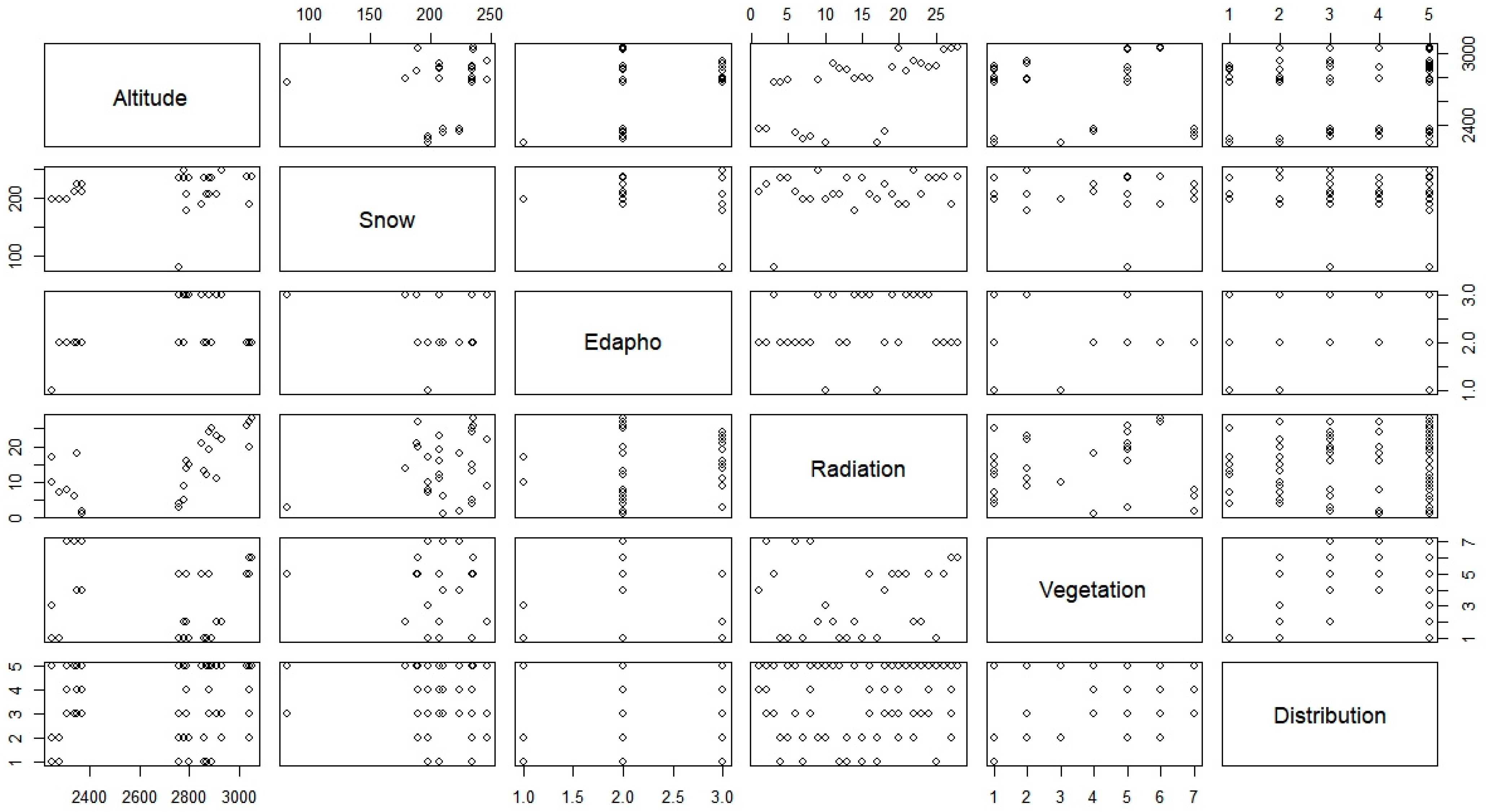
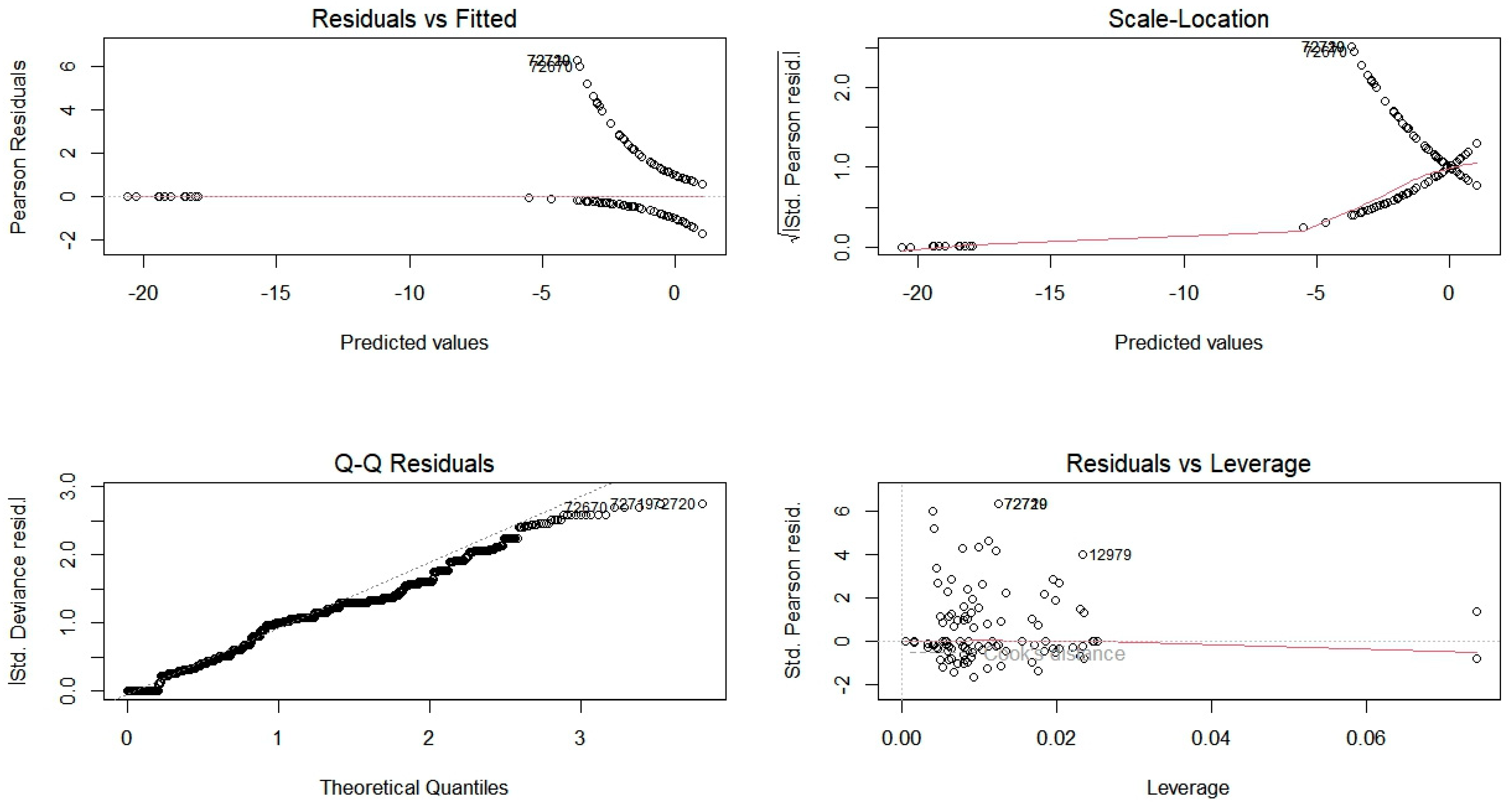
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Taxon | Family | Vegetation | Br-Bl | Total Cover | Altitude | Snow | Radiation | Distribution | Soil |
|---|---|---|---|---|---|---|---|---|---|
| Thymus serpylloides Bory subsp. serpylloides | Labiatae | La, Pp, To | 2, 1, + | 50–70% | 2270–2790 | 99–224 | 4058–7365 | Sierra Nevada s.l. (SN): Sierra Nevada y Sierra de Filabres | Dystric regosols, Stones and rocks |
| Arenaria armerina subsp. caesia (Boiss.) C. Díaz, C. Morales & F. Valle | Caryophyllaceae | La, Pp, Pv, To | 2, 1, + | 10–70% | 2270–3040 | 99–224 | 4058–7365 | Baetic ranges (Baetic): Eastern Andalusia | Humic cambisols, Dystric regosols, Stones and rocks |
| Senecio pyrenaicus subsp. granatensis (Boiss.) Rivas Mart. | Asteraceae | La, To | 2, 1, + | 20–40% | 2270–2370 | 99–224 | 4058–7365 | Sierra Nevada s.l. (SN): Sierra Nevada, Sierra de la Sagra y Sierra de Tejeda | Humic cambisols, Dystric regosols, Stones and rocks |
| Agrostis nevadensis Boiss. | Poaceae | Pv, Pp, Bo, To, Cpm, P | + | 50–65% | 2310–3050 | 82–247 | 4058–7186 | Sierra Nevada s.l. (SN): Sierra Nevada | Humic cambisols, Dystric regosols |
| Armeria splendens (Lag. & Rodr.) Webb | Plumbaginaceae | Bo, La | 2, 1 | 50–90% | 2270–2780 | 99–234 | 5251–7123 | Sierra Nevada s.l. (SN): Sierra Nevada | Dystric regosols, Stones and rocks |
| Hormathophylla spinosa (L.) P. Küpfer | Brassicaceae | To, P, Pp, Pv, Cpm | 2, 1, + | 15–20% | 2310–3040 | 82–247 | 4058–7074 | Mediterranean region (Med.): Western Oro-Mediterranean | Humic cambisols, Dystric regosols, Stones and rocks |
| Paronychia polygonifolia (Vill.) DC. | Caryophyllaceae | Pp, To | + | 30–80% | 2310–2790 | 82–224 | 4058–5410 | Mediterranean region (Med.) | Humic cambisols, Dystric regosols, Stones and rocks |
| Dactylis glomerata subsp. juncinella (Bory) Stebbins & Zohary | Poaceae | P, Pp, Pv | 1, + | 15–50% | 2350–3040 | 190–234 | 3821–7074 | Baetic ranges (Baetic): Betic-Maghrebi | Dystric regosols, Stones and rocks |
| Festuca clementei Lam. | Poaceae | P, Pp, Pv, Cpm | 3, 2, 1 | 10–75% | 2350–3040 | 190–247 | 3821–7074 | Sierra Nevada s.l. (SN): Sierra Nevada | Dystric regosols, Stones and rocks |
| Reseda complicata Bory | Resedaceae | To, P, Pp, Pv, Cpm, En | 3, 2, 1 | 10–75% | 2250–3050 | 179–247 | 3821–7186 | Sierra Nevada s.l. (SN): Sierra Nevada | Dystric regosols, Stones and rocks |
| Holcus caespitosus Boiss. | Poaceae | To, Pp, Pv, Cpm | 1, + | 2–15% | 2310–2930 | 179–236 | 4058–7186 | Sierra Nevada s.l. (SN): Sierra Nevada | Dystric regosols, Stones and rocks |
| Epilobium alsinifolium Vill. | Onagraceae | Bo | 1, + | 40–75% | 2250–2890 | 198–234 | 4679–6359 | Wide distribution (Amp.): European | Humic cambisols, Dystric regosols, Stones and rocks |
| Epilobium anagallidifolium Lam. | Onagraceae | Bo | + | 40–75% | 2250–2860 | 198–234 | 4679–5593 | Alpine species (Alp.): Circumboreal | Humic cambisols, Dystric regosols |
| Arabis alpina L. | Brassicaceae | En, Cpm, Pp, Pv | 1 | 5% | 2250–3040 | 190–247 | 4892–7074 | Alpine species (Alp.): Holartic | Humic cambisols, Dystric regosols, Stones and rocks |
| Trisetum glaciale (Bory) Boiss. | Poaceae | Pp, To | 1, + | 25–30% | 2340–3040 | 190–236 | 4765–6531 | Sierra Nevada s.l. (SN): Sierra Nevada | Dystric regosols, Stones and rocks |
References
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Walther, G.R.; Beißner, S.; Burga, C.A. Trends in the upward shift of alpine plants. J. Veg. Sci. 2005, 16, 541–548. [Google Scholar] [CrossRef]
- Cannone, N.; Diolaiuti, G.; Guglielmin, M.; Smiraglia, C. Accelerating climate change impacts on alpine glacier forefield ecosystems in the European Alps. Ecol. Appl. 2008, 18, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Stöckli, V.; Wipf, S.; Nilsson, C.; Rixen, C. Using historical plant surveys to track biodiversity on mountain summits. Plant Ecol. Divers. 2012, 4, 415–425. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.F.; et al. Recent plant diversity changes on Europe’s mountain summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Benito Alonso, J.L.; Coldea, G.; Dick, J.; Erschbamer, B.; Fernández Calzado, M.R.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Rixen, C.; Wipf, S.; Frei, E.; Stöckli, V. Faster, higher, more? Past, present and future dynamics of alpine and arctic flora under climate change. Alp. Bot. 2014, 124, 77–79. [Google Scholar] [CrossRef]
- Roth, T.; Plattner, M.; Amrhein, V. Plants, birds and butterflies: Short-term responses of species communities to Climate Warming vary by taxon and with altitude. PLoS ONE 2014, 9, e82490. [Google Scholar] [CrossRef]
- Giménez-Benavides, L.; Escudero, A.; García-Camacho, R.; García-Fernández, A.; Iriondo, J.M.; Lara-Romero, C.; Morente-López, J. How does climate change affect regeneration of Mediterranean high-mountain plants? An integration and synthesis of current knowledge. Plant Biol. 2018, 20, 50–62. [Google Scholar] [CrossRef]
- Kadereit, J.W. Adaptive evolutionary divergence of populations persisting in warming cold-stage refugia: Candidate examples from the periphery of the European Alps. Alp. Bot. 2023, 133, 1–10. [Google Scholar] [CrossRef]
- Lamprecht, A.; Pauli, H.; Fernández Calzado, M.R.; Lorite, J.; Molero Mesa, J.; Steinbauer, K.; Winkler, M. Changes in plant diversity in a water-limited and isolated high-mountain range (Sierra Nevada, Spain). Alp. Bot. 2021, 131, 27–39. [Google Scholar] [CrossRef]
- Pickering, C.M.; Harrington, J.; Worboys, G. Environmental Impacts of Tourism on the Australian Alps Protected Areas. Mt. Res. Dev. 2003, 23, 247–254. [Google Scholar] [CrossRef]
- Fort, M. Impact of climate change on mountain environment dynamics. Rev. Géographie Alp. 2015, 103, 2. [Google Scholar] [CrossRef]
- Nogués Bravo, D.; Araújo, M.B.; Lasanta, T.; López Moreno, J.I. Climate change in Mediterranean mountains during the 21st century. Ambio 2008, 37, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Zamora, R.; Hódar, J.A.; Gómez, J.M.; Gómez-Aparicio, L. Benefits of using shrubs as nurse plants for reforestation in Mediterranean mountains: A 4-year study. Restor. Ecol. 2004, 12, 352–358. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Zamora Rodríguez, R.; Gómez, J.M.; Hódar, J.A.; Castro, J.; Baraza, E. Applying plant facilitation to forest restoration: A meta-analysis of the use of shrubs as nurse plants. Ecol. Appl. 2004, 14, 1128–1138. [Google Scholar] [CrossRef]
- Giménez-Benavides, L.; Escudero, A.; Iriondo, J.M. Local adaptation enhances seedling recruitment along an altitudinal gradient in a high mountain mediterranean Plant. Ann. Bot. 2007, 99, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Köner, C. Alpine Plant Life, 2nd ed.; Springer: Heidelberg, Germany, 2003. [Google Scholar]
- Rumpf, S.B.; Semenchuk, P.R.; Dullinger, S.; Cooper, E.J. Idiosyncratic responses of high Artic plants to changing snow regimes. PLoS ONE 2014, 9, e86281. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.K. Adaptive management of natural resources-framework and issues. J. Environ. Manag. 2011, 92, 1346–1353. [Google Scholar] [CrossRef]
- Loftin, M.K. Truths and governance for adaptive management. Ecol. Soc. 2014, 19, 21. [Google Scholar] [CrossRef]
- Vázquez-Ramírez, J.; Venn, S.E. Seeds and seedlings in a changing world: A systematic review and meta-analysis from high altitude and high latitude ecosystems. Plants 2021, 10, 768. [Google Scholar] [CrossRef]
- Hock, R.; Rasul, G.; Adler, C.; Cáceres, B.; Gruber, S.; Hirabayashi, Y.; Jackson, M.; Kääb, A.; Kang, S.; Kutuzov, S.; et al. High mountains areas. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 131–202. [Google Scholar]
- Jones, H.G. The ecology of snow-covered systems: A brief overview of nutrient cycling and life in the cold. Hydrol. Process. 1999, 13, 2135–2147. [Google Scholar] [CrossRef]
- Aerts, R.; Cornelissen, J.H.C.; Dorrepaal, E.; van Logtestijn, R.S.P.; Callaghan, T.V. Effects of experimentally imposed climate scenarios on flowering phenology and flower production of subarctic bog species. Glob. Chang. Biol. 2004, 10, 1599–1609. [Google Scholar] [CrossRef]
- Vilagrosa, A.; Cortina, J.; Gil-Pelegrín, E.; Bellot, J. Suitability of drought-preconditioning techniques in Mediterranean climate. Restor. Ecol. 2003, 11, 208–216. [Google Scholar] [CrossRef]
- Laternser, M.; Schneebeli, M. Long-term snow climate trends of the Swiss Alps (1931-99). Int. J. Climatol. 2003, 23, 733–750. [Google Scholar] [CrossRef]
- Bonet, F.J.; Pérez-Luque, A.J.; Perez-Perez, R. 2.4. Trend analysis (2000–2014) of the snow cover by satellite (MODIS sensor). In Sierra Nevada Global-Change Observatory: Monitoring Methodologies; Aspizua, R., Barea, J.M., Bonet, F.J., Pérez-Luque, A.J., Zamora, R., Eds.; Junta de Andalucía, Consejería de Medio Ambiente y Ordenación del Territorio: Granada, Spain, 2016; pp. 43–47. [Google Scholar]
- Domisch, T.; Martz, F.; Repo, T.; Rautio, P. Winter survival of Scots pine seedlings under different snow conditions. Tree Physiol. 2018, 38, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.Z.; Corona, M.C.; Dentant, C.; Bonet, R.; Thuiller, W.; Choler, P. Observed long-term greening of alpine vegetation—A case study in the French Alps. Environ. Res. Lett. 2017, 12, 114006. [Google Scholar] [CrossRef]
- Gao, X.; Giorgi, F. Increased aridity in the Mediterranean Region under greenhouse gas forcing estimated from high resolution simulations with a regional climate model. Glob. Planet. Chang. 2008, 62, 195–209. [Google Scholar] [CrossRef]
- Boissier, P.E. Voyage Botanique dans le Midi de L’Espagne Pendant L´Année 1837; Tome, I., Ed.; Gide et Cie, Libraires-Éditeurs: París, France, 1839–1845. [Google Scholar]
- Willkomm, H.M. Grundzüge der Pflanzenverbreitung auf der Iberischen Halbinsel. In Die Vegetation der Erde; Engler, A., Drude, O., Eds.; Biodiversity Heritage Library: Leipzig, Germany, 1896. [Google Scholar]
- Clemente, S.R. Tentativa sobre la liquenología geográfica de Andalucía; por D Simón de Rojas Clemente. Rev. de los Prog. de las Cienc. Exactas Físicas y Nat. 1864, 14, 39–58. [Google Scholar]
- Ozenda, P. Sur les étages de végétation dans les montagnes du bassin méditerranéen. Doc. Cartogr. Ecol. 1975, 16, 75. [Google Scholar] [CrossRef]
- Quézel, P. La Région Méditerranéenne française et ses essences forestières. Signification écologique dans le contexte circum-méditerranéen. Forêt Méditerr. 1979, 1, 7–18. [Google Scholar]
- Rivas Martínez, S. Los pisos de la vegetación de la Sierra Nevada. Bol. Real Soc. Esp. Hist. Nat. Secc. Biol. 1961, 59, 55–64. [Google Scholar]
- Rivas Martínez, S. Etages bioclimatiques, secteurs chorologiques et series de vegetation de l’Espagne méditerranéene. Ecol. Mediterr. 1982, 8, 275–288. [Google Scholar] [CrossRef]
- Rivas Martínez, S. Pisos bioclimáticos de España. Lazaroa 1983, 5, 33–43. [Google Scholar]
- Rivas Martínez, S. Global Bioclimatics; Centro de Investigaciones Fitosociológicas: Madrid, Spain, 2004. [Google Scholar]
- Blanca, G.; Cueto, M.; Martinez-Lirola, M.J.; Molero, J. Threatened vascular flora of Sierra Nevada (Southern Spain). Biol. Conserv. 1998, 85, 269–285. [Google Scholar] [CrossRef]
- Blanca, G.; López Onieva, M.R.; Lorite, J.; Martínez Lirola, M.J.; Molero, J.; Quintas, S.; Ruíz Girela, M.; Varo, M.A.; Vidal, S. Flora Amenazada y Endémica de Sierra Nevada; Universidad de Granada, Consejería de Medio Ambiente, Junta de Andalucía: Granada, Spain, 2001. [Google Scholar]
- Cueto, M.; Blanca, G.; Salazar, C.; Cabezudo, B. Diversity and ecological characteristics of the vascular flora in the Western Mediterranean (Eastern Andalusia, Spain). Acta Bot. Malacit. 2014, 39, 81–97. [Google Scholar] [CrossRef][Green Version]
- Algarra, J.A.; Checa, R.; Irurita, J.M. Programa de Recuperación de Flora de Altas Cumbres de Andalucía, Tomo 1; Empresa de Gestión Medioambiental SA, Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2006.
- Wahren, C.H.; Walker, M.D.; Bret-Harte, M.S. Vegetation responses in Alaskan artic tundra after 8 years of a summer warming and winter snow manipulation experiment. Glob. Chang. Biol. 2005, 5, 537–552. [Google Scholar] [CrossRef]
- Billings, W.D.; Mooney, H.A. The ecology of Arctic and Alpine plants. Biol. Rev. 1968, 43, 481–529. [Google Scholar] [CrossRef]
- Wipf, S.; Rixen, C. A review of snow manipulation experiments in Artic and alpine tundra ecosystems. Polar Res. 2010, 29, 95–109. [Google Scholar] [CrossRef]
- Cabezudo, B.; Talavera, S.; Blanca, G.; Salazar, C.; Cueto, M.; Valdés, B.; Hernández Bermejo, J.E.; Herrera, C.M.; Rodríguez Hiraldo, M.C.; Navas, D. Lista Roja de la Flora Vascular de Andalucía; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2005.
- Moreno, J.C. Lista Roja 2008 de la Flora Vascular Española; Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente, y Medio Rural y Marino: Madrid, Spain, 2008.
- Kitajima, K.; Fenner, M. Ecology of seedling regeneration. In Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CAB International: Wallingford, UK, 2000; pp. 331–359. [Google Scholar] [CrossRef]
- Jonas, T.; Rixen, C.; Sturm, M.; Stöckli, V. How alpine plant growth is linked to snow cover and climate variability. J. Geophys. Res. Biogeosci. 2008, 113, G03013. [Google Scholar] [CrossRef]
- Köner, C. The use of “altitude” in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Gégeut, J.C.; Marquet, P.A.; Ruffray, P.D.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Matteodo, M.; Wipf, S.; Stöckli, V.; Rixen, C.; Vittoz, P. Elevation gradient of successful plant traits for colonizing alpine summits under climate change. Environ. Res. Lett. 2013, 8, 024043. [Google Scholar] [CrossRef]
- Corripio, J.G. Snow surface albedo estimation using terrestrial photography. Int. J. Remote Sens. 2004, 25, 5705–5729. [Google Scholar] [CrossRef]
- Herrero, J. Modelo Físico de Acumulación y Fusión de la Nieve. Aplicación en Sierra Nevada (España). Master’s Thesis, Universidad de Granada, Granada, Spain, 2007. [Google Scholar]
- Lorite, J.; Ros-Candeira, A.; Alcaraz-Segura, D.; Salazar-Mendías, C. FloraSNevada: A trait database of the vascular flora of Sierra Nevada, southeast Spain. Ecology 2020, 101, e03091. [Google Scholar] [CrossRef] [PubMed]
- Instituo Geográfico Nacional. Vértices de Las Redes Geodésicas REGENTE y ROI; Red de Infraestructuras Geodésicas: Madrid, Spain, 2023. [Google Scholar]
- Pohl, M.; Alig, D.; Körner, C.; Rixen, C. Higer plant diversity enhances soil stability in disturbed alpine ecosystems. Plant Soil 2009, 324, 91–102. [Google Scholar] [CrossRef]
- Schupp, E.W. Seed-Seedling Conflicts, Habitat Choice, and Patterns of Plant Recruitment. Am. J. Bot. 1995, 82, 399–409. [Google Scholar] [CrossRef]
- Kameswara Rao, N.K.; Hanson, J.; Dulloo, M.E.; Ghosh, K.; Nowell, D.; Larinde, M. Manual of Seed Handling in Genebanks. Handbooks for Genebanks; Genebanks, No. 8; Bioversity International: Rome, Italy, 2006. [Google Scholar]
- Grossnickle, S.C. Why seedlings survive: Influence of plant attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- Blanca, G.; Cabezudo, B.; Cueto, M.; Salazar, C.; Morales, C. Flora Vascular de Andalucía Oriental; Granada, Universidades de Almería, Granada, Jaén y Málaga: Granada, Spain, 2011. [Google Scholar]
- Eriksson, O. Seedling Dynamics and Life Histories in Clonal Plants. Oikos 1989, 55, 231–238. [Google Scholar] [CrossRef]
- Wookey, P.A.; Robinson, C.H.; Parsons, A.N.; Welker, J.M.; Press, M.C.; Callaghan, T.V.; Lee, J.A. Environmental constraints on the growth, photosynthesis and reproductive development of Dryas octopetala at a high Arctic polar semi-desert, Svalbard. Oecologia 1995, 102, 478–489. [Google Scholar] [CrossRef]
- Tremblay, M.F.; Bergeron, Y.; Lalonde, D.; Mauffette, Y. The potential effects of sexual reproduction and seedling recruitment on the maintenance of red maple (Acer rubrum L.) populations at the northern limit of the species range. J. Biogeogr. 2002, 29, 365–373. [Google Scholar] [CrossRef]
- Molero Mesa, J.; Pérez Raya, F.; López Nieto, J.M.; El Aallali, A.; Hita Fernández, J.A. Cartografía y Evaluación de la Vegetación del Parque Natural de Sierra Nevada. Segunda Fase; Universidad de Granada, Consejería de Medio Ambiente de la Junta de Andalucía: Granada, Spain, 2001. [Google Scholar]
- Tripathi, R.S.; Khan, M.L. Effects of seed weight and microsite characteristics on germination and seedling fitness in two species of Quercus in a subtropical Wet Hill Forest. Oikos 1990, 57, 289–296. [Google Scholar] [CrossRef]
- HilleRisLambers, J.; Harsch, M.A.; Ettinger, A.K.; Ford, K.R.; Theobald, E.J. How will biotic interactions influence climate change-induced range shifts? Ann. N. Y. Acad. Sci. 2013, 1297, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Algarra, J.A.; Herrero, J. Snow cover dynamics at the summits of Sierra Nevada. In Sierra Nevada Global-Change Observatory: Monitoring Methodologies; Aspizua, R., Barea, J.M., Bonet, F.J., Pérez-Luque, A.J., Zamora, R., Eds.; Junta de Andalucía. Consejería de Medio Ambiente y Ordenación del Territorio: Granada, Spain, 2014; pp. 32–37. [Google Scholar]
- Algarra, J.A.; Herrero, J. Analysis of temporal changes in the cryosphere. In Global Change Impacts in Sierra Nevada: Challenges for Conservation; Zamora, R., Pérez-Luque, A.J., Bonet, F.J., Barea-Azcón, J.M., Aspizua, R., Eds.; Consejería de Medio Ambiente y Ordenación del Territorio: Granada, Spain, 2016; pp. 34–38. [Google Scholar]
- Gómez Ortiz, A.; Palacios Estremera, D.; Ramos Sainz, M. Permafrost, evolución de formas asociadas y comportamiento térmico en el Corral del Veleta (Sierra Nevada, España). Últimos resultados. Real Soc. Española Hist. Nat. (Secc. Geol.) 2004, 99, 47–63. [Google Scholar]
- ICA. Mapa Topográfico de Andalucía Ráster Color a Escala 1:10.000; Consejería de Vivienda y Ordenación del Territorio: Sevilla, Spain, 2004.
- Aguilar, J.; Martín Peinado, F.; Diez Ortíz, M.; Sierra, M.; Fernández García, J.; de la Fuente, C.S.R.; Ortega, E.; Oyonate, C. Proyecto LUCDEME. Mapa Digital de Suelos. Provincia de Granada; Dirección General para la Biodiversidad, Ministerio de Medio Ambiente, Universidad de Granada: Madrid, Spain, 2006. [Google Scholar]
- CAPMA. Mapa de Radiación Solar de Andalucía a Escala de Detalle: Radiación Total Media Anual Para el Año 2010; Servicio de Información y Evaluación Ambiental, Consejería de Agricultura, Pesca y Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2010.
- Martínez Parras, J.M.; Lorca, M.P.; Alcaraz Ariza, F. Comunidades Vegetales de Sierra Nevada (España); Universidad de Alcalá de Henares: Madrid, Spain, 1987. [Google Scholar]
- Pérez Raya, F.A.; López Nieto, J.M.; Molero, J.; Valle, F. Vegetación de Sierra Nevada. Guía Geobotánica de la Excursión de las X Jornadas de Fitosociología; Ayuntamiento de Granada, Universidad de Granada: Granada, Spain, 1990. [Google Scholar]
- Rivas Martínez, S.; Díaz, T.E.; Fernández-González, F.; Izco, J.; Loidi, J.; Lousã, M.; Penas, A. Vascular plant communities of Spain and Portugal. Addenda to the syntaxonomical checklist of 2001. Itinera Geobot. 2002, 15, 5–922. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. QGIS Association. Available online: https://qgis.org (accessed on 1 June 2022).
- McCullagh, P.; Nelder, J.A. Generalized Linear Models; Chapman and Hall: New York, NY, USA, 1989. [Google Scholar]
- Algarra, J.A.; Cariñanos, P.; Herrero, J.; Delgado-Capel, M.; Ramos-Lorente, M.M.; Díaz de la Guardia, C. Tracking Montane Mediterranean grasslands: Analysis of the effects of snow with other related hydro-meteorological variables and land-use change on pollen emissions. Sci. Total Environ. 2019, 649, 889–901. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]

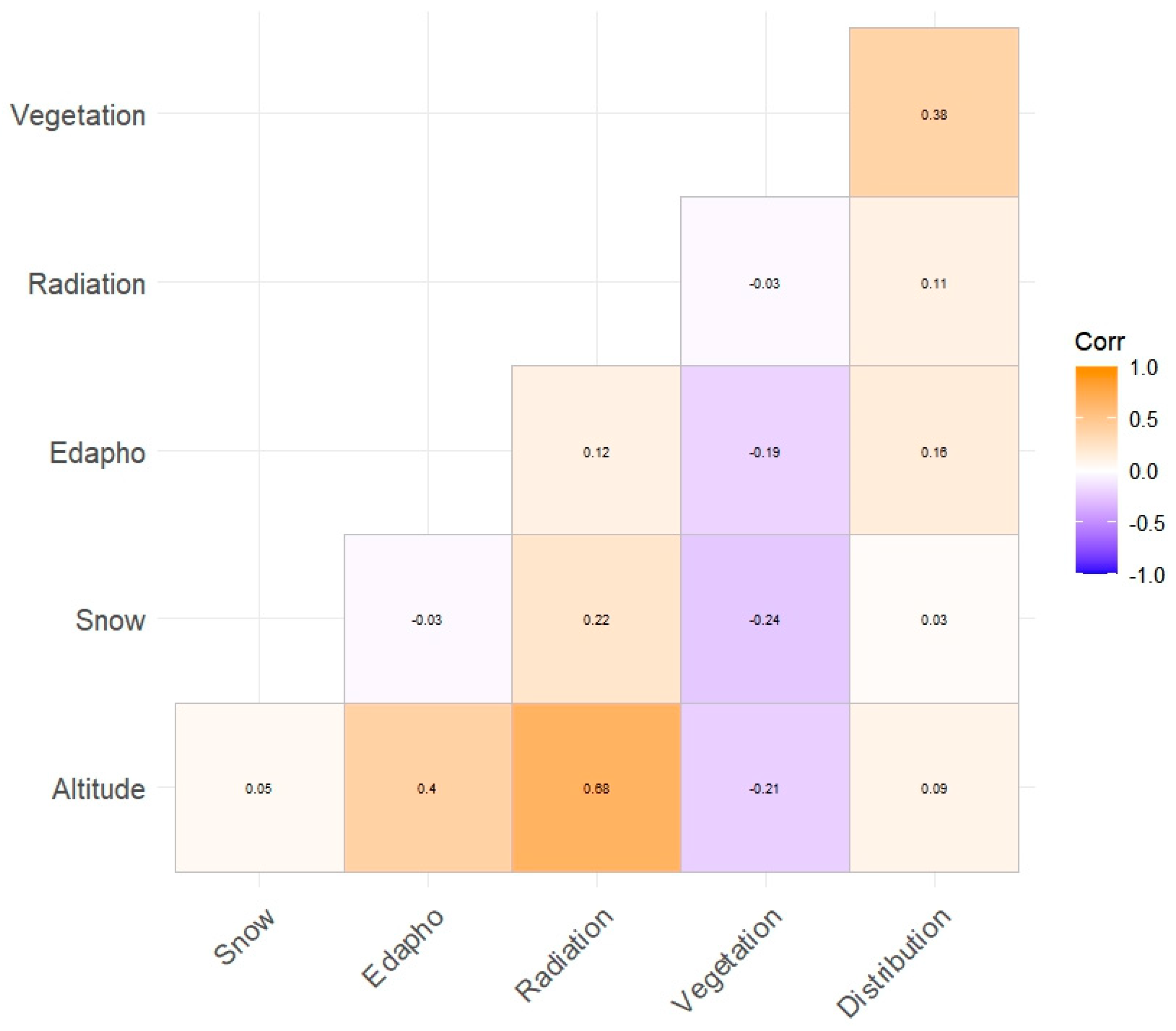
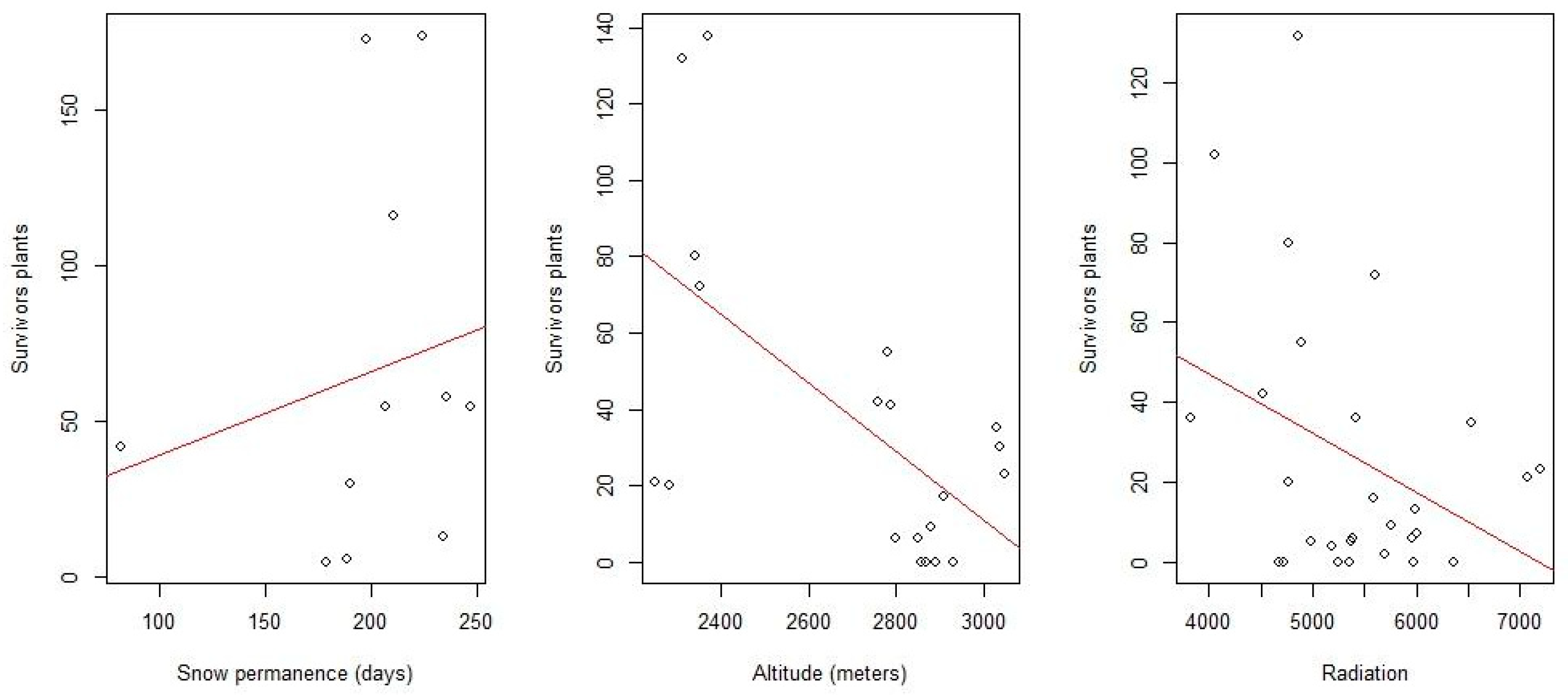
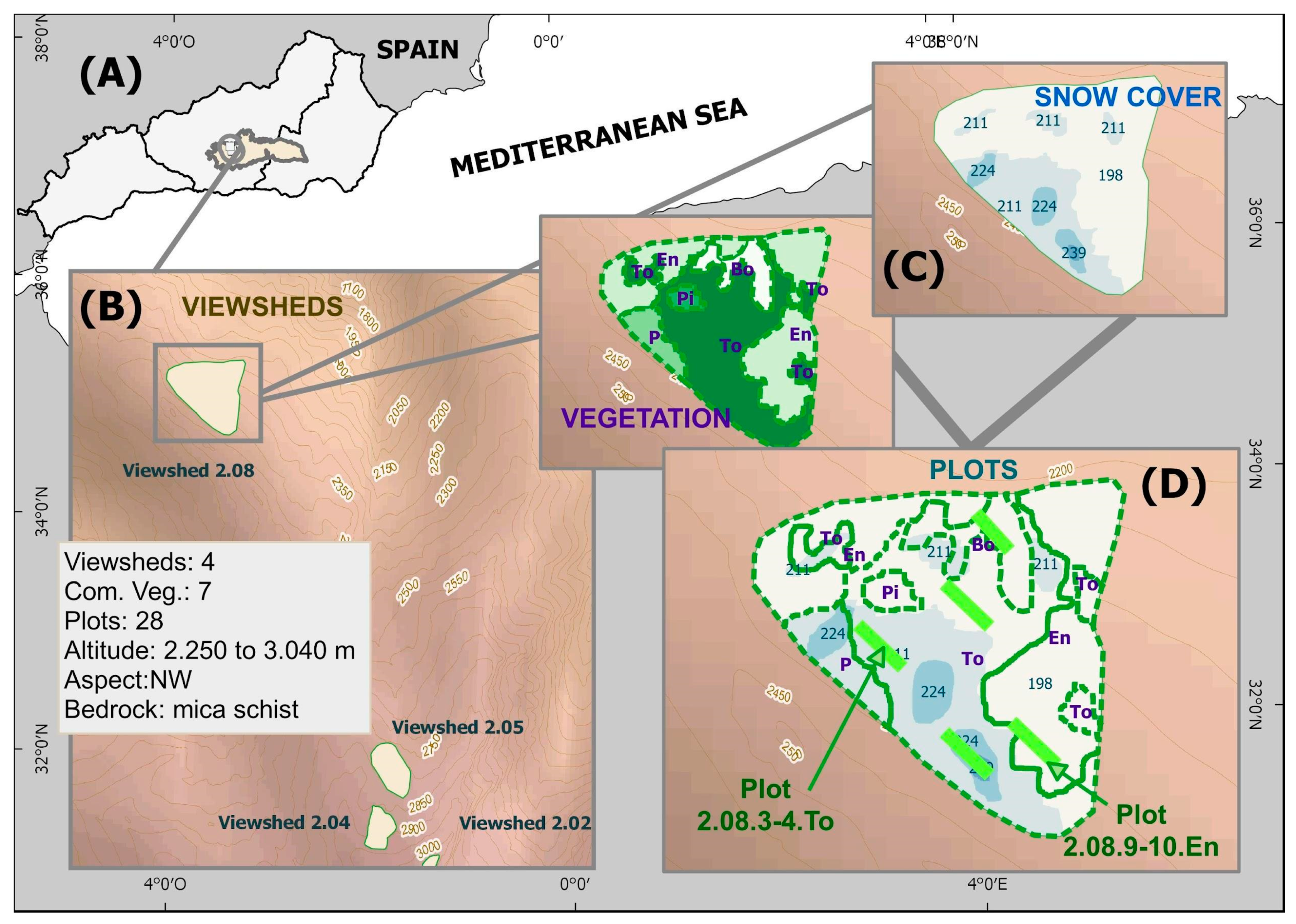

| Variable | Normality | |
|---|---|---|
| Snow | 0.17564 | *** |
| Altitude | 0.25439 | *** |
| Edaphology | 426.47 | · |
| Plant Community | 3690.3 | * |
| Radiation | 15,701 | *** |
| Distribution Range | 1531.9 | · |
| Variable | χ2 Tests | |
|---|---|---|
| Snow | 649.5425, df = 10 | *** |
| Altitude | 728.9778, df = 19 | *** |
| Edaphology | 114.3517, df = 2 | *** |
| Plant Community | 85.31395, df = 4 | *** |
| Radiation | 820.7352, df = 27 | *** |
| Distribution Range | 591.4444; df = 6 | *** |
| Variable | Df | Deviance | Resid. Df | Resid. Dev | |
|---|---|---|---|---|---|
| NULL | 3458 | 3557.168008 | - | ||
| Altitude | 1 | 479.069 | 3457 | 3078.098671 | *** |
| Snow | 1 | 10.032 | 3456 | 3068.066032 | ** |
| Edaphology | 2 | 51.033 | 3454 | 3017.032695 | *** |
| Radiation | 23 | 349.725 | 3431 | 2667.307451 | *** |
| Plant Community | 7 | 217.1368 | 4416 | 4212.44011 | - |
| Distribution | 4 | 46.521 | 3427 | 2620.786436 | *** |
| Estimate | Std. Error | z Value | ||
|---|---|---|---|---|
| (Intercept) | 0.55799 | 1.77291 | 0.31473 | |
| Altitude | −0.00276 | 0.00045 | −6.15907 | *** |
| Snow | 0.02445 | 0.00739 | 3.30855 | *** |
| Edaphology: Stones and Rocks | −3.39597 | 0.86870 | −3.90925 | *** |
| Radiation: 4514 | 6.11479 | 1.21077 | 5.05031 | *** |
| Radiation: 4855 | 0.57840 | 0.32850 | 1.76073 | · |
| Radiation: 4892 | 2.74985 | 0.44423 | 6.19013 | *** |
| Radiation: 5177 | 1.27587 | 0.68270 | 1.86885 | · |
| Radiation: 5386 | 2.73445 | 0.84363 | 3.24129 | *** |
| Radiation: 5410 | 1.68823 | 0.46968 | 3.59439 | *** |
| Radiation: 5595 | −0.71829 | 0.32168 | −2.23293 | * |
| Radiation: 5755 | −0.87135 | 0.41315 | −2.10906 | * |
| Radiation: 5955 | 2.63749 | 0.68570 | 3.84639 | *** |
| Radiation: 5981 | 2.40640 | 0.53763 | 4.47590 | *** |
| Distribution: Alp. | −0.75441 | 0.36463 | −2.06897 | * |
| Distribution: Med. | 1.80713 | 0.68356 | 2.64369 | ** |
| Distribution: Baetic | 2.14136 | 0.69423 | 3.08449 | ** |
| Distribution: SN | 1.46485 | 0.67081 | 2.18372 | * |
| Data Levels and Range | Units | |
|---|---|---|
| Snow | 82–247 | days |
| Altitude | 2250–3050 | meters |
| Edaphology | Dystric regosols, humic cambisols, stones and rocks | type |
| Plant Community | Mire (Bo); scree vegetation (dwarf shrub) 2 (Cpm); shrub (juniper) (En); Festuca pastures (grassland) (P); psycro-xerophilous pastures (grassland) (Pp); snowdrift grasslands (Pv); dwarf shrubs (To) | type |
| Radiation | 3821–7365 (10 m accuracy in data) | MJ·m−2 |
| Distribution Range | Wide distribution (Amp.); alpine species (Alp); Mediterranean region (Med.); Baetic ranges (Baetic); Sierra Nevada s.l. 1 (SN) | areas |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algarra, J.A.; Cariñanos, P.; Ramos-Lorente, M.M. The Role of Snow-Related Environmental Variables in Plant Conservation Plans in the Mediterranean Mountains. Plants 2024, 13, 783. https://doi.org/10.3390/plants13060783
Algarra JA, Cariñanos P, Ramos-Lorente MM. The Role of Snow-Related Environmental Variables in Plant Conservation Plans in the Mediterranean Mountains. Plants. 2024; 13(6):783. https://doi.org/10.3390/plants13060783
Chicago/Turabian StyleAlgarra, Jose A., Paloma Cariñanos, and María M. Ramos-Lorente. 2024. "The Role of Snow-Related Environmental Variables in Plant Conservation Plans in the Mediterranean Mountains" Plants 13, no. 6: 783. https://doi.org/10.3390/plants13060783
APA StyleAlgarra, J. A., Cariñanos, P., & Ramos-Lorente, M. M. (2024). The Role of Snow-Related Environmental Variables in Plant Conservation Plans in the Mediterranean Mountains. Plants, 13(6), 783. https://doi.org/10.3390/plants13060783







