Assembly and Repair of Photosystem II in Chlamydomonas reinhardtii
Abstract
:1. Introduction
2. Discussion
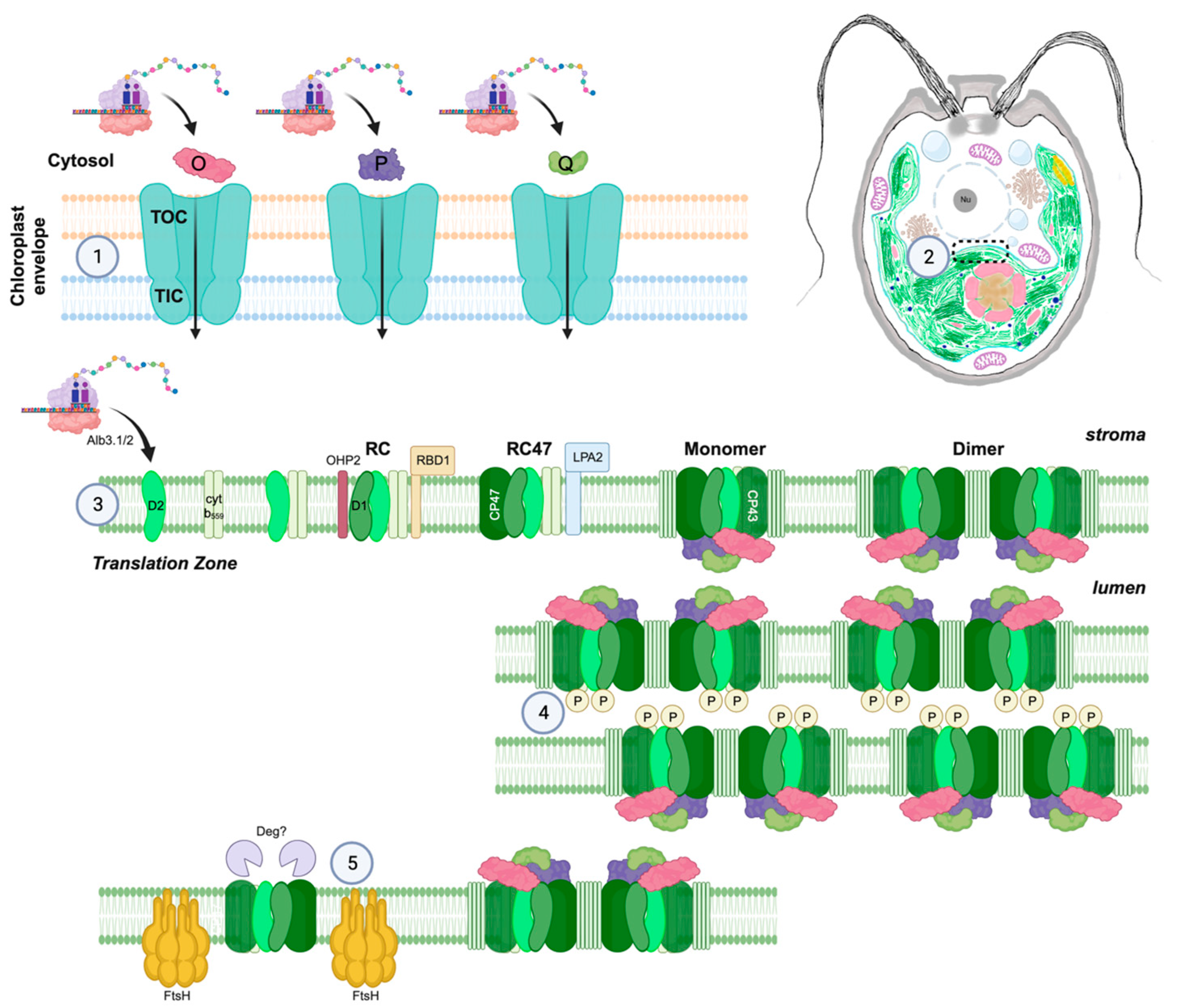
2.1. Architecture of the Chlamydomonas chloroplast
2.2. Transcription of PSII Subunits in the Chloroplast
2.3. Translation of PSII Subunits in the Chloroplast—Control by Epistasy of Synthesis
2.4. Translation of PSII Subunits in the Chloroplast—Regulatory Elements
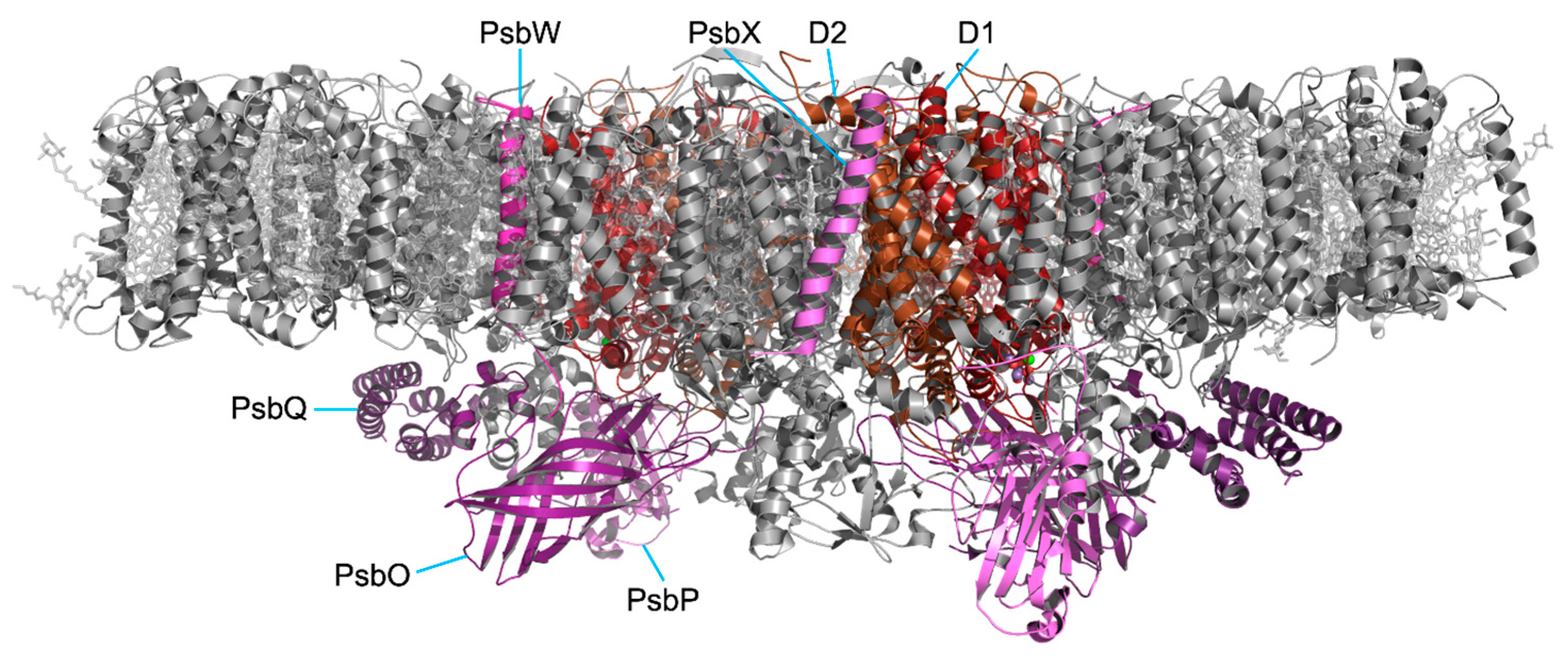
2.5. Assembly of Protein Subunits and Cofactors
2.6. PSII Phosphorylation and Dephosphorylation
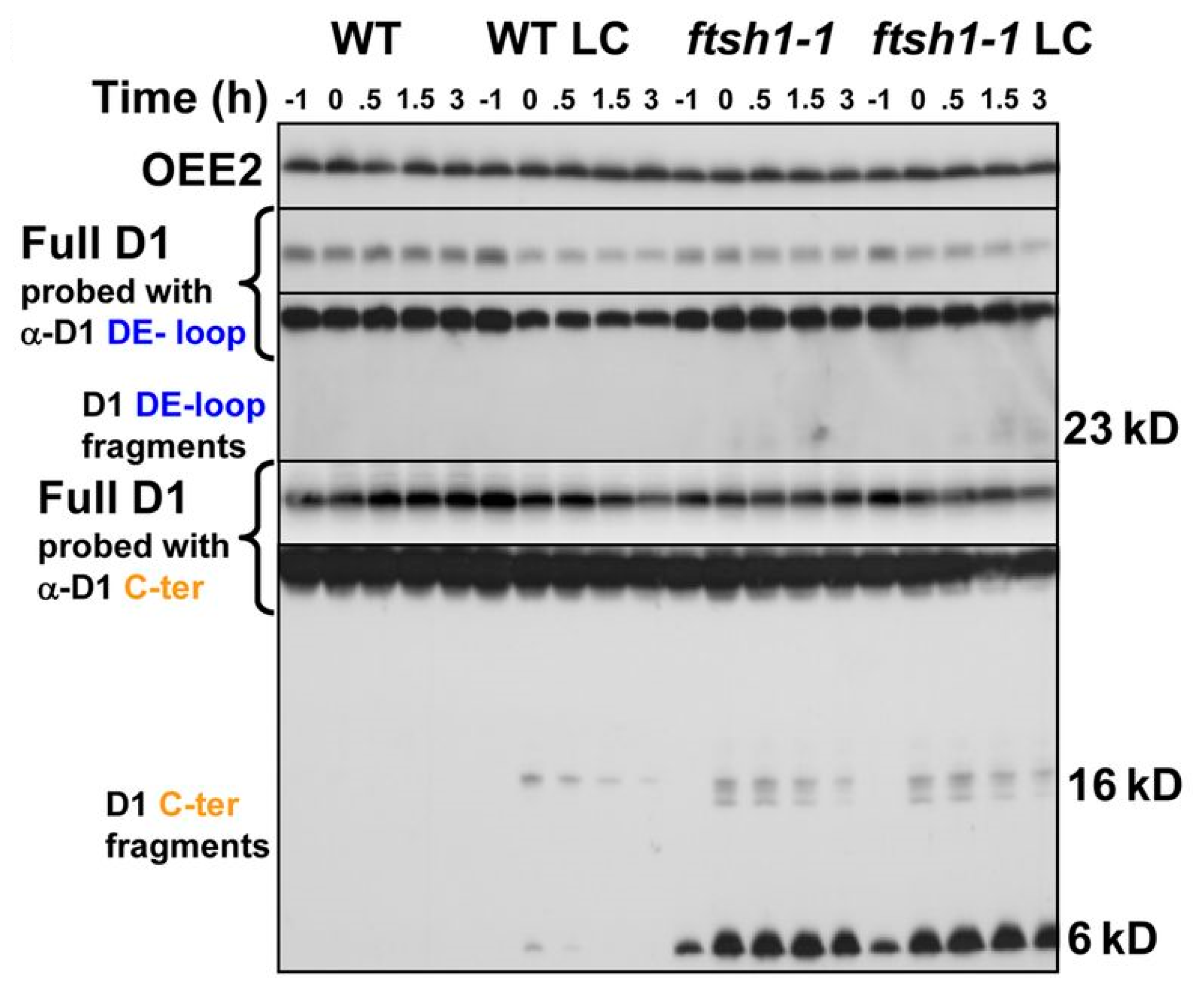
2.7. Proteolysis of the D1 Subunit
2.8. PSII Repair
3. Conclusions
- PSII assembly in Chlamydomonas provides an excellent model system for the evolution and interplay between nuclear and organellar genomes.
- The CES mechanism, which is well studied in terms of PSII assembly in Chlamydomonas, is applicable to multiple protein complexes in the chloroplast and other systems.
- Analogously, the extensive translational control of PSII subunits in the Chlamydomonas chloroplast has revealed gene regulation strategies.
- The PSII phosphorylation, dephosphorylation, and degradation pathways in Chlamydomonas show intermediate mechanisms between cyanobacteria and plants, thus providing insights into evolution of photosynthetic organisms.
4. Remaining Questions
- What are the molecular mechanisms that allow chloroplast protein import and chloroplast protein synthesis to be coordinated?
- Why are the genes that encode cytochrome b559 separated in the Chlamydomonas chloroplast genome?
- What is the full suite of regulatory elements that control the translation of PSII subunits in the chloroplast?
- What are the specific triggers for PSII core subunit phosphorylation and dephosphorylation?
- Which protease(s) degrades D1 into fragments before FtsH processing?
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis, 3rd, ed.; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Shevela, D.; Kern, J.F.; Govindjee, G.; Whitmarsh, J.; Messinger, J. Photosystem II. In eLS; Wiley: Hoboken, NJ, USA, 2021; pp. 1–16. [Google Scholar]
- Redding, K.E.; Santabarbara, S. Photosystems I and II. In The Chlamydomonas Sourcebook; Elsevier: Amsterdam, The Netherlands, 2023; pp. 525–560. [Google Scholar]
- Shen, J.-R. The Structure of Photosystem II and the Mechanism of Water Oxidation in Photosynthesis. Annu. Rev. Plant Biol. 2015, 66, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, W.; Chrysina, M.; Cox, N. Water oxidation in photosystem II. Photosynth. Res. 2019, 142, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Vinyard, D.J.; Brudvig, G.W. Progress toward a Molecular Mechanism of Water Oxidation in Photosystem II. Annu. Rev. Phys. Chem. 2017, 68, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Nickelsen, J.; Rengstl, B. Photosystem II Assembly: From Cyanobacteria to Plants. Annu. Rev. Plant Biol. 2013, 64, 609–635. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.M.; Pakrasi, H.B. Advances in the Understanding of the Lifecycle of Photosystem II. Microorganisms 2022, 10, 836. [Google Scholar] [CrossRef]
- Young, K.J.; Brennan, B.J.; Tagore, R.; Brudvig, G.W. Photosynthetic Water Oxidation: Insights from Manganese Model Chemistry. Acc. Chem. Res. 2015, 48, 567–574. [Google Scholar] [CrossRef]
- Chiu, Y.-F.; Chu, H.-A. New Structural and Mechanistic Insights into Functional Roles of Cytochrome b559 in Photosystem II. Front. Plant Sci. 2022, 13, 914922. [Google Scholar] [CrossRef] [PubMed]
- Müh, F.; Zouni, A. The nonheme iron in photosystem II. Photosynth. Res. 2013, 116, 295–314. [Google Scholar] [CrossRef]
- Sirohiwal, A.; Pantazis, D.A. Reaction Center Excitation in Photosystem II: From Multiscale Modeling to Functional Principles. Acc. Chem. Res. 2023, 56, 2921–2932. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.F. Why chloroplasts and mitochondria retain their own genomes and genetic systems: Colocation for redox regulation of gene expression. Proc. Natl. Acad. Sci. USA 2015, 112, 10231–10238. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Jiao, Q.; Jia, T.; Hu, X. The photosystem-II repair cycle: Updates and open questions. Planta 2023, 259, 20. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.; Kim, T.D.; Trinugroho, J.P.; Cordón-Preciado, V.; Wijayatilake, N.; Bhatia, A.; Rutherford, A.W.; Cardona, T. The Evolution and Evolvability of Photosystem II. Annu. Rev. Plant Biol. 2023, 74, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.W.; Hemp, J.; Johnson, J.E. Evolution of Oxygenic Photosynthesis. Annu. Rev. Earth Planet. Sci. 2016, 44, 647–683. [Google Scholar] [CrossRef]
- Moore, G.F.; Brudvig, G.W. Energy Conversion in Photosynthesis: A Paradigm for Solar Fuel Production. Annu. Rev. Condens. Matter Phys. 2011, 2, 303–327. [Google Scholar] [CrossRef]
- Berthold, D.A.; Babcock, G.T.; Yocum, C.F. A highly resolved, oxygen-evolving photosystem II preparation from spinach thylakoid membranes. FEBS Lett. 1981, 134, 231–234. [Google Scholar] [CrossRef]
- Greife, P.; Schönborn, M.; Capone, M.; Assunção, R.; Narzi, D.; Guidoni, L.; Dau, H. The electron–proton bottleneck of photosynthetic oxygen evolution. Nature 2023, 617, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Mino, H.; Asada, M. Location of two Mn2+ affinity sites in photosystem II detected by pulsed electron–electron double resonance. Photosynth. Res. 2022, 152, 289–295. [Google Scholar] [CrossRef]
- Wang, J.; Perez-Cruet, J.M.; Huang, H.-L.; Reiss, K.; Gisriel, C.J.; Banerjee, G.; Kaur, D.; Ghosh, I.; Dziarski, A.; Gunner, M.R.; et al. Identification of a Na+-Binding Site near the Oxygen-Evolving Complex of Spinach Photosystem II. Biochemistry 2020, 59, 2823–2831. [Google Scholar] [CrossRef]
- Debus, R.J. Protein ligation of the photosynthetic oxygen-evolving center. Coord. Chem. Rev. 2008, 252, 244–258. [Google Scholar] [CrossRef]
- Nixon, P.J.; Michoux, F.; Yu, J.; Boehm, M.; Komenda, J. Recent advances in understanding the assembly and repair of photosystem II. Ann. Bot. 2010, 106, 1–16. [Google Scholar] [CrossRef]
- Williams, J.G.K. [85] Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1988; Volume 167, pp. 766–778. [Google Scholar]
- Ghosh, I.; Khan, S.; Banerjee, G.; Dziarski, A.; Vinyard, D.J.; Debus, R.J.; Brudvig, G.W. Insights into Proton-Transfer Pathways during Water Oxidation in Photosystem II. J. Phys. Chem. B 2019, 123, 8195–8202. [Google Scholar] [CrossRef] [PubMed]
- Avramov, A.P.; Hwang, H.J.; Burnap, R.L. The role of Ca2+ and protein scaffolding in the formation of nature’s water oxidizing complex. Proc. Natl. Acad. Sci. USA 2020, 117, 28036–28045. [Google Scholar] [CrossRef]
- Russell, B.P.; Vinyard, D.J. Conformational changes in a Photosystem II hydrogen bond network stabilize the oxygen-evolving complex. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2024, 1865, 149020. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Tabata, S. Synechocystis sp. PCC 6803—A useful tool in the study of the genetics of cyanobacteria. Photosynth. Res. 2001, 70, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Vermaas, W. Molecular genetics of the cyanobacterium Synechocystis sp. PCC 6803: Principles and possible biotechnology applications. J. Appl. Phycol. 1996, 8, 263–273. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Guskov, A.; Kern, J.; Gabdulkhakov, A.; Broser, M.; Zouni, A.; Saenger, W. Cyanobacterial photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride. Nat. Struct. Mol. Biol. 2009, 16, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.N.; Iverson, T.M.; Maghlaoui, K.; Barber, J.; Iwata, S. Architecture of the Photosynthetic Oxygen-Evolving Center. Science 2004, 303, 1831–1838. [Google Scholar] [CrossRef]
- Kato, Y.; Noguchi, T. Redox properties and regulatory mechanism of the iron-quinone electron acceptor in photosystem II as revealed by FTIR spectroelectrochemistry. Photosynth. Res. 2022, 152, 135–151. [Google Scholar] [CrossRef]
- Noguchi, T. Fourier transform infrared difference and time-resolved infrared detection of the electron and proton transfer dynamics in photosynthetic water oxidation. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2015, 1847, 35–45. [Google Scholar] [CrossRef]
- Sugiura, M.; Boussac, A. Variants of photosystem II D1 protein in Thermosynechococcus elongatus. Res. Chem. Intermed. 2014, 40, 3219–3229. [Google Scholar] [CrossRef]
- Sugiura, M.; Inoue, Y. Highly Purified Thermo-Stable Oxygen-Evolving Photosystem II Core Complex from the Thermophilic Cyanobacterium Synechococcus elongatus Having His-Tagged CP43. Plant Cell Physiol. 1999, 40, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; Junge, W. Structure and Energy Transfer in Photosystems of Oxygenic Photosynthesis. Annu. Rev. Biochem. 2015, 84, 659–683. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, U. The Chlamydomonas Sourcebook: Volume 1: Introduction to Chlamydomonas and Its Laboratory Use, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Dupuis, S.; Merchant, S.S. Chlamydomonas reinhardtii: A model for photosynthesis and so much more. Nat. Methods 2023, 20, 1441–1442. [Google Scholar] [CrossRef] [PubMed]
- Sager, R.; Palade, G.E. Structure and development of the chloroplast in Chlamydomonas. I. The normal green cell. J. Cell Biol. 1957, 3, 463–488. [Google Scholar] [CrossRef]
- Gaffal, K.; Arnold, C.-G.; Friedrichs, G.; Gemple, W. Morphodynamical changes of the chloroplast of Chlamydomonas reinhardtii during the 1st round of division. Arch. Für Protistenkd. 1995, 145, 10–23. [Google Scholar] [CrossRef]
- Vallon, O.; Wollman, F.A.; Olive, J. Laterial distribution of the main protein complexes of the photosynthetic apparatus in Chlamydomonas reinhardtii and in spinach: An immunocytochemical study using intact thylakoid membranes and a PS II enriched membrane preparation. Photobiochem. Photobiophys. 1986, 12, 203–220. [Google Scholar]
- Wietrzynski, W.; Schaffer, M.; Tegunov, D.; Albert, S.; Kanazawa, A.; Plitzko, J.M.; Baumeister, W.; Engel, B.D. Charting the native architecture of Chlamydomonas thylakoid membranes with single-molecule precision. Elife 2020, 9, e53740. [Google Scholar] [CrossRef] [PubMed]
- Maul, J.E.; Lilly, J.W.; Cui, L.; DePamphilis, C.W.; Miller, W.; Harris, E.H.; Stern, D.B. The Chlamydomonas reinhardtii plastid chromosome: Islands of genes in a sea of repeats. Plant Cell 2002, 14, 2659–2679. [Google Scholar] [CrossRef] [PubMed]
- Uniacke, J.; Zerges, W. Photosystem II assembly and repair are differentially localized in Chlamydomonas. Plant Cell 2007, 19, 3640–3654. [Google Scholar] [CrossRef]
- Uniacke, J.; Zerges, W. Chloroplast protein targeting involves localized translation in Chlamydomonas. Proc. Natl. Acad. Sci. USA 2009, 106, 1439–1444. [Google Scholar] [CrossRef]
- Sun, Y.; Valente-Paterno, M.; Bakhtiari, S.; Law, C.; Zhan, Y.; Zerges, W. Photosystem Biogenesis Is Localized to the Translation Zone in the Chloroplast of Chlamydomonas. Plant Cell 2019, 31, 3057–3072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Paakkarinen, V.; van Wijk, K.J.; Aro, E.M. Co-translational assembly of the D1 protein into photosystem II. J. Biol. Chem. 1999, 274, 16062–16067. [Google Scholar] [CrossRef]
- Gohre, V.; Ossenbühl, F.; Crevecoeur, M.; Eichacker, L.A.; Rochaix, J.-D. One of two alb3 proteins is essential for the assembly of the photosystems and for cell survival in Chlamydomonas. Plant Cell 2006, 18, 1454–1466. [Google Scholar] [CrossRef]
- Ossenbühl, F.; Gohre, V.; Meurer, J.; Krieger-Liszkay, A.; Rochaix, J.-D.; Eichacker, L.A. Efficient assembly of photosystem II in Chlamydomonas reinhardtii requires Alb3. 1p, a homolog of Arabidopsis ALBINO3. Plant Cell 2004, 16, 1790–1800. [Google Scholar] [CrossRef]
- Wang, L.; Patena, W.; Van Baalen, K.A.; Xie, Y.; Singer, E.R.; Gavrilenko, S.; Warren-Williams, M.; Han, L.; Harrigan, H.R.; Hartz, L.D.; et al. A chloroplast protein atlas reveals punctate structures and spatial organization of biosynthetic pathways. Cell 2023, 186, 3499–3518.e3414. [Google Scholar] [CrossRef] [PubMed]
- Delepelaire, P. Partial characterization of the biosynthesis and integration of the Photosystem II reaction centers in the thylakoid membrane of Chlamydomonas reinhardtii. EMBO J. 1984, 3, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.L.; Purton, S.; Nugent, J.H. Molecular analysis of the Chlamydomonas nuclear gene encoding PsbW and demonstration that PsbW is a subunit of photosystem II, but not photosystem I. Plant Mol. Biol. 2003, 52, 285. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Watanabe, A.; Li, A.; Kim, E.; Song, C.; Murata, K.; Song, D.; Minagawa, J.; Liu, Z. Structural insight into light harvesting for photosystem II in green algae. Nat. Plants 2019, 5, 1320–1330. [Google Scholar] [CrossRef]
- Westhoff, P.; Jansson, C.; Klein-Hitpaß, L.; Berzborn, R.; Larsson, C.; Bartlett, S.G. Intracellular coding sites of polypeptides associated with photosynthetic oxygen evolution of photosystem II. Plant Mol. Biol. 1985, 4, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Bruce, B.D. Chloroplast transit peptides: Structure, function and evolution. Trends Cell Biol. 2000, 10, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Theg, S.M.; Bauerle, C.; Olsen, L.J.; Selman, B.R.; Keegstra, K. Internal ATP Is the Only Energy Requirement for the Translocation of Precursor Proteins across Chloroplastic Membranes. J. Biol. Chem. 1989, 264, 6730–6736. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-X.; Theg, S.M. The chloroplast protein import system: From algae to trees. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Ramundo, S.; Asakura, Y.; Salomé, P.A.; Strenkert, D.; Boone, M.; Mackinder, L.C.; Takafuji, K.; Dinc, E.; Rahire, M.; Crèvecoeur, M. Coexpressed subunits of dual genetic origin define a conserved supercomplex mediating essential protein import into chloroplasts. Proc. Natl. Acad. Sci. USA 2020, 117, 32739–32749. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, A.; Rochaix, J.-D.; Liu, Z. Architecture of chloroplast TOC-TIC translocon supercomplex. Nature 2023, 615, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wan, L.; Zhang, Y.; Li, X.; Cao, Y.; Liu, H.; Fan, S.; Cao, D.; Wang, Z.; Li, X.; et al. Structure of a TOC-TIC supercomplex spanning two chloroplast envelope membranes. Cell 2022, 185, 4788–4800.e4713. [Google Scholar] [CrossRef] [PubMed]
- Schottkowski, M.; Peters, M.; Zhan, Y.; Rifai, O.; Zhang, Y.; Zerges, W. Biogenic membranes of the chloroplast in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2012, 109, 19286–19291. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Bakhtiari, S.; Valente-Paterno, M.; Wu, Y.; Law, C.; Dai, D.; Dhaliwal, J.; Bui, K.H.; Zerges, W. Chloroplast-localized translation for protein targeting in Chlamydomonas reinhardtii. bioRxiv 2021. [Google Scholar] [CrossRef]
- Willmund, F.; Hauser, C.; Zerges, W. Translation and protein synthesis in the chloroplast. In The Chlamydomonas Sourcebook; Elsevier: Amsterdam, The Netherlands, 2023; pp. 467–508. [Google Scholar]
- Gallaher, S.D.; Fitz-Gibbon, S.T.; Strenkert, D.; Purvine, S.O.; Pellegrini, M.; Merchant, S.S. High-throughput sequencing of the chloroplast and mitochondrion of Chlamydomonas reinhardtii to generate improved de novo assemblies, analyze expression patterns and transcript speciation, and evaluate diversity among laboratory strains and wild isolates. Plant J. 2018, 93, 545–565. [Google Scholar] [CrossRef]
- Erickson, J.M.; Rahire, M.; Rochaix, J.-D. Chlamydomonas reinhardii gene for the 32 000 mol. wt. protein of photosystem II contains four large introns and is located entirely within the chloroplast inverted repeat. EMBO J. 1984, 3, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Bedbrook, J.R.; Link, G.; Coen, D.M.; Bogorad, L. Maize plastid gene expressed during photoregulated development. Proc. Natl. Acad. Sci. USA 1978, 75, 3060–3064. [Google Scholar] [CrossRef] [PubMed]
- Minai, L.; Wostrikoff, K.; Wollman, F.-A.; Choquet, Y. Chloroplast biogenesis of photosystem II cores involves a series of assembly-controlled steps that regulate translation. Plant Cell 2006, 18, 159–175. [Google Scholar] [CrossRef]
- Pakrasi, H.B.; Williams, J.G.; Arntzen, C.J. Targeted mutagenesis of the psbE and psbF genes blocks photosynthetic electron transport: Evidence for a functional role of cytochrome b559 in photosystem II. EMBO J. 1988, 7, 325–332. [Google Scholar] [CrossRef]
- Cantrell, A.; Bryant, D.A. Nucleotide sequence of the genes encoding cytochrome b-559 from the cyanelle genome of Cyanophora paradoxa. Photosynth. Res. 1988, 16, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Cushman, J.C.; Christopher, D.A.; Little, M.C.; Hallick, R.B.; Price, C.A. Organization of the psbE, psbF, orf38, and orf42 gene loci on the Euglena gracilis chloroplast genome. Curr. Genet. 1988, 13, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, R.G.; Alt, J.; Schiller, B.; Widger, W.R.; Cramer, W.A. Nucleotide sequence of the gene for apocytochrome b-559 on the spinach plastid chromosome: Implications for the structure of the membrane protein. FEBS Lett. 1984, 176, 239–244. [Google Scholar] [CrossRef]
- Mor, T.S.; Ohad, I.; Hirschberg, J.; Pakrasi, H.B. An unusual organization of the genes encoding cytochrome b559 in Chlamydomonas reinhardtii: psbE and psbF genes are separately transcribed from different regions of the plastid chromosome. Mol. Gen. Genet. MGG 1995, 246, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Nechushtai, R.; Barber, J.; Nixon, P. Nucleotide sequence of the psbE, psbF and trnM genes from the chloroplast genome of Chlamydomonas reinhardtii. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1994, 1188, 439–442. [Google Scholar] [CrossRef]
- Liu, X.Q.; Gillham, N.W.; Boynton, J.E. Chloroplast Ribosomal Protein Gene rps12 of Chlamydomonas reinhardtii: Wild-type sequence, mutation to streptomycin resistance and dependence, and function in escherichia coli. J. Biol. Chem. 1989, 264, 16100–16108. [Google Scholar] [CrossRef] [PubMed]
- Cavaiuolo, M.; Kuras, R.; Wollman, F.A.; Choquet, Y.; Vallon, O. Small RNA profiling in Chlamydomonas: Insights into chloroplast RNA metabolism. Nucleic Acids Res. 2017, 45, 10783–10799. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, Y.; Hu, Z.; Huang, X.; Zhang, B.; Lu, Q.; Wen, X.; Wang, Y.; Lu, C. mTERF5 Acts as a Transcriptional Pausing Factor to Positively Regulate Transcription of Chloroplast psbEFLJ. Mol. Plant 2019, 12, 1259–1277. [Google Scholar] [CrossRef]
- Wollman, F.-A.; Minai, L.; Nechushtai, R. The biogenesis and assembly of photosynthetic proteins in thylakoid membranes. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1999, 1411, 21–85. [Google Scholar] [CrossRef]
- Choquet, Y.; Wollman, F.-A. The assembly of photosynthetic proteins. In The Chlamydomonas Sourcebook; Elsevier: Amsterdam, The Netherlands, 2023; pp. 615–646. [Google Scholar]
- Erickson, J.M.; Rahire, M.; Malnoë, P.; Girard-Bascou, J.; Pierre, Y.; Bennoun, P.; Rochaix, J.-D. Lack of the D2 protein in a Chlamydomonas reinhardtii psbD mutant affects photosystem II stability and D1 expression. EMBO J. 1986, 5, 1745–1754. [Google Scholar] [CrossRef]
- de Vitry, C.; Olive, J.; Drapier, D.; Recouvreur, M.; Wollman, F.-A. Posttranslational events leading to the assembly of photosystem II protein complex: A study using photosynthesis mutants from Chlamydomonas reinhardtii. J. Cell Biol. 1989, 109, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Morais, F.; Barber, J.; Nixon, P.J. The Chloroplast-encoded α Subunit of Cytochromeb-559 Is Required for Assembly of the Photosystem Two Complex in both the Light and the Dark in Chlamydomonas reinhardtii. J. Biol. Chem. 1998, 273, 29315–29320. [Google Scholar] [CrossRef] [PubMed]
- Bohne, A.-V.; Nickelsen, J. Control of organellar gene expression by nucleus-encoded proteins. In The Chlamydomonas Sourcebook; Elsevier: Amsterdam, The Netherlands, 2023; pp. 443–466. [Google Scholar]
- Mayfield, S.P.; Rahire, M.; Frank, G.; Zuber, H.; Rochaix, J.-D. Expression of the nuclear gene encoding oxygen-evolving enhancer protein 2 is required for high levels of photosynthetic oxygen evolution in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1987, 84, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Nickelsen, J.; van Dillewijn, J.; Rahire, M.; Rochaix, J.-D. Determinants for stability of the chloroplast psbD RNA are located within its short leader region in Chlamydomonas reinhardtii. EMBO J. 1994, 13, 3182–3191. [Google Scholar] [CrossRef] [PubMed]
- Rochaix, J.-D.; Kuchka, M.; Mayfield, S.; Schirmer-Rahire, M.; Girard-Bascou, J.; Bennoun, P. Nuclear and chloroplast mutations affect the synthesis or stability of the chloroplast psbC gene product in Chlamydomonas reinhardtii. EMBO J. 1989, 8, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Danon, A.; Mayfield, S.P. Light regulated translational activators: Identification of chloroplast gene specific mRNA binding proteins. EMBO J. 1991, 10, 3993–4001. [Google Scholar] [CrossRef] [PubMed]
- Yohn, C.B.; Cohen, A.; Danon, A.; Mayfield, S.P. A poly(A) binding protein functions in the chloroplast as a message-specific translation factor. Proc. Natl. Acad. Sci. USA 1998, 95, 2238–2243. [Google Scholar] [CrossRef] [PubMed]
- Yohn, C.B.; Cohen, A.; Rosch, C.; Kuchka, M.R.; Mayfield, S.P. Translation of the Chloroplast psbA mRNA Requires the Nuclear-encoded Poly(A)-binding Protein, RB47. J. Cell Biol. 1998, 142, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mayfield, S.P. Protein disulfide isomerase as a regulator of chloroplast translational activation. Science 1997, 278, 1954–1957. [Google Scholar] [CrossRef] [PubMed]
- Somanchi, A.; Barnes, D.; Mayfield, S.P. A nuclear gene of Chlamydomonas reinhardtii, Tba1, encodes a putative oxidoreductase required for translation of the chloroplast psbA mRNA. Plant J. 2005, 42, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.; Cohen, A.; Bruick, R.K.; Kantardjieff, K.; Fowler, S.; Efuet, E.; Mayfield, S.P. Identification and Characterization of a Novel RNA Binding Protein That Associates with the 5‘-Untranslated Region of the Chloroplast psbA mRNA. Biochemistry 2004, 43, 8541–8550. [Google Scholar] [CrossRef] [PubMed]
- Yohn, C.B.; Cohen, A.; Danon, A.; Mayfield, S.P. Altered mRNA binding activity and decreased translational initiation in a nuclear mutant lacking translation of the chloroplast psbA mRNA. Mol. Cell. Biol. 1996, 16, 3560–3566. [Google Scholar] [CrossRef] [PubMed]
- Ossenbühl, F.; Hartmann, K.; Nickelsen, J. A chloroplast RNA binding protein from stromal thylakoid membranes specifically binds to the 5′ untranslated region of the psbA mRNA. Eur. J. Biochem. 2002, 269, 3912–3919. [Google Scholar] [CrossRef] [PubMed]
- Bohne, A.-V.; Schwarz, C.; Schottkowski, M.; Lidschreiber, M.; Piotrowski, M.; Zerges, W.; Nickelsen, J. Reciprocal Regulation of Protein Synthesis and Carbon Metabolism for Thylakoid Membrane Biogenesis. PLoS Biol. 2013, 11, e1001482. [Google Scholar] [CrossRef] [PubMed]
- Neusius, D.; Kleinknecht, L.; Teh, J.T.; Ostermeier, M.; Kelterborn, S.; Eirich, J.; Hegemann, P.; Finkemeier, I.; Bohne, A.-V.; Nickelsen, J. Lysine acetylation regulates moonlighting activity of the E2 subunit of the chloroplast pyruvate dehydrogenase complex in Chlamydomonas. Plant J. 2022, 111, 1780–1800. [Google Scholar] [CrossRef]
- Kafri, M.; Patena, W.; Martin, L.; Wang, L.; Gomer, G.; Ergun, S.L.; Sirkejyan, A.K.; Goh, A.; Wilson, A.T.; Gavrilenko, S.E.; et al. Systematic identification and characterization of genes in the regulation and biogenesis of photosynthetic machinery. Cell 2023, 186, 5638–5655.e5625. [Google Scholar] [CrossRef]
- Kuchka, M.R.; Mayfield, S.P.; Rochaix, J.-D. Nuclear mutations specifically affect the synthesis and/or degradation of the chloroplast-encoded D2 polypeptide of photosystem II in Chlamydomonas reinhardtii. EMBO J. 1988, 7, 319–324. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Rosch, C.; Kuchka, M.R. Cloning and characterization of the nuclear AC115 gene of Chlamydomonas reinhardtii. Plant Mol. Biol. 1999, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kuchka, M.R.; Goldschmidt-Clermont, M.; van Dillewijn, J.; Rochaix, J.-D. Mutation at the chlamydomonas nuclear NAC2 locus specifically affects stability of the chloroplast psbD transcript encoding polypeptide D2 of PS II. Cell 1989, 58, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Ossenbühl, F.; Nickelsen, J. cis- and trans-Acting Determinants for Translation of psbD mRNA in Chlamydomonas reinhardtii. Mol. Cell. Biol. 2000, 20, 8134–8142. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Elles, I.; Kortmann, J.; Piotrowski, M.; Nickelsen, J. Synthesis of the D2 protein of photosystem II in Chlamydomonas is controlled by a high molecular mass complex containing the RNA stabilization factor Nac2 and the translational activator RBP40. Plant Cell 2007, 19, 3627–3639. [Google Scholar] [CrossRef] [PubMed]
- Monod, C.; Goldschmidt-Clermont, M.; Rochaix, J.-D. Accumulation of chloroplast psbB RNA requires a nuclear factor in Chlamydomonas reinhardtii. Mol. Gen. Genet. 1992, 231, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Vaistij, F.E.; Goldschmidt-Clermont, M.; Wostrikoff, K.; Rochaix, J.-D. Stability determinants in the chloroplast psbB/T/H mRNAs of Chlamydomonas reinhardtii. Plant J. 2000, 21, 469–482. [Google Scholar] [CrossRef]
- Zerges, W.; Rochaix, J.-D. The 5′ leader of a chloroplast mRNA mediates the translational requirements for two nucleus-encoded functions in Chlamydomonas reinhardtii. Mol. Cell. Biol. 1994, 14, 5268–5277. [Google Scholar] [CrossRef] [PubMed]
- Zerges, W. Translation of the psbC mRNA and incorporation of its polypeptide product into photosystem II is controlled by interactions between the psbC 5′leader and the NCT loci in Chlamydomonas reinhardtii. Mol. Cell. Biol. 1997, 17, 3440–3448. [Google Scholar] [CrossRef] [PubMed]
- Zoschke, R.; Bock, R. Chloroplast Translation: Structural and Functional Organization, Operational Control, and Regulation. Plant Cell 2018, 30, 745–770. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A.; Hihara, Y. Transcriptional and posttranscriptional regulation of cyanobacterial photosynthesis. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2016, 1857, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dischinger, K.; Westrich, L.D.; Meindl, I.; Egidi, F.; Trösch, R.; Sommer, F.; Johnson, X.; Schroda, M.; Nickelsen, J.; et al. One-helix protein 2 is not required for the synthesis of photosystem II subunit D1 in Chlamydomonas. Plant Physiol. 2023, 191, 1612–1633. [Google Scholar] [CrossRef] [PubMed]
- Calderon, R.H.; de Vitry, C.; Wollman, F.-A.; Niyogi, K.K. Rubredoxin 1 promotes the proper folding of D1 and is not required for heme b559 assembly in Chlamydomonas photosystem II. J. Biol. Chem. 2023, 299, 102968. [Google Scholar] [CrossRef]
- Calderon, R.H.; García-Cerdán, J.G.; Malnoë, A.; Cook, R.; Russell, J.J.; Gaw, C.; Dent, R.M.; de Vitry, C.; Niyogi, K.K. A Conserved Rubredoxin Is Necessary for Photosystem II Accumulation in Diverse Oxygenic Photoautotrophs. J. Biol. Chem. 2013, 288, 26688–26696. [Google Scholar] [CrossRef] [PubMed]
- García-Cerdán, J.G.; Furst, A.L.; McDonald, K.L.; Schünemann, D.; Francis, M.B.; Niyogi, K.K. A thylakoid membrane-bound and redox-active rubredoxin (RBD1) functions in de novo assembly and repair of photosystem II. Proc. Natl. Acad. Sci. USA 2019, 116, 16631–16640. [Google Scholar] [CrossRef] [PubMed]
- Spaniol, B.; Lang, J.; Venn, B.; Schake, L.; Sommer, F.; Mustas, M.; Geimer, S.; Wollman, F.-A.; Choquet, Y.; Mühlhaus, T. Complexome profiling on the Chlamydomonas lpa2 mutant reveals insights into PSII biogenesis and new PSII associated proteins. J. Exp. Bot. 2022, 73, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Cecchin, M.; Jeong, J.; Son, W.; Kim, M.; Park, S.; Zuliani, L.; Cazzaniga, S.; Pompa, A.; Young Kang, C.; Bae, S. LPA2 protein is involved in photosystem II assembly in Chlamydomonas reinhardtii. Plant J. 2021, 107, 1648–1662. [Google Scholar] [CrossRef] [PubMed]
- Oliver, N.; Avramov, A.P.; Nürnberg, D.J.; Dau, H.; Burnap, R.L. From manganese oxidation to water oxidation: Assembly and evolution of the water-splitting complex in photosystem II. Photosynth. Res. 2022, 152, 107–133. [Google Scholar] [CrossRef]
- Rova, E.M.; McEwen, B.; Fredriksson, P.O.; Styring, S. Photoactivation and photoinhibition are competing in a mutant of Chlamydomonas reinhardtii lacking the 23-kDa extrinsic subunit of photosystem II. J. Biol. Chem. 1996, 271, 28918–28924. [Google Scholar] [CrossRef]
- Vinyard, D.J.; Sun, J.S.; Gimpel, J.; Ananyev, G.M.; Mayfield, S.P.; Charles Dismukes, G. Natural isoforms of the Photosystem II D1 subunit differ in photoassembly efficiency of the water-oxidizing complex. Photosynth. Res. 2016, 128, 141–150. [Google Scholar] [CrossRef]
- Mayfield, S.P.; Bennoun, P.; Rochaix, J.-D. Expression of the nuclear encoded OEE1 protein is required for oxygen evolution and stability of photosystem II particles in Chlamydomonas reinhardtii. EMBO J. 1987, 6, 313–318. [Google Scholar] [CrossRef]
- Popelkova, H.; Yocum, C.F. PsbO, the manganese-stabilizing protein: Analysis of the structure–function relations that provide insights into its role in photosystem II. J. Photochem. Photobiol. B Biol. 2011, 104, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Rova, M.; Franzen, L.G.; Fredriksson, P.O.; Styring, S. Photosystem II in a mutant of Chlamydomonas reinhardtii lacking the 23 kDa psbP protein shows increased sensitivity to photoinhibition in the absence of chloride. Photosynth. Res. 1994, 39, 75–83. [Google Scholar] [CrossRef]
- Enami, I.; Okumura, A.; Nagao, R.; Suzuki, T.; Iwai, M.; Shen, J.-R. Structures and functions of the extrinsic proteins of photosystem II from different species. Photosynth. Res. 2008, 98, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Gisriel, C.J.; Wang, J.; Liu, J.; Flesher, D.A.; Reiss, K.M.; Huang, H.-L.; Yang, K.R.; Armstrong, W.H.; Gunner, M.R.; Batista, V.S.; et al. High-resolution cryo-electron microscopy structure of photosystem II from the mesophilic cyanobacterium, Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. USA 2022, 119, e2116765118. [Google Scholar] [CrossRef] [PubMed]
- Knoppová, J.; Yu, J.; Konik, P.; Nixon, P.J.; Komenda, J. CyanoP is Involved in the Early Steps of Photosystem II Assembly in the Cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2016, 57, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, J.; Takahashi, Y. Structure, function and assembly of Photosystem II and its light-harvesting proteins. Photosynth. Res. 2004, 82, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, S.; Kim, M.; Bellamoli, F.; Jeong, J.; Lee, S.; Perozeni, F.; Pompa, A.; Jin, E.; Ballottari, M. Photosystem II antenna complexes CP26 and CP29 are essential for nonphotochemical quenching in Chlamydomonas reinhardtii. Plant Cell Environ. 2020, 43, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Swiatek, M.; Kuras, R.; Sokolenko, A.; Higgs, D.; Olive, J.; Cinque, G.; Muller, B.; Eichacker, L.A.; Stern, D.B.; Bassi, R.; et al. The chloroplast gene ycf9 encodes a photosystem II (PSII) core subunit, PsbZ, that participates in PSII supramolecular architecture. Plant Cell 2001, 13, 1347–1367. [Google Scholar] [CrossRef] [PubMed]
- Delosme, R.; Olive, J.; Wollman, F.-A. Changes in light energy distrubution upon state transitions: An in vivo photoacoustic study of the wild type and photosynthesis mutants from Chlamydomonas reinhardtii. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1996, 1273, 150–158. [Google Scholar] [CrossRef]
- Tikkanen, M.; Aro, E.-M. Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2012, 1817, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Vainonen, J.P.; Hansson, M.; Vener, A.V. STN8 protein kinase in Arabidopsis thaliana is specific in phosphorylaton of photosystem II core proteins J. Biol. Chem. 2005, 280, 33679–33686. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, V.; Pesaresi, P.; Becker, T.; Schleiff, E.; Wagner, R.; Pfannschmidt, T.; Jahns, P.; Leister, D. Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature 2005, 437, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Samol, I.; Shapiguzov, A.; Ingelsson, B.; Fucile, G.; Crèvecoeur, M.; Vener, A.V.; Rochaix, J.-D.; Goldschmidt-Clermont, M. Identification of a Photosystem II Phosphatase Involved in Light Acclimation in Arabidopsis. Plant Cell 2012, 24, 2596–2609. [Google Scholar] [CrossRef] [PubMed]
- Bellafiore, S.; Barneche, F.; Peltier, G.; Rochaix, J.-D. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 2005, 433, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Nurmi, M.; Kangasjärvi, S.; Aro, E.-M. Core protein phosphorylation facilitates the repair of photodamaged photosystem II at high light. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2008, 1777, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Shapiguzov, A.; Ingelsson, B.; Samol, I.; Andres, C.; Kessler, F.; Rochaix, J.-D.; Vener, A.V.; Goldschmidt-Clermont, M. The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 4782–4787. [Google Scholar] [CrossRef] [PubMed]
- Pribil, M.; Pesaresi, P.; Hertle, A.; Barbato, R.; Leister, D. Role of Plastid Protein Phosphatase TAP38 in LHCII Dephosphorylation and Thylakoid Electron Flow. PLoS Biol. 2010, 8, e1000288. [Google Scholar] [CrossRef]
- de Vitry, C.; Diner, B.A.; Popot, J.-L. Photosystem II Particle from Chlamydomonas reinhardtii. J. Biol. Chem. 1991, 266, 16614–16621. [Google Scholar] [CrossRef] [PubMed]
- Lemeille, S.; Turkina, M.V.; Vener, A.V.; Rochaix, J.-D. Stt7-dependent phosphorylation during state transitions in the green alga Chlamydomonas reinhardtii. Mol. Cell Proteom. 2010, 9, 1281–1295. [Google Scholar] [CrossRef]
- Depège, N.; Bellafiore, S.; Rochaix, J.-D. Role of Chloroplast Protein Kinase Stt7 in LHCII Phosphorylation and State Transition in Chlamydomonas. Science 2003, 299, 1572–1575. [Google Scholar] [CrossRef]
- Cariti, F.; Chazaux, M.; Lefebvre-Legendre, L.; Longoni, P.; Ghysels, B.; Johnson, X.; Goldschmidt-Clermont, M. Regulation of Light Harvesting in Chlamydomonas reinhardtii Two Protein Phosphatases Are Involved in State Transitions. Plant Physiol. 2020, 183, 1749–1764. [Google Scholar] [CrossRef]
- Terentyev, V.V.; Shukshina, A.K.; Shitov, A.V. Carbonic anhydrase CAH3 supports the activity of photosystem II under increased pH. Biochim. Et Biophys. Acta (BBA) Bioenerg. 2019, 1860, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Terentyev, V.V.; Shukshina, A.K.; Ashikhmin, A.A.; Tikhonov, K.G.; Shitov, A.V. The Main Structural and Functional Characteristics of Photosystem-II-Enriched Membranes Isolated from Wild Type and cia3 Mutant Chlamydomonas reinhardtii. Life 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Reisman, S.; Ohad, I. Light-dependent degradation of the thylakoid 32 kDa QB protein in isolated chloroplast membranes of Chlamydomonas reinhardtii. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1986, 849, 51–61. [Google Scholar] [CrossRef]
- Murata, N.; Nishiyama, Y. ATP is a driving force in the repair of photosystem II during photoinhibition. Plant Cell Environ. 2018, 41, 285–299. [Google Scholar] [CrossRef]
- Schuster, G.; Timberg, R.; Ohad, I. Turnover of thylakoid photosystem II proteins during photoinhibition of Chlamydomonas reinhardtii. Eur. J. Biochem. 1988, 177, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Haussuhl, K.; Andersson, B.; Adamska, I. A chloroplast DegP2 protease performs the primary cleavage of the photodamaged D1 protein in plant photosystem II. EMBO J. 2001, 20, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Huesgen, P.F.; Schuhmann, H.; Adamska, I. Photodamaged D1 protein is degraded in Arabidopsis mutants lacking the Deg2 protease. FEBS Lett. 2006, 580, 6929–6932. [Google Scholar] [CrossRef] [PubMed]
- Kapri-Pardes, E.; Naveh, L.; Adam, Z. The thylakoid lumen protease Deg1 is involved in the repair of photosystem II from photoinhibition in Arabidopsis. Plant Cell 2007, 19, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Peng, L.; Guo, J.; Chi, W.; Ma, J.; Lu, C.; Zhang, L. Formation of DEG5 and DEG8 complexes and their involvement in the degradation of photodamaged photosystem II reaction center D1 protein in Arabidopsis. Plant Cell 2007, 19, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.; de Vries, R.; Nield, J.; Komenda, J.; Nixon, P.J. The Deg Proteases Protect Synechocystis sp. PCC 6803 during Heat and Light Stresses but Are Not Essential for Removal of Damaged D1 Protein during the Photosystem Two Repair Cycle. J. Biol. Chem. 2006, 281, 30347–30355. [Google Scholar] [CrossRef]
- Malnoë, A.; Wang, F.; Girard-Bascou, J.; Wollman, F.-A.; de Vitry, C. Thylakoid FtsH Protease Contributes to Photosystem II and Cytochrome b 6 f Remodeling in Chlamydomonas reinhardtii under Stress Conditions. Plant Cell 2014, 26, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Schroda, M.; de Vitry, C. Molecular chaperones, proteases, and unfolded protein responses. In The Chlamydomonas Sourcebook; Elsevier: Amsterdam, The Netherlands, 2023; pp. 647–689. [Google Scholar]
- Theis, J.; Lang, J.; Spaniol, B.; Ferté, S.; Niemeyer, J.; Sommer, F.; Zimmer, D.; Venn, B.; Mehr, S.F.; Mühlhaus, T.; et al. The Chlamydomonas deg1c Mutant Accumulates Proteins Involved in High Light Acclimation1 [OPEN]. Plant Physiol. 2019, 181, 1480–1497. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.; Schroda, M. Revisiting the photosystem II repair cycle. Plant Signal. Behav. 2016, 11, e1218587. [Google Scholar] [CrossRef] [PubMed]
- Muranaka, L.S.; Rütgers, M.; Bujaldon, S.; Heublein, A.; Geimer, S.; Wollman, F.-A.; Schroda, M. TEF30 Interacts with Photosystem II Monomers and Is Involved in the Repair of Photodamaged Photosystem II in Chlamydomonas reinhardtii. Plant Physiol. 2015, 170, 821–840. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Khamai, P.; Garcia-Cerdan, J.G.; Melis, A. REP27, a Tetratricopeptide Repeat Nuclear-Encoded and Chloroplast-Localized Protein, Functions in D1/32-kD Reaction Center Protein Turnover and Photosystem II Repair from Photodamage. Plant Physiol. 2007, 143, 1547–1560. [Google Scholar] [CrossRef]
- Dewez, D.; Park, S.; García-Cerdán, J.G.; Lindberg, P.; Melis, A. Mechanism of REP27 Protein Action in the D1 Protein Turnover and Photosystem II Repair from Photodamage. Plant Physiol. 2009, 151, 88–99. [Google Scholar] [CrossRef]
| PSII Subunit Affected | Translation Factor | Mechanism | References |
|---|---|---|---|
| psbA (D1) | RB47 | Binds to A-rich region in the psbA 5′ UTR; required for D1 synthesis | [87,88,89] |
| RB60 | Protein disulfide isomerase that redox regulates RB47 | [87,90] | |
| TBA1 | Oxidoreductase that facilitates binding of RB47 to psbA transcript | [91] | |
| RB55 | Observed to bind psbA mRNA but not characterized | [92,93] | |
| RBP63 | Binds to psbA 5′ UTR; essential for D1 synthesis; subunit of chloroplast pyruvate dehydrogenase complex that becomes a translational regulator upon acetylation | [94,95,96] | |
| CrHCF173 | Homolog of Arabidopsis HCF173; affects D1 accumulation | [97] | |
| psbD (D2) | NAC1 | Promotes psbD translation at a step that is likely after initiation | [98,99] |
| AC115 | |||
| NAC2 | Promotes psbD stability by binding to its 5′ UTR | [85,100] | |
| RBP40 (RB38) | Binds to U-rich region of psbD 5′ UTR; forms a complex with NAC2 to control psbD mRNA stability and initiation; also observed to bind psbA mRNA although this interaction may not be specific | [92,101,102] | |
| psbB (CP47) | Mbb1 | Promotes psbB mRNA stability by interacting with its 5′ UTR; also affects psbH mRNA maturation | [103,104] |
| psbC (CP43) | TBC1 | Facilitates psbC translation by binding to its 5′ UTR | [86,105,106] |
| TBC2 | |||
| TBC3 | |||
| MBCI | Stabilizes psbC mRNA | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehra, H.S.; Wang, X.; Russell, B.P.; Kulkarni, N.; Ferrari, N.; Larson, B.; Vinyard, D.J. Assembly and Repair of Photosystem II in Chlamydomonas reinhardtii. Plants 2024, 13, 811. https://doi.org/10.3390/plants13060811
Mehra HS, Wang X, Russell BP, Kulkarni N, Ferrari N, Larson B, Vinyard DJ. Assembly and Repair of Photosystem II in Chlamydomonas reinhardtii. Plants. 2024; 13(6):811. https://doi.org/10.3390/plants13060811
Chicago/Turabian StyleMehra, Himanshu S., Xiaozhuo Wang, Brandon P. Russell, Nidhi Kulkarni, Nicholas Ferrari, Brent Larson, and David J. Vinyard. 2024. "Assembly and Repair of Photosystem II in Chlamydomonas reinhardtii" Plants 13, no. 6: 811. https://doi.org/10.3390/plants13060811
APA StyleMehra, H. S., Wang, X., Russell, B. P., Kulkarni, N., Ferrari, N., Larson, B., & Vinyard, D. J. (2024). Assembly and Repair of Photosystem II in Chlamydomonas reinhardtii. Plants, 13(6), 811. https://doi.org/10.3390/plants13060811






