Exogenous 24-Epibrassinolide Enhanced Drought Tolerance and Promoted BRASSINOSTEROID-INSENSITIVE2 Expression of Quinoa
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. ERB Treatment and Drought Stress
2.3. Scan of Roots
2.4. Determination of Lipid Peroxidation
2.5. Determination of Soluble Protein, Free Proline, and Antioxidant Enzyme Activities
2.6. BR and ABA Content
2.7. Transcriptome Sequencing and Data Analysis
2.8. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.9. Virus-Induced Gene Silencing (VIGS) of Quinoa Seedlings
2.10. Statistical Analysis
3. Results
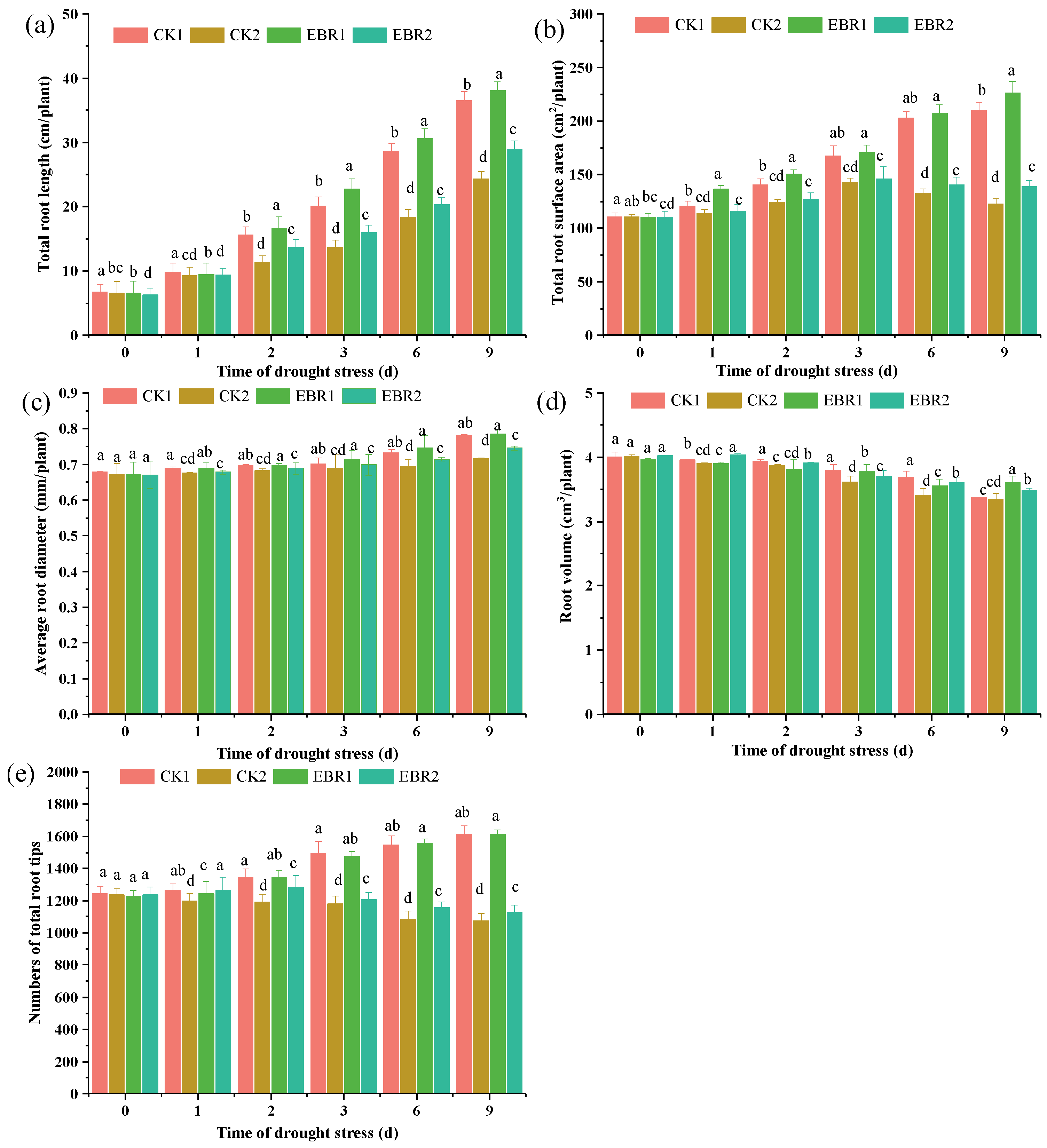
3.1. Growth Parameters
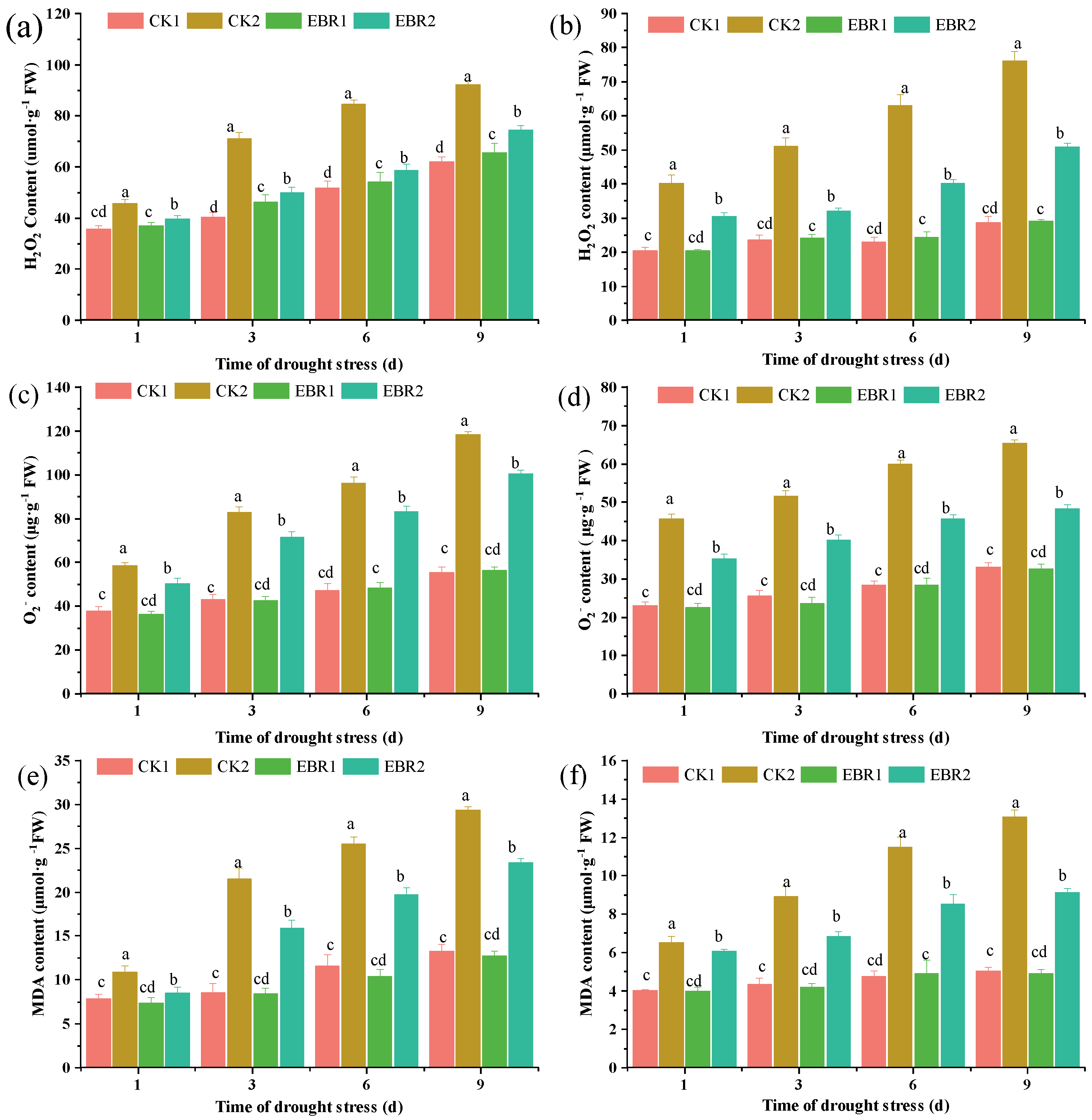
3.2. H2O2, , and MDA Content
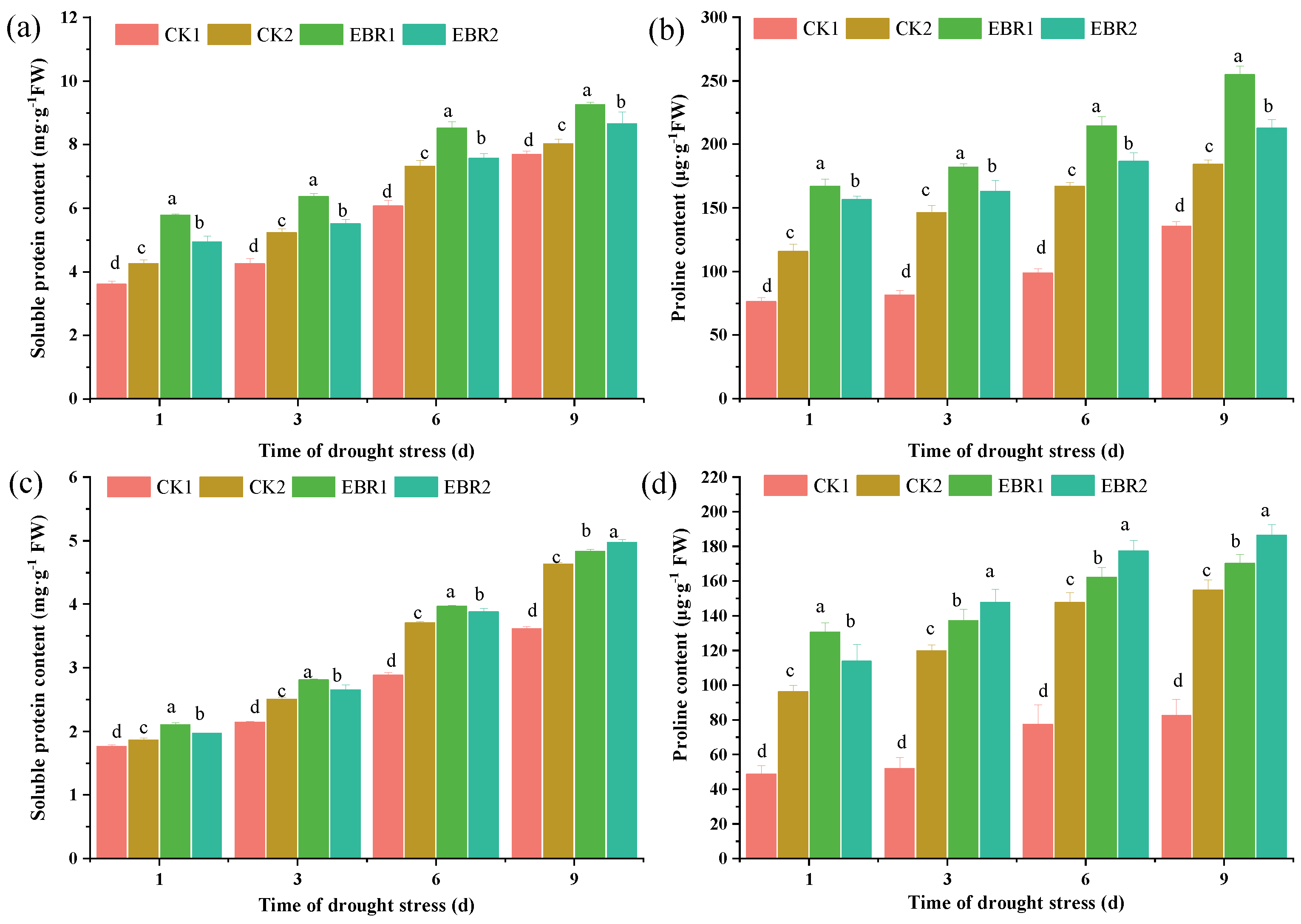
3.3. Soluble Protein and Proline Content
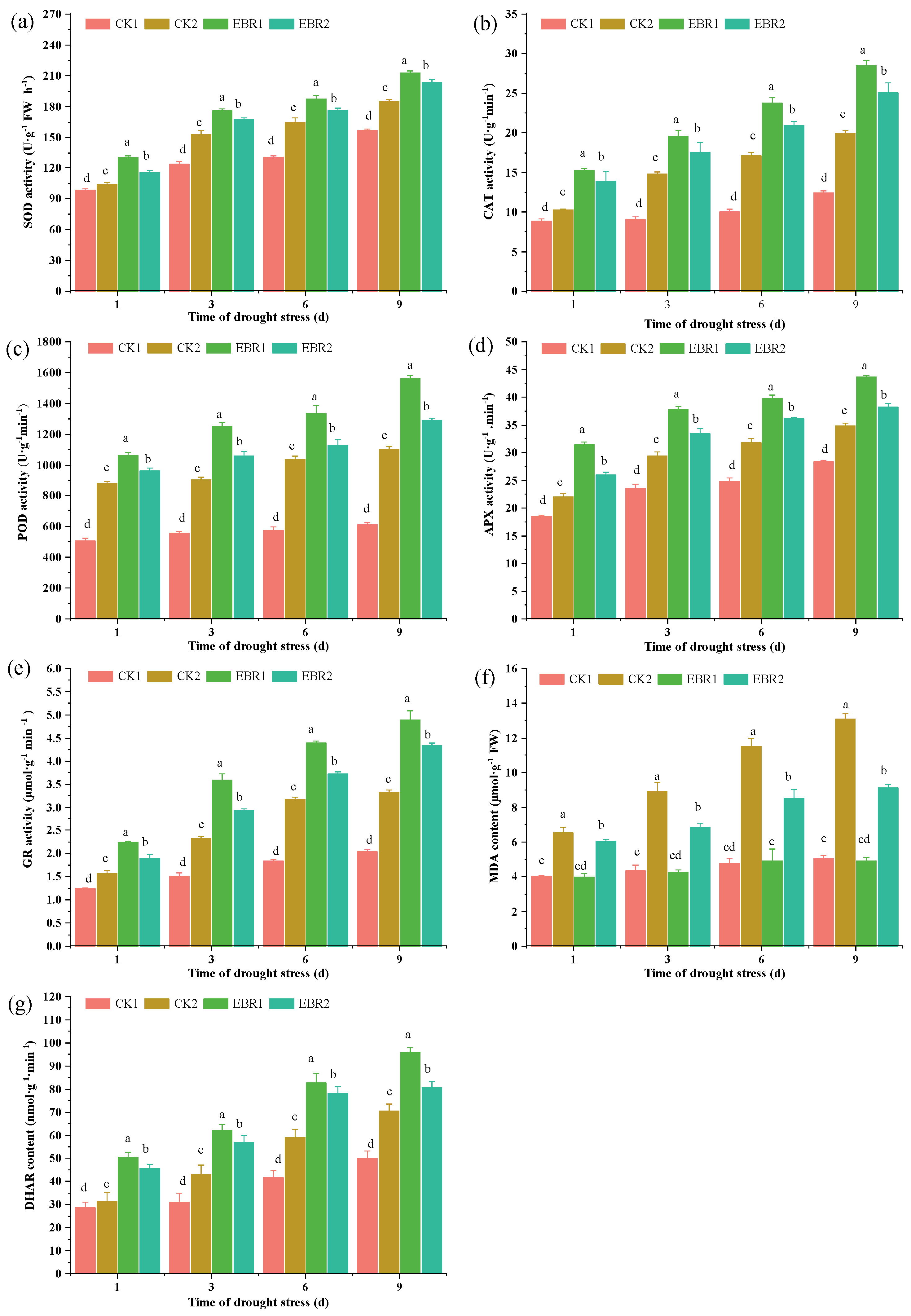
3.4. Antioxidant Enzyme Activities
3.5. ABA and BR Content
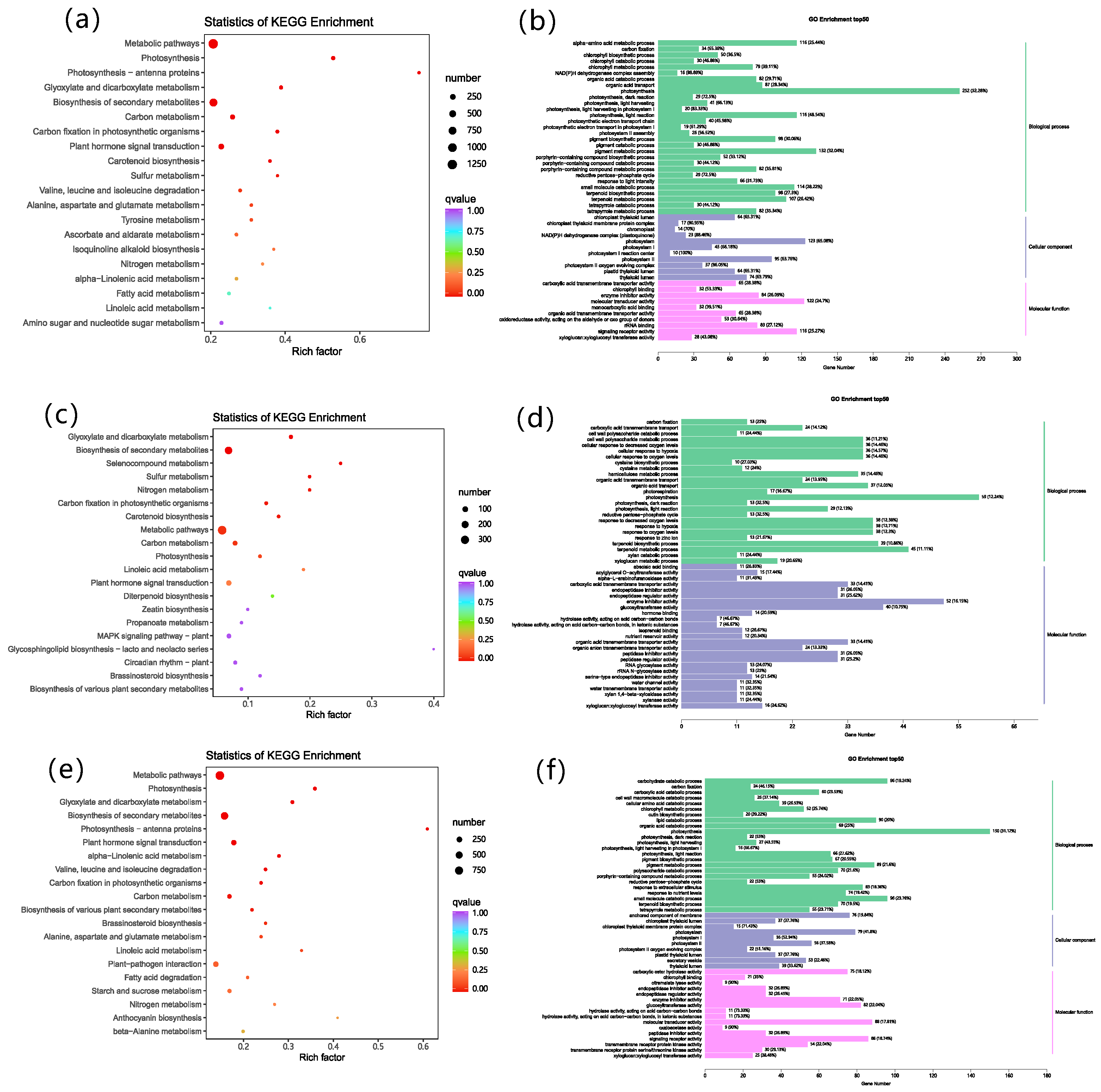
3.6. Transcriptomic Analysis
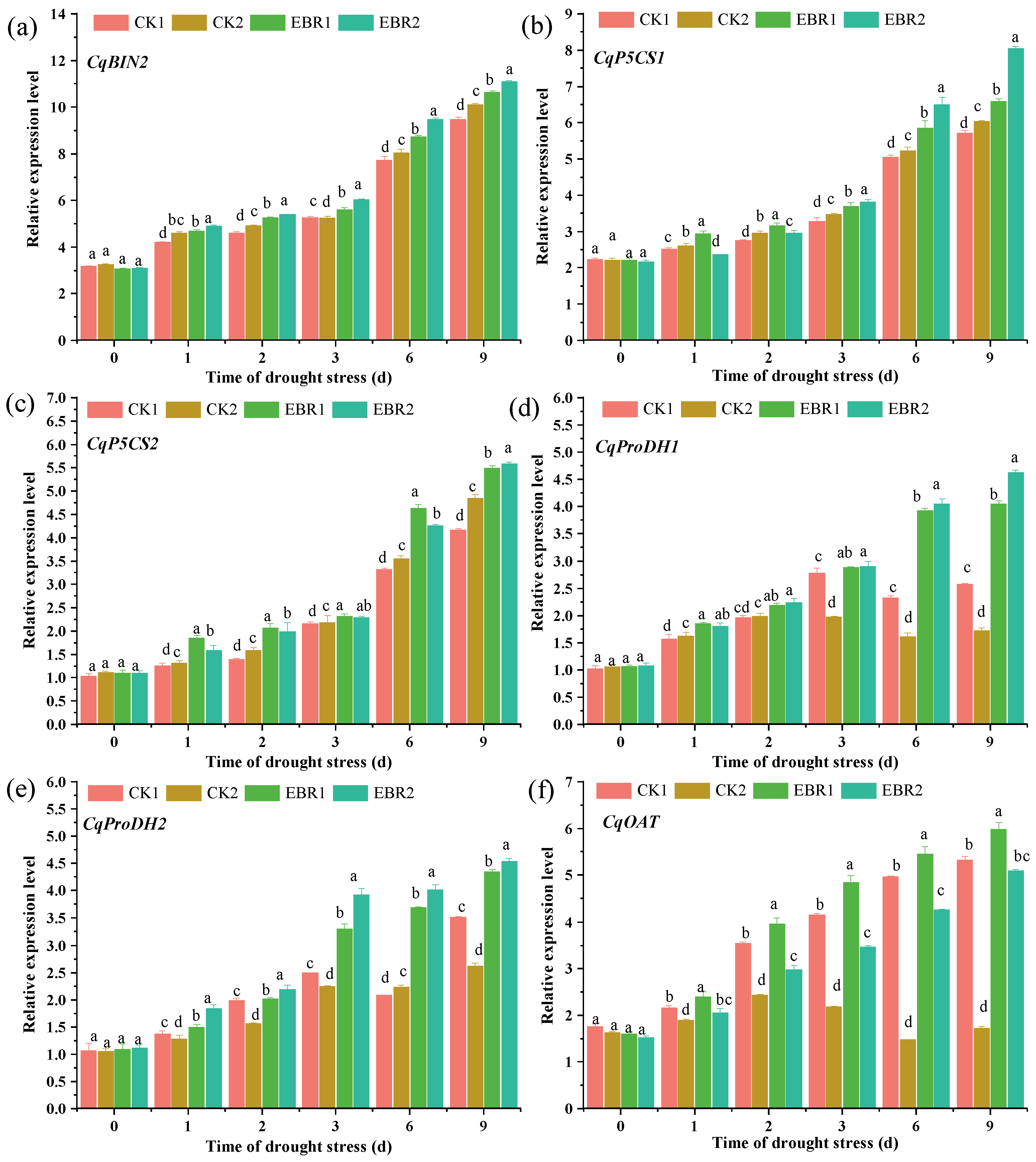
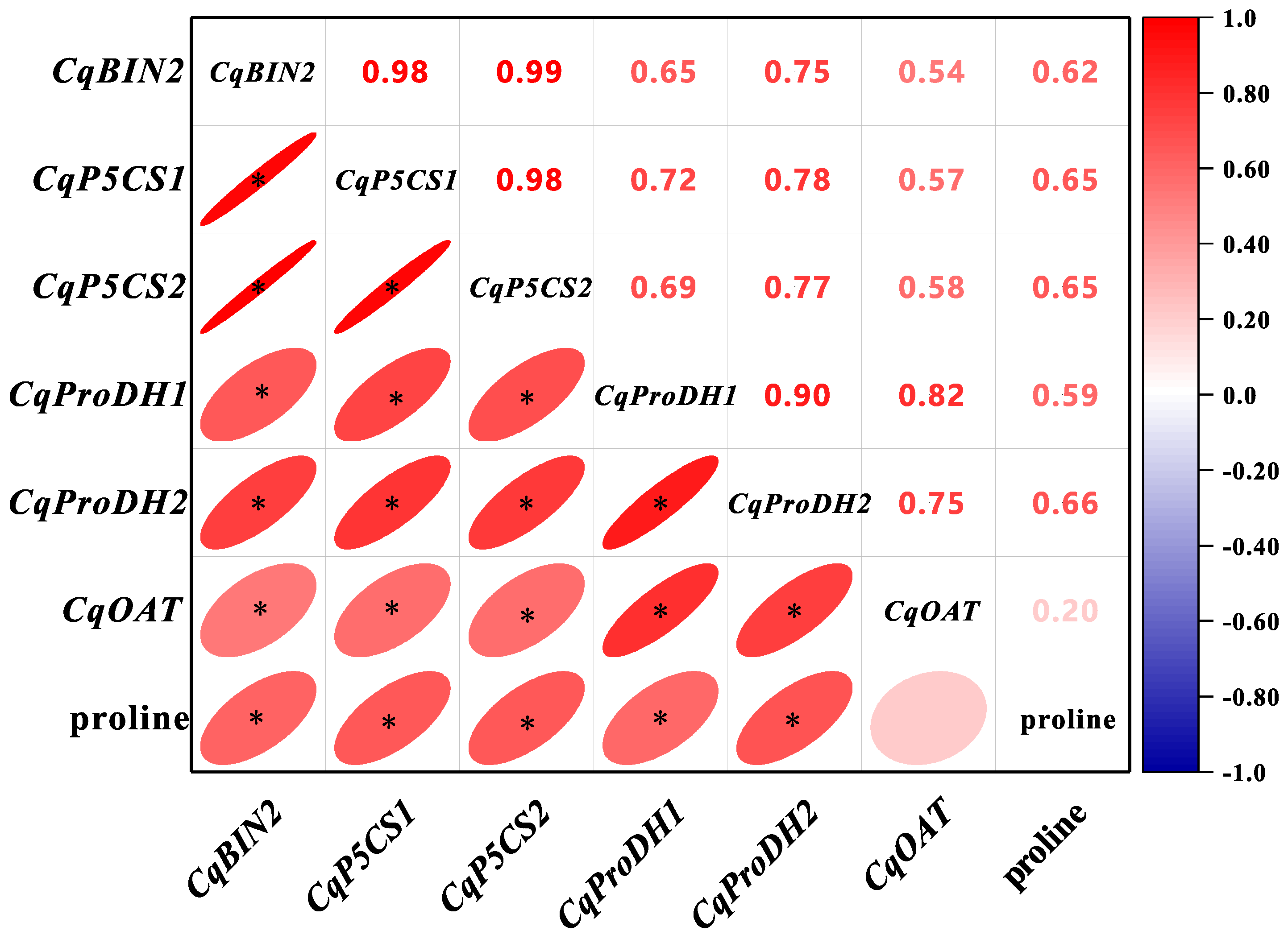
3.7. Expression Patterns of CqBIN2 and Proline Biosynthesis Genes in Quinoa Seedlings
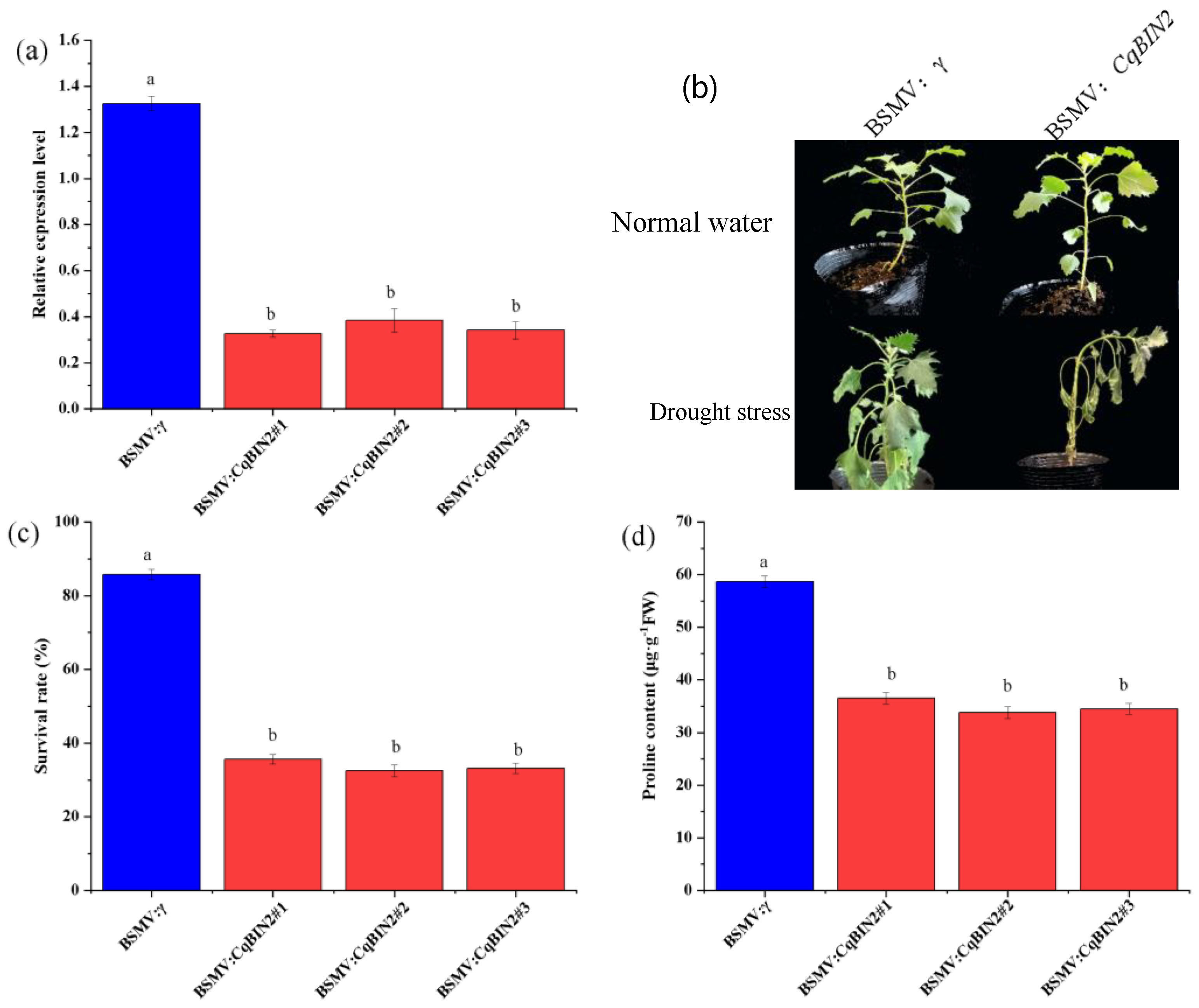
3.8. Silencing of CqBIN2 Improves Drought Tolerance in Quinoa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hussain, M.I.; Muscolo, A.; Ahmed, M.; Asghar, M.A.; Al-Dakheel, A.J. Agro-morphological, yield and quality traits and interrelationship with yield stability in Quinoa (Chenopodium quinoa Willd.) genotypes under saline marginal environment. Plants 2020, 9, 1763. [Google Scholar] [CrossRef]
- Villacres, E.; Quelal, M.; Galarza, S.; Iza, D.; Silva, E. Nutritional Value and Bioactive Compounds of Leaves and Grains from Quinoa (Chenopodium quinoa Willd.). Plants 2022, 11, 213. [Google Scholar] [CrossRef]
- Pereira, E.; Cadavez, V.; Barros, L.; Encina-Zelada, C.; Ferreira, I. Chenopodium quinoa Willd. (quinoa) grains: A good source of phenolic compounds. Food Res. Int. 2020, 137, 109574. [Google Scholar] [CrossRef]
- López-Moreno, M.; Jiménez-Moreno, E.; Gallego, A.M.; Pasamontes, G.V.; Ocio, J.A.U.; Garcés-Rimón, M.; Miguel-Castro, M. Red quinoa hydrolysates with antioxidant properties improve cardiovascular health in spontaneously hypertensive rats. Antioxidants 2023, 12, 1291. [Google Scholar] [CrossRef]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone signaling and crosstalk in regulating drought stress response in plants. Plant Cell Rep. 2021, 40, 1305–1329. [Google Scholar] [CrossRef]
- Xia, H.; Liu, X.; Wang, Y.; Lin, Z.; Deng, H.; Wang, J.; Lin, L.; Deng, Q.; Lv, X.; Xu, K. 24-Epibrassinolide and nitric oxide combined to improve the drought tolerance in kiwifruit seedlings by proline pathway and nitrogen metabolism. Sci. Hortic. 2022, 297, 110929. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Wu, S.T.; Gao, H.; Tang, W.Q.; Wu, X.D.; Zhang, B.W. The BR signaling pathway regulates primary root development and drought stress response by suppressing the expression of PLT1 and PLT2 in Arabidopsis thaliana. Front. Plant Sci. 2023, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Awan, S.A.; Ikram, R.; Rizwan, M.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Ali, S.; Ilyas, N. Effects of 24-epibrassinolide on plant growth, antioxidants defense system, and endogenous hormones in two wheat varieties under drought stress. Physiol. Plant. 2021, 172, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Khamsuk, O.; Sonjaroon, W.; Suwanwong, S.; Jutamanee, K.; Suksamrarn, A. Effects of 24-epibrassinolide and the synthetic brassinosteroid mimic on chili pepper under drought. Acta Physiol. Plant. 2018, 40, 106. [Google Scholar] [CrossRef]
- Behnamnia, M.; Kalantari, K.M.; Rezanejad, F. Exogenous application of brassinosteroid alleviates drought-induced oxidative stress in Lycopersicon esculentum L. Gen. Appl. Plant Physiol. 2009, 35, 22–34. [Google Scholar]
- Mahesh, K.; Balaraju, P.; Ramakrishna, B.; Rao, S. Effect of Brassinosteroids on Germination and Seedling Growth of Radish (Raphanus sativus L.) under PEG-6000 Induced Water Stress. Am. J. Plant Sci. 2013, 4, 2305–2313. [Google Scholar] [CrossRef]
- Duan, F.; Ding, J.; Lu, X.; Feng, Y.; Song, W. Overexpression of SoCYP85A1, a spinach cytochrome p450 gene in transgenic tobacco enhances root development and drought stress tolerance. Front. Plant Sci. 2017, 8, 1909. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Prasad, B.D.; Liu, Q.; Grbic, V.; Sharpe, A.; Singh, S.P.; Krishna, P. Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci. Rep. 2016, 6, 28298. [Google Scholar] [CrossRef]
- Liu, L.; Xiang, Y.; Yan, J.W.; Di, P.C.; Li, J.; Sun, X.J.; Han, G.Q.; Ni, L.; Jiang, M.Y.; Yuan, J.H.; et al. Brassinosteroid-signaling kinase 1 phosphorylating calcium/calmodulin-dependent protein kinase functions in drought tolerance in maize. New Phytol. 2021, 231, 695–712. [Google Scholar] [CrossRef]
- Cui, X.Y.; Gao, Y.; Guo, J.; Yu, T.F.; Zheng, W.J.; Liu, Y.W.; Chen, J.; Xu, Z.S.; Ma, Y.Z. BES/BZR transcription factor TaBZR2 positively regulates drought responses by activation of TaGST1. Plant Physiol. 2019, 180, 605–620. [Google Scholar] [CrossRef]
- Wang, Z.; Nakano, T.; Gendron, J.; Junxian, H.; Meng, C.; Vafeados, D.; Yang, Y.; Fujioka, S.; Yoshida, S.; Asami, T. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2002, 2, 505–513. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, B.; Xie, Z.; Nolan, T.; Ye, H.; Song, G.-Y.; Walley, J.; Yin, Y. GSK3-like kinase BIN2 phosphorylates RD26 to potentiate drought signaling in Arabidopsis. Plant J. 2019, 100, 923–937. [Google Scholar] [CrossRef]
- Xie, Z.; Nolan, T.; Jiang, H.; Tang, B.; Yin, Y. The AP2/ERF transcription factor TINY modulates brassinosteroid-regulated plant growth and drought responses in Arabidopsis. Plant Cell 2019, 31, 1788–1806. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Li, H.; Ding, Y.; Shi, Y.; Yang, S. BRASSINOSTEROID-INSENSITIVE2 negatively regulates the stability of transcription factor ICE1 in response to cold stress in Arabidopsis. Plant Cell 2019, 31, 2682–2696. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, J.; Wang, H.; Yang, C.; Chen, Y.; Li, Y.; Pan, S.; Dong, R.; Tang, G.; Barajas-Lopez, J.d.D.; et al. GSK3-like kinases positively modulate abscisic acid signaling through phosphorylating subgroup III SnRK2s in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 9651–9656. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Du, L.; Huang, X.; Ding, L.; Wang, Z.; Tang, D.; Chen, B.; Ao, L.; Liu, Y.; Kang, Z.; Mao, H. TaERF87 and TaAKS1 synergistically regulate TaP5CS1/TaP5CR1-mediated proline biosynthesis to enhance drought tolerance in wheat. New Phytol. 2023, 237, 232–250. [Google Scholar] [CrossRef]
- Sohag, A.; Tahjib-Ul-Arif, M.; Brestic, M.; Afrin, S.; Hossain, M.A. Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant Soil Environ. 2020, 66, 7–13. [Google Scholar] [CrossRef]
- Oh, M.H.; Honey, S.H.; Tax, F.E. The Control of Cell Expansion, Cell Division, and Vascular Development by Brassinosteroids: A Historical Perspective. Int. J. Mol. Sci. 2020, 21, 1743. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Zahra, N.; Zahra, K.; Raza, A.; Khan, S. Brassinosteroids: Molecular and physiological responses in plant growth and abiotic stresses. Plant Stress 2021, 2, 100029. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Shulaev, V.; Blumwald, E.; Suzuki, N. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar]
- Khan, R.; Ma, X.; Hussain, Q.; Asim, M.; Iqbal, A.; Ren, X.; Shah, S.; Chen, K.; Shi, Y. Application of 2,4-Epibrassinolide Improves Drought Tolerance in Tobacco through Physiological and Biochemical Mechanisms. Biology 2022, 11, 1192. [Google Scholar] [CrossRef] [PubMed]
- Barros Junior, U.O.; Lima, M.D.; Alsahli, A.A.; Lobato, A.K. Unraveling the roles of brassinosteroids in alleviating drought stress in young Eucalyptus urophylla plants: Implications on redox homeostasis and photosynthetic apparatus. Physiol. Plant. 2021, 172, 748–761. [Google Scholar] [CrossRef]
- Talaat, N.B.; Ibrahim, A.S.; Shawky, B.T. Enhancement of the expression of ZmBZR1 and ZmBES1 regulatory genes and antioxidant defense genes triggers water stress mitigation in maize (Zea mays L.) plants treated with 24-Epibrassinolide in combination with spermine. Agronomy 2022, 12, 2517. [Google Scholar] [CrossRef]
- Wei, T.-L.; Wang, Z.-X.; He, Y.-F.; Xue, S.; Zhang, S.-Q.; Pei, M.-S.; Liu, H.-N.; Yu, Y.-H.; Guo, D.-L. Proline synthesis and catabolism-related genes synergistically regulate proline accumulation in response to abiotic stresses in grapevines. Sci. Hortic. 2022, 305, 111373. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Yadav, V.; Zhao, W.; He, Y.; Zhang, X.; Wei, C. Drought-induced proline is mainly synthesized in leaves and transported to roots in watermelon under water deficit. Hortic. Plant J. 2022, 8, 615–626. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, F.; Xie, Q. Balancing growth and adaptation to stress: Crosstalk between brassinosteroid and abscisic acid signaling. Plant Cell Environ. 2020, 43, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, Y.; Zhang, Y.; Dong, Y.; Liu, Y.; Liu, L.; Wan, S.; He, J.; Yu, Y. Accumulation of galactinol and ABA is involved in exogenous EBR-induced drought tolerance in tea plants. J. Agric. Food Chem. 2022, 70, 13391–13403. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ma, J.; Huang, W.L.; Di, H.M.; Xia, X.; Ma, W.; Ma, J.; Yang, J.; Li, X.M.; Lian, H.S.; et al. Physiological and Comparative Transcriptome Analysis Reveals the Mechanism by Which Exogenous 24-Epibrassinolide Application Enhances Drought Resistance in Potato (Solanum tuberosum L.). Antioxidants 2022, 11, 1701. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.F.; Jia, C.G.; Li, Z.; Sun, B.; Zhang, L.P.; Liu, N.; Wang, Q.M. Effect of brassinosteroids on drought resistance and abscisic acid concentration in tomato under water stress. Sci. Hortic. 2010, 126, 103–108. [Google Scholar] [CrossRef]
- Wang, L.S.; Chen, Q.S.; Xin, D.W.; Qi, Z.M.; Zhang, C.; Li, S.N.; Jin, Y.M.; Li, M.; Mei, H.Y.; Su, A.Y.; et al. Overexpression of GmBIN2, a soybean glycogen synthase kinase 3 gene, enhances tolerance to salt and drought in transgenic Arabidopsis and soybean hairy roots. J. Integr. Agric. 2018, 17, 1959–1971. [Google Scholar] [CrossRef]
- Nie, S.; Huang, S.; Wang, S.; Mao, Y.; Liu, J. Enhanced brassinosteroid signaling intensity via SlBRI1 overexpression negatively regulates drought resistance in a manner opposite of that via exogenous BR application in tomato. Plant Physiol. Biochem. 2019, 138, 36–47. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Yu, H. GSK3s: Nodes of multilayer regulation of plant development and stress responses. Trends Plant Sci. 2021, 26, 1286–1300. [Google Scholar] [CrossRef]
- Mao, J.; Li, W.X.; Liu, J.; Li, J.M. Versatile Physiological Functions of Plant GSK3-Like Kinases. Genes 2021, 12, 697. [Google Scholar] [CrossRef]
- Lu, Q.; Houbaert, A.; Ma, Q.; Huang, J.J.; Sterck, L.; Zhang, C.; Benjamins, R.; Coppens, F.; Van Breusegem, F.; Russinova, E. Adenosine monophosphate deaminase modulates BIN2 activity through hydrogen peroxide-induced oligomerization. Plant Cell 2022, 34, 3844–3859. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.-L.; You, X.-Y.; Wang, X.-Y.; Cui, L.-H.; Jiang, Z.-H.; Zhang, K.-P. Exogenous 24-Epibrassinolide Enhanced Drought Tolerance and Promoted BRASSINOSTEROID-INSENSITIVE2 Expression of Quinoa. Plants 2024, 13, 873. https://doi.org/10.3390/plants13060873
Zhou Y-L, You X-Y, Wang X-Y, Cui L-H, Jiang Z-H, Zhang K-P. Exogenous 24-Epibrassinolide Enhanced Drought Tolerance and Promoted BRASSINOSTEROID-INSENSITIVE2 Expression of Quinoa. Plants. 2024; 13(6):873. https://doi.org/10.3390/plants13060873
Chicago/Turabian StyleZhou, Ya-Li, Xin-Yong You, Xing-Yun Wang, Li-Hua Cui, Zhi-Hui Jiang, and Kun-Peng Zhang. 2024. "Exogenous 24-Epibrassinolide Enhanced Drought Tolerance and Promoted BRASSINOSTEROID-INSENSITIVE2 Expression of Quinoa" Plants 13, no. 6: 873. https://doi.org/10.3390/plants13060873
APA StyleZhou, Y.-L., You, X.-Y., Wang, X.-Y., Cui, L.-H., Jiang, Z.-H., & Zhang, K.-P. (2024). Exogenous 24-Epibrassinolide Enhanced Drought Tolerance and Promoted BRASSINOSTEROID-INSENSITIVE2 Expression of Quinoa. Plants, 13(6), 873. https://doi.org/10.3390/plants13060873




