Functional Analysis of the GhIQD1 Gene in Cotton Resistance to Verticillium Wilt
Abstract
:1. Introduction
2. Results
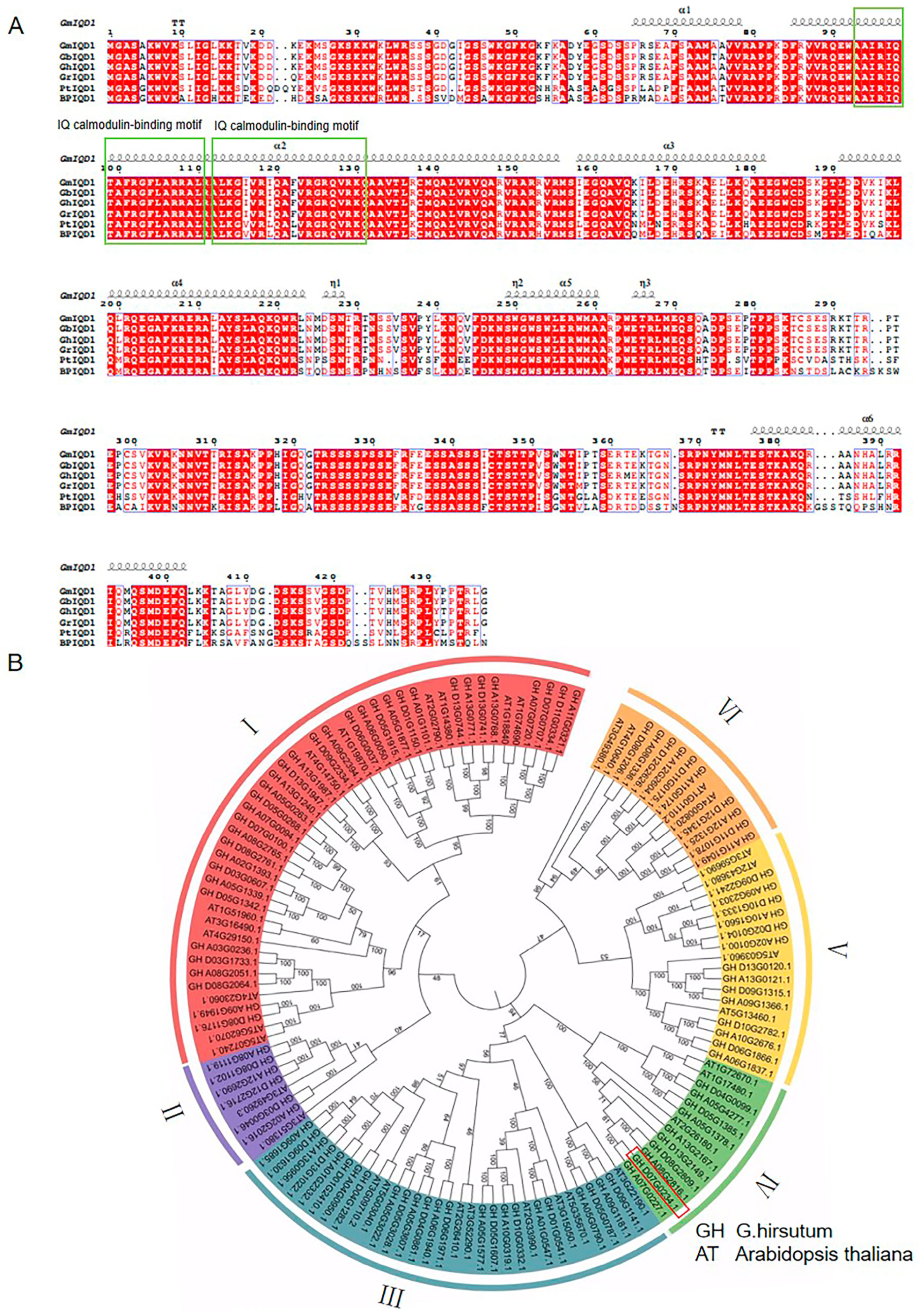
2.1. GhIQD1 Sequence Analysis
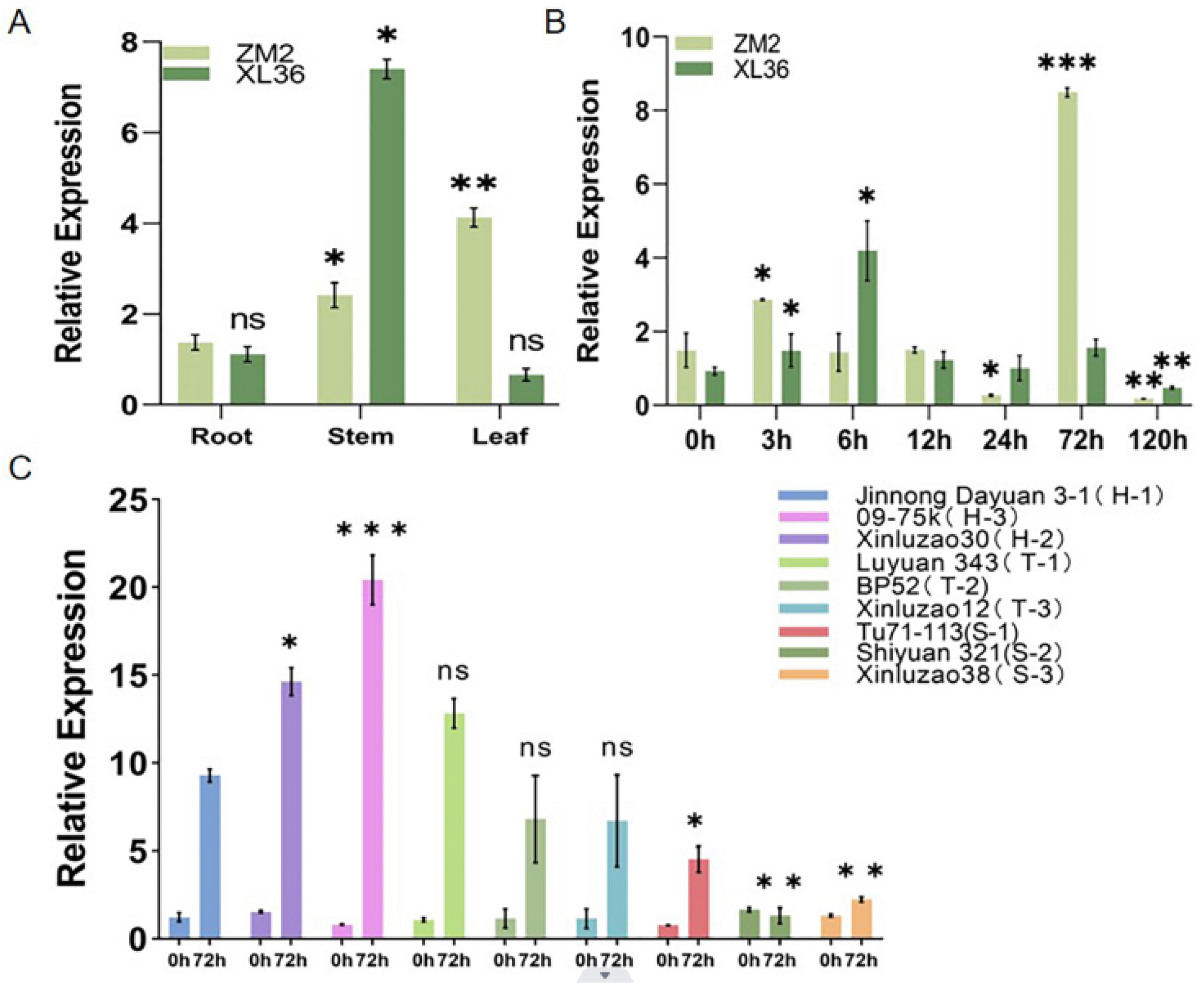
2.2. Analysis of GhIQD1 Expression Patterns
2.3. Construction and Silencing Efficiency Examination of the VIGS Vector of the GhIQD1 Gene
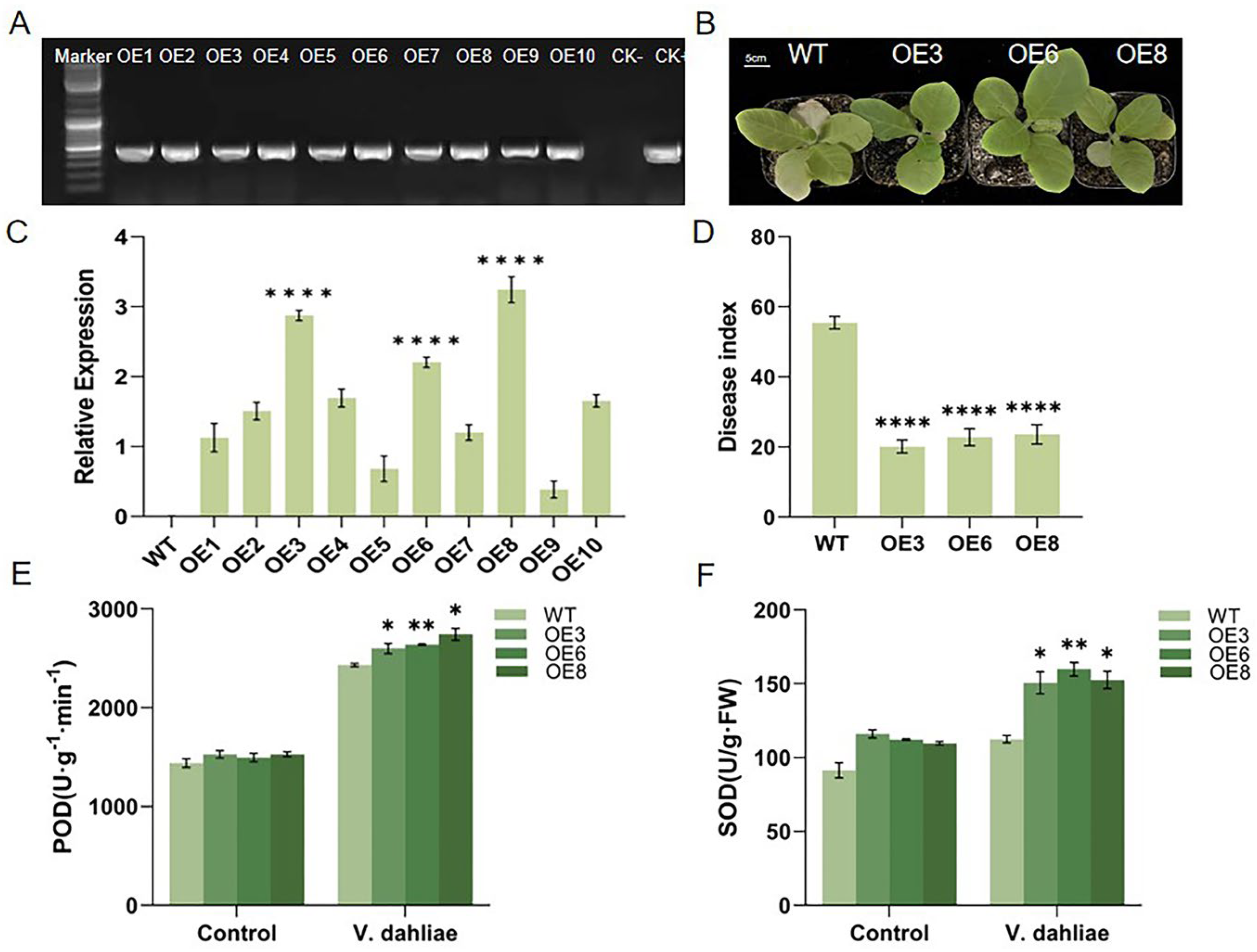
2.4. Overexpression of GhIQD1 Improved the Resistance of Tobacco to V. dahliae
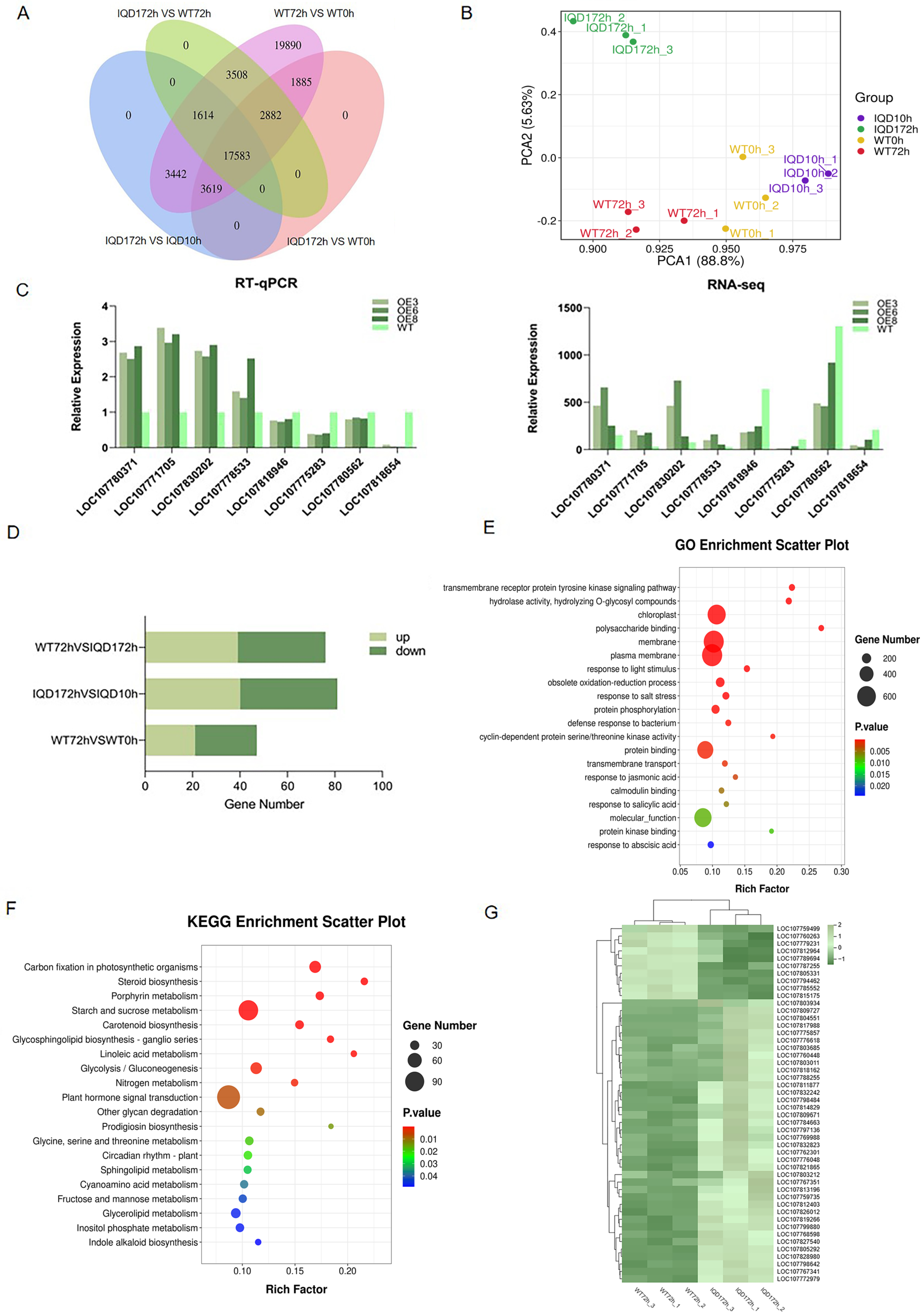
2.5. Transcriptome Analysis of the Resistance Mechanism of GhIQD1
2.6. The GhIQD1 Gene Is Associated with the Regulation of JA, ABA, and SA Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions and Culture of Test Strains
4.2. Cloning and Sequence Analysis of the GhIQD1 Gene
4.3. Analysis of GhIQD1 Expression Patterns
4.4. A VIGS Vector Was Constructed for Infection, and Trypan Blue Staining
4.5. Transgenic Tobacco
4.6. Determination of SOD and POD Activity in Transgenic Tobacco
4.7. Transcriptome Sequencing and Data Analysis
4.8. Expression Analysis of Defense Marker Genes and Characterization of Plant Hormones
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, R.; Li, J.; Xie, C.; Jian, W.; Yang, X. An overview of the molecular genetics of plant resistance to the Verticillium wilt pathogen Verticillium dahliae. Int. J. Mol. Sci. 2020, 21, 1120. [Google Scholar] [CrossRef] [PubMed]
- Ayele, A.G.; Wheeler, T.A.; Dever, J.K. Impacts of Verticillium wilt on photosynthesis rate, lint production, and fiber quality of greenhouse-grown cotton (Gossypium hirsutum). Plants 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Land, C.J.; Lawrence, K.S.; Burmester, C.H.; Meyer, B. Cultivar, irrigation, and soil contribution to the enhancement of Verticillium wilt disease in cotton. Crop Prot. 2017, 96, 1–6. [Google Scholar] [CrossRef]
- Jiang, Y.; Ding, P. Calcium signaling in plant immunity: A spatiotemporally controlled symphony. Trends Plant Sci. 2023, 28, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Xu, L.; Singh, A.; Du, L.; Poovaiah, B. Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front. Plant Sci. 2015, 6, 155259. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Jauregui, E.; Du, L.; Tanaka, K.; Poovaiah, B. Calcium signatures and signaling events orchestrate plant–microbe interactions. Curr. Opin. Plant Biol. 2017, 38, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, L.; Poovaiah, B. Calcium signaling and biotic defense responses in plants. Plant Signal. Behav. 2014, 9, e973818. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Ali, G.S.; Simons, K.A.; Hou, J.; Yang, T.; Reddy, A.; Poovaiah, B. Ca2+/calmodulin regulates salicylic-acid-mediated plant immunity. Nature 2009, 457, 1154–1158. [Google Scholar] [CrossRef]
- Galon, Y.; Nave, R.; Boyce, J.M.; Nachmias, D.; Knight, M.R.; Fromm, H. Calmodulin-binding transcription activator (CAMTA) 3 mediates biotic defense responses in Arabidopsis. FEBS Lett. 2008, 582, 943–948. [Google Scholar] [CrossRef]
- Laluk, K.; Prasad, K.; Savchenko, T.; Celesnik, H.; Dehesh, K.; Levy, M.; Mitchell-Olds, T.; Reddy, A. The calmodulin-binding transcription factor SIGNAL RESPONSIVE1 is a novel regulator of glucosinolate metabolism and herbivory tolerance in Arabidopsis. Plant Cell Physiol. 2012, 53, 2008–2015. [Google Scholar] [CrossRef]
- Qiu, Y.; Xi, J.; Du, L.; Suttle, J.C.; Poovaiah, B. Coupling calcium/calmodulin-mediated signaling and herbivore-induced plant response through calmodulin-binding transcription factor AtSR1/CAMTA3. Plant Mol. Biol. 2012, 79, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Peng, Z.; Li, H.; Qin, G.; Jia, Y.; Pan, Z.; He, S.; Qayyum, A.; Du, X. Genome wide analysis of IQD gene family in diploid and tetraploid species of cotton (Gossypium spp.). Int. J. Biol. Macromol. 2021, 184, 1035–1061. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Wang, Q.; Kaspi, R.; Parrella, M.P.; Abel, S. Arabidopsis IQD1, a novel calmodulin-binding nuclear protein, stimulates glucosinolate accumulation and plant defense. Plant J. 2005, 43, 79–96. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, T.; Yu, Z.; Li, Y.; Ren, H.; Hou, X.; Li, Y. Genome-wide analysis of the Chinese cabbage IQD gene family and the response of BrIQD5 in drought resistance. Plant Mol. Biol. 2019, 99, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; Bürstenbinder, K.; Müller, J. The emerging function of IQD proteins as scaffolds in cellular signaling and trafficking. Plant Signal. Behav. 2013, 8, e24369. [Google Scholar] [CrossRef]
- Li, Y.; Deng, M.; Liu, H.; Li, Y.; Chen, Y.; Jia, M.; Xue, H.; Shao, J.; Zhao, J.; Qi, Y. ABNORMAL SHOOT 6 interacts with KATANIN 1 and SHADE AVOIDANCE 4 to promote cortical microtubule severing and ordering in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 16. [Google Scholar] [CrossRef]
- Xing, L.; Li, W.; Yupeng, C.; Chen, L.; Yujie, L.; Lili, L.; Ming, L. The cotton protein GhIQD21 interacts with GhCaM7 and modulates organ morphogenesis in Arabidopsis by influencing microtubule stability. Plant Cell Rep. 2023, 42, 1025–1038. [Google Scholar]
- Xiu, Y.; Kirungu, J.N.; Magwanga, R.O.; Xu, Y.; Liu, F. Knockdown of GhIQD31 and GhIQD32 increases drought and salt stress sensitivity in Gossypium hirsutum. Plant Physiol. Biochem. 2019, 144, 166–177. [Google Scholar]
- Ranf, S.; Eschen-Lippold, L.; Pecher, P.; Lee, J.; Scheel, D. Interplay between calcium signalling and early signalling elements during defence responses to microbe-or damage-associated molecular patterns. Plant J. 2011, 68, 100–113. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Xu, H.; Yi, H.; Yang, L.; Kong, Z.; Zhang, L.; Xue, S.; Jia, H.; Ma, Z. Resistance to Hemi-Biotrophic F. graminearum Infection Is Associated with Coordinated and Ordered Expression of Diverse Defense Signaling Pathways. PLoS ONE 2011, 6, e19008. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Yang, Z.; Wang, X.; Butt, H.I.; Chen, E.; He, S.; Zhang, C.; Zhang, X.; Li, F. Salicylic acid-related cotton (Gossypium arboreum) ribosomal protein GaRPL18 contributes to resistance to Verticillium dahliae. BMC Plant Biology 2017, 17, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Hewedy, O.A.; Elsheery, N.I.; Karkour, A.M.; Elhamouly, N.; Arafa, R.A.; Mahmoud, G.A.-E.; Dawood, M.F.-A.; Hussein, W.E.; Mansour, A.; Amin, D.H. Jasmonic acid regulates plant development and orchestrates stress response during tough times. Environ. Exp. Bot. 2023, 208, 105260. [Google Scholar] [CrossRef]
- Wu, M.; Li, Y.; Chen, D.; Liu, H.; Zhu, D.; Xiang, Y. Genome-wide identification and expression analysis of the IQD gene family in moso bamboo (Phyllostachys edulis). Sci. Rep. 2016, 6, 24520. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Z.; Ma, H.; Chen, X.; Li, Y.; Wang, Y.; Xiang, Y. The IQD gene family in soybean: Structure, phylogeny, evolution and expression. PLoS ONE 2014, 9, e110896. [Google Scholar] [CrossRef] [PubMed]
- Barda, O.; Levy, M. IQD1 involvement in hormonal signaling and general defense responses against Botrytis cinerea. Cold Spring Harb. Lab. 2021, 13, 845140. [Google Scholar] [CrossRef] [PubMed]
- Bürstenbinder, K.; Savchenko, T.; Müller, J.; Adamson, A.W.; Stamm, G.; Kwong, R.; Zipp, B.J.; Dinesh, D.C.; Abel, S. Arabidopsis Calmodulin-binding Protein IQ67-Domain 1 Localizes to Microtubules and Interacts with Kinesin Light Chain-related Protein-1. J. Biol. Chem. 2013, 288, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, Z.; Ma, P.; Chen, Q. Integrative transcriptomic and gene co-expression network analysis of host responses upon Verticillium dahliae infection in Gossypium hirsutum. Nat. Publ. Group 2021, 11, 20586. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, X.; Sun, Y.; Wang, P.; Li, X.; Pei, Y.; Li, F.; Hou, Y. Molecular evidence for the involvement of a polygalacturonase-inhibiting protein, GhPGIP1, in enhanced resistance to Verticillium and Fusarium wilts in cotton. Sci. Rep. 2017, 7, 39840. [Google Scholar] [CrossRef]
- Eichmann, R.; Richards, L.; Schfer, P. Hormones as go-betweens in plant microbiome assembly. Plant J. 2020, 105, 518–541. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Li, X.; Fan, T.; Luo, H.; Xie, C.; Zhou, Y.; Tian, C.-E. The calmodulin-binding protein IQM1 interacts with CATALASE2 to affect pathogen defense. Plant Physiol. 2019, 181, 1314–1327. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Miyoshi, S.; Tamaoki, D.; Yamada, S.; Tanaka, K.; Uji, Y.; Tanaka, S.; Akimitsu, K.; Gomi, K. Isolation of jasmonate-induced sesquiterpene synthase of rice: Product of which has an antifungal activity against Magnaporthe oryzae. J. Plant Physiol. 2014, 171, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Moeder, W.; Yoshioka, K.; Shan, L. A tale of many families: Calcium channels in plant immunity. Plant Cell 2022, 34, 1551–1567. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Smigel, A.; Tsai, Y.-C.; Braam, J.; Berkowitz, G.A. Innate immunity signaling: Cytosolic Ca2+ elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein. Plant Physiol. 2008, 148, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Robe, E.; Jomat, L.; Aldon, D.; Mazars, C.; Galaud, J.-P. CML8, an Arabidopsis calmodulin-like protein, plays a role in Pseudomonas syringae plant immunity. Plant Cell Physiol. 2017, 58, 307–319. [Google Scholar] [PubMed]
- Zhang, L.; Wu, Y.; Yu, Y.; Zhang, Y.; Wei, F.; Zhu, Q.H.; Zhou, J.; Zhao, L.; Zhang, Y.; Feng, Z. Acetylation of GhCaM7 enhances cotton resistance to Verticillium dahliae. Plant J. 2023, 114, 1405–1424. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.M.; Panstruga, R. Cytoskeleton functions in plant–microbe interactions. Physiol. Mol. Plant Pathol. 2007, 71, 135–148. [Google Scholar] [CrossRef]
- Campe, R.; Langenbach, C.; Leissing, F.; Popescu, G.V.; Popescu, S.C.; Goellner, K.; Gerold, J.M.; Conrath, U. ABC transporter PEN3/PDR8/ABCG36 interacts with calmodulin that, like PEN3, is required for Arabidopsis nonhost resistance. New Phytol. 2016, 209, 294–306. [Google Scholar] [CrossRef]
- Hettenhausen, C.; Baldwin, I.T.; Wu, J. Nicotiana attenuata MPK4 suppresses a novel jasmonic acid (JA) signaling-independent defense pathway against the specialist insect Manduca sexta, but is not required for the resistance to the generalist Spodoptera littoralis. New Phytol. 2013, 199, 787–799. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, X.; Wang, P.; Wang, H.; Ge, X.; Li, F.; Hou, Y. GhODO1, an R2R3-type MYB transcription factor, positively regulates cotton resistance to Verticillium dahliae via the lignin biosynthesis and jasmonic acid signaling pathway. Int. J. Biol. Macromol. 2022, 201, 580–591. [Google Scholar] [CrossRef]
- Yan, C.; Fan, M.; Yang, M.; Zhao, J.; Zhang, W.; Su, Y.; Xiao, L.; Deng, H.; Xie, D. Injury Activates Ca2+/Calmodulin-Dependent Phosphorylation of JAV1-JAZ8-WRKY51 Complex for Jasmonate Biosynthesis. Mol. Cell 2018, 70, 136–149.e137. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhu, L.; Guan, Q.; Xiao, S.; Min, L.; Zhang, X. GhCPK33 Negatively Regulates Defense against Verticillium dahliae by Phosphorylating GhOPR3. Plant Physiol. 2018, 178, 876. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Kim, S.H.; Kim, K.E.; Bahk, S.; Nguyen, X.C.; Kim, M.G.; Hong, J.C.; Chung, W.S. Ca2+/CaM increases the necrotrophic pathogen resistance through the inhibition of a CaM-regulated dual-specificity protein phosphatase 1 in Arabidopsis. Plant Biotechnol. Rep. 2022, 16, 71–78. [Google Scholar] [CrossRef]
- Cao, M.-J.; Zhang, Y.-L.; Liu, X.; Huang, H.; Zhou, X.E.; Wang, W.-L.; Zeng, A.; Zhao, C.-Z.; Si, T.; Du, J. Combining chemical and genetic approaches to increase drought resistance in plants. Nat. Commun. 2017, 8, 1183. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zheng, Z.; Chinnusamy, V.; Zhu, J.; Cui, X.; Iida, K.; Zhu, J.-K. RAS1, a quantitative trait locus for salt tolerance and ABA sensitivity in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 5669–5674. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.I.; Hagiwara, S. Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proc. Natl. Acad. Sci. USA 1990, 87, 9305–9309. [Google Scholar] [CrossRef]
- Lulu, B.; Lin, W.; Zhuyan, J.; Han, X. The tomato IQD gene SUN24 regulates seed germination through ABA signaling pathway. Planta 2018, 248, 919–931. [Google Scholar]
- Guo, Y.; Xiong, L.; Song, C.P.; Gong, D.; Halfter, U.; Zhu, J.K. A calcium sensor and its interacting protein kinase are global regulators of abscisic acid signaling in Arabidopsis. Dev. Cell 2002, 3, 233–244. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, X.F.; Zhang, D.P. CPK12: A Ca2+-dependent protein kinase balancer in abscisic acid signaling. Plant Signal. Behav. 2011, 6, 1687–1690. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, Z.; Shah, S.H.A.; Xiao, D.; Hou, X.; Li, Y.; Luo, Z.B. Ectopic expression of BrIQD35 promotes drought stress tolerance in Nicotiana benthamiana. Plant Biol. 2022, 24, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Rong, W.; Yang, J.; Ma, Z. Island Cotton Enhanced Disease Susceptibility 1 Gene Encoding a Lipase-Like Protein Plays a Crucial Role in Response to Verticillium dahliae by Regulating the SA Level and H2O2 Accumulation. Front. Plant Sci. 2016, 7, 1830. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yang, L.; Li, B.; Zheng, X.-Y.; Li, J.; Yang, M.; Dong, X.; He, G.; An, C.; Deng, X.W. Salicylic acid biosynthesis is enhanced and contributes to increased biotrophic pathogen resistance in Arabidopsis hybrids. Nat. Commun. 2015, 6, 7309. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ding, Y.; Tang, X.; Wang, G.; Tang, X. Effect of L-Arginine on Maintaining Storage Quality of the White Button Mushroom (Agaricus bisporus). Food Bioprocess Technol. 2019, 12, 563–574. [Google Scholar] [CrossRef]
- Hernández-Plaza, A.; Szklarczyk, D.; Botas, J.; Cantalapiedra, C.P.; Giner-Lamia, J.; Mende, D.R.; Kirsch, R.; Rattei, T.; Letunic, I.; Jensen, L.J.; et al. eggNOG 6.0: Enabling comparative genomics across 12 535 organisms. Nucleic Acids Res. 2022, 51, D389–D394. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics-A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, T.; Sun, Y.; Zhang, Y.; Zhang, Y. Diverse Roles of the Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Plant Immunity. Plant Cell 2020, 32, 4002–4016. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, C.; Zheng, H.; Sun, M.; Ding, Z. Antagonistic Interaction between Auxin and SA Signaling Pathways Regulates Bacterial Infection through Lateral Root in Arabidopsis. Cell Rep. 2020, 32, 108060. [Google Scholar] [CrossRef]
- Yang, L.; Sun, Q.; Geng, B.; Shi, J.; Zhu, H.; Sun, Y.; Yang, Q.; Yang, B.; Guo, Z. Jasmonate biosynthesis enzyme allene oxide cyclase 2 mediates cold tolerance and pathogen resistance. Plant Physiol. 2023, 193, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhang, T.; Huangfu, J.; Li, R.; Lou, Y. Both allene oxide synthases genes are involved in the biosynthesis of herbivore-induced jasmonic acid and herbivore resistance in rice. Plants 2021, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhao, Y.; Gao, J.; Xiang, C.; Zhu, J. The ABA receptor PYL9 together with PYL8 plays an important role in regulating lateral root growth. Sci. Rep. 2016, 6, 27177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Cheng, J.; Sun, Q.; Zhang, Y.; Liu, J.; Li, H.; Zhang, Z.; Wang, P.; Cai, C.; et al. lncRNA7 and lncRNA2 modulate cell wall defense genes to regulate cotton resistance to Verticillium wilt. Plant Physiol. 2022, 189, 264–284. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhou, T.; Wang, Y.; Yang, Y.; Pu, Y.; Chen, Q.; Zheng, K.; Sun, G. Functional Analysis of the GhIQD1 Gene in Cotton Resistance to Verticillium Wilt. Plants 2024, 13, 1005. https://doi.org/10.3390/plants13071005
Xu J, Zhou T, Wang Y, Yang Y, Pu Y, Chen Q, Zheng K, Sun G. Functional Analysis of the GhIQD1 Gene in Cotton Resistance to Verticillium Wilt. Plants. 2024; 13(7):1005. https://doi.org/10.3390/plants13071005
Chicago/Turabian StyleXu, Jianglin, Ting Zhou, Yongqiang Wang, Yejun Yang, Yuanchun Pu, Quanjia Chen, Kai Zheng, and Guoqing Sun. 2024. "Functional Analysis of the GhIQD1 Gene in Cotton Resistance to Verticillium Wilt" Plants 13, no. 7: 1005. https://doi.org/10.3390/plants13071005




