Plant-Based Biostimulants for Seeds in the Context of Circular Economy and Sustainability
Abstract
:1. Introduction
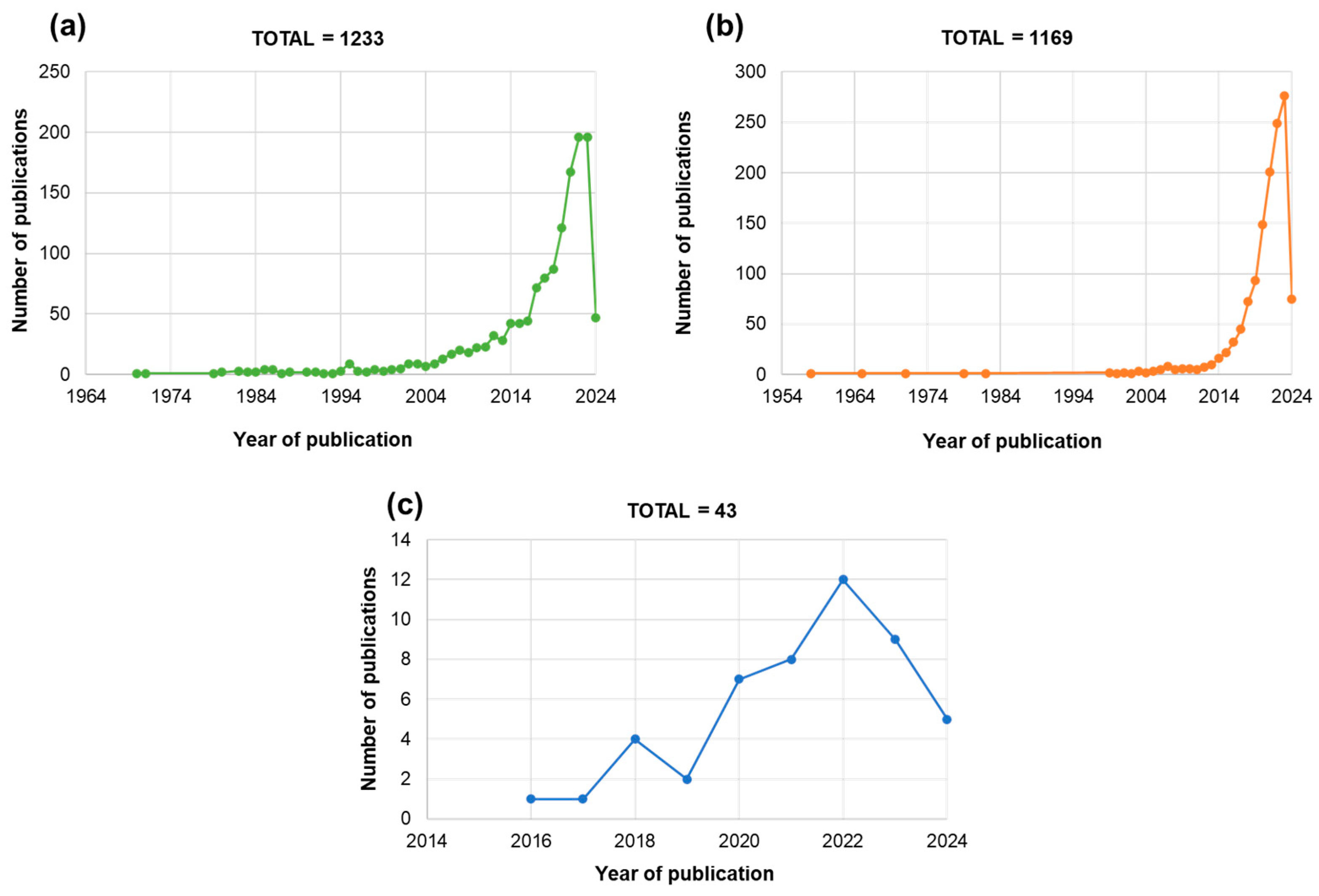
2. Agri-Food Market Trends Regarding Seeds and Biostimulants
3. Sustainable Methods for the Production of Plant-Based Biostimulants
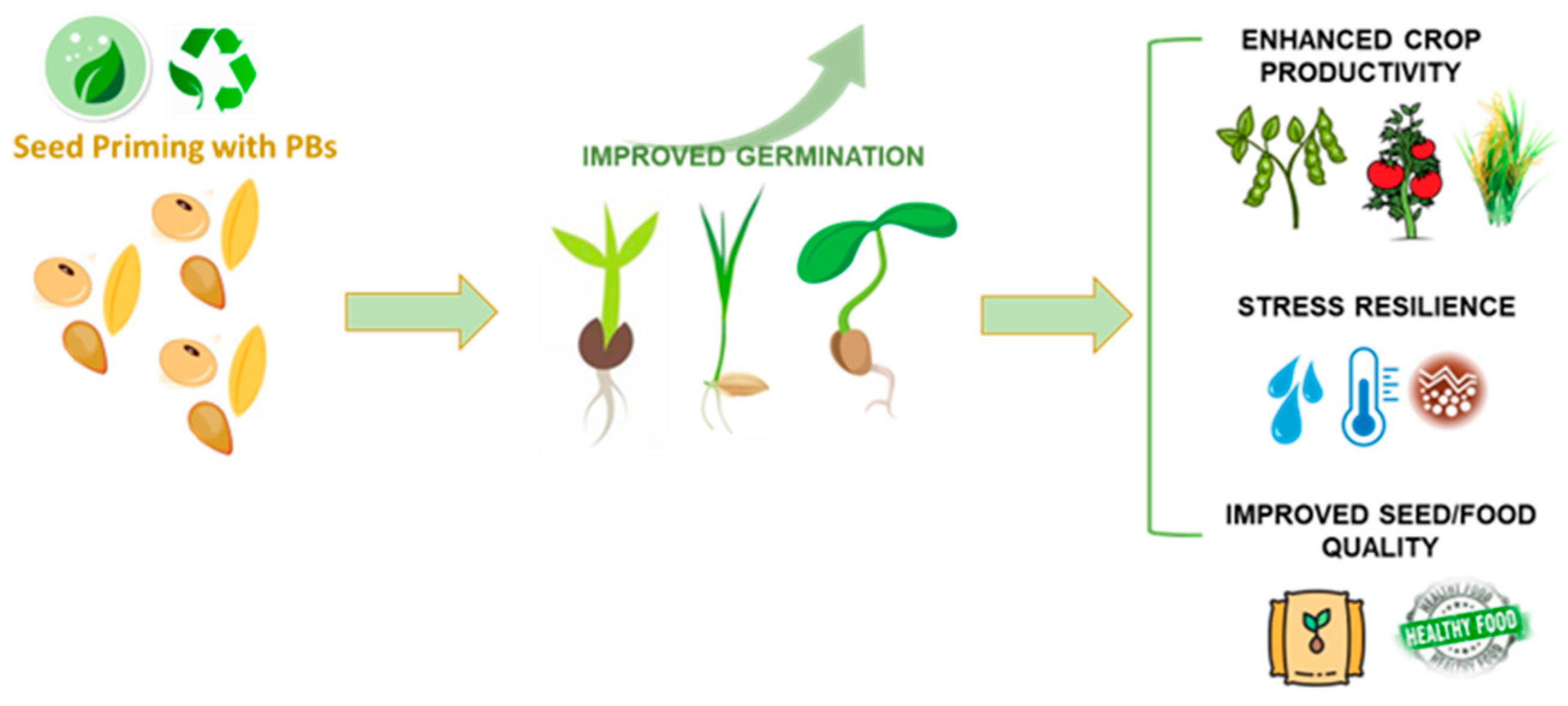
4. Why Use Biostimulants as Seed Treatments?
5. Examples of the Application of Plant-Based Biostimulants as Seeds Treatments
| Type | Product | Source | Application on Seeds | Advantages | References |
|---|---|---|---|---|---|
| Commercial | Kelpak® | Ecklonia maxima | Abelmoschus esculentus | Improved germination indexes (FGP, GI, GRI) | [57] |
| Ceratotheca triloba | Improved germination under drought and salinity stress | [58] | |||
| KIEM® | Lignins, amino acids, molybdenum | Cucumis sativus Glycine max | Increased germination and biomass, improved antioxidant defense under heat stress | [59,60] | |
| Algae | Microalgae extracts | Acutodesmus dimorphus | Solanum lycopersicum | Enhanced germination and plant growth | [62] |
| Scenedesmus obliquus | Lepidium sativum | Increased germination index | [63] | ||
| Chlorella vulgaris | Solanum lycopersicum Hordeum vulgare | Increased germination percentage and germination index, decreased germination time | [64] | ||
| Brown alga | Sargassum polycystum | Vicia faba Helianthus annuus | Improved SVI, radicle and seedling length, fresh weight, increase in phenolic and flavonoid contents and the total antioxidant capacity | [65] | |
| Green alga | Cladophora glomerata | Lupinus angustifolius | Enhanced germination percentage, root, hypocotyl and epicotyl length | [66] | |
| Plant Extracts | Leaf extracts | Moringa oleifera | Capsicum annuum | Improved germination and seedling growth under heavy metal and salinity stress | [68] |
| Atriplex halimus | Sorghum bicolor | Cadmium stress alleviation Enhanced GP, SVI, decreased MGT, stimulated antioxidant enzymes | [72] | ||
| Fresh biomass | Cuscuta reflexa | Triticum aestivum | Water stress mitigation | [69] | |
| Brown juice | Medicago sativa | Tagetes patula | Increased germination parameters, seedling growth and biomass | [70] | |
| Dry biomass | Posidonia oceanica | Solanum lycopersicum | Increased germination index and root length | [71] | |
| Helianthus annuus | Pisum sativum | Boosted SG, GI and VI | [73] | ||
| Bioactive Compouds | Bioflavonoids | Citrus fruits | Brassica napus Glycine max | Increased germination under salinity stress | [75] |
| Melatonin (MEL) | Monocot and dicot families | Stevia rebaudiana | Improved germination and salinity stress alleviation | [77] | |
| Zea mays | Improved germination and embryonic axis growth during chilling stress | [78] | |||
| Garlic extracts | Allium sativum | Solanum lycopersicum | Improvement of seed germination and alleviation of salinity stress | [13] | |
| CSL protein hydrolysate | Zea mays | Triticum aestivum | Increase in germination parameters | [81] | |
| Thymoquinone | Nigella sativa | Lens culinaris | Increased germination indexes and alleviation of cadmium stress | [83] | |
| Thyme essential oil | Thymbra capitata | Triticum turgidum | Enhanced germination and drought tolerance | [84] | |
| Saponins | Chenopodium quinoa | Raphanus sativus | Improved germination indexes with no phytotoxicity | [86] | |
| Lignin nanoparticles | Alkali lignin | Zea mays | Increased germination, radicle length, fresh weight, shoots and roots length | [87] | |
| Plant Waste Material | Hydrochar | Saccharum officinarum | Zea mays | Increased germination rate | [88] |
| Spent coffee grounds | Coffea arabica | Brassica spp. | Improvement of germination | [89] | |
| Compost material | Artichoke (Cynara cardunculus var. scolymus) | Zea mays | Increased germination rate, primary and lateral root length | [90] |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agriculture Organization). The Future of Food and Agriculture—Alternative Pathways to 2050. 2018. Available online: http://www.fao.org/3/I8429EN/i8429en.pdf (accessed on 29 March 2024).
- ISTA (International Seed Testing Association). International Rules for Seed Testing. Basserdorf, Switzerland. 2018. Available online: http://www.seedtest.org/en/home.html (accessed on 29 March 2024).
- Forti, C.; Ottobrino, V.; Bassolino, L.; Toppino, L.; Rotino, G.L.; Pagano, A.; Macovei, A.; Balestrazzi, A. Molecular dynamics of pre-germinative metabolism in primed eggplant (Solanum melongena L.) seeds. Hortic. Res. 2020, 7, 87. [Google Scholar] [CrossRef]
- Forti, C.; Shankar, A.; Singh, A.; Balestrazzi, A.; Prasad, V.; Macovei, A. Hydropriming and biopriming improve Medicago truncatula seed germination and upregulate DNA repair and antioxidant genes. Genes 2020, 11, 242. [Google Scholar] [CrossRef]
- Pagano, A.; Folini, G.; Pagano, P.; Sincinelli, F.; Rossetto, A.; Macovei, A.; Balestrazzi, A. ROS accumulation as a hallmark of dehydration stress in primed and overprimed Medicago truncatula seeds. Agronomy 2022, 12, 268. [Google Scholar] [CrossRef]
- Pagano, A.; Macovei, A.; Balestrazzi, A. Molecular dynamics of seed priming at the crossroads between basic and applied research. Plant Cell Rep. 2023, 42, 657–688. [Google Scholar] [CrossRef] [PubMed]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Gerna, D.; Roach, T.; Arc, E.; Stöggl, W.; Limonta, M.; Vaccino, P.; Kranner, I. Redox poise and metabolite changes in bread wheat seeds are advanced by priming with hot steam. Biochem. J. 2018, 475, 3725–3743. [Google Scholar] [CrossRef]
- Devika, O.S.; Singh, S.; Sarkar, D.; Barnwal, P.; Suman, J.; Rakshit, A. Seed priming: A potential supplement in integrated resource management under fragile intensive ecosystems. Front. Sustain. Food Syst. 2021, 5, 654001. [Google Scholar] [CrossRef]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed priming: A feasible strategy to enhance drought tolerance in crop plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Quan, W.; Bartels, D. Stress memory responses and seed priming correlate with drought tolerance in plants: An overview. Planta 2022, 255, 45. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.; Acosta, Y.; Nápoles, L.; Carvajal, C.; Linares, C.; Sershen, N.; Lorenzo, J.C.; Pérez, A. Pineapple stem-derived bromelain based priming improves pepper seed protein reserve mobilization, germination, emergence and plant growth. Physiol. Mol. Biol. Plants 2021, 27, 1651–1657. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, H.; Nasir, M.; Khan, M.N.; Ali, M.; Hayat, K.; Khan, M.A.; Khan, F.; Ma, Y.; Cheng, Z. Some physiological and biochemical mechanisms during seed-to-seedling transition in tomato as influenced by garlic allelochemicals. Antioxidants 2020, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The opportunity of valorizing agricultural waste, through its conversion into biostimulants, biofertilizers, and biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gama-Arachchige, N.S.; Zhao, M. Trends in Seed Priming Research in the Past 30 Years Based on Bibliometric Analysis. Plants 2023, 12, 3483. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent Advances in the Molecular Effects of Biostimulants in Plants: An Overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Gatto, F.; Re, I. Circular bioeconomy business models to overcome the Valley of Death. A systematic statistical analysis of studies and projects in emerging bio-based technologies and trends linked to the SME instrument support. Sustainability 2021, 13, 1899. [Google Scholar] [CrossRef]
- Meena, R.S.; Lal, R.; Yadav, G.S. Long-term impacts of topsoil depth and amendments on soil physical and hydrological properties of an Alfisol in central Ohio, USA. Geoderma 2020, 363, 114164. [Google Scholar] [CrossRef]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. Top. Curr. Chem. 2018, 376, 3. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Galanakis, C.M. Food Waste Recovery: Processing Technologies and Industrial Techniques; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Varzakas, T.; Zakynthinos, G.; Verpoort, F. Plant Food Residues as a Source of Nutraceuticals and Functional Foods. Foods 2016, 5, 88. [Google Scholar] [CrossRef]
- Nguyen, V.T. Potential, Uses and Future Perspectives of Agricultural Wastes. In Recovering Bioactive Compounds from Agricultural Wastes; Wiley: Hoboken, NJ, USA, 2017; pp. 1–32. [Google Scholar]
- Pham, H.N.T. Recovering Bioactive Compounds from Fruit and Vegetable Wastes. In Recovering Bioactive Compounds from Agricultural Wastes; Nguyen, V.T., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 81–99. [Google Scholar]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed]
- Abad-Garcia, B.; Barrueta, L.A.; Lopez-Marquez, D.M.; Crespo-Ferrer, I.; Gallo, B.; Vicente, F. Optimization and validation of a methodology based on solvent extraction and liquid chromatography for the simultaneous determination of several polyphenolic families in fruit juices. J. Chromatogr. A 2007, 1154, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tsakona, S.; Galanakis, C.M.; Gekas, V. Hydro-ethanolic mixtures for the recovery of phenols from Mediterranean plant material. Food Bioprocess Technol. 2012, 5, 1384–1393. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Effect of extraction parameters on the carotenoid recovery from tomato waste. Int. J. Food Sci. Technol. 2011, 46, 23–29. [Google Scholar] [CrossRef]
- Cerpa, M.G.; Rafael, B.; Mato, M.; Cocero, J.; Ceriani, R.; Meirelles, A.J.A.; Prado, J.M.; Patrícia, F.L.; Takeuchi, T.M.; Meireles, M.A.A. Steam distillation applied to the food industry. In Extracting Bioactive Compounds for Food Products: Theory and Applications, 1st ed.; Meireles, M.A.A., Ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Akyüz, A.; Ersus, S. Optimization of Enzyme Assisted Extraction of Protein from the Sugar Beet (Beta vulgaris L.) Leaves for Alternative Plant Protein Concentrate Production. Food Chem. 2021, 335, 127673. [Google Scholar] [CrossRef] [PubMed]
- Shinwari, K.J. Emerging technologies for the recovery of bioactive compounds from saffron species. In Saffron.; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 143–182. [Google Scholar]
- Garavand, F.; Rahaee, S.; Vahedikia, N.; Jafari, S.M. Different techniques for extraction and micro/nanoencapsulation of saffron bioactive ingredients. Trends Food Sci. Technol. 2019, 89, 26–44. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Munoz, O.; Sepulveda, M.; Schwartz, M. Effects of enzymatic treatment on anthocyanic pigments from grapes skin from Chilean wine. Food Chem. 2004, 87, 487–490. [Google Scholar] [CrossRef]
- Mishra, D.; Shukla, A.K.; Dixit, A.K.; Singh, K. Aqueous enzymatic extraction of oil from mandarin peels. J. Oleo Sci. 2005, 54, 355–359. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M. Extraction of phenolics from citrus peels: II. Enzyme-assisted extraction method. Sep. Purif. Technol. 2006, 8, 189–196. [Google Scholar] [CrossRef]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Singhal, R.S. Enzyme-Assisted Extraction of Bioactives. In Food Bioactives; Puri, M., Ed.; Springer: Cham, Switzerland, 2017; pp. 171–201. [Google Scholar]
- Cui, W.; Han, L.; Suo, F.; Liu, Z.; Zhou, L.; Zhou, Z. Exploitation of Bacillus subtilis as a robust workhorse for production of heterologous proteins and beyond. World J. Microbiol. Biotechnol. 2018, 34, 145. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Doria, E.; Buonocore, D.; Marra, A.; Bontà, V.; Gazzola, A.; Dossena, M.; Verri, M.; Calvio, C. Bacterial-Assisted Extraction of Bioactive Compounds from Cauliflower. Plants 2022, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants Application: A low input cropping management tool for sustainable farming of vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Rouphael, Y.; Spíchal, L.; Panzarová, K.; Casa, R.; Colla, G. High-Throughput plant phenotyping for developing novel biostimulants: From lab to field or from field to lab? Front. Plant Sci. 2018, 9, 1197. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Baffi, C.; Colla, G. A vegetal biopolymer-based biostimulant promoted root growth in melon while triggering brassinosteroids and stress-related compounds. Front. Plant Sci. 2018, 9, 472. [Google Scholar] [CrossRef]
- Andreotti, C.; Rouphael, Y.; Colla, G.; Basile, B. Rate and Timing of Application of Biostimulant Substances to Enhance Fruit Tree Tolerance toward Environmental Stresses and Fruit Quality. Agronomy 2022, 12, 603. [Google Scholar] [CrossRef]
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Efficacy of microalgal extracts as biostimulants through seed treatment and foliar spray for tomato cultivation. Ind. Crops Prod. 2020, 151, 112453. [Google Scholar]
- Macovei, A.; Pagano, A.; Leonetti, P.; Carbonera, D.; Balestrazzi, A.; De Sousa Araújo, S. Systems biology and genome-wide approaches to unveil the molecular players involved in the pre-germinative metabolism: Implications on seed technology traits. Plant Cell Rep. 2016, 36, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Makhaye, G.; Mofokeng, M.A.; Tesfay, S.; Aremu, A.O.; Van Staden, J.; Amoo, S.O. Influence of plant biostimulant application on seed germination. In Biostimulants for Crops from Seed Germination to Plant Development; Gupta, S., Van Staden, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 109–135. [Google Scholar]
- Paul, S.; Dey, S.; Kundu, R. Seed priming: An emerging tool towards sustainable agriculture. Plant Growth Regul. 2021, 97, 215–234. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 26, 2049. [Google Scholar] [CrossRef] [PubMed]
- Ogunsanya, H.Y.; Motti, P.; Li, J.; Trinh, H.K.; Xu, L.; Bernaert, N.; Van Droogenbroeck, B.; Murvanidze, N.; Werbrouck, S.P.O.; Mangelinckx, S.; et al. Belgian endive-derived biostimulants promote shoot and root growth in vitro. Sci. Rep. 2022, 12, 8792. [Google Scholar] [CrossRef] [PubMed]
- Makhaye, G.; Aremu, A.O.; Gerrano, A.S.; Tesfay, S.; Du Plooy, C.P.; Amoo, S.O. Biopriming with seaweed extract and microbial-based commercial biostimulants influences seed germination of five Abelmoschus esculentus genotypes. Plants 2021, 10, 1327. [Google Scholar] [CrossRef] [PubMed]
- Masondo, N.A.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Influence of biostimulants-seed-priming on Ceratotheca triloba germination and seedling growth under low temperatures, low osmotic potential and salinity stress. Ecotoxicol. Environ. Saf. 2018, 147, 43–48. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Grange, E.; Mannino, G.; Van Arkel, J.; Beekwilder, J.; Karlova, R.; Garabello, C.; Contartese, V.; Bertea, C.M. A biostimulant seed treatment improved heat stress tolerance during cucumber seed germination by acting on the antioxidant system and glyoxylate cycle. Front. Plant Sci. 2021, 11, 836. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Mannino, G.; Agliassa, C.; Acquadro, A.; Contartese, V.; Garabello, C.; Bertea, C.M. Transcriptome analyses and antioxidant activity profiling reveal the role of a lignin-derived biostimulant seed treatment in enhancing heat stress tolerance in soybean. Plants 2020, 9, 1308. [Google Scholar] [CrossRef] [PubMed]
- Rachidi, F.; Benhima, R.; Kasmi, Y.; Sbabou, L.; Arroussi, H.E. Evaluation of microalgae polysaccharides as biostimulants of tomato plant defense using metabolomics and biochemical approaches. Sci. Rep. 2021, 11, 930. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Navarro-López, E.; Ruíz-Nieto, A.; Ferreira, A.; Acién, F.G.; Gouveia, L. Biostimulant potential of Scenedesmus obliquus grown in brewery wastewater. Molecules 2020, 25, 664. [Google Scholar] [CrossRef] [PubMed]
- Alling, T.; Funk, C.; Gentili, F.G. Nordic microalgae produce biostimulant for the germination of tomato and barley seeds. Sci. Rep. 2023, 13, 3509. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; El-Sheekh, M.M.; Hamed Aly, S.; Al-Harbi, M.; Elkelish, A.; Nagah, A. Inductive role of the brown alga Sargassum polycystum on growth and biosynthesis of imperative metabolites and antioxidants of two crop plants. Front. Plant Sci. 2023, 14, 1136325. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, S.; Dziergowska, K.; Galek, R.; Michalak, I. Cladophora glomerata extracts produced by ultrasound-assisted extraction support early growth and development of lupin (Lupinus angustifolius L.). Sci. Rep. 2023, 13, 17867. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, K.; Ronga, D.; Michalak, I. Plant extracts—Importance in sustainable agriculture. Ital. J. Agron. 2021, 16, 1851. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef]
- Ali, Q.; Perveen, R.; El-Esawi, M.A.; Ali, S.; Hussain, S.M.; Amber, M.; Iqbal, N.; Rizwan, M.; Alyemeni, M.N.; El-Serehy, H.A.; et al. Low doses of Cuscuta reflexa extract act as natural biostimulants to improve the germination vigor, growth, and grain yield of wheat grown under water stress: Photosynthetic pigments, antioxidative defense mechanisms, and nutrient acquisition. Biomolecules 2020, 10, 1212. [Google Scholar] [CrossRef]
- Barna, D.; Kisvarga, S.; Kovács, S.; Csatári, G.; Tóth, I.O.; Fári, M.G.; Alshaal, T.; Bákonyi, N. Raw and fermented alfalfa brown juice induces changes in the germination and development of French marigold (Tagetes patula L.) plants. Plants 2021, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Ferrández-Gómez, B.; Jordá, J.D.; Cerdán, M.; Sánchez, A. Valorization of Posidonia oceanica biomass: Role on germination of cucumber and tomato seeds. Waste Manag. 2023, 171, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Ennoury, A.; Nhhala, N.; Kchikich, A.; Roussi, Z.; Asri, S.E.; Zouaoui, Z.; Nhiri, M. Saltbuch extract: A bio-solutionfor cadmium stress sorghum plants in germination and maturation. Biometals 2023, 36, 997–1012. [Google Scholar] [CrossRef] [PubMed]
- Janusauskaite, D. The allelopathic activity of aqueous extracts of Helianthus annuus L., grown in boreal conditions, on germination, development, and physiological indices of Pisum sativum L. Plants 2023, 12, 1920. [Google Scholar] [CrossRef] [PubMed]
- Dincheva, I.; Badjakov, I.; Galunska, B. New insights into the research of bioactive compounds from plant origins with nutraceutical and pharmaceutical potential. Plants 2023, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Subramanian, S.; Smith, D.L. Seed priming with Devosia sp. cell-free supernatant (CFS) and citrus bioflavonoids enhance canola and soybean seed germination. Molecules 2022, 27, 3410. [Google Scholar] [CrossRef] [PubMed]
- Back, K. Melatonin metabolism, signaling and possible roles in plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef] [PubMed]
- Simlat, M.; Szewczyk, A.; Ptak, A. Melatonin promotes seed germination under salinity and enhances the biosynthesis of steviol glycosides in Stevia rebaudiana Bertoni leaves. PLoS ONE 2020, 15, e0230755. [Google Scholar] [CrossRef]
- Kołodziejczyk, I.; Kaźmierczak, A.; Posmyk, M.M. Melatonin application modifies antioxidant defense and induces endoreplication in maize seeds exposed to chilling stress. Int. J. Mol. Sci. 2022, 22, 8628. [Google Scholar] [CrossRef]
- Ali, M.; Ahmad, H.; Hayat, S.; Ghani, M.I.; Amin, B.; Atif, M.J.; Wali, K.; Cheng, Z. Application of garlic allelochemicals improves growth and induces defense responses in eggplant (Solanum melongena) against Verticillium dahliae. Ecotoxicol. Environ. Saf. 2021, 215, 112132. [Google Scholar] [CrossRef]
- Luo, Y.; Niu, L.; Li, D.; Xiao, J. Synergistic effects of plant protein hydrolysates and xanthan gum on the short- and long-term retrogradation of rice starch. Int. J. Biol. Macromol. 2020, 144, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Trakselyte-Rupsiene, K.; Juodeikiene, G.; Cernauskas, D.; Bartkiene, E.; Klupsaite, D.; Zadeike, D.; Bendoraitiene, J.; Damasius, J.; Ignatavicius, J.; Sikorskaite-Gudziuniene, S. Integration of Ultrasound into the Development of Plant-Based Protein Hydrolysate and Its Bio-Stimulatory Effect for Growth of Wheat Grain Seedlings In Vivo. Plants 2021, 10, 1319. [Google Scholar] [CrossRef]
- Gupta, B.; Ghosh, K.K.; Gupta, R.C. Thymoquinone. In Nutraceuticals; Gupta, R.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 541–550. [Google Scholar]
- Ben Mrid, R.; Ennoury, A.; Roussi, Z.; Naboulsi, I.; Benmrid, B.; Kchikich, A.; El Omari, R.; Nhiri, M.; Yasri, A. Thymoquinone alleviates cadmium induced stress in germinated Lens culinaris seeds by reducing oxidative stress and increasing antioxidative activities. Life 2022, 12, 1779. [Google Scholar] [CrossRef]
- Ben-Jabeur, M.; Vicente, R.; López-Cristoffanini, C.; Alesami, N.; Djébali, N.; Gracia-Romero, A.; Serret, M.D.; López-Carbonell, M.; Araus, J.L.; Hamada, W. A novel aspect of essential oils: Coating seeds with thyme essential oil induces drought resistance in wheat. Plants 2019, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, R.; Alkhatib, B.; Abdo, N. Effect of Fe3O4 nanoparticles on seed germination in tobacco. Environ. Sci. Pollut. Res. 2021, 28, 53568–53577. [Google Scholar] [CrossRef]
- Segura, R.; Vásquez, G.; Colson, E.; Gerbaux, P.; Frischmon, C.; Nesic, A.; García, D.E.; Cabrera-Barjas, G. Phytostimulant properties of highly stable silver nanoparticles obtained with saponin extract from Chenopodium quinoa. J. Sci. Food Agric. 2020, 100, 4987–4994. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, D.; Luzi, F.; Puglia, D. 2021. Lignin nanoparticles: A promising tool to improve maize physiological, biochemical, and chemical traits. Nanomaterials 2021, 11, 846. [Google Scholar] [CrossRef]
- Bento, L.R.; Spaccini, R.; Cangemi, S.; Mazzei, P.; De Freitas, B.B.; De Souza, A.E.O.; Moreira, A.B.; Ferreira, O.P.; Piccolo, A.; Bisinoti, M.C. Hydrochar obtained with by-products from the sugarcane industry: Molecular features and effects of extracts on maize seed germination. J. Environ. Manag. 2021, 281, 111878. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Xylia, P.; Petropoulos, S.; Tzortzakis, N. The use of spent coffee grounds in growing media for the production of Brassica seedlings in nurseries. Environ. Sci. Pollut. Res. 2021, 28, 24279–24290. [Google Scholar] [CrossRef]
- Monda, H.; Cozzolino, V.; Vinci, G.; Spaccini, R.; Piccolo, A. Molecular characteristics of water-extractable organic matter from different composted biomasses and their effects on seed germination and early growth of maize. Sci. Total Environ. 2017, 590–591, 40–49. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wazeer, H.; Shridhar Gaonkar, S.; Doria, E.; Pagano, A.; Balestrazzi, A.; Macovei, A. Plant-Based Biostimulants for Seeds in the Context of Circular Economy and Sustainability. Plants 2024, 13, 1004. https://doi.org/10.3390/plants13071004
Wazeer H, Shridhar Gaonkar S, Doria E, Pagano A, Balestrazzi A, Macovei A. Plant-Based Biostimulants for Seeds in the Context of Circular Economy and Sustainability. Plants. 2024; 13(7):1004. https://doi.org/10.3390/plants13071004
Chicago/Turabian StyleWazeer, Hisham, Shraddha Shridhar Gaonkar, Enrico Doria, Andrea Pagano, Alma Balestrazzi, and Anca Macovei. 2024. "Plant-Based Biostimulants for Seeds in the Context of Circular Economy and Sustainability" Plants 13, no. 7: 1004. https://doi.org/10.3390/plants13071004







