Physio-Biochemical, Anatomical, and Molecular Analysis of Resistant and Susceptible Wheat Cultivars Infected with TTKSK, TTKST, and TTTSK Novel Puccinia graminis Races
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Wheat Cultivars against Stem Rust at the Adult Stage
2.2. Evaluation of 41 Stem Rust Resistance Genes (Sr,s)
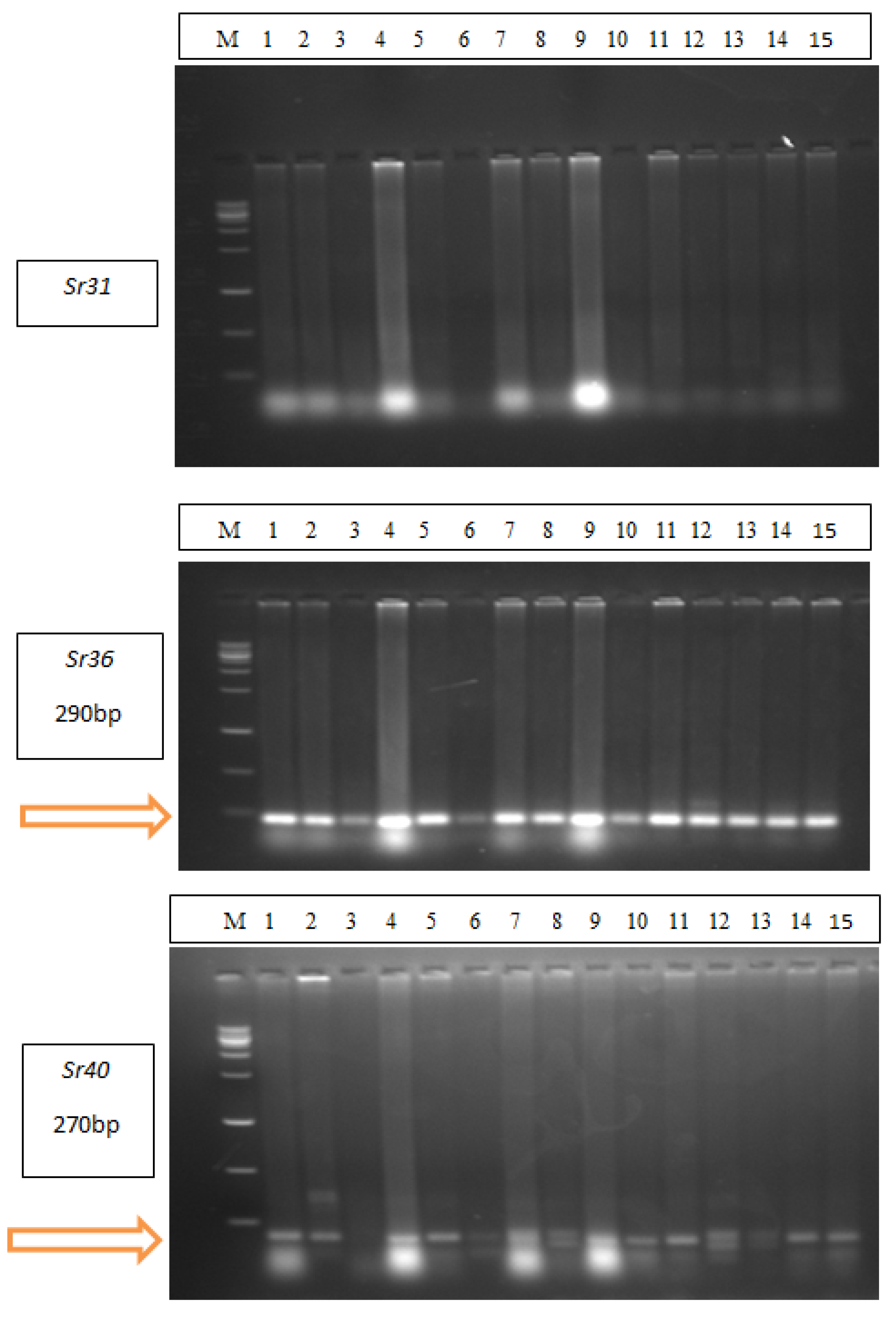
2.3. Identification of Stem Rust Resistance Genes in Wheat Cultivars
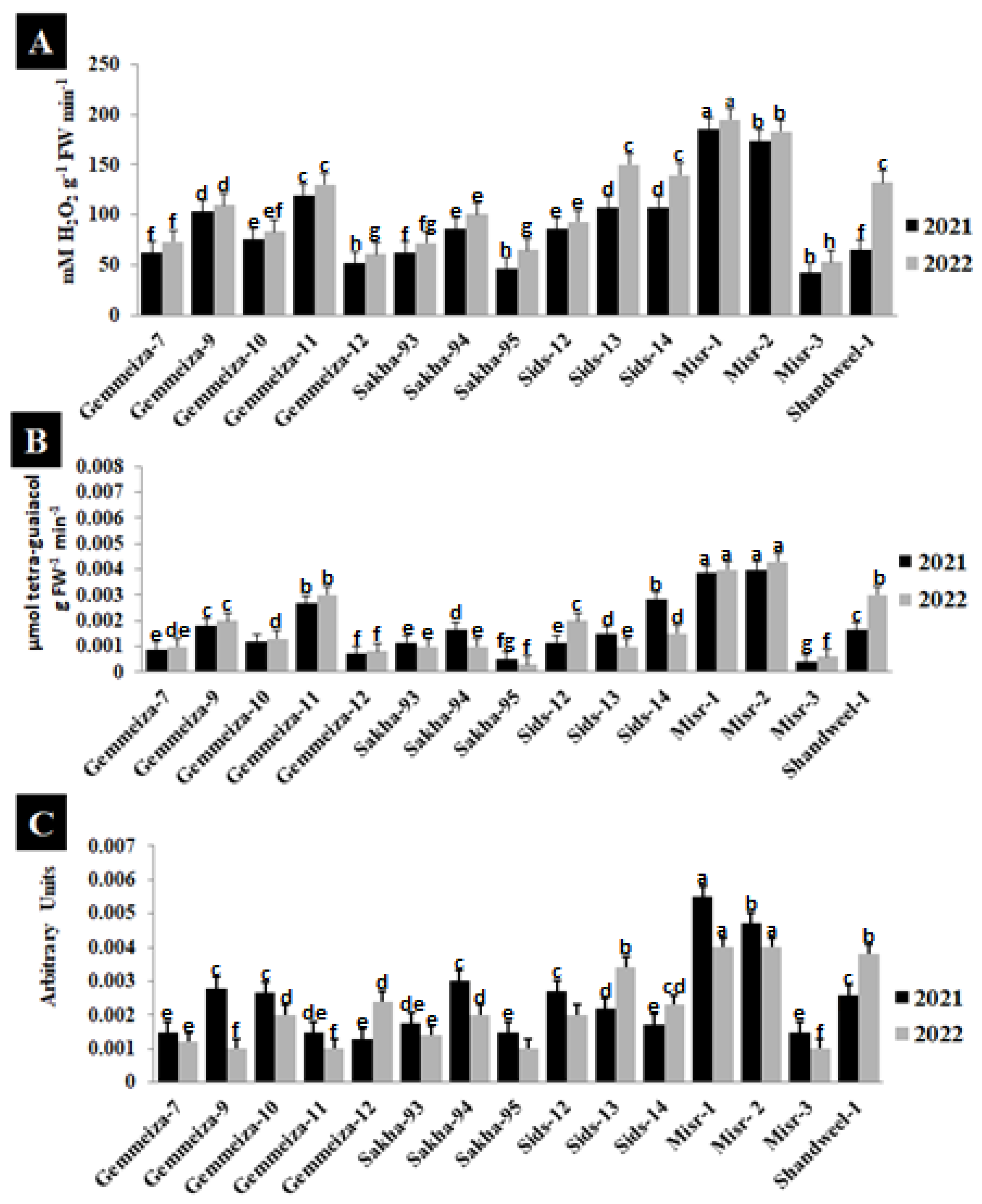
2.4. Effect of Stem Rust Infection on Enzyme Activity in Different Wheat Cultivars
2.5. Effect of Stem Rust on Electrolyte Leakage, Chlorophyll a, and Chlorophyll b of Different Cultivars
2.6. Effect of Stem Rust on Reactive Oxygen Species (ROS) of Four Wheat Cultivars
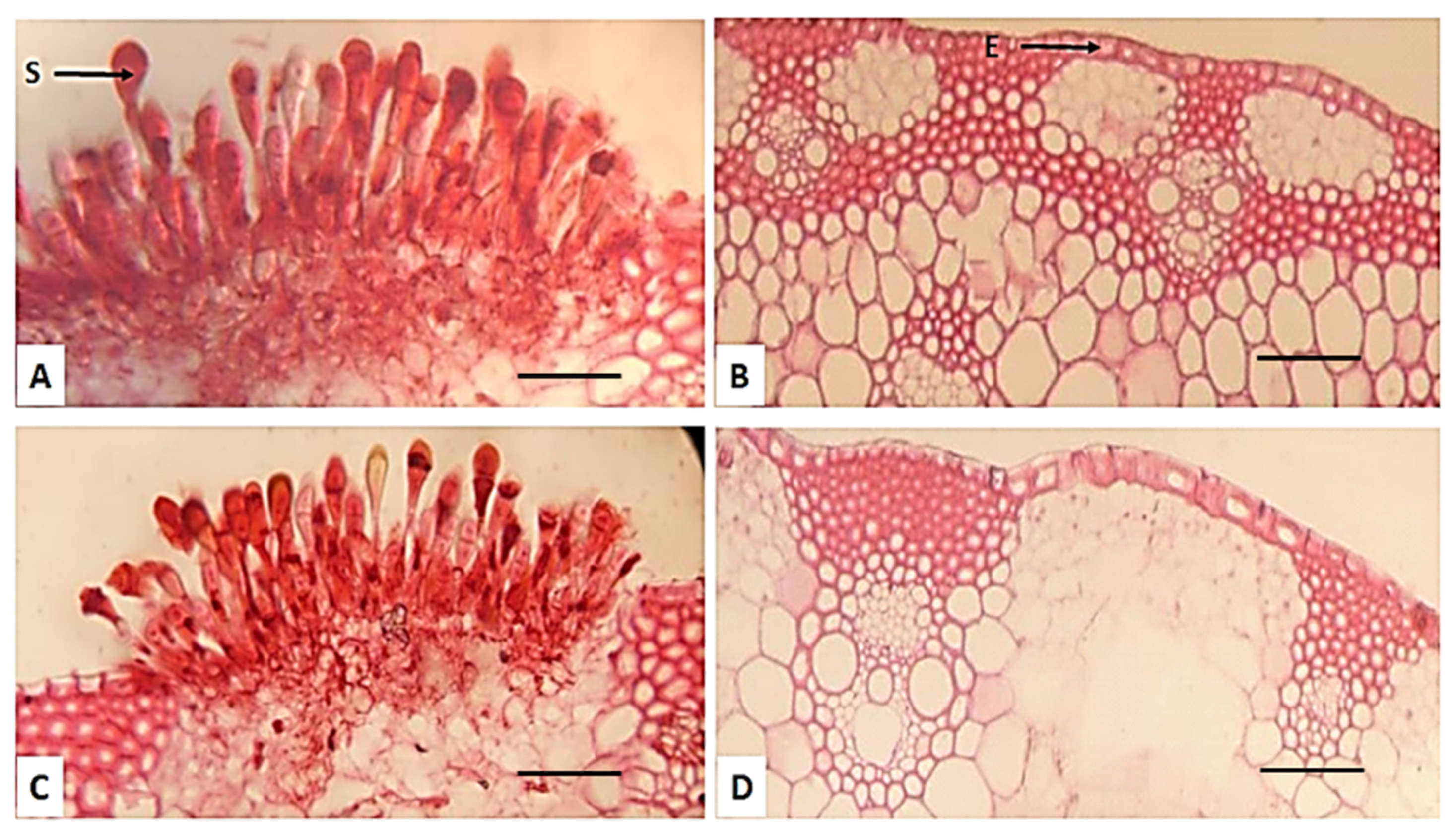
2.7. Effect of P. graminis f. sp. tritici on the Anatomical Characters of Four Wheat Cultivars
2.8. Distribution of P. graminis f. sp. tritici Races in Egypt and Other Countries
3. Discussion
4. Materials and Methods
4.1. Field Experiments
Evaluation of Some Wheat Cultivars and Stem Rust Resistance Genes (Sr,s) at the Adult Stage
4.2. Laboratory Experiments
4.2.1. Molecular Markers
4.2.2. Determination of O2- and H2O2
4.2.3. Assays of Antioxidant Enzymes
4.2.4. Electrolyte Leakage (EL%)
4.2.5. Chlorophyll a and b Content
4.2.6. Anatomical Studies
4.2.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alnusairi, G.S.H.; Mazrou, Y.; Qari, S.H.; Elkelish, A.A.; Soliman, M.H.; Eweis, M.; Abdelaal, K.; El-Samad, G.A.; Ibrahim, M.F.M.; ElNahhas, N. Exogenous Nitric Oxide Reinforces Photosynthetic Efficiency, Osmolyte, Mineral Uptake, Antioxidant, Expression of Stress-Responsive Genes and Ameliorates the Effects of Salinity Stress in Wheat. Plants 2021, 10, 1693. [Google Scholar] [CrossRef] [PubMed]
- Khedr, R.; Aboukhadrah, S.; El-Hag, D.; Elmohamady, E.; Abdelaal, K. Ameliorative effects of nano silica and some growth stimulants on water relations, biochemical and productivity of wheat under saline soil conditions. Fresenius Environ. Bull. 2023, 32, 375–384. [Google Scholar]
- Gui, Y.W.; Batool, A.; El-Keblawy, A.; Sheteiwy, M.S.; Yang, Y.M.; Zhao, L.; Duan, H.X.; Chang, S.J.; Xiong, Y.C. Response of source-sink relationship to progressive water deficit in the domestication of dryland wheat. Plant Physiol. Biochem. 2024, 1, 207–108380. [Google Scholar] [CrossRef]
- Li, J.; Liang, Z.; Li, Y.; Wang, K.; Nangia, V.; Mo, F.; Liu, Y. Advantageous spike-to-stem competition for assimilates contributes to the reduction in grain number loss in wheat spikes under water deficit stress. Agric. Water Manag. 2024, 292, 108675. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, D.; Zhang, Z.; Li, Y.; Shi, S.; Yang, Y. Calcium signal regulated carbohydrate metabolism in wheat seedlings under salinity stress. Physiol. Mol. Biol. Plants 2024, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.; Hafez, Y.; Badr, M.M.; Youseef, W.A.; Esmail, S. Biochemical, histological and molecular changes in susceptible and resistant wheat cultivars inoculated with stripe rust fungus Puccinia striiformis f. sp. tritici. Egypt. J. Biol. Pest Control 2014, 24, 421–429. [Google Scholar]
- Nazarov, T.; Liu, Y.; Chen, X.; See, D.R. Molecular Mechanisms of the Stripe Rust Interaction with Resistant and Susceptible Wheat Genotypes. Int. J. Mol. Sci. 2024, 25, 2930. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Bhardwaj, S.C.; Gangwar, O.P.; Prasad, P.; Kumar, S.; Singh, G.P. Identification of adult plant rust resistance genes in some pre and post-green revolution Indian bread-wheat varieties. Phytoparasitica 2024, 52, 32. [Google Scholar] [CrossRef]
- Shahin, A.; Esmaeil, R.A.; Badr, M.; Abdelaal, K.A.A.; Hassan, F.A.S.; Hafez, Y.M. Phenotypic characterization of race-specific and slow rusting resistance to stem rust disease in promising wheat genotypes. Fresenius Environ. Bull. 2021, 30, 6223–6236. [Google Scholar]
- Omara, R.I.; Abdelaal, K. Molecular and Genetic Analysis of Leaf Rust Resistance Genes in Two New Egyptian Wheat Cultivars. Egypt. J. Phytopathol. 2017, 45, 33–52. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Omara, R.; Hafez, Y.M.; Esmail, S.; EL Sabagh, A. Anatomical, biochemical and physiological changes in some Egyptian wheat cultivars inoculated with Puccinia graminis f.sp. tritici. Fresenius Environ. Bull. 2018, 27, 296–305. [Google Scholar]
- Singh, R.P.; Hodson, D.P.; Huerta-Espino, J.; Jin, Y.; Bhavani, S.; Njau, P.; Herrera-Foessel, S.; Singh, P.K.; Singh, S.; Govindan, V. The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu. Rev. Phytopathol. 2011, 49, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Q.; Wu, L.R.; Liu, T.G.; Xu, S.C.; Jin, S.L.; Peng, Y.L.; Wang, B.T. Race dynamics, diversity, and virulence evolution in Puccinia striiformis f. sp. tritici, the causal agent of wheat stripe rust in China from 2003 to 2007. Plant Dis. 2009, 93, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Pretorius, Z.A.; Singh, R.P.; Fetch, T., Jr. Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici. Plant Dis. 2008, 92, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Njau, P.N.; Jin, Y.; Huerta-Espino, J.; Keller, B.; Singh, R.P. Identification and evaluation of sources of resistance to stem rust race Ug99 in wheat. Plant Dis. 2010, 94, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Wanyera, R.; Kinyua, M.G.; Jin, Y.; Singh, R.P. The spread of stem rust caused by Puccinia graminis f. sp. tritici, with virulence on Sr31 in wheat in East Africa. Plant Dis. 2006, 90, 113. [Google Scholar] [CrossRef] [PubMed]
- Omara, R.I.; Abdelaal, K. Biochemical, histopathological and genetic analysis associated with leaf rust infection in wheat plants (Triticum aestivum L.). Physiol. Mol. Plant Pathol. 2018, 104, 48–57. [Google Scholar] [CrossRef]
- Pathan, A.K.; Park, R.F. Evaluation of seedling and adult plant resistance to stem rust in European wheat cultivars. Euphytica 2007, 155, 87–105. [Google Scholar] [CrossRef]
- El Nahhas, N.; AlKahtani, M.; Abdelaal, K.A.A.; Al Husnain, L.; AlGwaiz, H.; Hafez, Y.M.; Attia, K.; El-Esawi, M.; Ibrahim, M.; Elkelish, A. Biochar and jasmonic acid application attenuates antioxidative systems and improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water. Plant Physiol. Biochem. 2021, 166, 807–817166. [Google Scholar] [CrossRef] [PubMed]
- El-Egami, H.M.; Hegab, R.H.; Montaser, H.; El-Hawary, M.M.; Hasanuzzaman, M. Impact of Potassium-Solubilizing Microorganisms with Potassium Sources on the Growth, Physiology, and Productivity of Wheat Crop under Salt-Affected Soil Conditions. Agronomy 2024, 14, 423. [Google Scholar] [CrossRef]
- Nyaupane, S.; Poudel, M.R.; Panthi, B.; Dhakal, A.; Paudel, H.; Bhandari, R. Drought stress effect, tolerance, and management in wheat–a review. Cogent Food Agric. 2024, 10, 2296094. [Google Scholar]
- Omara, R.I.; El-Kot, G.; Fadel, F.; Abdelaal, K.; Saleh, E. Efficacy of certain bioagents on patho-physiological characters of wheat plants under wheat leaf rust stress. Physiol. Mol. Plant Pathol. 2019, 106, 102–108. [Google Scholar] [CrossRef]
- Hafez, Y.M.; Abdelaal, K.A.A.; Eid, M.E.; Mehiar, F.F. Morpho-physiological and Biochemical Responses of Barley Plants (Hordeum vulgare L.) Against Barley Net Blotch Disease with Application of Non-traditional Compounds and Fungicides. Egypt. J. Biol. Pest Control 2016, 26, 261–268. [Google Scholar]
- Al-Shammari, W.B.; Altamimi, H.R.; Abdelaal, K. Improvement in Physiobiochemical and Yield Characteristics of Pea Plants with Nano Silica and Melatonin under Salinity Stress Conditions. Horticulturae 2023, 9, 711. [Google Scholar] [CrossRef]
- Ashmmawy, M.A.; El-Orabey, W.M.; Nazim, M.; Shahin, A.A. Effect of stem rust infection on grain yield and yield components of some wheat cultivars in Egypt. Int. J. Phytopathol. 2013, 2, 171–178. [Google Scholar] [CrossRef]
- Hamada, A.A.; Abd El-Majeed, S.E.; El-Saied, E.A.; Tawfeles, M.B.; El-Borhamy, H.S. Misr 1 and Misr 2: Tow new high yielding and rust resistant bread wheat cultivar. Egypt. Menoufia J. Plant Prot. 2017, 2, 177–192. [Google Scholar] [CrossRef]
- Abdel-Majeed, S.E.; Hamada, A.A.; El-Saied, E.A.M.; Toweefles, M.B. Misr 3: A new Egyptian bread wheat cultivar. In Proceedings of the Seventh Field Crops Conference, Giza, Egypt, 18–19 December 2018; pp. 191–207. [Google Scholar]
- Hare, R.A.; McIntosh, R.A. Genetic and cytogenetic studies of the durable adult plant resistance in ‘Hope’ and related cultivars to wheat rusts. Z. Pflanzenzuchtg 1979, 83, 350–367. [Google Scholar]
- McIntosh, R.A.; Wellings, C.; Park, R. Wheat Rusts, An Atlas of Resistance Genes; CSIRO: East Melbourne, Australia, 1995; 200p.
- Cao, Y.Y.; Han, J.D.; Zhu, G.Q.; Zhang, L. Ug99, a new virulent race of Puccinia graminis f. sp. tritici and its effect on China. Plant Prot. 2007, 6, 86–89. [Google Scholar]
- Gao, F.; Wu, X.; Sun, H.; Wang, Z.; Chen, S.; Zou, L.; Yang, J.; Wei, Y.; Ni, X.; Sun, Q.; et al. Identification of Stem Rust Resistance Genes in Triticum Wheat Cultivars and Evaluation of Their Resistance to Puccinia graminis f. sp. tritici. Agriculture 2024, 14, 198. [Google Scholar] [CrossRef]
- Kokhmetova, A.; Morgounov, A.; Rsaliev, S.; Rsaliev, A.; Yessenbekova, G.; Typina, L. Wheat germplasm screening for stem rust resistance using conventional and molecular techniques. Czech J. Genet. Plant Breed. 2011, 47, 146–154. [Google Scholar] [CrossRef]
- Letta, A.; Tilahun, A. Stability analysis for selecting stem rust resistance in some Ethiopian wheat varieties. Proc. Afr. Crop Sci. Conf. 2007, 8, 853–856. [Google Scholar]
- Pretorius, Z.A.; Singh, R.P.; Wagoire, W.W.; Payne, T.S. Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Plant Dis. 2000, 84, 203. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Deadly new wheat disease threatens Europe’s crops. Nature 2017, 542, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Nazari, K.; Mafi, M.; Yahyaoui, A.; Singh, R.P.; Park, R.F. Detection of wheat stem rust (Puccinia graminis f. sp. tritici) race TTKSK (Ug99) in Iran. Plant Dis. 2009, 93, 317. [Google Scholar] [CrossRef] [PubMed]
- Patpour, M.; Hovmøller, M.S.; Shahin, A.A.; Newcomb, M.; Olivera, P.; Jin, Y.; Luster, D.; Hodson, D.; Nazari, K.; Azab, M. First report of the Ug99 race group of wheat stem rust, Puccinia graminis f. sp. tritici, in Egypt in 2014. Plant Dis. 2016, 100, 863. [Google Scholar] [CrossRef]
- Singh, R.P.; Hodson, D.P.; Huerta-Espino, J.; Jin, Y.; Njau, P. Will stem rust destroy the world’s wheat crop? Adv. Agron. 2008, 98, 271–309. [Google Scholar]
- Shahin, A.; Youssif, W.; EL-Naggar, D. Detection of Ug99 (TTKSK) of wheat stem rust fungus and new virulence races of Puccinia graminis f. sp. tritici in Egypt. Egypt. J. Phyto. 2020, 48, 14–28. [Google Scholar]
- Jin, Y.; Szabo, L.J.; Rouse, M.N.; Fetch, T., Jr.; Pretorius, Z.A.; Wanyera, R.; Njau, P. Wheat detection of virulence to resistance gene Sr36 within the TTKS Rrace lineage of Puccinia graminis f. sp. tritici. Plant Dis. 2009, 93, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Cui, J.M.; Su, Y.Q.; Yuan, S.; Yuan, M.; Zhang, H.Y. Influence of stripe rust infection on the photosynthetic characteristics and antioxidant system of susceptible and resistant wheat cultivars at the adult plant stage. Front. Plant Sci. 2015, 6, 779. [Google Scholar] [CrossRef] [PubMed]
- Marzec-Schmidt, K.; Hura, K.; Płażek, A. Changes in antioxidants activity in grasses from complex Lolium-Festuca as a response to Microdochium nivale infection. Physiol. Mol. Plant Pathol. 2018, 104, 40–47. [Google Scholar] [CrossRef]
- Kononenko, N.; Baranova, E.; Dilovarova, T.; Akanov, E.; Fedoreyeva, L. Oxidative Damage to Various Root and Shoot Tissues of Durum and Soft Wheat Seedlings during Salinity. Agriculture 2020, 10, 55. [Google Scholar] [CrossRef]
- Ning, D.; Zhang, Y.; Li, X.; Qin, A.; Huang, C.; Fu, Y.; Gao, Y.; Duan, A. The Effects of Foliar Supplementation of Silicon on Physiological and Biochemical Responses of Winter Wheat to Drought Stress during Different Growth Stages. Plants 2023, 12, 2386. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Yakupova, A.; Avtushenko, I.; Lastochkin, A.; Yuldashev, R. Effect of Seed Priming with Endophytic Bacillus subtilis on Some Physio-Biochemical Parameters of Two Wheat Varieties Exposed to Drought after Selective Herbicide Application. Plants 2023, 12, 1724. [Google Scholar] [CrossRef] [PubMed]
- El-Shawa, G.M.; Rashwan, E.M.; Abdelaal, K. Mitigating salt stress effects by exogenous application of proline and yeast extract on morphophysiological, biochemical and anatomical charaters of calendula plants. Sci. J. Flowers Ornam. Plants 2020, 7, 461–482. [Google Scholar] [CrossRef]
- Mandal, K.; Saravanan, R.; Maiti, S.; Kothari, I.L. Effect of downy mildew disease on photosynthesis and chlorophyll fluorescence in Plantago ovata Forsk. J. Plant Dis. Prot. 2009, 116, 164–168. [Google Scholar] [CrossRef]
- Yu, L.X.; Lorenz, A.; Rutkoski, J.; Singh, R.P.; Bhavani, S.; Huerta-Espino, J.; Sorrells, M.E. Association mapping and gene-gene interaction for stem rust resistance in CIMMYT spring wheat germplasm. Theor. Appl. Genet. 2011, 123, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Bryan, A.; Korolev, A.; Bergmann, S.; Boshoff, W.H.; Flath, K.; Justesen, A.F.; Schulz, P.; Visser, B.; Saunders, D.G. Comparative genomics identifies genetic markers linked to structural variations that differentiate Puccinia graminis tritici and secalis formae speciales. Plant Pathol. 2024. [Google Scholar] [CrossRef]
- Peterson, R.F.; Campbell, A.B.; Hannah, A.E. A diagrammatic scale for estimating rust severity on leaves and stems of cereals. Can. J. Res. 1948, 26, 496–500. [Google Scholar] [CrossRef]
- Stakman, E.C.; Stewart, D.M.; Loedering, W.Q. Identification of physiologic races of Pucccinia graminis f. sp. tritici, A.R.S. USA Agric. Res. Serv. Bull. 1962, E-617, 53. [Google Scholar]
- Saari, E.E.; Wilcoxson, R.D. Plant disease situation of high yielding durum wheat in Asia and Africa. Annu. Rev. Phytopathol. 1974, 2, 49–68. [Google Scholar] [CrossRef]
- Pandey, H.N.; Menon, T.C.; Rao, M.V. A simple formula for calculating area under disease progress curve. Rachis 1989, 8, 38–39. [Google Scholar]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Devos, K.M.; Gale, M.D. The genetic maps of wheat and their potential in plant breeding. Outlook Agric. 1992, 22, 93–99. [Google Scholar] [CrossRef]
- Mago, R.; Brown-Guedira, G.; Dreisigacker, S.; Breen, J.; Jin, Y.; Singh, R.; Appels, R.; Lagudah, E.S.; Ellis, J.; Spielmeyer, W. An accurate DNA marker assay for stem rust resistance gene Sr2 in wheat. Theor. Appl. Genet. 2011, 122, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Abate, Z.; Chao, S.; Zhang, W.; Rouse, M.; Jin, Y.; Elias, E.; Dubcovsky, J. Genetic mapping of stem rust resistance gene Sr13 in tetraploid wheat (Triticum turgidum ssp. durum L.). Theor. Appl. Genet. 2011, 122, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Liu, S.; Anderson, J.A.; Singh, R.P.; Jin, Y.; Dubcovsky, J.; Brown-Guidera, G.; Bhavani, S.; Morgounov, A.; He, Z.; et al. Haplotype diversity of stem rust resistance loci in uncharacterized wheat lines. Mol. Breed. 2010, 26, 667–680. [Google Scholar] [CrossRef]
- Singh, R.P.; Hodson, D.P.; Jin, Y.; Lagudah, E.S.; Ayliffe, M.A.; Bhavani, S.; Rouse, M.N.; Pretorius, Z.A.; Szabo, L.J.; Huerta-Espino, J.; et al. Emergence and spread of new races of wheat stem rust fungus: Continued threat to food security and prospects of genetic control. Phytopathology 2015, 105, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Baranova, O.; Solyanikova, V.; Kyrova, E.; Kon’kova, E.; Gaponov, S.; Sergeev, V.; Shevchenko, S.; Mal’chikov, P.; Dolzhenko, D.; Bespalova, L.; et al. Evaluation of Resistance to Stem Rust and Identification of Sr Genes in Russian Spring and Winter Wheat Cultivars in the Volga Region. Agriculture 2023, 13, 635. [Google Scholar] [CrossRef]
- Hückelhoven, R.; Fodor, J.; Preis, C.; Kogel, K.-H. Hypersensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with H2O2 but not with salicylic acid accumulation. Plant Physiol. 1999, 119, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Malik, C.P.; Singh, M.B. Indian and printed in Navin. Shanndara. In Plant Emynology and Histoenzymology; Kalyani Publishers: Delhi, India, 1980; pp. 54–56. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Szalai, G.; Janda, T.; Pa ldi, E.; Szigeti, Z. Role of light in post chilling symptoms in maize. J. Plant Physiol. 1996, 148, 378–383. [Google Scholar] [CrossRef]
- Whitlow, T.H.; Bassuk, N.L.; Ranney, T.G.; Reichert, D.L. An improved method for using electrolyte leakage to assess membrane competence in plant tissues. Plant Physiol. 1992, 98, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Moran, R.; Porath, D. Chlorophyll determination in intact tissues using N,N-Dimethyl formamide. Plant Physiol. 1982, 69, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, M. Improved staining procedures for photographic documentation of phenolic deposits in semi thin sections of plant tissue. J. Microsc. 1995, 179, 277–281. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Micro Techniques and Microscopy, 1st ed.; Oxford University Press: Oxford, MS, USA, 1999; p. 322. [Google Scholar]




| NO. | Sr Gene | Kafrelsheikh Governorate | |
|---|---|---|---|
| 2021 | 2022 | ||
| 1 | 2 | 5.63 | 6.00 |
| 2 | 5 | 73.33 | 76.67 |
| 3 | 6 | 36.67 | 93.33 |
| 4 | 7b | 53.33 | 66.67 |
| 5 | 8a | 63.33 | 83.33 |
| 6 | 9a | 46.67 | 76.67 |
| 7 | 9b | 43.33 | 66.67 |
| 8 | 9e | 63.33 | 86.67 |
| 9 | 9g | 43.33 | 86.67 |
| 10 | 9d | 66.67 | 76.67 |
| 11 | 10 | 63.33 | 66.67 |
| 12 | 11 | 36.67 | 46.67 |
| 13 | 13 | 0.00 | 0.00 |
| 14 | 17 | 2.66 | 6.00 |
| 15 | 17 | 76.67 | 46.67 |
| 16 | 21 | 66.67 | 83.33 |
| 17 | 22 | 0.00 | 5.00 |
| 18 | 24 | 1.33 | 26.00 |
| 19 | 26 | 0.00 | 5.00 |
| 20 | 28 | 0.00 | 7.66 |
| 21 | 30 | 66.67 | 36.67 |
| 22 | 31 | 2.33 | 18.67 |
| 23 | 31 | 2.66 | 4.33 |
| 24 | 31 | 2.133 | 1.33 |
| 25 | 31 | 0.00 | 2.33 |
| 26 | 31 | 1.13 | 2.8 |
| 27 | 31 | 0.00 | 2.00 |
| 28 | 35 | 0.00 | 6.00 |
| 29 | 36 | 1.133 | 9.333 |
| 30 | 37 | 0.00 | 0.00 |
| 31 | 38 | 2.43 | 4.33 |
| 32 | 40 | 0.00 | 0.00 |
| 33 | Tmp | 76.67 | 76.67 |
| 34 | GT | 0.00 | 0.00 |
| 35 | MCN | 66.67 | 70.00 |
| Combined genes | |||
| 36 | 24+31 | 1.06 | 9.00 |
| 37 | 36+31 | 0.00 | 7.66 |
| 38 | Sr36, 6 | 8.33 | 3.66 |
| 39 | Sr6, 24, 36, 1RS-Am | 0.73 | 1.46 |
| 40 | Sr7a, Sr12, Sr6 | 0.00 | 1.06 |
| 41 | SRTT3, SR10 | 0.00 | 0.00 |
| No. | Wheat Cultivar | Pedigree |
|---|---|---|
| 1 | Sakha-93 | SAKHA92/TR810328 S.8871-1S-2S-1S-0S. |
| 2 | Sakha-94 | OPATA/RAYON//KAUZ. CMBW90Y3180-OTOPM-3Y-010M-010M-010Y-10M-015Y-0Y-0AP-0S. |
| 3 | Sakha-95 | PASTOR//SITE/MO/3/CHEN/AEGILOPS SQUARROSA(TAUS)//BCN/4/WBLL1CMSA01Y00158S-040P0Y-040M030ZTM-040SY-26M-0Y-0SY-0S |
| 4 | Gemmeiza-7 | CMH74A.630/5X//SERI82/3/AGENT. GM4611-2GM-3GM-1GM-0GM. |
| 5 | Gemmeiza-9 | ALD”S”/HUAS//CMH74A.630/SX.GCM4583-5GM-1GM-0GM. |
| 6 | Gemmeiza-10 | MAYA74”S”/ON / 1160- 147 /3/ BB/ G11/4/ CHAT “S”/5/ CROW “S”GCM 5820- 3GM- 1GM- 2GM- 0GM. |
| 7 | Gemmeiza-11 | BOW”S” /KVZ”S”// 7C/SERI82/3/GIZA168 /SKHA61. GM7892-2GM-1GM-2GM-1GM-0GM. |
| 8 | Gemmeiza-12 | OTUS/3/SARA/THB//VEE. CCMSS97Y00227S-5Y-010M-010Y -010M-2Y-1M-0Y-0GM |
| 9 | Sids-12 | BUC//7C/ALD/5/MAYA74/ON//1160147/3/BB/GLL/4/CHAT”S”/6/MAYA/VUL//CMH74A.630//4*SX. SD7096-4SD-1SD-1SD-0SD. |
| 10 | Sids-13 | AMAZ19=KAUZ”S”//TSI/SNB”S”. ICW94-0375-4AP-2AP-030AP-0APS-3AP-0APS-050AP-0AP-0SD. |
| 11 | Sids-14 | SW8488*2/KUKUNACGSS01Y00081T-099M-099Y-099M-099B-9Y-0B-0SD |
| 12 | Misr-1 | OASIS/SKAUZ//4*BCN/3/2*PASTOR.CMSSOYO1881T-050M-030YO3OM-30WGY-33M-0Y-0S |
| 13 | Misr-2 | SKAUZ/BAV92. CMSS96M0361S-1M-010SY-010M-010SY-8M -0Y-0S |
| 14 | Misr-3 | ATTILA*2/PBW65*2//KACHU CMSS06Y00582T-099TOPM-099Y-099ZTM-099Y-099M-10WGY-0B-0EGY |
| 15 | Shandweel-1 | SITE/MO/4/NAC/TH.AC//3*PVN/3/MIRLO/BUC CMSS93B00567S-72Y-010M-010Y-010M-3Y-0M-0THY-0SH |
| No. | Sr Gene | Tester | No. | Sr Gene | Tester |
|---|---|---|---|---|---|
| 1 | 2 | CnS(Hope3B) | 22 | 31 | Sr31 (Benno)/6*LMPG-6 DK42 |
| 2 | 5 | ISr5-Ra | 23 | 31 | Kavkaz |
| 3 | 6 | ISr6-Ra | 24 | 31 | Federation 4/Kavkaz |
| 4 | 7b | ISr7b-Ra | 25 | 31 | Brigardier |
| 5 | 8a | ISr8a-Ra | 26 | 31 | Clement |
| 6 | 9a | ISr9a-Ra | 27 | 31 | PBW343 |
| 7 | 9b | W2691Sr9b | 28 | 35 | W3763-SR35 |
| 8 | 9e | Vernstein | 29 | 36 | W2691SrTt-1 |
| 9 | 9d | ISr9d-Ra | 30 | 37 | W2691 SR37TT2 |
| 10 | 9g | CnsSr 9g | 31 | 38 | VPM-1 |
| 11 | 10 | W2691Sr10 | 32 | 40 | RL 6087 Dyck |
| 12 | 11 | ISr11-Ra | 33 | Tmp | CnsSr Tmp |
| 13 | 13 | W2691SR13 | 34 | GT | BT-SrGt |
| 14 | 17 | Combination | 35 | MCN | McNair 701 |
| 15 | 17 | LC/KENYA HUNTER-SR17 | 36 | 24+31 | Siouxland |
| 16 | 21 | CnS_T_monococcum | 37 | 36+31 | Sisson |
| 17 | 22 | SWSR22T.B. | 38 | Sr36, 6 | Roughrider |
| 18 | 24 | LcSr24Ag | 39 | Sr6, 24, 36, 1RS-Am | Fleming |
| 19 | 26 | EAGLE-SR26,SR9G | 40 | Sr7a, Sr12, Sr6 | Chris |
| 20 | 28 | W2691 SR28KT | 41 | SRTT3, SR10 | FR*2/SRTT3-SRTT3, SR10 |
| 21 | 30 | BtSr30Wst |
| Genes | Primer Sequence | Annealing Temp. (°C) | Amplicon Size (bp) | Reference | |
|---|---|---|---|---|---|
| Sr2 | F R | CAA GGG TTG CTA GGA TTG GAA AAC AGA TAA CTC TTA TGA TCT TAC ATT TTT CTG | 55 °C | 350 bp | [55] |
| Sr13 | F R | CGGAGCAAGGACGATAGG CACCACACCAATCAGGAACC | 54 °C | 320 bp | [56] |
| Sr24 | F R | CAC CCG TGA CAT GCT CGT A AAC AGG AAA TGA GCA ACG ATG T | 58 °C | 480 bp | [57] |
| Sr31 | F R | CTCTGTGGATAGTTACTTGATCGA CCTAGAACATGCATGGCTGTTACA | 55 °C | 1200 bp | [58] |
| Sr36 | F R | CGT CGA AAA CCG TAC ACT CTC C GCG AAA CAG AAT AGC CCT GAT G | 61 °C | 290 bp | [59] |
| Sr40 | F R | CAAGGAAATAGGCGGTAACT ATTTGAGTCTGAAGTTTGCA | 51 °C | 270 bp | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alafari, H.A.; Hafez, Y.; Omara, R.; Murad, R.; Abdelaal, K.; Attia, K.; Khedr, A. Physio-Biochemical, Anatomical, and Molecular Analysis of Resistant and Susceptible Wheat Cultivars Infected with TTKSK, TTKST, and TTTSK Novel Puccinia graminis Races. Plants 2024, 13, 1045. https://doi.org/10.3390/plants13071045
Alafari HA, Hafez Y, Omara R, Murad R, Abdelaal K, Attia K, Khedr A. Physio-Biochemical, Anatomical, and Molecular Analysis of Resistant and Susceptible Wheat Cultivars Infected with TTKSK, TTKST, and TTTSK Novel Puccinia graminis Races. Plants. 2024; 13(7):1045. https://doi.org/10.3390/plants13071045
Chicago/Turabian StyleAlafari, Hayat Ali, Yaser Hafez, Reda Omara, Rasha Murad, Khaled Abdelaal, Kotb Attia, and Amr Khedr. 2024. "Physio-Biochemical, Anatomical, and Molecular Analysis of Resistant and Susceptible Wheat Cultivars Infected with TTKSK, TTKST, and TTTSK Novel Puccinia graminis Races" Plants 13, no. 7: 1045. https://doi.org/10.3390/plants13071045
APA StyleAlafari, H. A., Hafez, Y., Omara, R., Murad, R., Abdelaal, K., Attia, K., & Khedr, A. (2024). Physio-Biochemical, Anatomical, and Molecular Analysis of Resistant and Susceptible Wheat Cultivars Infected with TTKSK, TTKST, and TTTSK Novel Puccinia graminis Races. Plants, 13(7), 1045. https://doi.org/10.3390/plants13071045







