Proline Spray Relieves the Adverse Effects of Drought on Wheat Flag Leaf Function
Abstract
1. Introduction
2. Results
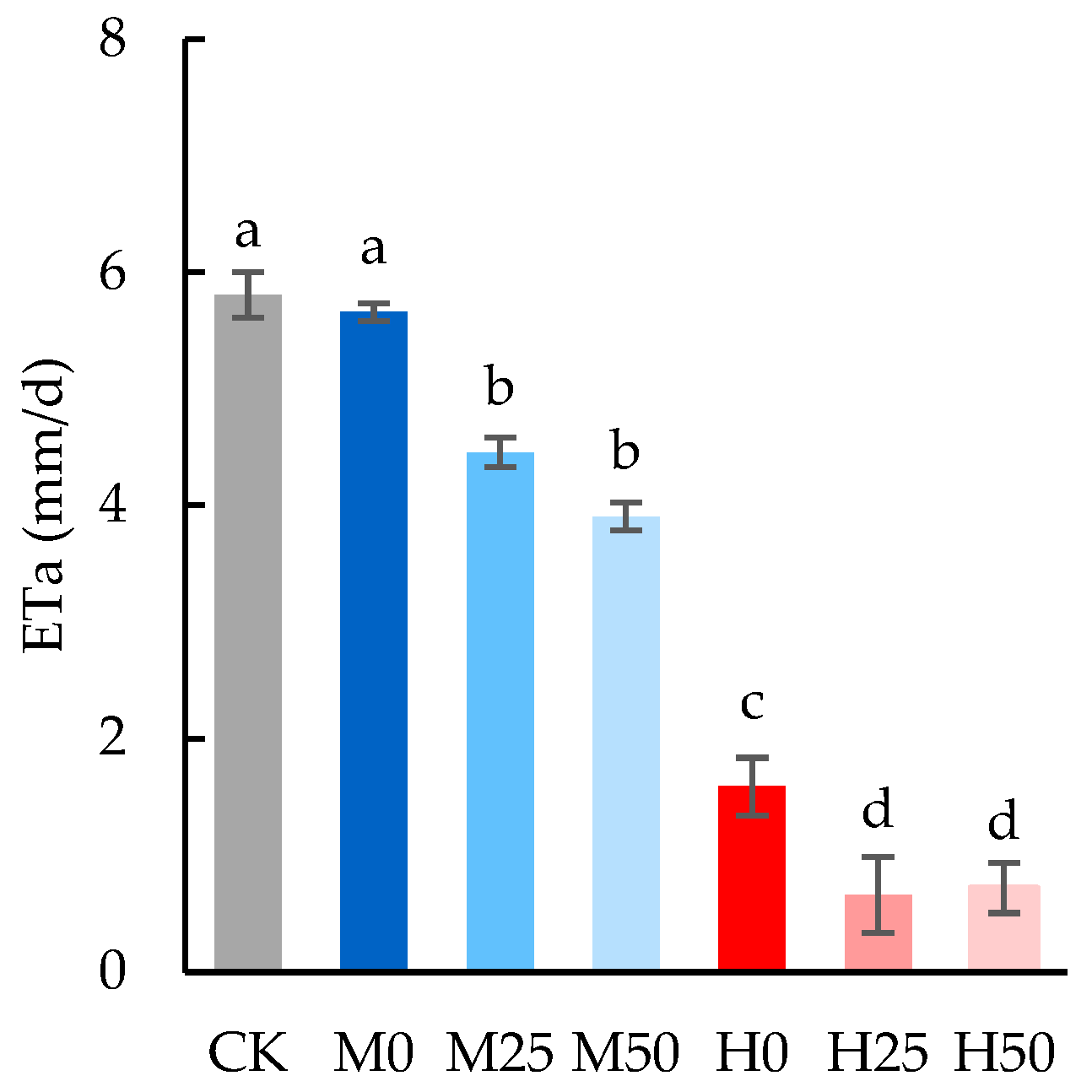
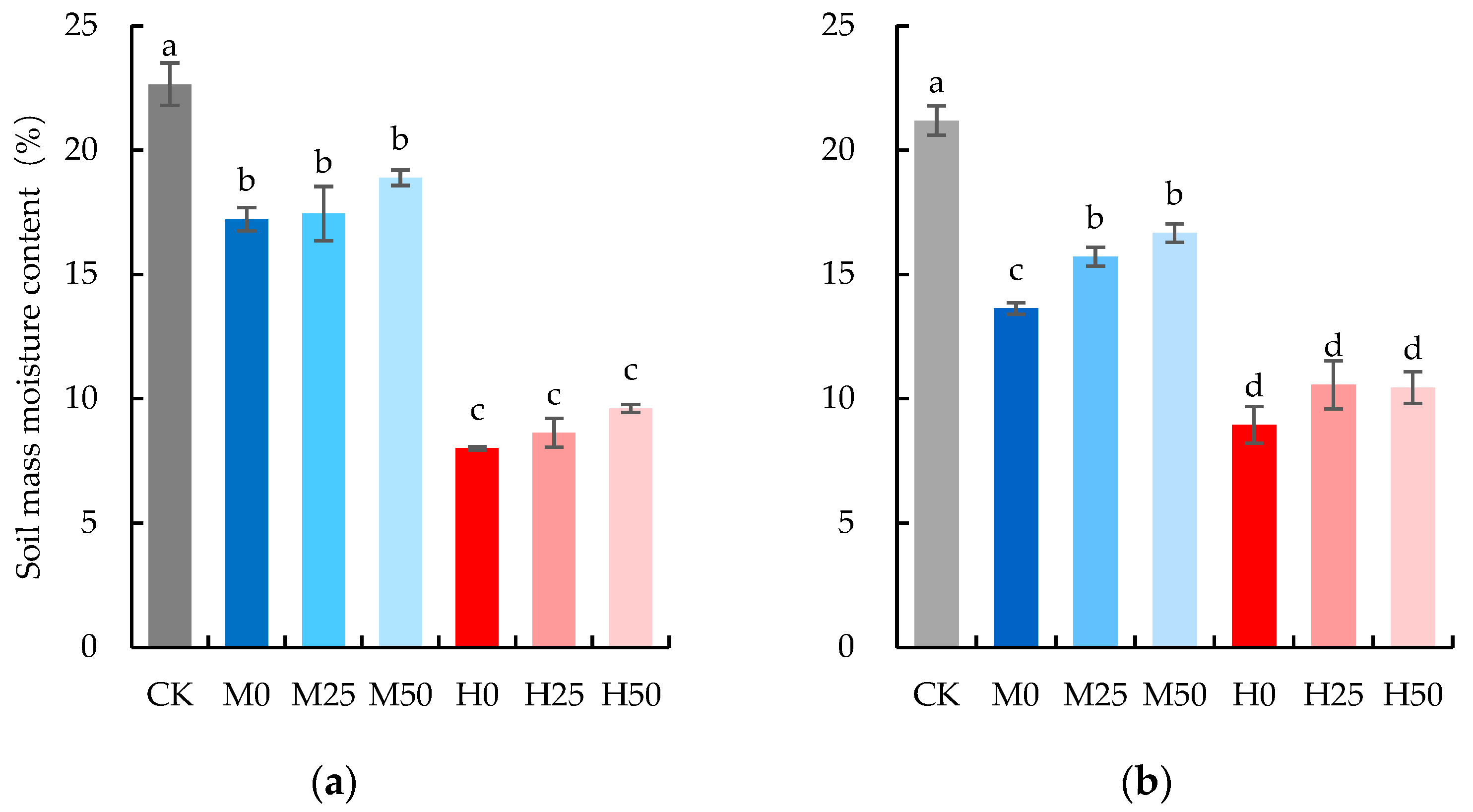
2.1. Drought Lowers Evapotranspiration
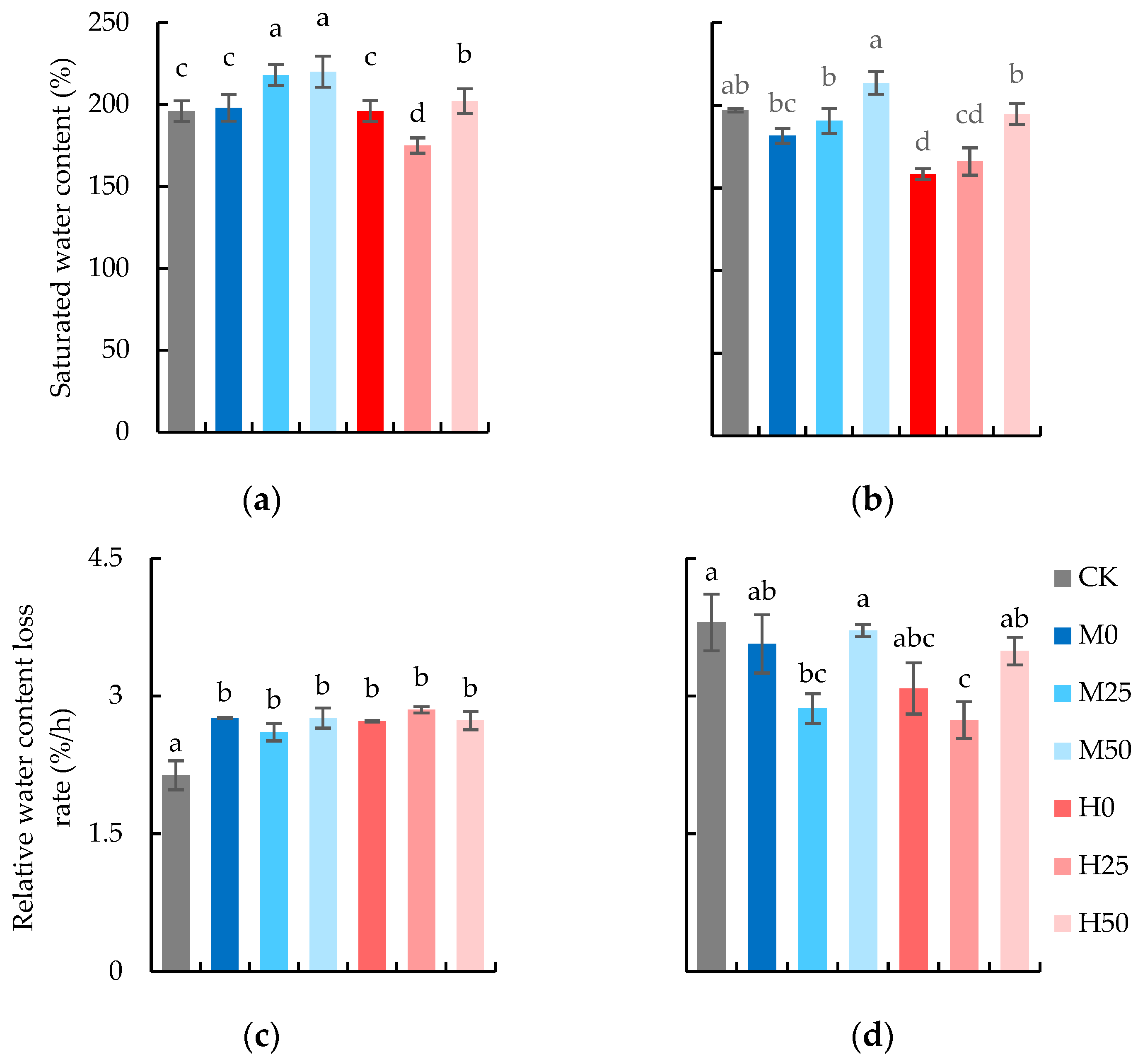
2.2. Proline Spraying Promotes Water-Holding Capacity and SPAD of Wheat Flag Leaves
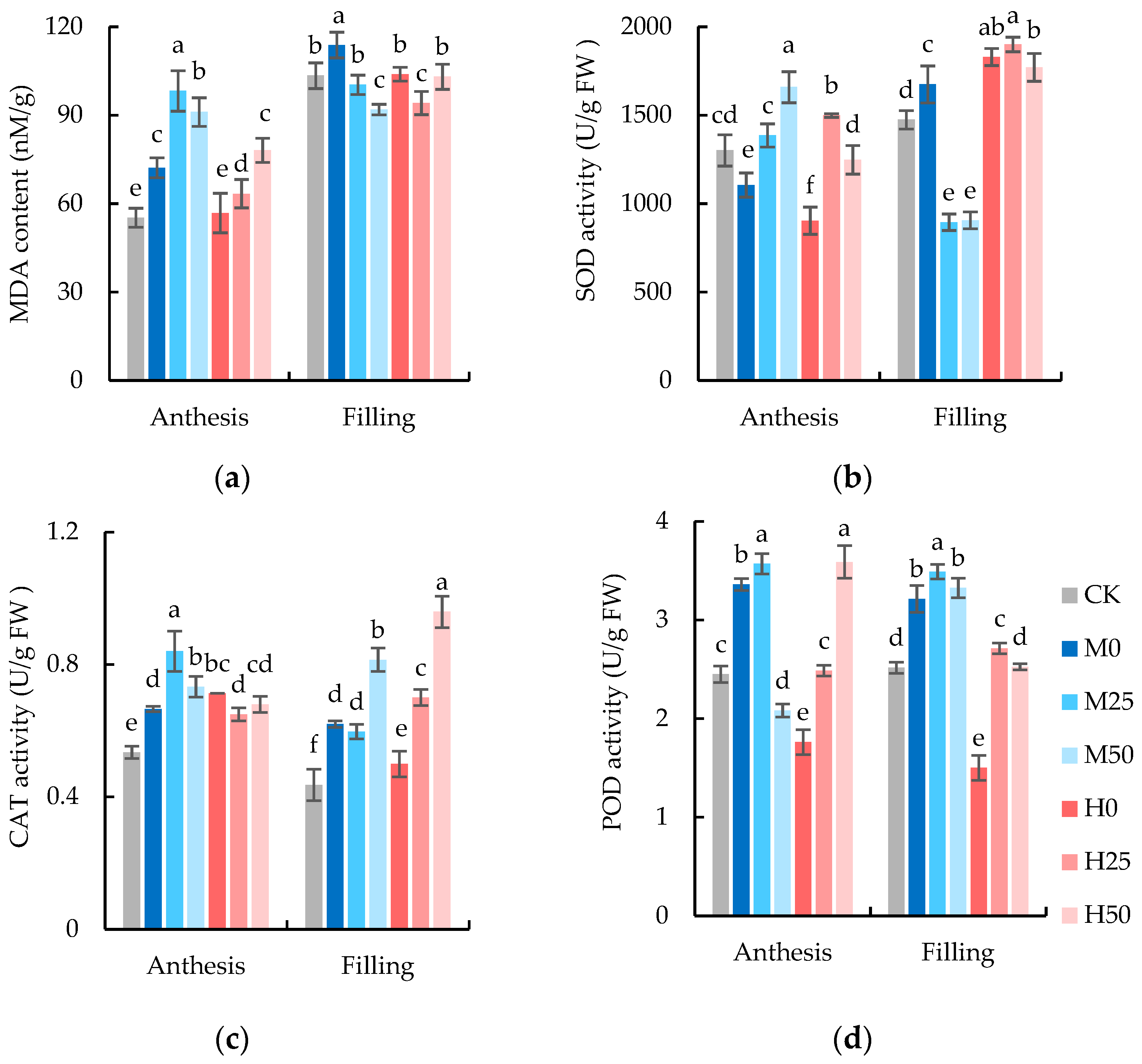
2.3. Exogenous Proline Improves the Performance of Wheat Antioxidant Enzyme System
2.4. Exogenous Proline Significantly Increases the Content of Proline in the Flag Leaves of Wheat
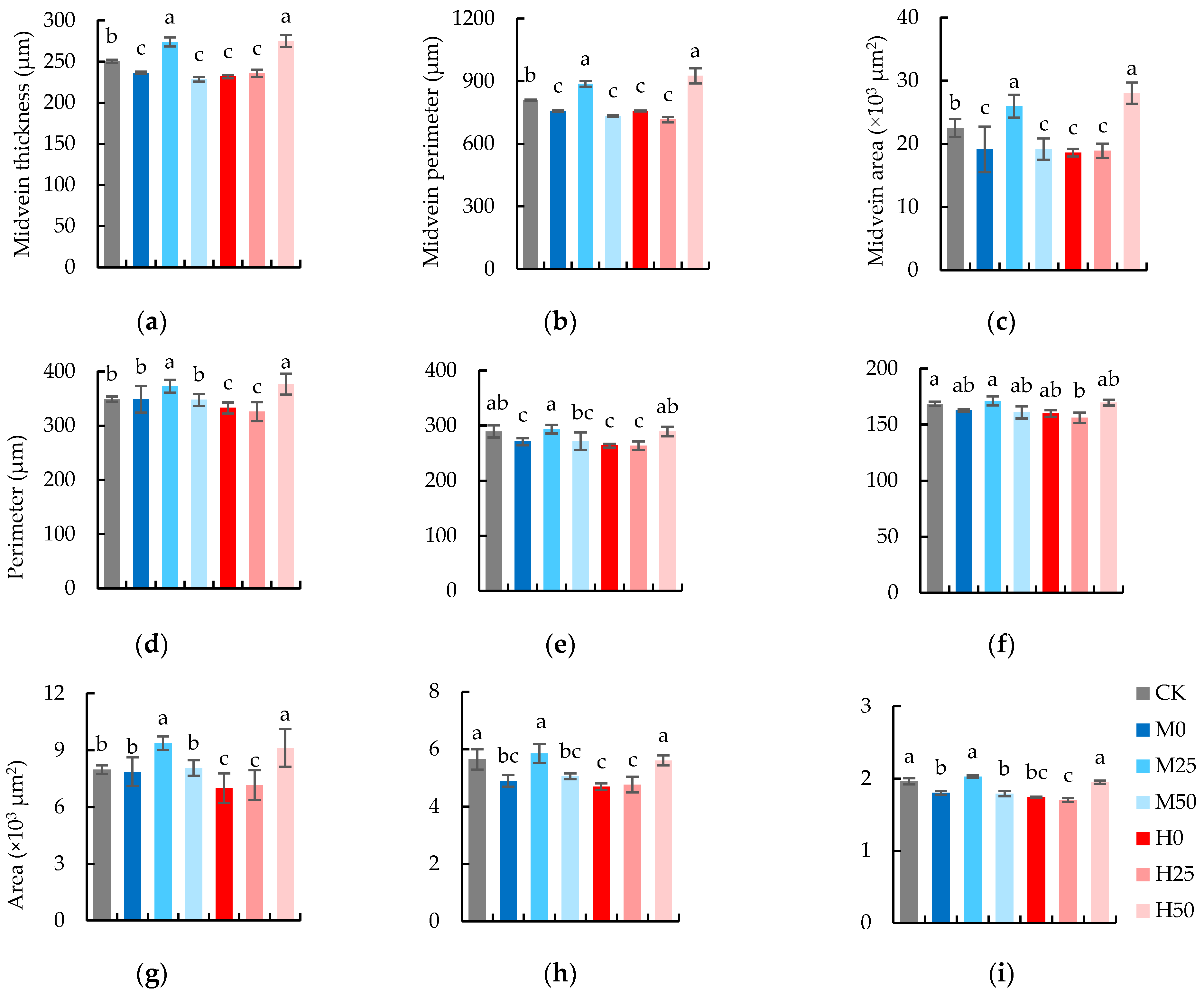
2.5. The Anatomic Characteristics of Flag Leaves Are Elevated by the Suitable Concentration of Proline Spray
2.6. Exogenous Proline Significantly Increased Wheat Yield under Severe Drought Conditions
3. Discussion
3.1. Effects of Exogenous Proline on the Function of Flag Leaves of Wheat under Drought Stress
3.2. Effects of Exogenous Proline on Antioxidant System of Flag Leaves of Wheat under Drought Stress
3.3. Effects of Exogenous Proline on Anatomical Characteristics of Flag Leaves of Wheat under Drought Stress
3.4. Effects of Exogenous Proline on Wheat Yield under Drought Stress
4. Materials and Methods
4.1. Experiment Site
4.2. Experiment Design
4.2.1. Soil Moisture Control
4.2.2. Foliar Spraying Method
4.3. Item Measurement
4.3.1. Evapotranspiration
4.3.2. Leaf Mass Moisture Content
4.3.3. Chlorophyll Measurement
4.3.4. Antioxidant-System Determination
4.3.5. Anatomical Feature Qualification
4.3.6. Grain Yield Characterization
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Qingqing, Z.; Xingyuan, M.; Cang, H.; Feng, G.; Fang, O. Wheat yield losses from pests and pathogens in china. Agric. Ecosyst. Environ. 2022, 326, 107821. [Google Scholar]
- NBS. Announcement of the National Bureau of Statistics on 2023 Summer Grain Production Data. Available online: https://www.stats.gov.cn/sj/zxfb/202307/t20230715_1941239.html (accessed on 24 March 2024).
- Flohr, B.M.; Hunt, J.R.; Kirkegaard, J.A.; Evans, J.R.; Swan, A.; Rheinheimer, B. Genetic gains in nsw wheat cultivars from 1901 to 2014 as revealed from synchronous flowering during the optimum period. Eur. J. Agron. 2018, 98, 1–13. [Google Scholar] [CrossRef]
- IPCC. Ar5 Synthesis Report: Climate Change 2014. Available online: https://www.ipcc.ch/report/ar5/syr/.2024 (accessed on 7 February 2024).
- Jian-Yong, W.; You-Cai, X.; Feng-Min, L.; Kadambot, H.M.S.; Neil, C.T. Chapter three—Effects of drought stress on morphophysiological traits, biochemical characteristics, yield, and yield components in different ploidy wheat: A meta-analysis. Adv. Agron. 2017, 143, 139–173. [Google Scholar]
- Nikolaeva, M.K.; Maevskaya, S.N.; Shugaev, A.G.; Bukhov, N.G. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russ. J. Plant Physiol. 2010, 57, 87–95. [Google Scholar] [CrossRef]
- Keyvan, S. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J. Anim. Plant Sci. (JAPS) 2010, 8, 1051–1060. [Google Scholar]
- Centritto, M.; Lauteri, M.; Monteverdi, M.C.; Serraj, R. Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage. J. Exp. Bot. 2009, 60, 2325–2339. [Google Scholar] [CrossRef] [PubMed]
- Akram, H.M.; Ali, A.; Sattar, A.; Rehman, H.S.U.; Bibi, A. Impact of water deficit stress on various physiological and agronomic traits of three basmati rice (Oryza sativa L.) cultivars. J. Anim. Plant Sci. 2013, 23, 1415–1423. [Google Scholar]
- Ali, Z.; Merrium, S.; Habib-ur-Rahman, M.; Hakeem, S.; Saddique, M.A.B.; Sher, M.A. Wetting mechanism and morphological adaptation; leaf rolling enhancing atmospheric water acquisition in wheat crop-a review. Environ. Sci. Pollut. Res. 2022, 29, 30967–30985. [Google Scholar] [CrossRef] [PubMed]
- Mathew, I.; Shimelis, H.; Mutema, M.; Clulow, A.; Zengeni, R.; Mbava, N.; Chaplot, V. Selection of wheat genotypes for biomass allocation to improve drought tolerance and carbon sequestration into soils. J. Agron. Crop Sci. 2019, 205, 385–400. [Google Scholar] [CrossRef]
- Hameed, A.; Bibi, N.; Akhter, J.; Iqbal, N. Differential changes in antioxidants, proteases, and lipid peroxidation in flag leaves of wheat genotypes under different levels of water deficit conditions. Plant Physiol. Biochem. 2011, 49, 178–185. [Google Scholar] [CrossRef]
- Erezhetova, U.; Sultangalyeva, G.M.; Terletskaya, N.V.; Kurmanbayeva, M.S.; Hoffmann, M.H. Anatomical parameters of the flag leaf of alloplasmic lines and their parental forms in drought conditions. Eurasian J. Ecol. 2021, 67, 78–84. [Google Scholar] [CrossRef]
- Maqbool, M.M.; Ali, A.; Tanveer-ul-Haq, T.U.H.; Majeed, M.N.; Lee, D.; Lee, D.J. Response of spring wheat (triticum aestivum l.) to induced water stress at critical growth stages. Sarhad J. Agric. 2015, 31, 53–58. [Google Scholar]
- Loutfy, N.; El-Tayeb, M.A.; Hassanen, A.M.; Moustafa, M.F.M.; Sakuma, Y.; Inouhe, M. Changes in the water status and osmotic solute contents in response to drought and salicylic acid treatments in four different cultivars of wheat (Triticum aestivum). J. Plant Res. 2012, 125, 173–184. [Google Scholar] [CrossRef]
- Pei, Z.F.; Ming, D.F.; Liu, D.; Wan, G.L.; Geng, X.X.; Gong, H.J.; Zhou, W.J. Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J. Plant Growth Regul. 2010, 29, 106–115. [Google Scholar] [CrossRef]
- Marcinska, I.; Czyczylo-Mysza, I.; Skrzypek, E.; Filek, M.; Grzesiak, S.; Grzesiak, M.T.; Janowiak, F.; Hura, T.; Dziurka, M.; Dziurka, K.; et al. Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta Physiol. Plant. 2013, 35, 451–461. [Google Scholar] [CrossRef]
- Gou, W.; Tian, L.; Ruan, Z.; Zheng, P.; Chen, F.C.; Zhang, L.; Cui, Z.Y.; Zheng, P.F.; Li, Z.; Gao, M.; et al. Accumulation of choline and glycinebetaine and drought stress tolerance induced in maize (Zea mays) by three plant growth promoting rhizobacteria (pgpr) strains. Pak. J. Bot. 2015, 47, 581–586. [Google Scholar]
- Kang, G.Z.; Li, G.Z.; Liu, G.Q.; Xu, W.; Peng, X.Q.; Wang, C.Y.; Zhu, Y.J.; Guo, T.C. Exogenous salicylic acid enhances wheat drought tolerance by influence on the expression of genes related to ascorbate-glutathione cycle. Biol. Plant. 2013, 57, 718–724. [Google Scholar] [CrossRef]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Morgan, J.M. Differences in osmoregulation between wheat genotypes. Nature 1977, 270, 234–235. [Google Scholar] [CrossRef]
- Morgan, J.V. Osmoregulation as a selection criterion for drought tolerance in wheat. Crop Pasture Sci. 1983, 34, 607–614. [Google Scholar] [CrossRef]
- Mikołajczak, K.; Kuczyńska, A.; Krajewski, P.; Kempa, M.; Witaszak, N. Global proteome profiling revealed the adaptive reprogramming of barley flag leaf to drought and elevated temperature. Cells 2023, 12, 1685. [Google Scholar] [CrossRef] [PubMed]
- Yong, B.; Xie, H.; Li, Z.; Li, Y.-P.; Zhang, Y.; Nie, G.; Zhang, X.-Q.; Ma, X.; Huang, L.-K.; Yan, Y.-H.; et al. Exogenous application of gaba improves peg-induced drought tolerance positively associated with gaba-shunt, polyamines, and proline metabolism in white clover. Front. Physiol. 2017, 8, 1107. [Google Scholar] [CrossRef] [PubMed]
- Veroneze, V.; Martins, M.; Mc Leod, L.; Souza, K.R.D.; Santos, P.R.; Magalhaes, P.C.; Carvalho, D.T.; Santos, M.H.; Souza, T.C. Leaf application of chitosan and physiological evaluation of maize hybrids contrasting for drought tolerance under water restriction. Braz. J. Biol. 2020, 80, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, X.; Dai, M. Improving crop drought resistance with plant growth regulators and rhizobacteria: Mechanisms, applications, and perspectives. Plant Commun. 2022, 3, 100228. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Shabala, L. Ion transport and osmotic adjustment in plants and bacteria. Biomol. Concepts 2011, 2, 407–419. [Google Scholar] [CrossRef]
- Kemble, A.R.; Macpherson, H.T. Liberation of amino acids in perennial rye grass during wilting. Biochem. J. 1954, 58, 46–49. [Google Scholar] [CrossRef]
- Roy, D.; Basu, N.; Bhunia, A.; Banerjee, S.K. Counteraction of exogenous l-proline with nacl in salt-sensitive cultivar of rice. Biol. Plant. 1993, 35, 69–72. [Google Scholar] [CrossRef]
- Hussain, R.; Ayyub, C.M.; Shaheen, M.R.; Rashid, S.H.; Nafees, M.; Ali, S.; Butt, M.; Ali, M.; Maqsood, A.; Fiaz, S.; et al. Regulation of osmotic balance and increased antioxidant activities under heat stress in Abelmoschus esculentus L. Triggered by exogenous proline application. Agronomy 2021, 11, 685. [Google Scholar] [CrossRef]
- Ali, Q.; Ashraf, M.; Athar, H.U.R. Exogenously applied proline at different growth stages enhances growth of two maize cultivars grown under water deficit conditions. Pak. J. Bot. 2007, 39, 1133–1144. [Google Scholar]
- Ali, Q.; Ashraf, M.; Shahbaz, M.; Humera, H. Ameliorating effect of foliar applied proline on nutrient uptake in water stressed maize (Zea mays L.) plants. Pak. J. Bot. 2008, 40, 211–219. [Google Scholar]
- Kamran, M.; Shahbaz, M.; Ashraf, M.; Akram, N.A. Alleviation of drought-induced adverse effects on spring wheat (Triticum aestivum L.) using proline as a pre-sowing seed treatment. Pak. J. Bot. 2009, 41, 621–632. [Google Scholar]
- Molla, M.R.; Ali, M.R.; Hasanuzzaman, M.; Al-Mamun, M.H.; Ahmed, A.; Nazim-Ud-Dowla, M.A.N.; Rohman, M.M. Exogenous proline and betaine-induced upregulation of glutathione transferase and glyoxalase i in lentil (Lens culinaris) under drought stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 73–80. [Google Scholar] [CrossRef]
- Morgan, J.M.; Hare, R.A.; Fletcher, R.J. Genetic variation in osmoregulation in bread and durum wheats and its relationship to grain yield in a range of field environments. Aust. J. Agric. Res. 1986, 37, 449–457. [Google Scholar] [CrossRef]
- Johnson, K.M.; Jordan, G.J.; Brodribb, T.J. Wheat leaves embolized by water stress do not recover function upon rewatering. Plant Cell Environ. 2018, 41, 2704–2714. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Sperry, J.S. Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 19–38. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Márquez, S.; Conde-Martínez, V.; Trejo, C.; Delgado-Alvarado, A.; Carballo, A.; Suárez, R.; Mascorro, J.; Trujillo, A.R. Effects of water deficit on radicle apex elongation and solute accumulation in zea mays l. Plant Physiol. Biochem. 2015, 96, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Lastochkina, O.; Ivanov, S.; Petrova, S.; Garshina, D.; Lubyanova, A.; Yuldashev, R.; Kuluev, B.; Zaikina, E.; Maslennikova, D.; Allagulova, C.; et al. Role of endogenous salicylic acid as a hormonal intermediate in the bacterial endophyte bacillus subtilis-induced protection of wheat genotypes contrasting in drought susceptibility under dehydration. Plants 2022, 11, 3365. [Google Scholar] [CrossRef]
- Houda, H.; Nadia, Y.; Bouldjedj, R.; Belbekri, N. Mitigation of peg-induces drought stress in wheat (Triticum durum) by exogenous application of proline. Rom. Agric. Res. 2023, 40, 105–116. [Google Scholar] [CrossRef]
- Hanif, S.; Saleem, M.F.; Sarwar, M.; Irshad, M.; Shakoor, A.; Wahid, M.A.; Khan, H.Z. Biochemically triggered heat and drought stress tolerance inrice by proline application. J. Plant Growth Regul. 2021, 40, 305–312. [Google Scholar] [CrossRef]
- Said, C.O.; Boulahia, K.; Eid, M.A.M.; Rady, M.M.; Djebbar, R.; Abrous-Belbachir, O. Exogenously used proline offers potent antioxidative and osmoprotective strategies to re-balance growth and physio-biochemical attributes in herbicide-stressed (Trigonella foenum-graecum). J. Soil Sci. Plant Nutr. 2021, 21, 3254–3268. [Google Scholar] [CrossRef]
- Ozden, M.; Demirel, U.; Kahraman, A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 2009, 119, 163–168. [Google Scholar] [CrossRef]
- Merwad, A.-R.M.A.; Desoky, E.-S.M.; Rady, M.M. Response of water deficit-stressed (Vigna unguiculata) performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.A.; Okuma, E.; Murata, Y. Exogenous proline and glycinebetaine increase nacl-induced a scorbate-glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco bright yellow-2 suspension-cu ltured cells. J. Plant Physiol. 2007, 164, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Hoque, A.; Okuma, E.; Nasrin, M.; Banu, A.; Shimoishi, Y.; Nakamura, Y.; Murata, Y. Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J. Plant Physiol. 2009, 166, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Kishor, P.B.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Ghafoor, R.; Akram, N.A.; Rashid, M.; Ashraf, M.; Iqbal, M.; Lixin, Z. Exogenously applied proline induced changes in key anatomical features and physio-biochemical attributes in water stressed oat (Avena sativa L.) plants. Physiol. Mol. Biol. Plants 2019, 25, 1121–1135. [Google Scholar] [CrossRef]
- Wyka, T.P.; Bagniewska-Zadworna, A.; Kuczynska, A.; Mikolajczak, K.; Ogrodowicz, P.; Zytkowiak, M.; Surma, M.; Adamski, T. Drought-induced anatomical modifications of barley (Hordeum vulgare L.) leaves: An allometric perspective. Environ. Exp. Bot. 2019, 166, 103798. [Google Scholar] [CrossRef]
- Moulia, B.; Fournier, M. Mechanics of the maize leaf: A composite beam model of the midrib. J. Mater. Sci. 1997, 32, 2771–2780. [Google Scholar] [CrossRef]
- Niklas, K.J. A mechanical perspective on foliage leaf form and function. New Phytol. 1999, 143, 19–31. [Google Scholar] [CrossRef]
- Limochi, K.; Farahvash, F.; Davodi, S.; Fateminik, F. The impact of different planting methods on yield and cluster characters wheat (cultivar of Chamran) under different conditions of irrigation in the northern khuzestan climate. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 2038–2044. [Google Scholar]
- Limouchi, K.; Siadat, S.A. Investigating the effect of different planting methods and different levels of hormone distribution on flag leaf anatomy of rice genotypes under salinity stress in northern khuzestan. Appl. Res. Field Crops 2021, 34, fa1–fa29. [Google Scholar]
- Wheeler, J.K.; Sperry, J.S.; Hacke, U.G.; Hoang, N. Inter-vessel pitting and cavitation in woody rosaceae and other vesselled plants: A basis for a safety versus efficiency trade-off in xylem transport. Plant Cell Environ. 2005, 28, 800–812. [Google Scholar] [CrossRef]
- Cattivelli, L.; Rizza, F.; Badeck, F.-W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Mare, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crops Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
- Dolferus, R.; Ji, X.; Richards, R.A. Abiotic stress and control of grain number in cereals. Plant Sci. 2011, 181, 331–341. [Google Scholar] [CrossRef]
- Farooq, M.; Nawaz, A.; Chaudhry, M.A.M.; Indrasti, R.; Rehman, A. Improving resistance against terminal drought in bread wheat by exogenous application of proline and gamma-aminobutyric acid. J. Agron. Crop Sci. 2017, 203, 464–472. [Google Scholar] [CrossRef]
- Rady, M.M.; Kuşvuran, A.; Alharby, H.F.; Alzahrani, Y.; Kuşvuran, S. Pretreatment with proline or an organic bio-stimulant induces salt tolerance in wheat plants by improving antioxidant redox state and enzymatic activities and reducing the oxidative stress. J. Plant Growth Regul. 2019, 38, 449–462. [Google Scholar] [CrossRef]
- Alam, R.; Das, D.K.; Islam, M.R.; Murata, Y.; Hoque, M.A. Exogenous proline enhances nutrient uptake and confers tolerance to salt stress in maize (Zea mays L.). Progress. Agric. 2017, 27, 409–417. [Google Scholar] [CrossRef]
- Kibria, M.G.; Kaniz, F.; Matin, M.A.; Hoque, M.A. Mitigating water stress in wheat (bari gom-26) by exogenous application of proline. Fundam. Appl. Agric. 2016, 1, 118–123. [Google Scholar]
- Schönherr, J. Calcium chloride penetrates plant cuticles via aqueous pores. Planta 2000, 212, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Gao, J. Determination of water-holding capacity of plant leaves. In Experimental supervision of plant physiology, 1st ed.; Tian, G.L.J., Ed.; Higher Education Press: Beijing, China, 2006; pp. 16–17. [Google Scholar]

| Growth Stage | Source of Variation | Saturated Water Content (%) | Relative Water-Content Loss Rate (%/h) | ||
|---|---|---|---|---|---|
| F | p | F | p | ||
| Anthesis | Drought degree | 5.04 | 0.0102 * | 1.16 | 0.3818 |
| Proline concentration | 12.17 | 0.0045 ** | 1.04 | 0.3278 | |
| Interaction | 2.5 | 0.1235 | 0.04 | 0.9605 | |
| Grain Filling | Drought degree | 10 | 0.0006 *** | 3.65 | 0.0308 * |
| Proline concentration | 18.36 | 0.0011 ** | 1.43 | 0.2554 | |
| Interaction | 15.72 | 0.0004 *** | 7.99 | 0.0062 ** | |
| Growth Stage | Source of Variation | SPAD | |
|---|---|---|---|
| F | p | ||
| Anthesis | Drought degree | 7.09 | 0.0136 * |
| Proline concentration | 0.81 | 0.4561 | |
| Interaction | 0.64 | 0.5357 | |
| Seed formation | Drought degree | 21.42 | 0.0001 *** |
| Proline concentration | 1.94 | 0.1656 | |
| Interaction | 2.17 | 0.1363 | |
| Treatment | Heading Stage | Anthesis Stage | Seed-Formation Stage |
|---|---|---|---|
| CK | 46.22 ± 1.14 a | 49.79 ± 1.31 a | 50.43 ± 0.78 a |
| M0 | 44.70 ± 0.87 a | 43.33 ± 0.39 ab | 43.09 ± 0.60 b |
| M25 | 44.71 ± 1.07 a | 49.00 ± 3.91 a | 47.68 ± 0.84 a |
| M50 | 46.43 ± 1.11 a | 46.61 ± 2.08 ab | 43.38 ± 1.58 b |
| H0 | 45.47 ± 1.10 a | 39.98 ± 0.70 b | 39.80 ± 0.32 b |
| H25 | 45.17 ± 1.06 a | 40.16 ± 0.83 b | 39.82 ± 1.06 b |
| H50 | 43.55 ± 1.25 a | 42.11 ± 0.94 ab | 40.12 ± 0.76 b |
| Growth Stage | Source of Variation | MDA Content | SOD Activity | CAT Activity | POD Activity | Proline Content | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | ||
| Anthesis | Drought degree | 139.49 | <0.001 *** | 45.77 | <0.001 *** | 39.73 | <0.001 *** | 487.09 | <0.001 *** | 287.55 | <0.001 *** |
| Proline concentration | 47.82 | <0.001 *** | 144.01 | <0.001 *** | 9.9 | 0.0029 ** | 174.17 | <0.001 *** | 220.75 | <0.001 *** | |
| Interaction | 15.13 | 0.0005 ** | 38.29 | <0.001 *** | 44.23 | <0.001 *** | 83.54 | <0.001 *** | 187.21 | <0.001 *** | |
| Grain Filling | Drought degree | 1.87 | 0.1961 | 1035.49 | <0.001 *** | 14.62 | <0.001 *** | 1081.94 | <0.001 *** | 1.78 | 0.2072 |
| Proline concentration | 40.52 | <0.001 *** | 151.39 | <0.001 *** | 310.8 | 0.0024 ** | 181.29 | <0.001 *** | 13.87 | 0.0008 *** | |
| Interaction | 28.92 | <0.001 *** | 157.19 | <0.001 *** | 55.79 | <0.001 *** | 85.26 | <0.001 *** | 12.44 | 0.0012 ** | |
| Source of Variation | Midvein | Vascular Bundle of The Midvein | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Thickness | Perimeter | Area | Perimeter | Area | ||||||
| F | p | F | p | F | p | F | p | F | p | |
| Drought degree | 0.18 | 0.6791 | 0.25 | 0.6239 | 0.35 | 0.5661 | 17.8 | 0.0012 ** | 14.94 | 0.0022 ** |
| Proline concentration | 16.12 | 0.0004 *** | 10.12 | 0.0027 ** | 15.46 | 0.0005 *** | 22.36 | <0.0001 *** | 15.8 | 0.0004 *** |
| Interaction | 59.05 | <0.0001 *** | 63.57 | <0.0001 *** | 39.84 | <0.0001 *** | 69.83 | <0.0001 *** | 29.67 | <0.0001 *** |
| Source of Variation | Large Vascular Bundle | Small Vascular Bundle | ||||||
|---|---|---|---|---|---|---|---|---|
| Perimeter | Area | Perimeter | Area | |||||
| F | p | F | p | F | p | F | p | |
| Drought degree | 0.71 | 0.4166 | 0.67 | 0.429 | 1.27 | 0.2824 | 1.32 | 0.2728 |
| Proline concentration | 1.15 | 0.35 | 1.3 | 0.3082 | 1.67 | 0.229 | 1.95 | 0.1849 |
| Interaction | 3.05 | 0.0851 | 3.17 | 0.0782 | 3.61 | 0.0594 | 4.64 | 0.0321 * |
| Source of Variation | Ear Number | Grains Number Per Ear | Thousand-Grain Weight (g) | Yield | ||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| Drought degree | 214.05 | <0.0001 *** | 5.69 | 0.0257 * | 30.3 | <0.0001 *** | 85.32 | <0.0001 *** |
| Proline concentration | 3.59 | 0.0438 | 0.27 | 0.7674 | 2.62 | 0.0945 | 11.95 | 0.0003 ** |
| Interaction | 1.63 | 0.2187 | 0.2 | 0.82 | 3.09 | 0.0645 | 8.63 | 0.0016 ** |
| Treatments | Ear Number | Grains Number Per Ear | Thousand Grain Weight (g) | Yield (g/barrel) | Yield (t/ha) |
|---|---|---|---|---|---|
| CK | 51.60 ± 0.66 a | 40.02 ± 2.17 a | 37.69 ± 38.11 a | 40.46 ± 0.77 a | 5.36 ± 0.10 a |
| M0 | 46.40 ± 0.66 b | 38.12 ± 2.13 ab | 34.49 ± 31.78 ab | 35.72 ± 5.31 bc | 4.73 ± 0.70 bc |
| M25 | 49.40 ± 0.32 ab | 39.07 ± 1.50 ab | 36.08 ± 15.40 a | 39.58 ± 1.15 ab | 5.25 ± 0.15 ab |
| M50 | 48.00 ± 1.15 b | 39.19 ± 2.60 ab | 34.41 ± 71.87 ab | 36.75 ± 1.02 ab | 4.87 ± 0.14 ab |
| H0 | 33.20 ± 1.25 d | 37.00 ± 0.92 b | 26.42 ± 23.47 d | 20.94 ± 0.53 e | 2.78 ± 0.07 e |
| H25 | 35.60 ± 2.50 cd | 37.08 ± 1.25 b | 29.28 ± 28.41 cd | 28.04 ± 0.71 d | 3.72 ± 0.09 d |
| H50 | 37.00 ± 0.71 c | 37.16 ± 1.14 b | 32.07 ± 19.87 bc | 32.20 ± 0.85 c | 4.27 ± 0.11 c |
| Soil Layer (cm) | pH | EC (μS/cm) | AP (mg/kg) | TK (g/kg) | AN (mg/kg) | OM (g/kg) | TP (g/kg) |
|---|---|---|---|---|---|---|---|
| 0–10 | 8.43 | 443.00 | 81.23 | 10.70 | 71.16 | 10.16 | 0.11 |
| 10–20 | 8.83 | 174.10 | 177.69 | 11.30 | 29.79 | 17.11 | 0.60 |
| 20–30 | 8.86 | 185.30 | 127.37 | 11.60 | 28.13 | 13.33 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Liu, Y.; Zhen, B.; Lv, M.; Zhou, X.; Yong, B.; Niu, Q.; Yang, S. Proline Spray Relieves the Adverse Effects of Drought on Wheat Flag Leaf Function. Plants 2024, 13, 957. https://doi.org/10.3390/plants13070957
Li H, Liu Y, Zhen B, Lv M, Zhou X, Yong B, Niu Q, Yang S. Proline Spray Relieves the Adverse Effects of Drought on Wheat Flag Leaf Function. Plants. 2024; 13(7):957. https://doi.org/10.3390/plants13070957
Chicago/Turabian StyleLi, Huizhen, Yuan Liu, Bo Zhen, Mouchao Lv, Xinguo Zhou, Beibei Yong, Qinglin Niu, and Shenjiao Yang. 2024. "Proline Spray Relieves the Adverse Effects of Drought on Wheat Flag Leaf Function" Plants 13, no. 7: 957. https://doi.org/10.3390/plants13070957
APA StyleLi, H., Liu, Y., Zhen, B., Lv, M., Zhou, X., Yong, B., Niu, Q., & Yang, S. (2024). Proline Spray Relieves the Adverse Effects of Drought on Wheat Flag Leaf Function. Plants, 13(7), 957. https://doi.org/10.3390/plants13070957








