Habitat Degradation Facilitates the Invasion of Neophytes: A Resurvey Study Based on Permanent Vegetation Plots in Oak Forests in Slovenia (Europe)
Abstract
:1. Introduction
2. Results
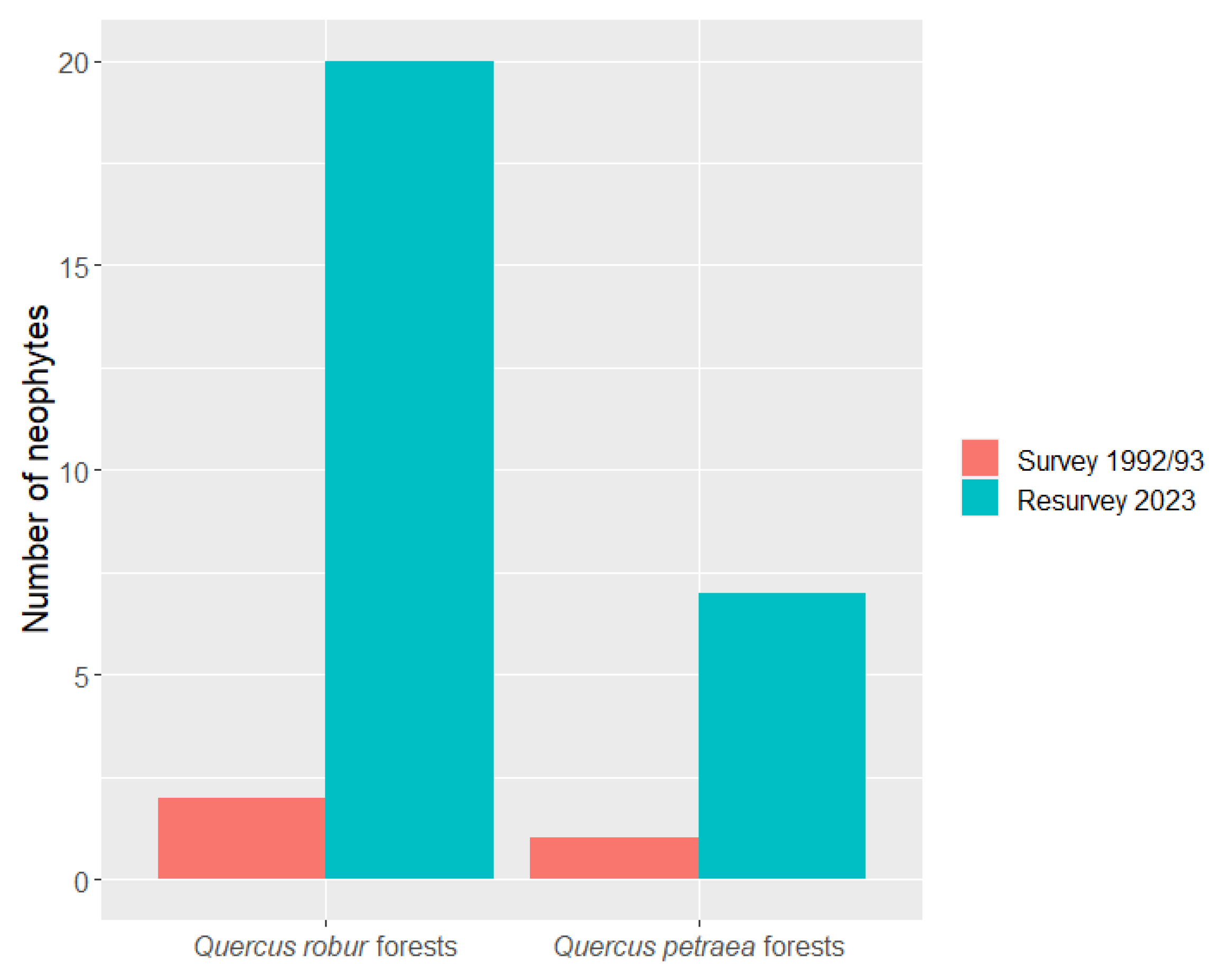
2.1. Patterns of Neophyte Occurrence
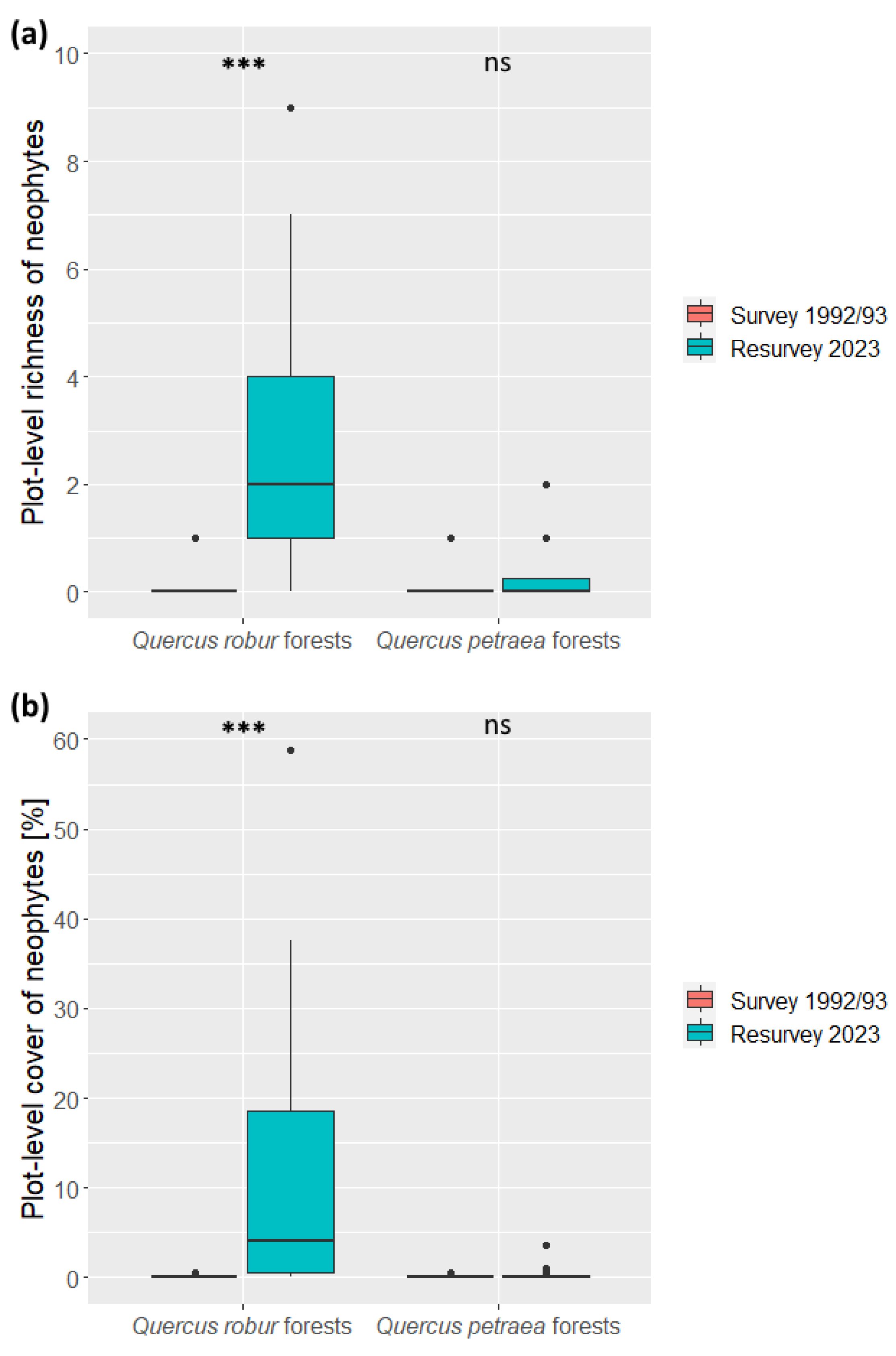
2.2. Changes in Richness and Cover of Neophytes
2.3. Neophytes in Relation to Tree Layer Cover and Soil Disturbance
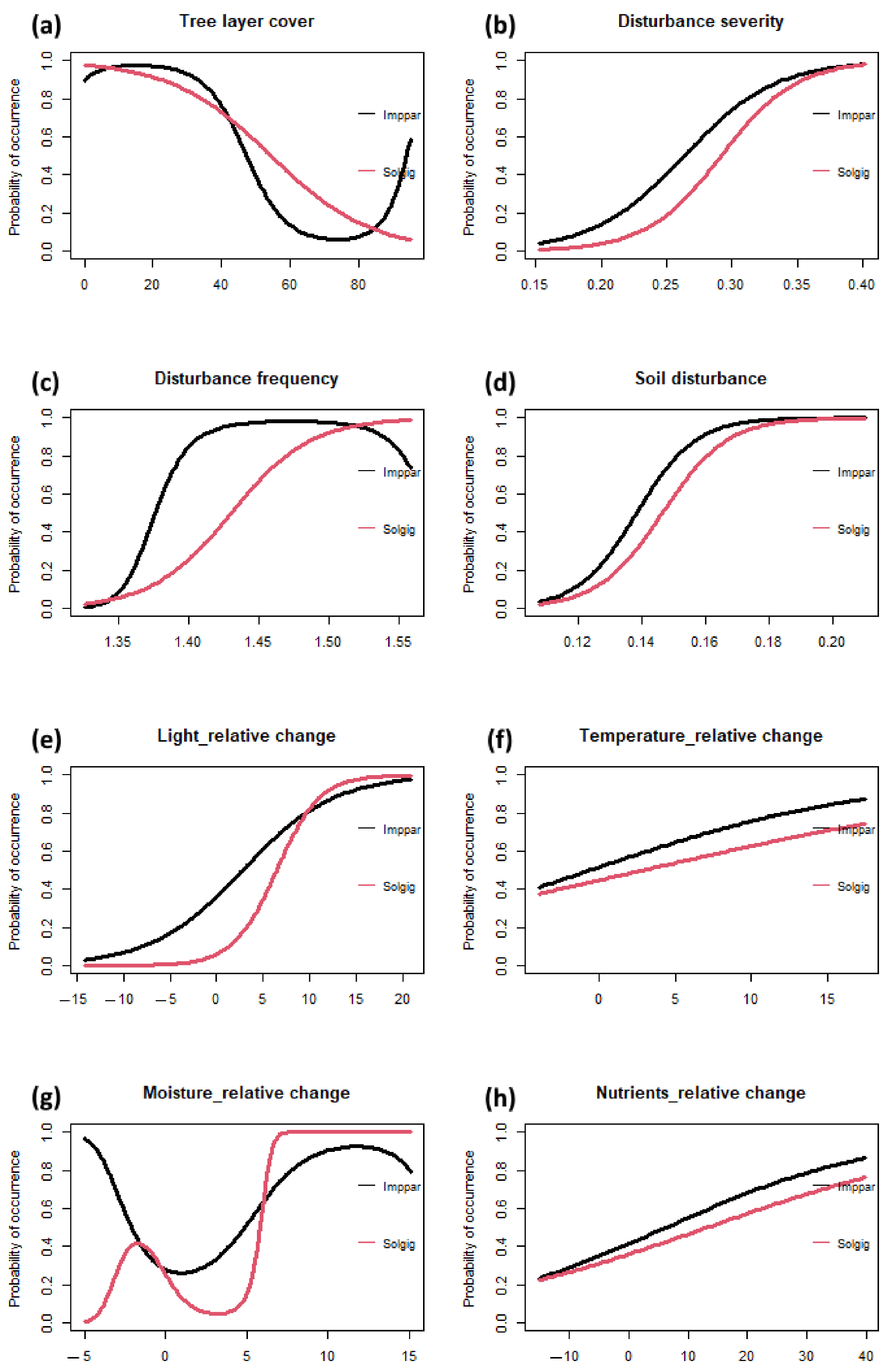
2.4. Species Response Curves
3. Discussion
3.1. Oak Mortality Promotes the Spread of Neophytes
3.2. Importance of Soil Disturbance and Moisture Conditions
3.3. Impatiens parviflora and Other Frequent Neophytes
4. Materials and Methods
4.1. Study Area
4.2. Sampling Design and Vegetation Survey
4.3. Definition of Neophytes
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simberloff, D. Sustainability of biodiversity under global changes, with particular reference to biological invasions. In Sustainability Science: The Emerging Paradigm and the Urban Environment; Weinstein, M., Turner, E., Eds.; Springer: New York, NY, USA, 2012; pp. 139–157. Available online: https://link.springer.com/book/10.1007/978-1-4614-3188-6 (accessed on 18 December 2023).
- Šipek, M.; Kutnar, L.; Marinšek, A.; Šajna, N. Contrasting responses of alien and ancient forest indicator plant species to fragmentation process in the temperate lowland forests. Plants 2022, 11, 3392. [Google Scholar] [CrossRef]
- FAO; UNEP. The State of the World’s Forests 2020, Forests, Biodiversity and People; FAO and UNEP: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Klimo, E.; Hager, H.; Matić, S.; Anić, I.; Kulhavý, J. Floodplain Forests of the Temperate Zone of Europe; Lesnická Práce s.r.o.: Kostelec nad Černými lesy, Czech Republic, 2008; 624p, ISBN 978-80-87154-16-8. [Google Scholar]
- Vojík, M.; Boublík, K. Fear of the dark: Decline in plant diversity and invasion of alien species due to increased tree canopy density and eutrophication in lowland woodlands. Plant Ecol. 2018, 219, 749–758. [Google Scholar] [CrossRef]
- Daneshgar, P.; Jose, S. Mechanisms of plant invasions: A review. In Invasive Plants and Forest Ecosystems; Kohli, R.K., Jose, S., Singh, H.P., Batish, D.R., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2009; pp. 11–27. [Google Scholar]
- Scherrer, D.; Bürgi, M.; Gessler, A.; Kessler, M.; Nobis, M.P.; Wohlgemuth, T. Abundance changes of neophytes and native species indicate a thermophilisation and eutrophisation of the Swiss flora during the 20th century. Ecol. Ind. 2022, 135, 108558. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Brockerhoff, E.G.; Kalisz, S.; Nuñez, M.A.; Wardle, D.A.; Wingfield, M.J. Biological invasions in forest ecosystems. Biol. Invasions 2017, 19, 3437–3458. [Google Scholar] [CrossRef]
- Marinšek, A.; Kutnar, L. Occurrence of invasive alien plant species in the floodplain forests along the Mura River in Slovenia. Period. Biol. 2017, 119, 251–260. [Google Scholar] [CrossRef]
- Kermavnar, J.; Kutnar, L.; Marinšek, A.; Kus Veenvliet, J.; De Groot, M. Invasive alien plant species kudzu (Pueraria montana var. lobata) as a potential threat for forests in Slovenia. Gozd Vestn. 2019, 77, 55–72, (In Slovenian with English summary). [Google Scholar]
- Langmaier, M.; Lapin, K. A systematic review of the impact of invasive alien plants on forest regeneration in European temperate forests. Front. Plant Sci. 2020, 11, 524969. [Google Scholar] [CrossRef]
- Jose, S.; Kohli, R.K.; Singh, H.P.; Batish, D.R.; Pieterson, E.C. Invasive plants: A Threat to the integrity and sustainability of forest ecosystems. In Invasive Plants and Forest Ecosystems; Kohli, R.K., Jose, S., Singh, H.P., Batish, D.R., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2009; pp. 3–10. [Google Scholar]
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Martin, P.H.; Canham, C.D.; Marks, P.L. Why forests appear resistant to exotic plant invasions: Intentional introductions, stand dynamics and the role of shade tolerance. Front. Ecol. Environ. 2009, 7, 142–149. [Google Scholar] [CrossRef]
- Rejmánek, M.; Richardson, D.; Pyšek, P. Plant invasions and invasibility of plant communities. In Vegetation Ecology; van der Maarel, E., Ed.; Blackwell Science Ltd.: Oxford, UK, 2005; pp. 332–355. [Google Scholar]
- Richardson, D.M.; Pyšek, P. Plant invasions: Merging the concepts of species invasiveness and community invasibility. Prog. Phys. Geog. 2006, 30, 409–431. [Google Scholar] [CrossRef]
- Jantsch, M.C.; Fischer, A.; Fischer, H.S.; Winter, S. Shifts in plant species composition reveals environmental changes during the last decades: A long-term study in beech (Fagus sylvatica) forests in Bavaria, Germany. Folia Geobot. 2013, 48, 467–491. [Google Scholar] [CrossRef]
- Aszalós, R.; Kovács, B.; Tinya, F.; Németh, C.; Horváth, C.V.; Ódor, P. Canopy gaps are less susceptible to disturbance-related and invasive herbs than clear-cuts: Temporal changes in the understorey after experimental silvicultural treatments. Forest Ecol. Manag. 2023, 549, 121438. [Google Scholar] [CrossRef]
- Essl, F.; Mang, T.; Moser, D. Ancient and recent alien species in temperate forests: Steady state and time lags. Biol. Invasions 2012, 14, 1331–1342. [Google Scholar] [CrossRef]
- Šebesta, J.; Rogers, P.C.; Maděra, P.; Koutecký, T.; Dufour, S.; Řepka, R. Long-term effects of mechanical site preparation on understorey plant communities in lowland floodplain forests. Forest Ecol. Manag. 2021, 480, 118651. [Google Scholar] [CrossRef]
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; p. e01c6df+. [Google Scholar]
- Mölder, A.; Meyer, P.; Nagel, R.V. Integrative management to sustain biodiversity and ecological continuity in Central European temperate oak (Quercus robur, Q. petraea) forests: An overview. Forest Ecol. Manag. 2019, 437, 324–339. [Google Scholar] [CrossRef]
- Čater, M. A 20-year overview of Quercus robur L. mortality and crown conditions in Slovenia. Forests 2015, 6, 581–593. [Google Scholar] [CrossRef]
- Brunet, J.; Felton, A.; Hedwall, P.O. Vegetation responses to pathogen-induced tree loss: Swedish elm and ash forests revisited after 32 years. Plant Ecol. 2023, 224, 875–884. [Google Scholar] [CrossRef]
- Hédl, R.; Bernhardt-Römermann, M.; Grytnes, J.A.; Jurasinski, G.; Ewald, J. Resurvey of historical vegetation plots: A tool for understanding long-term dynamics of plant communities. Appl. Veg. Sci. 2017, 20, 161–163. [Google Scholar] [CrossRef]
- Kapfer, K.; Hédl, R.; Jurasinski, G.; Kopecký, M.; Schei, F.H.; Grytnes, J.A. Resurveying historical vegetation data—Opportunities and challenges. Appl. Veg. Sci. 2017, 20, 164–171. [Google Scholar] [CrossRef]
- Mikulová, K.; Jarolímek, I.; Šibík, J.; Bacigál, T.; Šibíkova, M. Long term changes of softwood floodplain forests—Did the disappearance of wet vegetation accelerate the invasion process? Forests 2020, 11, 1218. [Google Scholar] [CrossRef]
- Medvecká, J.; Jarolímek, J.; Hegedüšová, K.; Škodová, I.; Bazalová, D.; Botková, K.; Šibíková, M. Forest habitat invasions—Who with whom, where and why. Forest Ecol. Manag. 2018, 409, 468–478. [Google Scholar] [CrossRef]
- Lapin, K.; Oettel, J.; Steiner, H.; Langmaier, M.; Sustic, D.; Starlinger, F.; Kindermann, G.; Frank, G. Invasive alien plant species in unmanaged forest reserves, Austria. NeoBiota 2019, 48, 71–96. [Google Scholar] [CrossRef]
- Petrášová, M.; Jarolímek, I.; Medvecká, J. Neophytes in Pannonian hardwood floodplain forests—History, present situation and trends. Forest Ecol. Manag. 2013, 308, 31–39. [Google Scholar] [CrossRef]
- ARSO—Slovenian Environment Agency, Geoportal Atlas Okolja. Available online: https://gis.arso.gov.si/atlasokolja/profile.aspx?id=Atlas_Okolja_AXL@Arso (accessed on 23 January 2024).
- Lanta, V.; Liancourt, P.; Altman, J.; Černý, T.; Dvorský, M.; Fibich, P.; Götzenberger, L.; Hornych, O.; Miklín, J.; Petřík, P.; et al. Determinants of invasion by single versus multiple plant species in temperate lowland forests. Biol. Invasions 2022, 24, 2513–2528. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, J.; Wang, Z.; Xing, C.; Chen, B.; Wang, X.; Wei, C.; Liu, J.; He, Z.; Xu, D. Canopy gaps control litter decomposition and nutrient release in Subtropical forests. Forests 2023, 14, 673. [Google Scholar] [CrossRef]
- Jauni, M.; Gripenberg, S.; Ramula, S. Non-native plant species benefit from disturbance: A meta-analysis. Oikos 2015, 124, 122–129. [Google Scholar] [CrossRef]
- Chmura, D. Penetration and naturalization of invasive alien plant species (neophytes) in woodlands of the Silesian upland (Southern Poland). Nat. Conserv. 2004, 60, 3–11. [Google Scholar]
- Bolton, N.W.; D’Amato, A.W. Herbaceous vegetation responses to gap size within natural disturbance-based silvicultural systems in Northeastern Minnesota, USA. Forests 2019, 10, 111. [Google Scholar] [CrossRef]
- Chmura, D.; Sierka, E. The invasibility of deciduous forest communities after disturbance: A case study of Carex brizoides and Impatiens parviflora invasion. Forest Ecol. Manag. 2007, 242, 487–495. [Google Scholar] [CrossRef]
- Spicer, M.E.; Royo, A.A.; Wenzel, J.W.; Carson, W.P. Understory plant growth forms respond independently to combined natural and anthropogenic disturbances. Forest Ecol. Manag. 2023, 543, 121077. [Google Scholar] [CrossRef]
- Kutnar, L.; Nagel, T.A.; Kermavnar, J. Effects of disturbance on understory vegetation across Slovenian forest ecosystems. Forests 2019, 10, 1048. [Google Scholar] [CrossRef]
- Closset-Kopp, D.; Hattab, T.; Decocq, G. Do drivers of forestry vehicles also drive herb layer changes (1970–2015) in a temperate forest with contrasting habitat and management conditions. J. Ecol. 2019, 107, 1439–1456. [Google Scholar] [CrossRef]
- Chudomelová, M.; Hédl, R.; Zouhar, V.; Szabó, P. Open oakwoods facing modern threats: Will they survive the next fifty years? Biol. Conserv. 2017, 210, 163–173. [Google Scholar] [CrossRef]
- Chabrerie, O.; Verheyen, K.; Saguez, R.; Decocq, G. Disentangling relationships between habitat conditions, disturbance history, plant diversity, and American black cherry (Prunus serotina Ehrh.) invasion in a European temperate forest. Divers. Distrib. 2008, 14, 204–212. [Google Scholar] [CrossRef]
- Dakskobler, I.; Kutnar, L.; Šilc, U. Floodplain Woods, Swamp Woods and Riverine Forests in Slovenia—Forests of Willows, Alders, White Elm, European and Narrow-LEAVED Ash, Pedunculate Oak and Scots Pine along Rivers and Streams; Silva Slovenica, Gozdarski Inštitut Slovenije and Zveza gozdarskih Društev Slovenije—Gozdarska Založba: Ljubljana, Slovenija, 2013; 127p, (In Slovenian with English abstract). [Google Scholar]
- Kus Veenvliet, J.; Veenvliet, P.; De Groot, M.; Kutnar, L. Terenski Priročnik za Prepoznavanje Tujerodnih Vrst v Gozdovih, 3rd ed.; Silva Slovenica, Gozdarski Inštitut Slovenije: Ljubljana, Slovenija, 2020; 202p, Available online: https://www.tujerodne-vrste.info/wp-content/uploads/2020/12/TERENSKI-PRIROCNIK-TUJERODNE-VRSTE-tretja-izdaja-WWW.pdf (accessed on 2 February 2024).
- Ogris, N. Spletna Aplikacija Invazivke: Različica 4.2. Ljubljana, Gozdarski Inštitut Slovenije. 2024. Available online: https://www.invazivke.si (accessed on 15 February 2024).
- Kermavnar, J. Impacts of Forest Management on Functional Properties of Vegetation and Ecological Conditions in the Dinaric Fir-Beech Forests. Ph.D. Thesis, University of Ljubljana, Biotechnical Faculty, Ljubljana, Slovenia, 26 January 2021. [Google Scholar]
- Wagner, V.; Chytrý, M.; Jiménez-Alfaro, B.; Pergl, J.; Hennekens, S.; Biurrun, I.; Knollová, I.; Berg, C.; Vassilev, K.; Rodwell, J.S.; et al. Alien plant invasions in European woodlands. Divers. Distrib. 2017, 23, 969–981. [Google Scholar] [CrossRef]
- Florianová, A.; Münzbergová, Z. Invasive Impatiens parviflora has negative impact on native vegetation in oak-hornbeam forests. Flora 2017, 226, 10–16. [Google Scholar] [CrossRef]
- Reczyńska, K.; Świerkosz, K.; Dajdok, Z. The spread of Impatiens parviflora DC. in Central European oak forests—Another stage of invasion? Acta Soc. Bot. Pol. 2015, 84, 401–411. [Google Scholar] [CrossRef]
- Kalusová, V.; Chytrý, M.; Večeřa, M.; Svenning, J.C.; Biurrun, I.; Kintrová, K.; Agrillo, E.; Carli, E.; Ecker, K.; Garbolino, E.; et al. Neophyte invasions in European heathlands and scrub. Biol. Invasions 2023, 25, 1739–1765. [Google Scholar] [CrossRef]
- Reczyńska, K.; Orczewska, A.; Yurchenko, V.; Wójcicka-Rosińska, A.; Świerkosz, K. Changes in species and functional diversity of the herb layer of riparian forest despite six decades of strict protection. Forests 2022, 13, 747. [Google Scholar] [CrossRef]
- von Oheimb, G.; Brunet, J. Dalby Söderskog revisited: Long-term vegetation changes in a south Swedish deciduous forest. Acta Oecol. 2007, 31, 229–242. [Google Scholar] [CrossRef]
- Kleyer, M.; Bekker, R.M.; Knevel, I.C.; Bakker, J.P.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; Van Groenendael, J.M.; Klimeš, L.; Klimešová, J.; et al. The LEDA Traitbase: A database of life-history traits of the Northwest European Flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Kaplan, Z.; Danihelka, J.; Šumberová, K.; Prančl, J.; Velebil, J.; Dřevojan, P.; Ducháček, M.; Businský, R.; Řepka, R.; Maděra, P.; et al. Distributions of vascular plants in the Czech Republic. Part 12. Preslia 2023, 95, 1–118. [Google Scholar] [CrossRef]
- Babij, V.; Kutnar, L.; Marinšek, A.; Kermavnar, J. Damage and development of soil and vegetation after a wildfire in the Karst. Gozd Vestn. 2024. submitted. [Google Scholar]
- Milanović, M.; Knapp, S.; Pyšek, P.; Kühn, I. Trait–environment relationships of plant species at different stages of the introduction process. NeoBiota 2020, 58, 55–74. [Google Scholar] [CrossRef]
- Smolej, I.; Hager, H. Oak Decline in Slovenia: Endbericht Über die Arbeiten 1994; Gozdarski Inštitut Slovenije, Institut für Waldökologie: Ljubljana, Slovenia, 1995; 213p. [Google Scholar]
- Smole, I.; Kutnar, L. Vegetacijske in Rastiščne Razmere na Trajnih Raziskovalnih Ploskvah Hrasta v Sloveniji; III. del: Povzetek I. in II. dela naloge; Slovenian Forestry Institute: Ljubljana, Slovenia, 1994; 50p. [Google Scholar]
- Azarov, E. Qualitative and quantitative characteristics of oaks on permanent research plots. In Oak Decline in Slovenia: Endbericht Über die Arbeiten 1995; Smolej, I., Hager, H., Eds.; Gozdarski Inštitut Slovenije, Institut für Waldökologie: Ljubljana, Slovenia, 1995; pp. 26–34. [Google Scholar]
- Kutnar, L. Plant diversity of selected Quercus robur L. and Quercus petraea (Matt.) Liebl. forests in Slovenia. Zb. Gozdarstva Lesar. 2006, 79, 37–52. Available online: http://dirros.openscience.si/IzpisGradiva.php?id=7654 (accessed on 30 November 2023).
- Braun-Blanquet, J. Pflanzensoziologie, 3rd ed.; Springer: Berlin, Germany, 1964. [Google Scholar]
- Martinčič, A.; Wraber, T.; Jogan, N.; Podobnik, A.; Turk, B.; Vreš, B.; Ravnik, B.; Frajman, B.; Strgulc Krajšek, S.; Trčak, B.; et al. Mala Flora Slovenije: Ključ za Določanje Praprotnic in Semenk; Tehniška Založba Slovenije: Ljubljana, Slovenia, 2007; 969p. [Google Scholar]
- FloraVeg. EU—Database of European Vegetation, Habitats and Flora. Available online: www.floraveg.eu (accessed on 8 November 2023).
- Richardson, D.M.; Pyšek, P.; Rejmánek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Midolo, G.; Herben, T.; Axmanova, I.; Marcenò, C.; Pätsch, R.; Bruelheide, H.; Karger, D.N.; Aćić, S.; Bergamini, A.; Bergmeier, E.; et al. Disturbance indicator values for European plants. Global. Ecol. Biogeogr. 2023, 32, 24–34. [Google Scholar] [CrossRef]
- Lavorel, S.; Grigulis, K.; McIntyre, S.; Williams, N.S.G.; Garden, D.; Dorrough, J.; Berman, S.; Quétier, F.; Thébault, A.; Bonis, A. Assessing functional diversity in the field—Methodology matters! Funct. Ecol. 2008, 22, 134–147. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, P. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0-12.3, 2014. Available online: https://cran.r-project.org/web/packages/FD/FD.pdf (accessed on 1 December 2023).
- von Lampe, F.; Schellenberg, J. Goeveg: Functions for Community Data and Ordinations. R Package Version 0.7.2, 2024. Available online: https://cran.r-project.org/web/packages/goeveg/goeveg.pdf (accessed on 9 January 2024).
- Dengler, J.; Jansen, F.; Chusova, O.; Hüllbusch, E.; Nobis, M.P.; Van Meerbeek, K.; Axmanová, I.; Bruun, H.H.; Chytrý, M.; Guarino, R.; et al. Ecological Indicator Values for Europe (EIVE) 1.0. Veg. Classif. Surv. 2023, 4, 7–29. [Google Scholar] [CrossRef]
- Diekmann, M. Species indicator values as an important tool in applied plant ecology—A review. Basic Appl. Ecol. 2003, 4, 493–506. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 13 December 2023).





| Species | Survey 1992/93 | Resurvey 2023 | ||
|---|---|---|---|---|
| Presence | Mean Cover (%) | Presence | Mean Cover (%) | |
| Impatiens parviflora | 0 | 0 | 14 | 5.1 |
| Solidago gigantea | 0 | 0 | 12 | 7.2 |
| Erechtites hieraciifolia | 0 | 0 | 7 | 1.6 |
| Erigeron annuus | 0 | 0 | 6 | 7.5 |
| Impatiens glandulifera | 0 | 0 | 4 | 4.8 |
| Epilobium ciliatum | 0 | 0 | 4 | 1.8 |
| Veronica persica | 3 | 0.5 | 1 | 0.5 |
| Conyza canadensis | 0 | 0 | 3 | 1.4 |
| Bidens frondosa | 0 | 0 | 3 | 0.5 |
| Phytollaca americana | 0 | 0 | 2 | 0.5 |
| Oxalis fontana | 0 | 0 | 2 | 0.5 |
| Pinus strobus | 1 | 0.5 | 1 | 0.5 |
| Duchesnea indica | 0 | 0 | 1 | 0.5 |
| Juglans nigra | 0 | 0 | 1 | 0.5 |
| Matricaria discoidea | 0 | 0 | 1 | 0.5 |
| Parthenocissus quinquefolia | 0 | 0 | 1 | 0.5 |
| Populus × canadensis | 0 | 0 | 1 | 0.5 |
| Robinia pseudacacia | 0 | 0 | 1 | 0.5 |
| Solidago canadensis | 0 | 0 | 1 | 0.5 |
| Quercus rubra | 0 | 0 | 1 | 0.5 |
| Species | Survey 1992/93 | Resurvey 2023 | ||
|---|---|---|---|---|
| Presence | Mean Cover (%) | Presence | Mean Cover (%) | |
| Conyza canadensis | 1 | 0.5 | 1 | 0.5 |
| Erigeron annuus | 0 | 0 | 1 | 0.5 |
| Galinsoga parviflora | 0 | 0 | 1 | 0.5 |
| Robinia pseudacacia | 0 | 0 | 1 | 0.5 |
| Solidago canadensis | 0 | 0 | 1 | 0.5 |
| Solidago gigantea | 0 | 0 | 1 | 0.5 |
| Spiraea japonica | 0 | 0 | 1 | 0.5 |
| Ecological Factor | Impatiens parviflora | Solidago gigantea |
|---|---|---|
| Tree layer cover | 41.9 ** | 52.5 *** |
| Disturbance severity | 50.7 *** | 61.7 *** |
| Disturbance frequency | 53.9 *** | 64.3 *** |
| Soil disturbance | 56.2 *** | 51.5 *** |
| Light—relative change | 40.5 *** | 68.0 *** |
| Temperature—relative change | 2.9 ns | 1.5 ns |
| Moisture—relative change | 21.5 * | 49.1 *** |
| Nutrients—relative change | 8.2 ns | 5.5 ns |
| Site | Latitude, Longitude | Elevation (m a.s.l.) | Dominant Tree Species (% in Growing Stock 1990s) | Study Period |
|---|---|---|---|---|
| 1. Krakovski gozd | 45.8819, 15.4159 | 150 | Quercus robur (70.5%) | 1992–2023 |
| 2. Cigonca | 46.3633, 15.5816 | 260 | Quercus robur (80.1%) | 1992–2023 |
| 3. Hraščica | 46.6439, 16.2780 | 180 | Quercus robur (83.7%) | 1992–2023 |
| 4. Dobrava | 45.9426, 15.6520 | 160 | Quercus robur (87.2%) | 1993–2023 |
| 5. Polom | 45.7483, 14.8616 | 370 | Quercus robur (77.5%) | 1992–2023 |
| 6. Bojanci | 45.4938, 15.2671 | 280 | Quercus petraea (98.4%) | 1992–2023 |
| 7. Panovec | 45.9492, 13.6750 | 140 | Quercus petraea (95.4%) | 1992–2023 |
| 8. Bukovnica | 46.6996, 16.3251 | 230 | Quercus petraea (82.3%) | 1993–2023 |
| 9. Pišece | 46.0128, 15.6538 | 470 | Quercus petraea (78.1%) | 1993–2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kermavnar, J.; Kutnar, L. Habitat Degradation Facilitates the Invasion of Neophytes: A Resurvey Study Based on Permanent Vegetation Plots in Oak Forests in Slovenia (Europe). Plants 2024, 13, 962. https://doi.org/10.3390/plants13070962
Kermavnar J, Kutnar L. Habitat Degradation Facilitates the Invasion of Neophytes: A Resurvey Study Based on Permanent Vegetation Plots in Oak Forests in Slovenia (Europe). Plants. 2024; 13(7):962. https://doi.org/10.3390/plants13070962
Chicago/Turabian StyleKermavnar, Janez, and Lado Kutnar. 2024. "Habitat Degradation Facilitates the Invasion of Neophytes: A Resurvey Study Based on Permanent Vegetation Plots in Oak Forests in Slovenia (Europe)" Plants 13, no. 7: 962. https://doi.org/10.3390/plants13070962
APA StyleKermavnar, J., & Kutnar, L. (2024). Habitat Degradation Facilitates the Invasion of Neophytes: A Resurvey Study Based on Permanent Vegetation Plots in Oak Forests in Slovenia (Europe). Plants, 13(7), 962. https://doi.org/10.3390/plants13070962






