Abstract
Opuntia ficus-indica has always interacted with many phytophagous insects; two of them are Dactylopius coccus and D. opuntiae. Fine cochineal (D. coccus) is produced to extract carminic acid, and D. opuntiae, or wild cochineal, is an invasive pest of O. ficus-indica in more than 20 countries around the world. Despite the economic and environmental relevance of this cactus, D. opuntiae, and D. coccus, there are few studies that have explored volatile organic compounds (VOCs) derived from the plant–insect interaction. The aim of this work was to determine the VOCs produced by D. coccus and D. opuntiae and to identify different VOCs in cladodes infested by each Dactylopius species. The VOCs (essential oils) were obtained by hydrodistillation and identified by GC-MS. A total of 66 VOCs from both Dactylopius species were identified, and 125 from the Esmeralda and Rojo Pelón cultivars infested by D. coccus and D. opuntiae, respectively, were determined. Differential VOC production due to infestation by each Dactylopius species was also found. Some changes in methyl salicylate, terpenes such as linalool, or the alcohol p-vinylguaiacol were related to Dactylopius feeding on the cladodes of their respective cultivars. Changes in these VOCs and their probable role in plant defense mechanisms should receive more attention because this knowledge could improve D. coccus rearing or its inclusion in breeding programs for D. opuntiae control in regions where it is a key pest of O. ficus-indica.
1. Introduction
Dactylopiidae, or cochineals, is a family of scale insects that includes only the genus Dactylopius and 11 recognized species [1] that are endemic to North and South America [2,3]. An important characteristic of these insects is that they produce carminic acid, probably as a defense mechanism against predation [4,5,6]. All the species of the genus are considered obligate parasites of Cactacea with high host specificity, particularly for the genera Nopalea Salm-Dyck and Opuntia Miller [7].
Because of the high carminic acid concentration (~20–25%) of Dactylopius coccus Costa, the true cochineal, it is the only species of commercial interest for production. It is reared on Opuntia ficus-indica (L.) Miller, the cactus pear. Carminic acid is recognized as a natural dye with cosmetic, food, pharmaceutical, textile, and plastic applications [8]. In addition, it is currently used in biomedicine [9] and as a photosynthesizing pigment in solar cells [10]. In contrast, Dactylopius opuntiae Cockerell, or wild cochineal, whose carminic acid content is less than 5%, is not considered useful for obtaining this substance. Rather, it is considered the key pest of O. ficus-indica in commercial plantations in Mexico [11,12], where plants and insects are native [7,13]. Additionally, D. opuntiae is an invasive pest in at least 20 countries in America, Europe, Africa, and Asia [14,15,16], where O. ficus-indica was adopted or naturalized and became one of the most important cultivated cactus species in the world because of its economic, environmental, and ecological benefits [13,14,17,18].
From a scientific perspective, most D. coccus research has focused on the basic biology of the species and the quest to understand the mechanisms of carminic acid production and its possible physiological or ecological functions [4,19,20]. On the other hand, research on D. opuntiae has focused on control tactics because it is a key pest of O. ficus-indica [14,15,21,22]. The different cultivars of O. ficus-indica used as hosts of both Dactylopius species are likely to have particular physical and chemical characteristics, as well as volatile organic compounds (VOCs) that influence the trophic plant–insect and plant–pest–natural enemy relationship, as has been shown in other models of tritrophic interactions where volatiles cause positive or negative responses in terms of attraction and establishment of insects of the same or different species [23,24].
Volatile organic compounds (VOCs) are synthesized as products of plant metabolism, and they are emitted into the environment [25] in response to biotic complexes or abiotic stresses [23,26]. These VOCs and essential oils are released from the leaves, flowers, and fruits into the atmosphere and from the roots into the soil [27,28]. This set of volatiles, essential in the defense mechanisms of plants against herbivores or in interspecific communication [23,24,27], is called the volatilome, and its analysis is carried out by gas chromatography-mass spectrometry (GC-MS) [29]. This is a field that is continuously growing with the development of analytical and data-processing methods [30]. In this regard, some research has been carried out on VOCs of O. ficus-indica emanating from cladodes, flowers, fruits, and the oils of its seeds [31,32,33,34,35,36]. These studies concluded that VOC composition is a function of the geographical area, species or cultivar, plant structure, state of development, and season, among other factors. However, none of these relatively recent papers included interaction with any of the important Dactylopius species, nor did they relate the production of VOCs to insect infestation. To our knowledge, there is only one study that evaluated VOCs in O. ficus-indica cladodes uninfested and infested by D. coccus [37]. This study reported eight types of compounds in uninfested cladodes and nine in cladodes infested by the insect. Furthermore, no other work is known to have explored VOCs of either Dactylopius species.
Because plant VOCs play an important role in interactions between insects and other organisms, e.g., pathogens or predators, and parasitoids [23,24,38], as well as in the plant’s response to insect attacks [39], the objectives of this work were (1) to determine the VOCs of D. coccus and D. opuntiae feeding on O. ficus-indica and (2) to establish the changes in the composition and proportion of VOCs in cladodes of O. ficus-indica uninfested and infested with Dactylopius. This information could contribute to understanding the variation between cultivars of both species of insects and to exploring the potential of the biological functions that these compounds play in interspecies communication.
2. Results
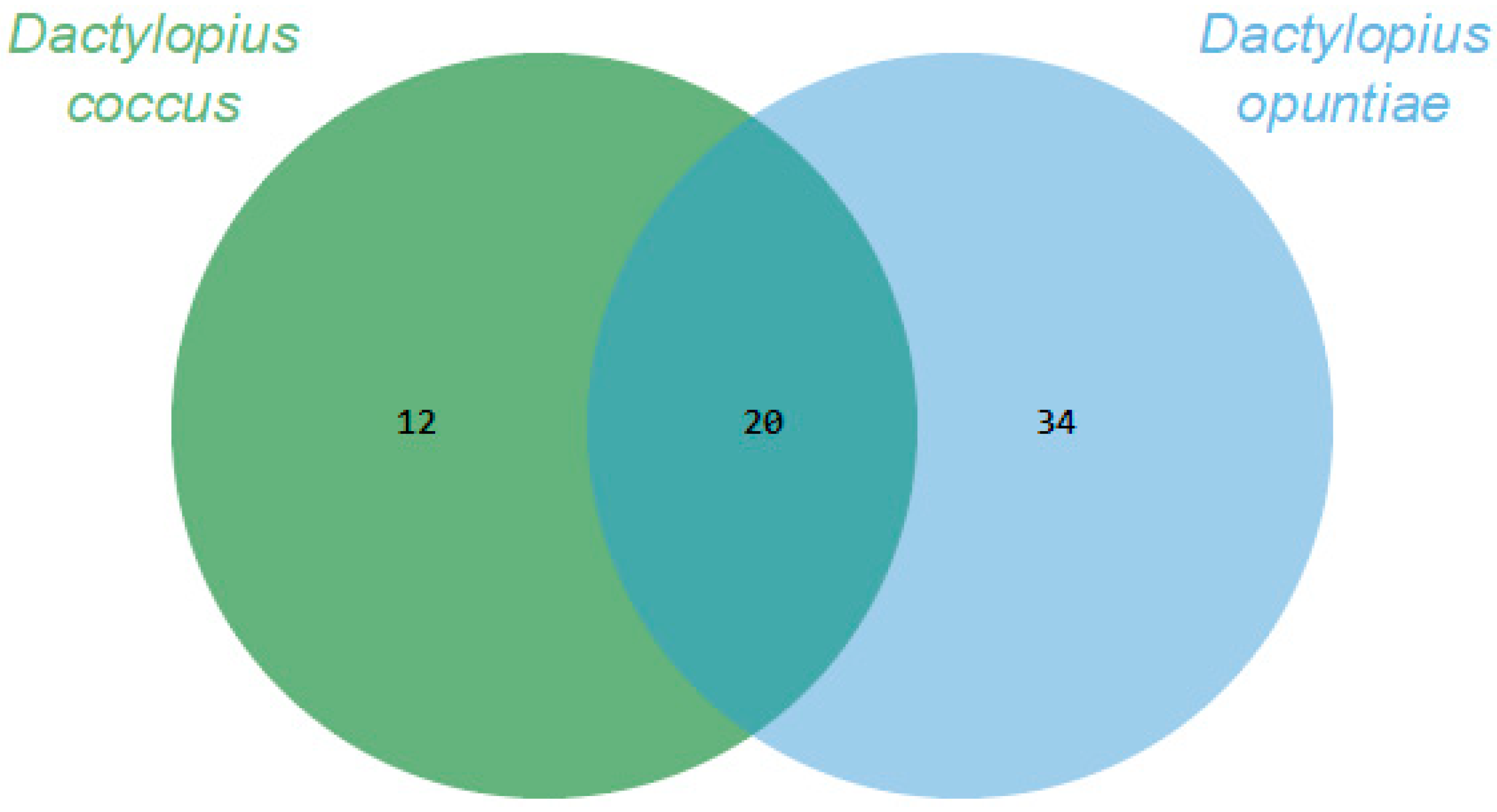
Through essential oils, it was possible to recover and identify about 80% and 90% of the volatile organic compounds (VOCs) of D. coccus and D. opuntiae, respectively. The Dactylopius species had 20 VOCs in common. In addition, 12 and 34 VOCs were specifically produced by D. coccus and D. opuntiae, respectively (Figure 1). Thus, the volatilome of each species was 32 or 54 compounds, and the proportion of each compound varied greatly between species (Table 1). The VOCs belonged to eight chemical groups, of which three had the highest relative abundance. Carboxylic acids and their derivatives were the most important group, accounting for 59.28% and 78.29% of the VOC abundance for D. coccus and D. opuntiae, respectively. The second group was alcohols only for D. coccus (12.15%), and the third group was aldehydes with 5.8% and 7.68% of the relative abundance for D. coccus and D. opuntiae, respectively. The alkanes recovered were less than 2.5% for both species. The remaining four groups of recovered compounds (ether, terpenes, ketones, and alkenes) had less than 0.55% relative abundance per group (Table 1).
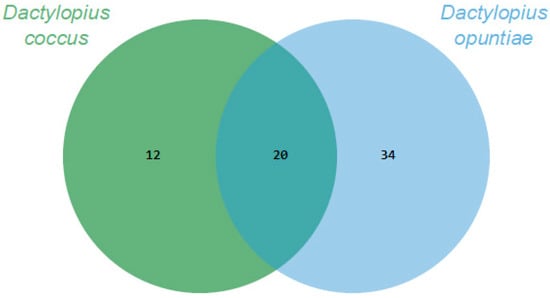
Figure 1.
Comparison of the Dactylopius volatilomes using Venn diagrams based on the number of Volatile organic compounds (VOCs) obtained through essential oils for each Dactylopius species.

Table 1.
Volatile organic compounds (VOCs) obtained through the essential oils of each Dactylopius species.
As mentioned above, the number and abundance of volatiles in each group of compounds also varied greatly in each Dactylopius species. For example, in the carboxylic acids and their derivatives, tetradecanoic acid was the most abundant in both species, but decanoic acid, lactic acid, and dodecanoic acid presented greater relative abundance in D. coccus. On the other hand, for D. opuntiae, 2-ethylhexanoic acid and cis-5-dodecenoic acid were detected only in this species in greater relative abundance. Hexadecanoic acid, (Z,Z)-9,12-octadecadienoic acid, (Z)-9-octadecenoic acid, and octadecanoic acid occurred in both species, but their abundance differed considerably between species; again, they were more abundant for D. opuntiae (Table 1).
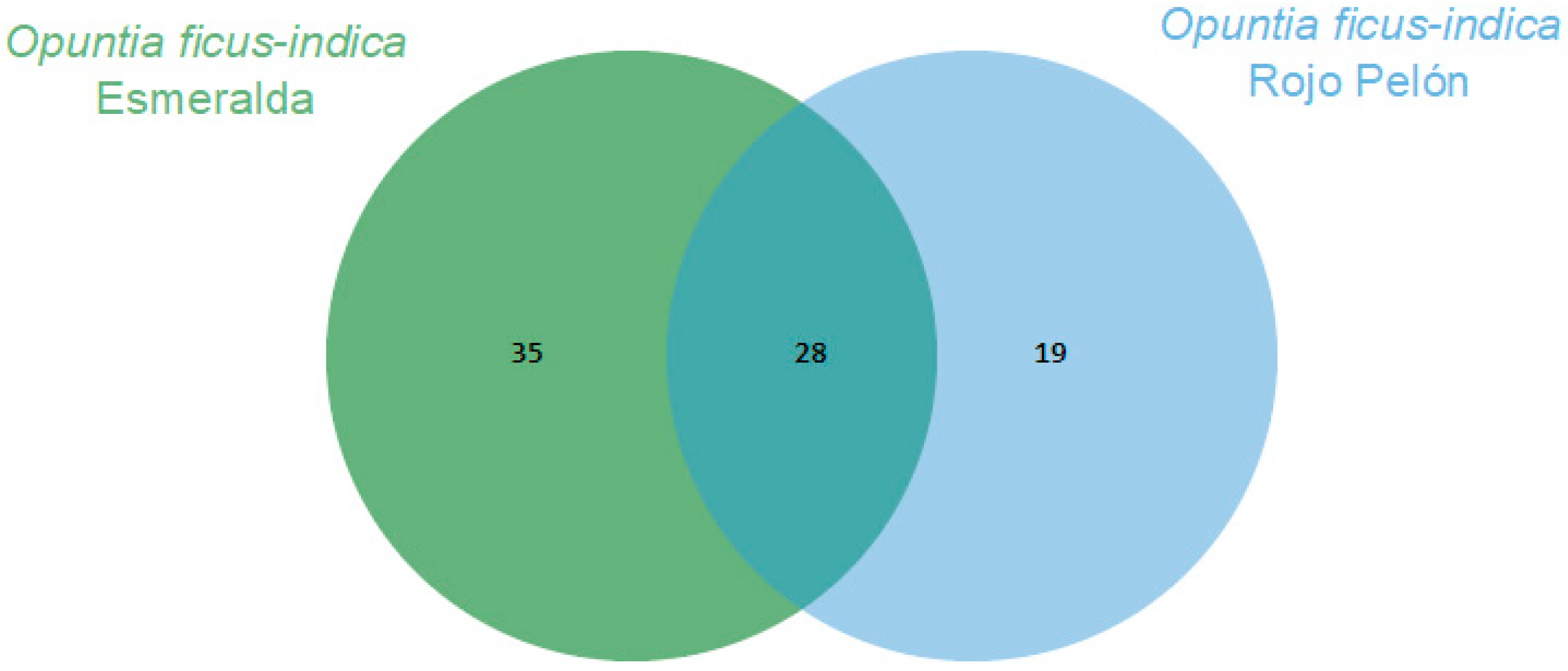
The Esmeralda and Rojo Pelón cultivars had VOC production profiles that differed before and after Dactylopius infestation. In both cultivars, 28 VOCs were commonly produced and identified. In addition, 35 specific compounds were identified in Esmeralda and 19 in Rojo Pelón (Figure 2). After infestation by each Dactylopius species in the respective O. ficus-indica cultivar, a contrasting difference occurred between uninfested and infested cladodes of each cultivar (Table 2). The changes were not only in the number of VOCs but also in their abundance and variation. Sometimes they decreased, sometimes they increased, sometimes some VOCs were no longer detected, and of course there were also some de novo compounds (Table 2). After infestation by D. coccus, the Esmeralda cultivar increased the number of volatiles from 63 (uninfested) to 87, of which 48 were produced de novo and belonged to nine chemical groups. In the case of Rojo Pelón D. opuntiae, uninfested cladodes produced 47 VOCs, and after infestation, they decreased to 38, 13 of which were identified as de novo, belonging to seven chemical groups (Table 2, Figures S1 and S2).
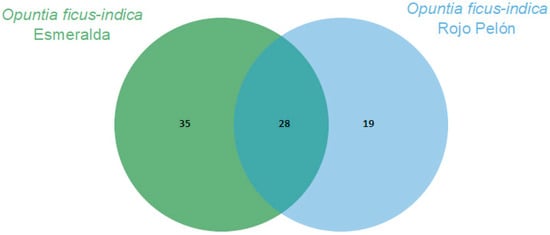
Figure 2.
Comparison of volatilomes of the Opuntia ficus-indica uninfested cladodes of each cultivar using a Venn diagram, based on the number of VOCs obtained through essential oils.

Table 2.
Volatilomes of Opuntia ficus-indica (OFI) cultivars before and after infestation by Dactylopius species.
Although there was an enormous variation between the number and proportion of VOCs before and after infestation, it was observed that four chemical groups maintained the highest abundance in both infested cultivars. These groups were (a) carboxylic acid and derivatives, (b) terpenes, (c) alcohols, and (d) aldehydes and their derivatives. Another group, the heterocycles, was only abundant for the uninfested Rojo Pelón cultivar (8.91%), but after D. opuntiae infestation, it decreased to less than 1.4%. The rest of the recovered chemical groups (ethers, ketones, aromatic derivatives, and alkanes) were less than 1.16% of the relative abundance per group in either cultivar infested by the respective Dactylopius species. Two of these groups (ethers and aromatic derivatives) were not detected in the infested Rojo Pelón cultivar (Table 2).
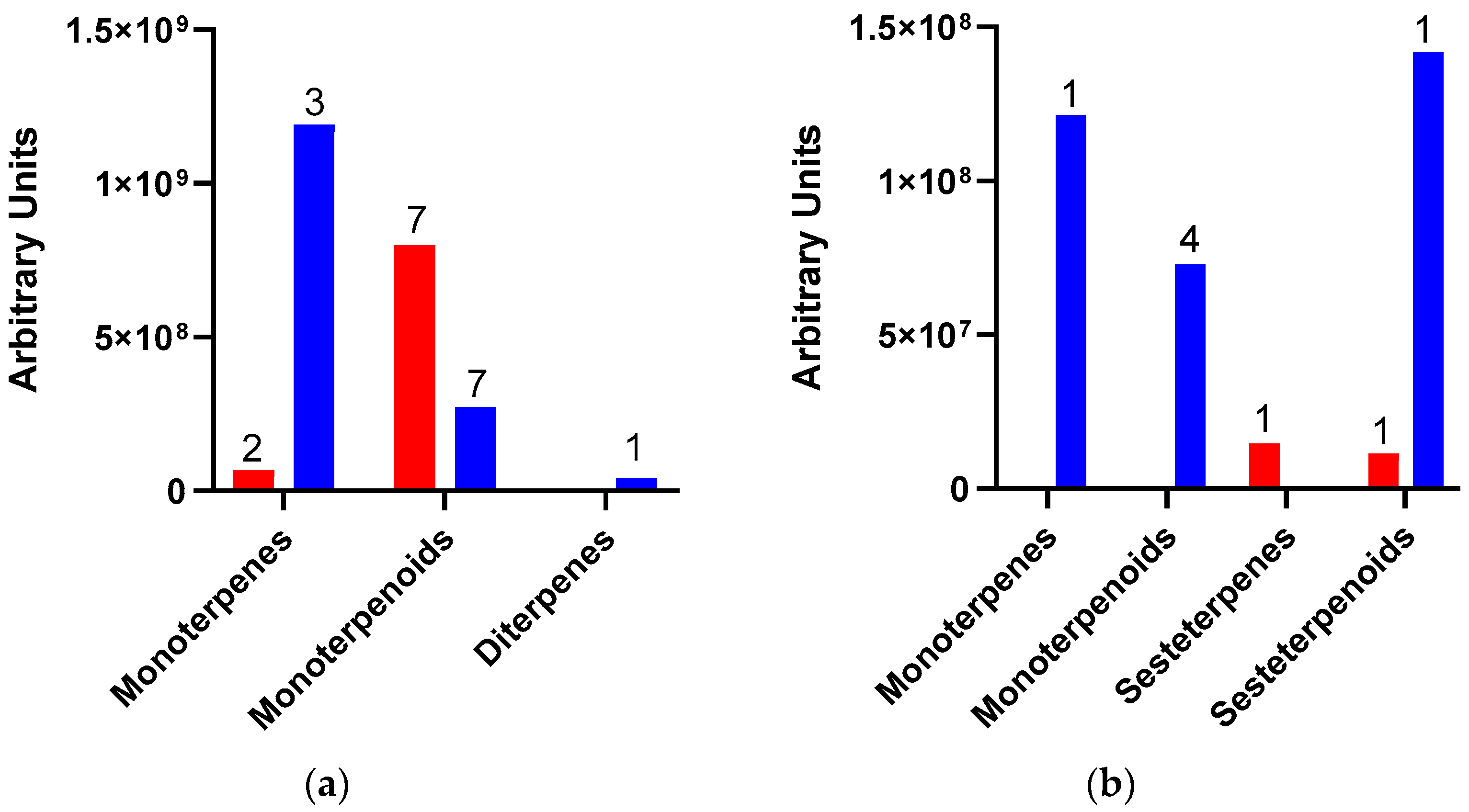
As indicated above, because of Dactylopius infestation in each cultivar, there were many changes in the relative abundance of compounds and the production of some de novo compounds. The de novo compounds were mostly of low relative abundance (equal to or less than 1.0%), except for some terpenes and alcohols. For example, in the uninfested Rojo Pelón cultivar, the relative abundance of terpenes was around 0.8%, but this relative abundance of terpenes changed to 15.5% after D. opuntiae infestation. On the other hand, the relative abundance of terpenes in the Esmeralda cultivar decreased from 18 to 13.9% due to D. coccus infestation (Table 2). The amount and type of terpenes were different between infested O. ficus-indica cultivars, but monoterpenes or their derivatives predominated in both cases (Figure 3).
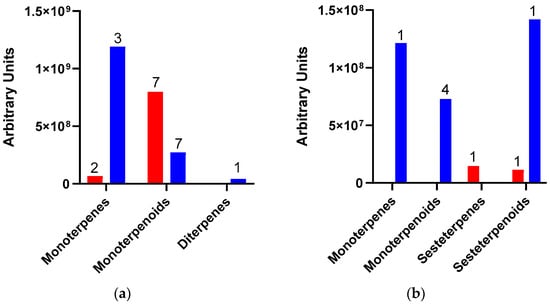
Figure 3.
Amount and type of terpenes released by Opuntia ficus-indica (OFI) after Dactylopius infestation. (a) OFI Esmeralda-D. coccus; (b) OFI Rojo Pelón-D. opuntiae. The red columns represent uninfested cladodes, and the blue columns represent cladodes infested by each Dactylopius species. Data are presented as means of the peak area of each terpene (grouped by type and number of compounds).
The terpenes linalool oxide, trans-linalool oxide, and the alcohol 3,7,11,15-tetramethyl-2-hexadecenol reached a relative abundance of 5.06%, 5.7%, and 1.8% in the Esmeralda cultivar infested by D. coccus. On the other hand, the terpenes linalool, geraniol, and the alcohol 3,7,11,15-tetramethyl-2-hexadecenol registered 5.6%, 1.84%, and 3.5% of the relative abundance in the Rojo Pelón cultivar infested by D. opuntiae, respectively. Also, p-vinylguaiacol increased 2.3% in relative abundance after D. opuntiae infestations (Table 2).
3. Discussion
Previous assays of Dactylopius VOCs extraction, such as Headspace (HS-SPME) and extraction by Autosampler Headspace coupled to CG-MS (HS-CG-MS), did not provide the results expected for GC-MS analysis. Thus, to identify the volatiles from Dactylopius and its cultivar hosts, we preferred to do so using their essential oils. Essential oils were obtained by the hydrodistillation method (Table S1), which is frequently used to obtain essential oils from plants that contain low-vapor pressure compounds or low-volatile compounds. This technique is also used for concentrating compounds with lower concentrations in the essential oil and allows working with a larger sample mass than microextraction techniques, which can potentially improve the characterization of insect VOCs [29].
In the volatilome of D. coccus and D. opuntiae, 32 and 54 VOCs were identified for each species, respectively. To our knowledge, neither of these volatilomes had been reported previously, and this may be the first contribution to this work. By their composition, these VOCs corresponded to eight different chemical groups, but there were three groups of greater abundance. These were (a) carboxylic acids and their derivatives, 59.28% and 78.29% abundance for D. coccus and D. opuntiae, respectively; (b) alcohols, which were abundant only for D. coccus (12%); and (c) aldehydes, 5.8% and 7.68% abundance for D. coccus and D. opuntiae, respectively (Figures S3 and S4). This composition could be one of the reasons that results were not obtained with the HS-SPME and HS-CG-MS techniques. The VOCs of Dactylopius species are mostly fatty acids, some of which may be part of the fat content of the insects or of the complexity of their waxy coat [40,41]. In fact, each VOC in those groups may have more than one role in structure, function, metabolism, and probably in intra- or interspecific communication. For example, D. coccus produces a sex pheromone [42], and D. opuntiae is suspected to do so as well [43]. Regarding tetradecanoic acid, which is one of the most abundant VOCs for both species of Dactylopius, and hexadecanoid acid, relevant to D. opuntiae, they have many functions in insect metabolism. One of these is to participate in the metabolic pathways of sex pheromones of some Lepidoptera, such as Spodoptera lottoralis Boisduval and Plodia interpunctella Hubner [44,45], but none of these compounds appear to have relevance in the pheromones of Coccoidea [46], which is the superfamily to which the Dactylopiidae belong. The methodology for identifying insect pheromones begins with live females at a particular moment of maturity and sexual behavior, and so much work remains to be carried out in order to decipher the main functions of the VOCs that turned out to be more abundant, which could lead to novel acids with shorter chains and perhaps more specific for each Dactylopius species.
The volatilomes of the Esmeralda and Rojo Pelón cultivars were different before and after Dactylopius infestation. The variation in compound production in cladodes of both cultivars prior to infestation (by Dactylopius) may be specific to each cultivar, as variations of other bioactive and volatile compounds have been reported in different cultivars of O. ficus-indica [31,35,47]. However, variation in the number and abundance of VOCs within each cultivar after infestation can be attributed to D. coccus or D. opuntiae feeding on its corresponding cultivar host, as has been demonstrated in other plants where the change in production of VOCs, particularly terpenes and sesquiterpenes, was directly associated with phytophagous insect feeding [23,24,48,49].
In the volatilomes of the Esmeralda and Rojo Pelón, before or after Dactylopius infestation, four chemical groups were identified as the most abundant: (a) carboxylic acid and derivatives, (b) terpenes, (c) alcohols, and (d) aldehydes and derivatives (Figures S5 and S6). The structural composition of the host, particularly the quantity of waxes, could be related to the abundance of some of these VOCs in both cultivars [47,50]. This suggestion is related to the anatomical and physiological adaptations of cacti to develop in arid environments, such as a thick and impermeable epidermis covered by a layer of waxy cuticle, a hypodermis with chollenchyma, plenty of cells with mucilage distributed in the parenchyma, and crassulaceae acid metabolism (CAM), among other characteristics [50]. Of the first and most abundant chemical groups (a), it is probable that we should mention methyl salicylate, which increased in abundance after infestation by D. coccus (about 5%) in the Esmeralda cultivar. The same compound was identified de novo in the Rojo Pelón cultivar infested by D. opuntiae, although it was low in abundance (0.3%). Methyl salicylate is a phenolic compound that has been reported to be an herbivore-induced plant volatile (HIPV) [49,51,52]. Some of these HIPVs can induce direct defense against the phytophagous insect and indirect defense by attracting their natural enemies. It is also useful for communication among plants damaged by phytophagy and others that are not yet damaged. For example, methyl salicylate emitted by plants with phytophagous mite damage was attractive to Phytoseiulus persimilis Athias-Henriot (Phytoseiidae) [51,52]. In the same way, it was observed that emission of this compound, after damage by psyllids in pear trees, was attractive to the predatory bug Anthocoris nemoralis F. (Hemiptera: Anthocoridae) [53].
In general, a slight decrease in terpene abundance (18 to 14%) was observed after D. coccus infestation, but a considerable increase (0.8 to 15%) occurred after D. opuntiae infestation. In the Esmeralda cultivar, β-linalool abundance decreased from 5.0 to 0.3%, but linalool oxide and trans-linalool oxide increased to 5.0 and 5.7%, respectively. On the other hand, in the cultivar Rojo Pelón infested by D. opuntiae, five de novo terpenes were identified, of which the most abundant was linalool (5.6%). Terpenes are one of the most studied groups of HIPVs, and it has been shown that some of them have a relevant role in the direct defense system against phytophages, and some volatile terpenes constitute indirect defenses of plants as they attract natural enemies such as predators and parasitoids [23,24,27,48,49].
Linalool is a monoterpene that occurs naturally in flowers and aromatic plants, but it is also produced in response to feeding by phytophagous insects, and it is part of the indirect defenses of plants [54]. For example, an increase in linalool production in tobacco plants caused by feeding Lepidoptera larvae increased the rate of egg predation and decreased the oviposition of another Lepidoptera [55]. Linalool also increased due to phytophages feeding on corn, bean, cotton, and potato plants [23], or by a zoophytophagous mirid feeding on pepper plants, and favored the action of natural enemies of their pests [49]. This can suggest that significant changes in the abundance of methyl salicylate from the above group and terpenes, particularly linalool, are probably related to each Dactylopius species feeding on its corresponding cultivar host.
The alcohol of greatest abundance and change was p-vinylguaiacol. This compound is common in plants and is part of many essential oils. In addition, it can be found in the guts of some insects, probably through the process of lignin degradation [56]. Regarding secondary plant defenses due to damage by phytophagous insects, p-vinylguaiacol stimulated the ovipositional behavior of the natural enemy Coleomegilla maculata [57], and it was also a deterrent to the oviposition of the cerambycid Monochamus alternatus [56]. Therefore, it is suggested that some changes in p-vinylguaiacol abundance may be a consequence of Dactylopius feeding.
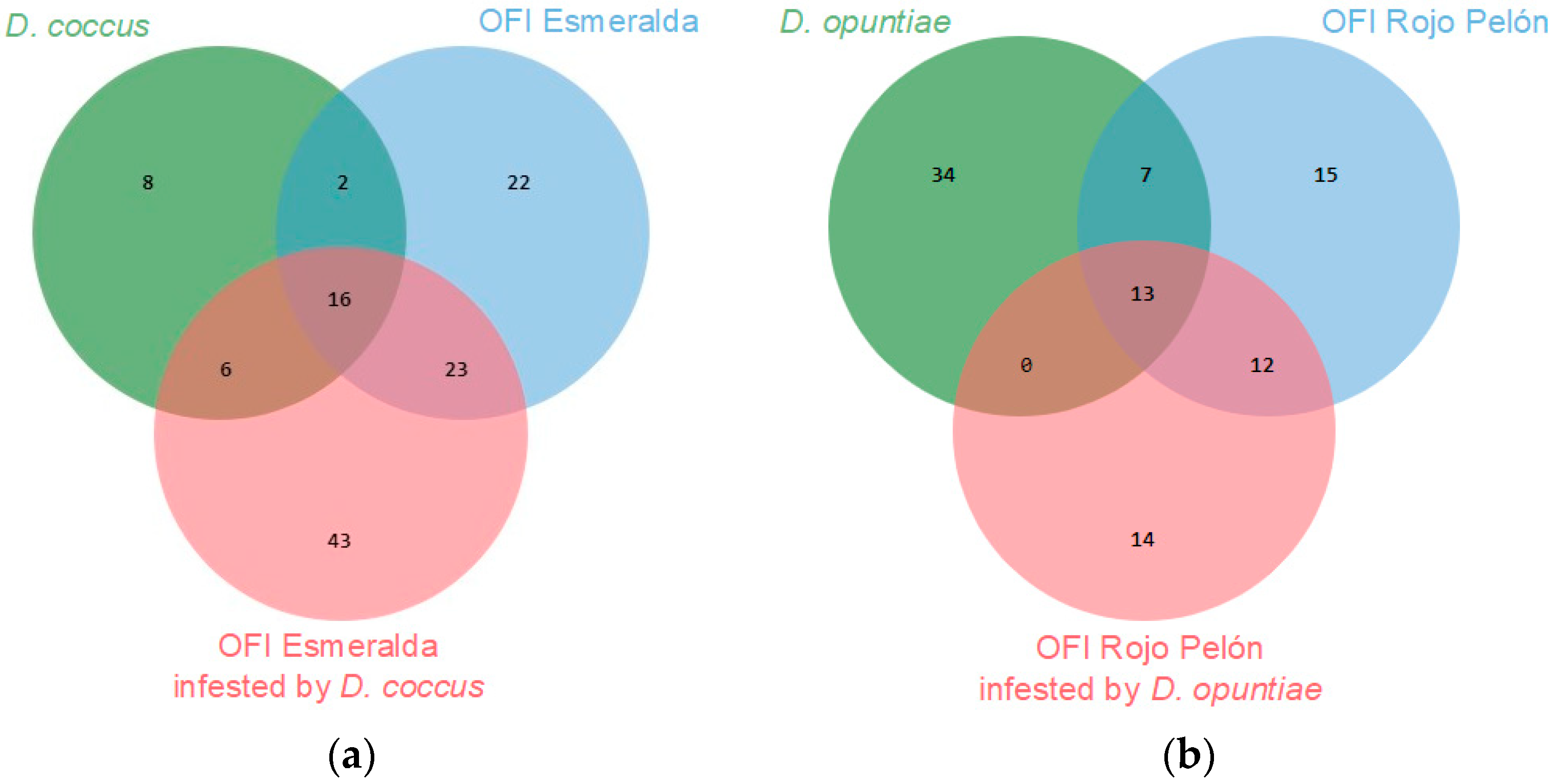
In this work, 66 VOCs of both Dactylopius species were identified, and 125 of the Esmeralda and Rojo Pelón cladodes were infested by D. coccus and D. opuntiae, respectively. A proportion of VOCs were commonly produced in both insect species or cultivars, but others were specific to each species or cultivar (Figure 4). This is a first approach to the diversity of VOCs produced by O. ficus-indica and the changes that occur due to D. coccus and D. opuntiae feeding on cultivars suitable for the development of each Dactylopius species. More time and work will now be needed to understand the functions performed by the most relevant compounds in these interactions.
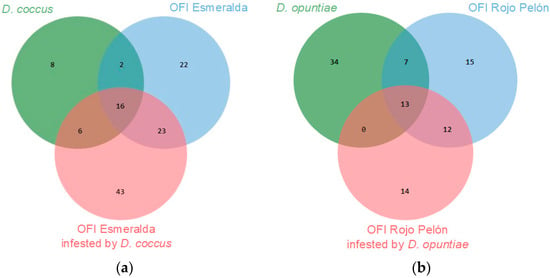
Figure 4.
Number of VOCs, obtained through its essential, identified as common or de novo compounds between uninfested and infested O. ficus-indica (OFI) cultivars, (a) by Dactylopius coccus, (b) by Dactylopius opuntiae.
If knowledge of the interaction is improved, for example, if it is confirmed that some terpenoids favor the direct or indirect defenses of O. ficus-indica against D. coccus or D. opuntiae, this information could be considered in breeding programs. These programs could be aimed at improving the rearing of D. coccus or inducing resistance to D. opuntiae. In this regard, breeding programs for O. ficus-indica resistant to D. opuntiae have already been developed in Brazil and Morocco, and these have focused on physical and biochemical defense mechanisms [15,21,58]. For example, selecting cultivars with high concentrations of calcium oxalates can physically and biochemically limit phytophagous insects [59,60]. However, there are no known breeding programs for O. ficus-indica that consider the abundance of terpenes in cultivars and the response this can trigger in the plant’s direct or indirect defenses. This mechanism would be classified as biochemical defense, and measuring terpenes in different cultivars could improve the direction and understanding of the response.
Besides, SIMPER analysis (Tables S2–S5) showed the components that are typical of each Dactylopius species and its hosts; these contribute a low percentage of each sample, so their contribution to the dissimilarity is low. This observation highlights the need to better understand the interaction between O. ficus-indica and Dactylopius because it can increase the possibilities of making proposals for sustainable management in the production of D. coccus or in the control of D. opuntiae.
4. Materials and Methods
4.1. Chemicals
The reagents used in this study were N, O-bis(trimethylsilyl) trifluoroacetamide (BSTFA), trimethylsilyl chloride (TMCS), boron trifluoride methanol solution (Sigma-Aldrich, St. Louis, MO, USA), and ethylic ether (JT Baker, Deventer, Holland).
4.2. Insects and Uninfested and Infested O. ficus-indica Cultivars
Dactylopius coccus and Opuntia ficus-indica Esmeralda cultivars (infested and uninfested) were originally obtained from a local provider in Jerez, Zacatecas, Mexico. Dactylopius opuntiae and O. ficus-indica Rojo Pelón cultivars (infested and uninfested) were collected from an experimental field at Colegio de Postgraduados, Campus San Luis Potosí (Salinas, SLP). These cactus pear cultivars were selected with the knowledge that each one is favorable for the development of the respective Dactylopius species [58]. The taxonomic identity of Dactylopuis species was corroborated by S. J. Méndez-Gallegos using De Lotto (1974) [40] and Ferris (1955) keys [61]. To increase material for the samples and analyses, D. coccus and D. opuntiae colonies were reared on the respective cultivars mentioned under greenhouse conditions (15 ± 2 °C, 22 ± 2 °C, and 50% RH).
4.3. Essential Oil of Dactylopius Species and Hosts
One hundred grams of adult females previous to the reproduction stage of D. coccus (80 to 85 d old) and D. opuntiae (30 to 35 d old) with their protective coverings (secretion substances) and 1000 g of infested and uninfested O. ficus-indica cladodes were used independently to obtain their essential oils by hydrodistillation. The Dactylopius species were manually separated from their hosts just before hydrodestillation, and the O. ficus-indica cladodes were cut into cubes just before hydrodestillation. The VOCs, which are components of essential oils, were obtained at boiling water temperature and extracted from the condensed water by liquid–liquid extraction with ethyl ether. The solvent was distillated, and the residual water was removed from the organic phase with anhydrous sodium sulfate. Each sample was then concentrated (to 1 mL) at 40 °C under vacuum, and the residual solvent was eliminated from each sample at atmospheric pressure at 0 °C.
4.4. Derivatization for Alcohol Detection
Essential oils were diluted to 2% in 500 µL heptane and introduced into a 10 mL microwave reaction tube with a gasket. Then, 100 µL of BSTFA/TMCS solution (9:1 v/v) was added to the same tube as a silanizing agent. The mixture was reacted at 90 °C under microwave irradiation (250 W microwave power) for 10 min using the Discover System 908,005 (CEM Corporation, Matthews, NC, USA) with autogenous pressure.
4.5. Derivatization for Aldehydes and Carboxylic Acid Detection
Essential oils were diluted to 2% in 500 µL heptane and introduced into a 10 mL microwave reaction tube with a gasket. Then, 500 µL of boron trifluoride (14% in methanol solution) was added to the same tube. The mixture reacted at 90 °C under microwave irradiation (250 W microwave power) for 10 min using the Discover System 908,005 with autogenous pressure.
4.6. Essential Oil GS-MS Analysis
Samples without derivatization were diluted to 2% in heptane, using 1 µL of each sample for the analysis, and each sample was analyzed in triplicate. GC-MS analysis was performed using a 7802A Network GC System coupled to a 5977E Network mass selective detector (MSD).
The separation was performed using an HP-5 capillary column (0.25 mm i.d., 30 mm, 0.25 mm film thickness) (J&W, Folsom, CA, USA). The injector was operated in splitless mode at 300 °C, with a flow of 1.0 mL/min, and the oven temperature was programmed to 40 °C for 3 min, and then heated at 3 °C/min to 300 °C with a holding time of 5 min at the final temperature. The MSD was operated at 70 eV; the ion source was set at 150 °C and the transfer line at 300 °C. VOCs were identified by interpreting their mass spectra as fragmentation in the mass range of 15 to 800 atomic mass units. The software MassHunter (Agilent B.07.01.1805, Santa Clara, CA, USA) was used for data recording. The compounds were identified by comparing the obtained mass spectra with those of reference compounds from the National Institute of Standards and Technology (NIST11) and Wiley 09. The identities of the compounds were confirmed by the Kovats retention index calculated for each peak with reference to the n-alkane standards (C7–C38) running under the same conditions.
4.7. Statistical Analysis
The relative percentage of each metabolite was calculated considering the peak area obtained by GC-MS of each metabolite in relation to the total area of peaks analyzed. The data represent the mean of the relative percentage of three repeats ± SD. Metabolites grouped by type for each essential oil were compared with the Mann–Whitney U test, considering the peak area of each metabolite and a p ≤ 0.05. The data in the graphics were expressed as the median and range of each group. GraphPad Prism 5 was used to perform the analysis. Venn diagrams were constructed using an online tool (http://jvenn.toulouse.inra.fr/app/example.html, accessed on 23 November 2023) [62]. PAST statistical software (version 4.09) was used to perform the SIMPER analysis [63].
5. Conclusions
This work presents an approach to better understanding the interaction between O. ficus-indica, D. coccus, and D. opuntiae by identifying volatile compounds in their essential oils. The abundance and proportion of VOCs of D. coccus and D. opuntiae were determined in the Esmeralda and Rojo Pelón cultivars, viable for the development of each insect species, respectively. Differential VOC production due to infestation by each Dactylopius species in each cultivar was also identified. Changes in methyl salicylate, terpenes, and p-vinylguaiacol and their likely role in plant defense mechanisms should receive more attention because they could contribute to the development of proposals to improve D. coccus rearing or for the control of D. opuntiae in those regions of the world where it is a key pest of O. ficus-indica.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13070963/s1, Table S1 shows the yields of essential oils of Dactylopius species and Opuntias varieties. Tables S2–S5 show the SIMPER analysis. Figures S1 and S2 show O. ficus-indica with Dactylopius species relationships by Venn diagrams. Figures S3–S6 show compound groups of Dactylopius species and O. ficus-indica cultivars.
Author Contributions
Conceptualization, M.M.G.-C. and S.d.J.M.-G.; methodology, E.G.-P., M.M.G.-C. and S.d.J.M.-G.; formal analysis, E.G.-P., E.R.-L., M.M.G.-C. and S.d.J.M.-G.; investigation, E.G.-P., E.R.-L., M.M.G.-C., S.d.J.M.-G., J.A.M.-R., J.C.P.-H., Á.B.-V. and A.F.-V.; writing—original draft preparation, E.R.-L. and S.d.J.M.-G.; writing—review and editing, E.G.-P., M.M.G.-C., J.A.M.-R., J.C.P.-H., Á.B.-V. and A.F.-V.; supervision, M.M.G.-C. and S.d.J.M.-G.; funding acquisition, M.M.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was financially supported by CONAHCYT from México (Ciencia de Frontera, funding number: 320298).
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank CONAHCYT for a graduate fellowship for Esperanza García-Pascual (CVU: 1146133). We also thank Vicente Rodríguez González (IPICYT) for access to GC-MS equipment and Brayan Arias-Alvarez, Juan J. Rodríguez-Silva, and María G. Ortega Salazar for the technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- García-Morales, M.; Denno, B.D.; Miller, D.R.; Miller, G.L.; Ben-Dov, Y.; Hardy, N.B. ScaleNet: A literature-based model of scale insect biology and systematics. Database 2016, 2016, bav118. [Google Scholar] [CrossRef] [PubMed]
- Van-Dam, A.R.; Martinez, L.P.; Chavez, A.J.; May, B.P. Range wide phylogeography of Dactylopius coccus (Hemiptera: Dactylopiidae). Ann. Entomol. Soc. Am. 2015, 108, 299–310. [Google Scholar] [CrossRef]
- Campana, M.G.; Robles, N.M.; Tuross, N. America’s red gold: Multiple lineages of cultivated cochineal in Mexico. Ecol. Evol. 2015, 5, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Barreto-García, O.A.; Rodríguez-Leyva, E.; Lomeli-Flores, J.R.; Vanegas-Rico, J.M.; Vigueras, A.L.; Portillo, L. Laetilia coccidivora feeding on two cochineal insect species, Does the prey affect the fitness of the predator? BioControl 2020, 65, 727–736. [Google Scholar] [CrossRef]
- Eisner, T.; Nowicki, S.; Goetz, M.; Meinwald, J. Red Cochineal Dye (Caminic Acid): Its Role in Nature. Science 1980, 208, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, S.; Rodríguez-Leyva, E.; Aranda-Ocampo, S.; Santillán-Galicia, M.T.; Hernández-López, A.; Guzmán-Franco, A.W. Bacteria associated with carminic acid metabolism in the intestinal tract of three predators of Dactylopius opuntiae. Entomol. Exp. Appl. 2023, 172, 183–192. [Google Scholar] [CrossRef]
- Chávez-Moreno, C.K.; Tecante, A.; Casas, A. The Opuntia (Cactaceae) and Dactylopius (Hemiptera: Dactylopiidae) in Mexico: A historical perspective of use, interaction and distribution. Biodivers. Conserv. 2009, 18, 3337–3355. [Google Scholar] [CrossRef]
- Borges, M.E.; Tejera, R.L.; Díaz, L.; Esparza, P.; Ibáñez, E. Natural dyes extraction from cochineal (Dactylopius coccus). New extraction methods. Food Chem. 2012, 132, 1855–1860. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Gras, C. The “Carmine Problem” and Potential Alternatives. In Handbook on Natural Pigments in Food and Beverages; Woodhead Publishing: Cambridge, UK, 2016; pp. 385–428. [Google Scholar] [CrossRef]
- Sánchez-García, M.A.; Bokhimi, X.; Velázquez, S.; Jiménez-González, A.E. Dye-Sensitized solar cells prepared with Mexican pre-hispanic dyes. J. Nanotechnol. 2018, 2018, 1236878. [Google Scholar] [CrossRef]
- Vanegas-Rico, J.M.; Rodríguez-Leyva, E.; Lomeli-Flores, J.R.; González-Hernández, H.; Pérez-Panduro, A.; Mora-Aguilera, G. Biology and life history of Hyperaspis trifurcata feeding on Dactylopius opuntiae. BioControl 2016, 61, 691–701. [Google Scholar] [CrossRef]
- Vanegas-Rico, J.M.; Lomeli-Flores, J.R.; Rodríguez-Leyva, E.; Mora-Aguilera, G.; Valdez, J.M. Natural enemies of Dactylopius opuntiae (Cockerell) on Opuntia ficus-indica (L.) Miller in Central Mexico. Acta Zool. Mex. 2010, 26, 415–433. [Google Scholar] [CrossRef]
- Griffith, M.P. The origins of an important cactus crop, Opuntia ficus-indica (Cactaceae): New molecular evidence. Am. J. Bot. 2004, 91, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, G.; Nucifora, S.; Russo, A.; Suma, P. Dactylopius opuntiae, a new prickly pear cactus pest in the Mediterranean: An overview. Entomol. Exp. Appl. 2019, 167, 59–72. [Google Scholar] [CrossRef]
- Torres, J.B.; Giorgi, J.A. Management of the false carmine cochineal Dactylopius opuntiae (Cockerell): Perspective from Pernambuco state, Brazil. Phytoparasitica 2018, 46, 331–340. [Google Scholar] [CrossRef]
- Mendel, Z.; Protasov, A.; Vanegas-Rico, J.M.; Lomeli-Flores, J.R.; Suma, P.; Rodríguez-Leyva, E. Classical and fortuitous biological control of the prickly pear cochineal, Dactylopius opuntiae. Biol. Control 2020, 142, 104157. [Google Scholar] [CrossRef]
- Silva, M.A.; Albuquerque, T.G.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.B.; Costa, H.S. Opuntia ficus-indica (L.) mill.: A multi-benefit potential to be exploited. Molecules 2021, 26, 951. [Google Scholar] [CrossRef] [PubMed]
- Kiesling, R. Origen, domesticación y distribución de Opuntia ficus-indica. J. Prof. Assoc. Cactus Dev. 1998, 3, 50–60. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, F.C.; García-Gil, F.; Rojas-Martínez, A.; Hernández-Martínez, S.; Lanz-Mendoza, H. Carminic acid dye from the homopteran Dactylopius coccus hemolymph is consumed during treatment with different microbial elicitors. Arch. Insect Biochem. Physiol. 2003, 54, 37–45. [Google Scholar] [CrossRef]
- Bravo-Vinaja, A.; Méndez-Gallegos, J. Emerging trends in scientific research on Dactylopius coccus Costa (Hemiptera: Dactylopiidae), carminic acid and its derivatives: A bibliometric analysis. Agric. Soc. Desarro. 2023, 20, 139–165. [Google Scholar] [CrossRef]
- Sabbahi, R.; Hock, V. Control of the prickly pear cochineal, Dactylopius opuntiae (Cockerell), in Morocco: An overview. J. Plant Dis. Prot. 2022, 129, 1323–1330. [Google Scholar] [CrossRef]
- García-Pascual, E.; González-Chávez, M.M.; Franco, A.; Rodríguez-Leyva, E.; Méndez-Gallegos, S.J.; Morales Rueda, J.A.; Bravo, A. Dactylopius opuntiae (Hemiptera: Dactylopiidae) control tactics: A bibliometric analysis. Investig. Bibl. 2023, 38, 13–29. [Google Scholar] [CrossRef]
- Paré, P.W.; Tumlinson, J.H. Update on plant-insect interactions plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2017, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Pophof, B.; Stange, G.; Abrell, L. Volatile organic compounds as signals in a plant-herbivore system: Electrophysiological responses in olfactory sensilla of the moth Cactoblastis cactorum. Chem. Senses 2005, 30, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.J.; Baig, N.; Cook, D.; Mahoney, N.E.; Marsico, T.D. Semiochemicals from ex situ abiotically stressed cactus tissue: A contributing role of fungal spores? J. Agric. Food Chem. 2014, 62, 12273–12276. [Google Scholar] [CrossRef] [PubMed]
- Rasmann, S.; Köllner, T.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Sites of synthesis, biochemistry and functional role of plant volatiles. S. Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef]
- Cázares-Samaniego, P.J.; Castillo, C.G.; Ramos-López, M.A.; González-Chávez, M.M. Volatilome and essential oil of Ulomoides dermestoides: A broad-spectrum medical insect. Molecules 2021, 26, 6311. [Google Scholar] [CrossRef]
- Kasote, D.; Lee, J.; Sreenivasulu, N. Editorial: Volatilomics in plant and agricultural research: Recent trends. Front. Plant Sci. 2023, 14, 1289998. [Google Scholar] [CrossRef]
- Flath, R.A.; Takahashi, J.M. Volatile Constituents of Prickly Pear (Opuntia ficus indica Mill., de Castilla Variety). J. Agric. Food Chem. 1971, 26, 835–837. [Google Scholar] [CrossRef]
- Chahdoura, H.; Barreira, J.C.M.; Fernández-Ruiz, V.; Morales, P.; Calhelha, R.C.; Flamini, G.; Soković, M.; Ferreira, I.C.F.R.; Achour, L. Bioactivity, proximate, mineral and volatile profiles along the flowering stages of Opuntia microdasys (Lehm.): Defining potential applications. Food Funct. 2016, 7, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Nounah, I.; Chbani, M.; Matthäus, B.; Charrouf, Z.; Hajib, A.; Willenberg, I. Profile of volatile aroma-active compounds of cactus seed oil (Opuntia ficus-indica) from different locations in morocco and their fate during seed roasting. Foods 2020, 9, 1280. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.R.; Setzer, W.N. Volatile compositions of two cactus species growing in the sonoran desert of Southern Arizona. Am. J. Essent. Oils Nat. Prod. 2013, 1, 41–47. Available online: https://www.researchgate.net/publication/270572666 (accessed on 23 March 2024).
- Apablaza, E.; Sáenz, C.; Prat, L.; Ubeda, C. Comprehensive characterization and volatile profile of cactus pear fruits from six different species and cultivars. ACS Food Sci. Technol. 2021, 1, 928–936. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; El-tantawy, M.E.; Rabeh, M.A.; Badr, W.K. Chemical composition and antimicrobial activity of volatile constituents of cladodes, fruits peel and fruits pulp from Opuntia ficus indica (L.) Mill. (Prickly pear) growing in Egypt. Egypt J. Chem. 2020, 64, 437–444. [Google Scholar] [CrossRef]
- Zhang, W.W.; Zheng, H.; Zhang, H.; Chen, X.M.; Guo, Y.H. Volatile changes of Opuntia ficus-indica Mill before and after parasitizing by Dactylopius coccus Costa. Chin. J. Appl. Entomol. 2012, 49, 986–991. [Google Scholar]
- Stierlin, É.; Nicolè, F.; Costes, T.; Fernandez, X.; Michel, T. Metabolomic study of volatile compounds emitted by lavender grown under open-field conditions: A potential approach to investigate the yellow decline disease. Metabolomics 2020, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Ling, P.; Zhu, H.; Keener, H.M. Plant pest detection using an artificial nose system: A review. Sensors 2018, 18, 378. [Google Scholar] [CrossRef]
- De Lotto, G. On the status and identity of the cochineal insects (Homoptera: Coccoidea: Dactylopiidae). J. Entomol. Soc. South. Afr. 1974, 37, 167–193. Available online: https://hdl.handle.net/10520/AJA00128789_2848 (accessed on 1 June 1974).
- Waku, Y.; Foldi, I. The fine structure of insect glands secreting waxy substances. In Insect Ultrastructure; King, R.C., Akai, H., Eds.; Springer: Boston, MA, USA, 1984; pp. 303–322. [Google Scholar] [CrossRef]
- Rodríguez, L.C.; Faúndez, E.H.; Niemeyer, H.M. Mate searching in the scale insect, Dactylopius coccus (Hemiptera: Coccoidea: Dactylopiidae). Eur. J. Entomol. 2005, 102, 305–306. [Google Scholar] [CrossRef]
- Palafox-Luna, J.A.; Rodríguez-Leyva, E.; Lomeli-Flores, J.R.; Vigueras-Guzmán, A.L.; Vanegas-Rico J., M. Ciclo de vida y fecundidad de Dactylopius opuntiae (Hemiptera: Dactylopiidae) en Opuntia ficus-indica (Caryophyllales: Cactaceae). Agrociencia 2018, 52, 103–114. [Google Scholar]
- Martinez, T.; Fabriás, G.; Camps, F. Sex pheromone biosynthetic pathway in Spodoptera littoralis and its activation by a neurohormone. J. Biol. Chem. 1990, 265, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Tsfadia, O.; Azrielli, A.; Falach, L.; Zada, A.; Roelofs, W.; Rafaeli, A. Pheromone biosynthetic pathways: PBAN-regulated rate-limiting steps and differential expression of desaturase genes in moth species. Insect Biochem. Mol. Biol. 2008, 38, 552–567. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Millar, J.G. Chemistry of the pheromones of mealybug and scale insects. Nat. Prod. Rep. 2015, 32, 1067–1113. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; de Paula, C.D.; Lahbouki, S.; Meddich, A.; Outzourhit, A.; Rashad, M.; Pari, L.; Coelhoso, I.; Fernando, A.L.; Souza, V.G.L. Opuntia spp.: An overview of the bioactive profile and food applications of this versatile crop adapted to arid lands. Foods 2023, 12, 1465. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Blande, J.D. Where do herbivore-induced plant volatiles go? Front. Plant Sci. 2013, 4, 48322. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hedo, M.; Bouagga, S.; Zhang, N.X.; Moerkens, R.; Messelink, G.; Jaques, J.A.; Flors, V.; Broufas, G.; Urbaneja, A.; Pappas, M.L. Induction of plant defenses: The added value of zoophytophagous predators. J. Pest Sci. 2022, 95, 1501–1517. [Google Scholar] [CrossRef]
- Terrazas, T.; Mauseth, J.D. Shoot Anatomy and Morphology. In Cacti: Biology and Uses; Nobel, P., Ed.; University of California Press: Oakland, CA, USA, 2002. [Google Scholar] [CrossRef]
- Dicke, M.; Sabelis, M.W.; Takabayashi, J.; Bruin, J.; Posthumus, M.A. Plant strategies of manipulating predator-prey interactions through allelochemicals: Prospects for application in pest control. J. Chem. Ecol. 1990, 16, 3091–3118. [Google Scholar] [CrossRef]
- Ozawa, R.; Arimura, G.I.; Takabayashi, J.; Shimoda, T.; Nishioka, T. Involvement of jasmonate-and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol. 2000, 41, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Drukker, B.; Bruin, J.; Sabelis, M.W. Anthocorid predators learn to associate herbivore-induced plant volatiles with presence or absence of prey. Physiol. Entomol. 2001, 25, 260–265. [Google Scholar] [CrossRef]
- Clavijo-McCormick, A.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Q.; Zhang, Z.N. The influence of volatiles from the hindgut of the pine sawyer, Monochamus alternatus (Coleoptera: Cerambycidae), on its oviposition behavior. Zool. Stud. 2007, 46, 726–733. [Google Scholar]
- Smith, B.C.; Williams, R.R. Temperature relations of adult Coleomegilla maculata lengi and C. m. medialzs (Coleoptera: Coccinellidae) and responses to ovipositional stimulants. Can. Entomol. 1976, 108, 925–930. [Google Scholar] [CrossRef]
- Berhe, Y.K.; Portillo, L.; Vigueras, A.L. Resistance of Opuntia ficus-indica cv ‘Rojo Pelon’ to Dactylopius coccus (Hemiptera: Dactylopiidae) under greenhouse condition. J. Prof. Assoc. Cactus Dev. 2022, 24, 293–309. [Google Scholar] [CrossRef]
- Nakata, P.A. An assessment of engineered calcium oxalate crystal formation on plant growth and development as a step toward evaluating its use to enhance plant defense. PLoS ONE 2015, 10, e0141982. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Buhroo, A.A.; Hussain, B.; Ahmad, T.; Nair, R.M.; Sharma, H.C. Plant defense and insect adaptation with reference to secondary metabolites. In Co-Evolution of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2020; pp. 795–822. [Google Scholar] [CrossRef]
- Ferris, G.F. Atlas of the Scale Insects of North America. Nature 1943, 151, 657. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of change in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).