Effect and Mechanism of L-Arginine against Alternaria Fruit Rot in Postharvest Blueberry Fruit
Abstract
:1. Introduction
2. Results
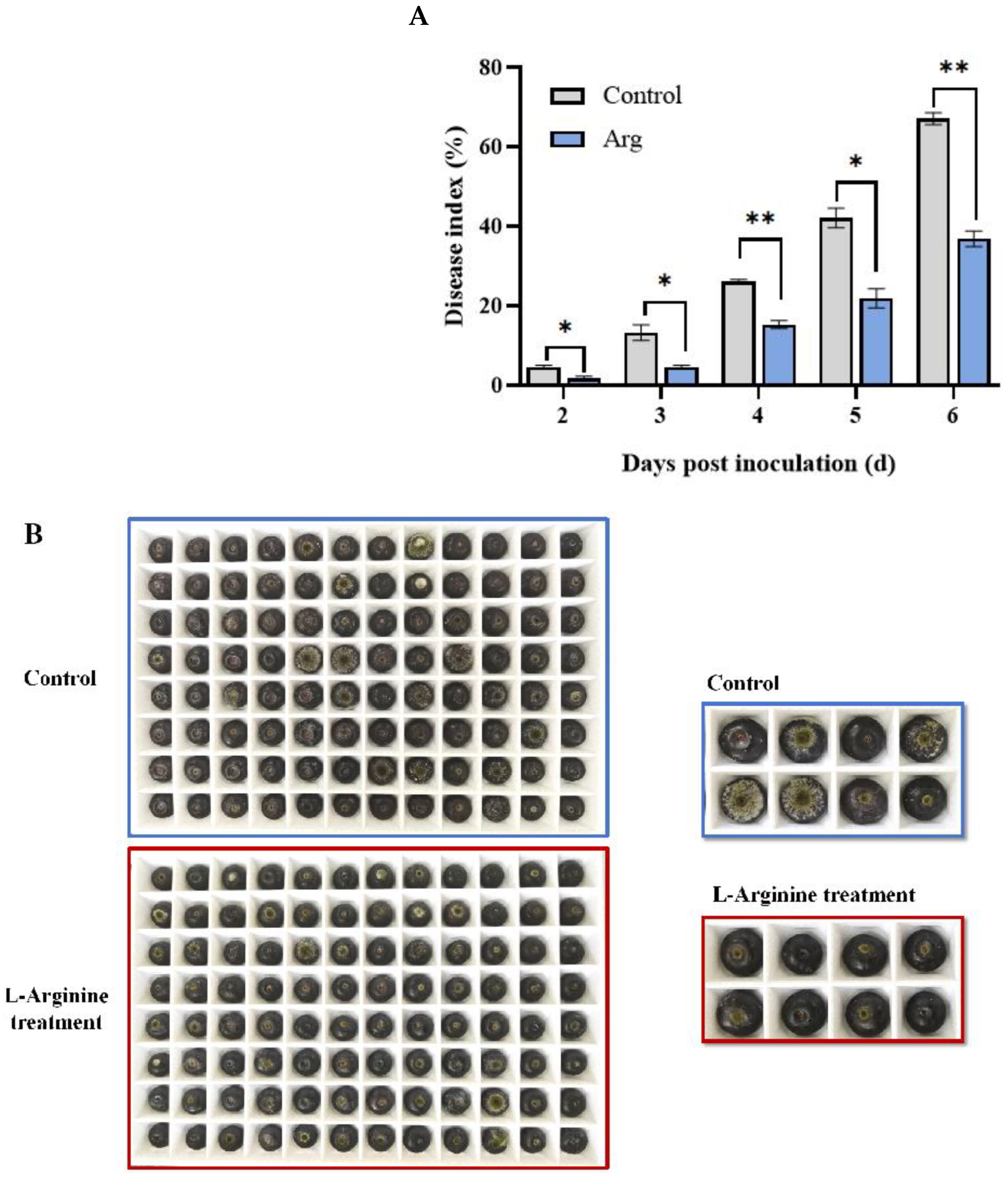
2.1. Arg Treatment Mitigated Disease Symptoms in Blueberries Inoculated with A. tenuissima
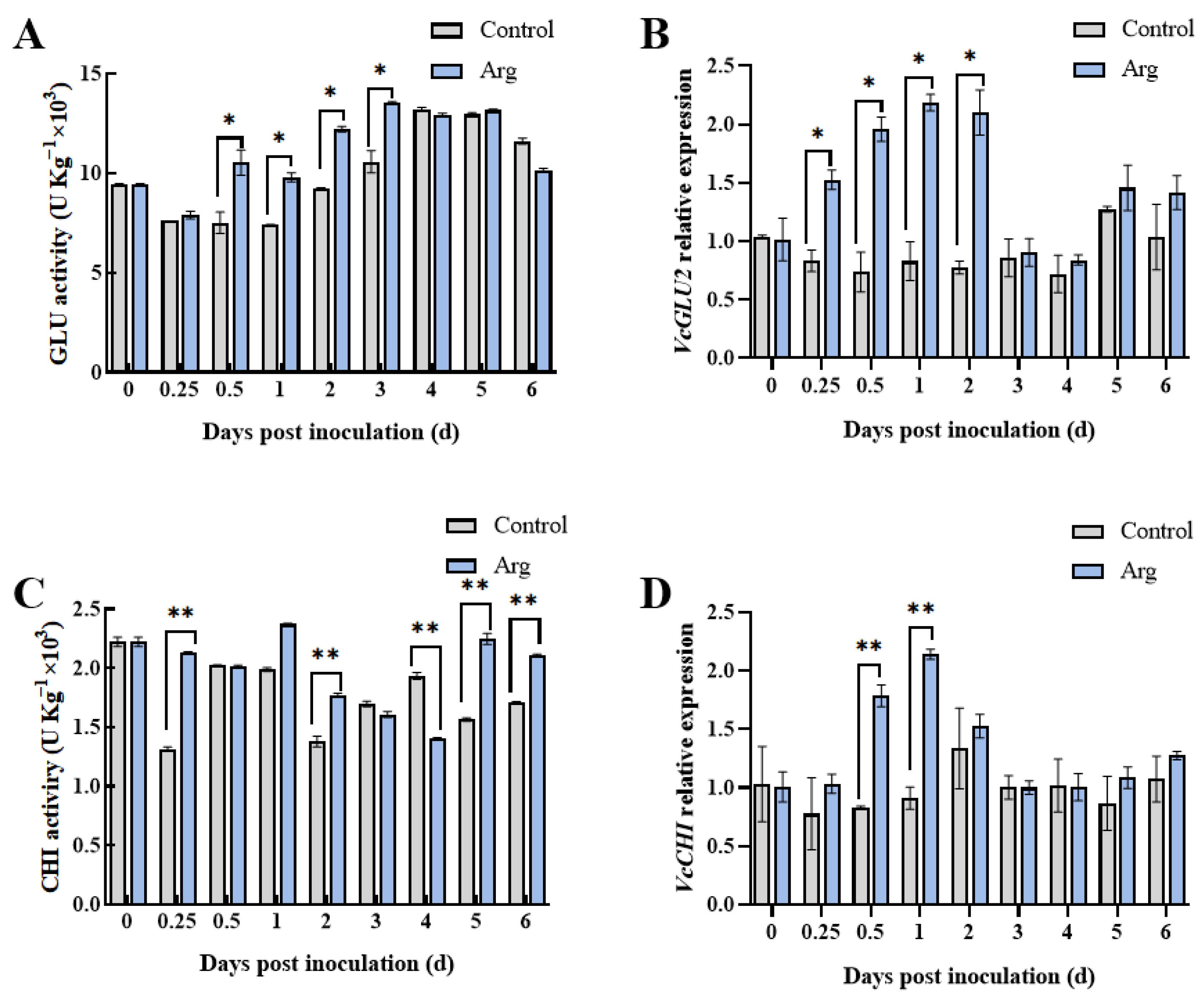
2.2. Effect of Arg Treatment on β-1,3-Glucanase (GLU) and Chitinase (CHI) Activities and Relative Gene Expression Levels in Inoculated Blueberries
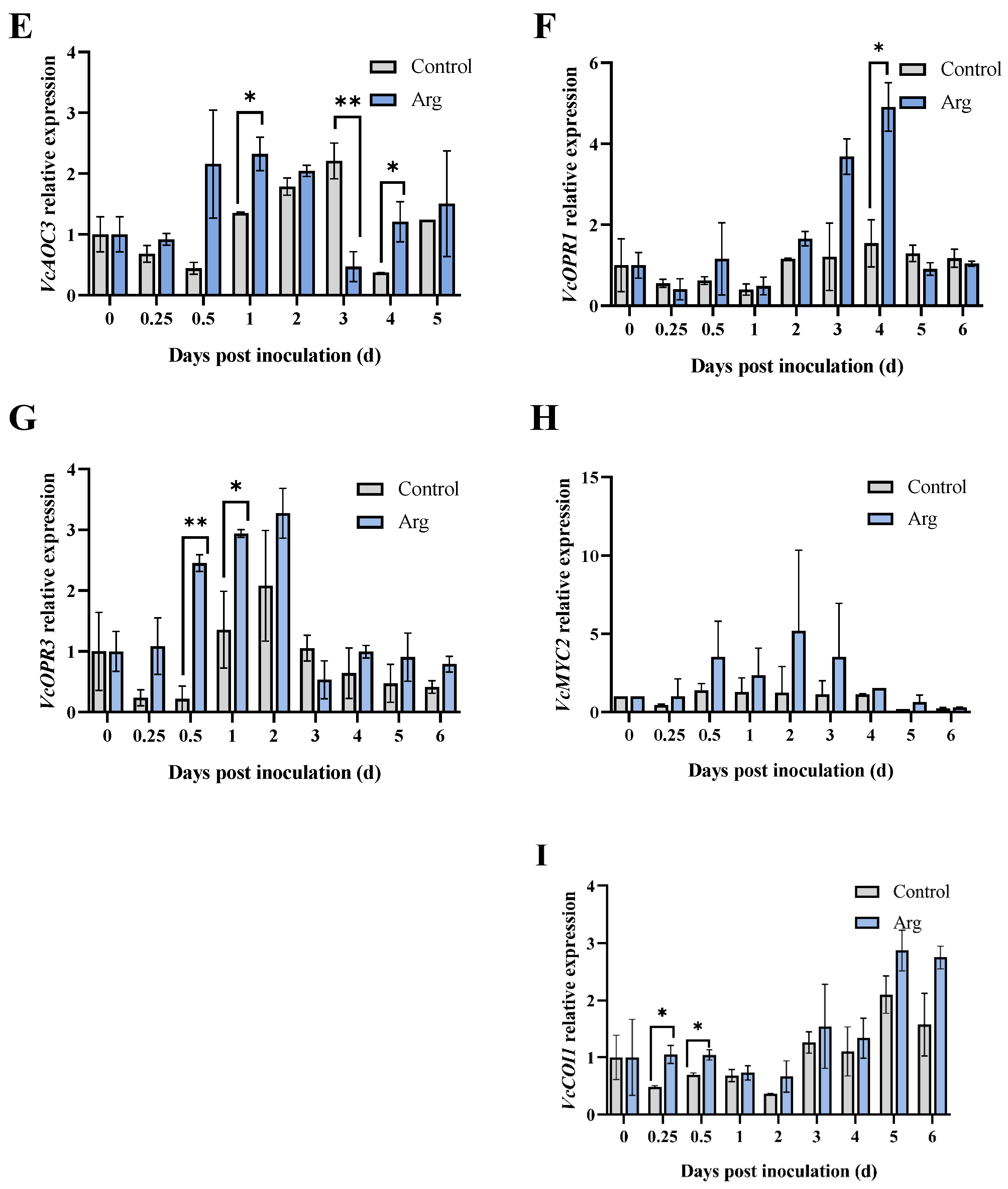
2.3. Effect of Arg Treatment on Endogenous JA Content and Gene Expression Levels Involved in JA Biosynthesis in Inoculated Blueberries
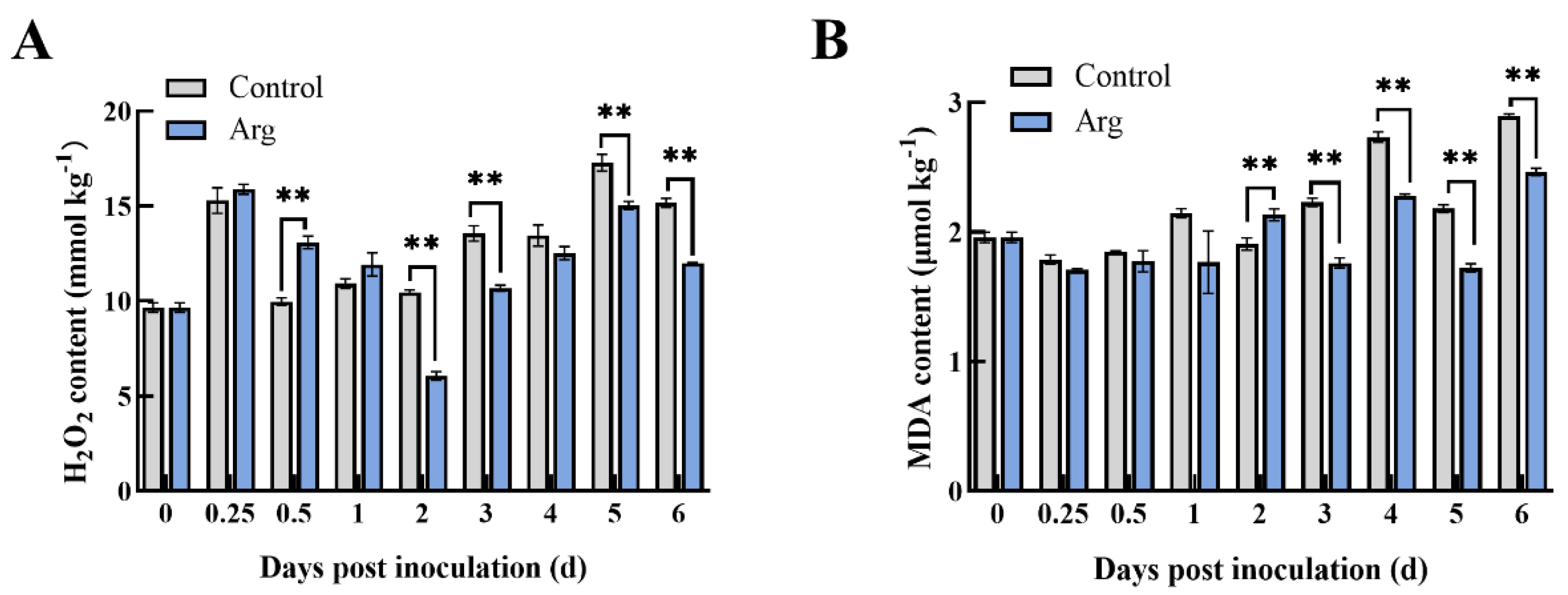
2.4. Effect of Arg Treatment on Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Content in Inoculated Blueberries
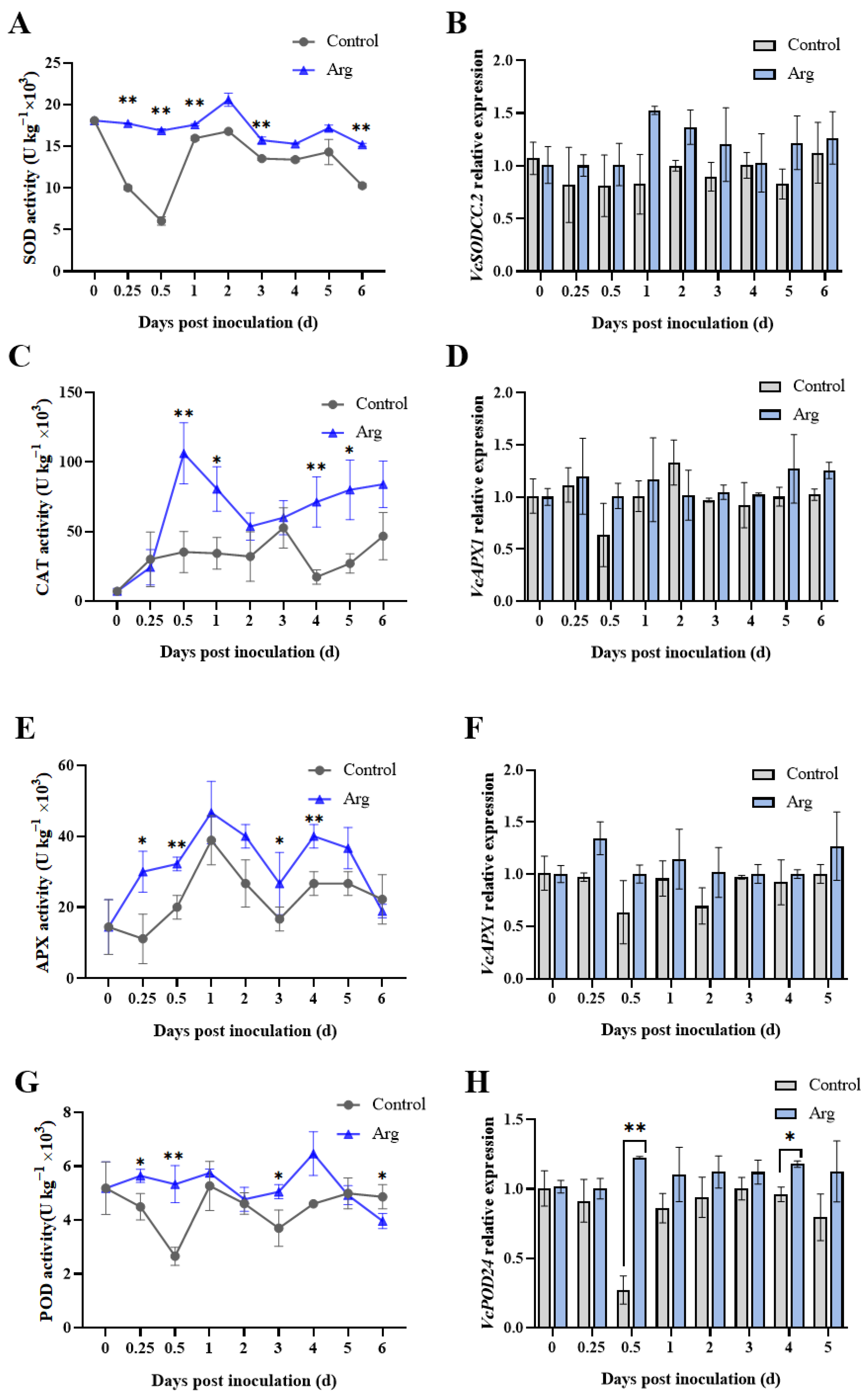
2.5. Effect of Arg Treatment on Antioxidant Enzyme Activities and Related Gene Expression in Inoculated Blueberries
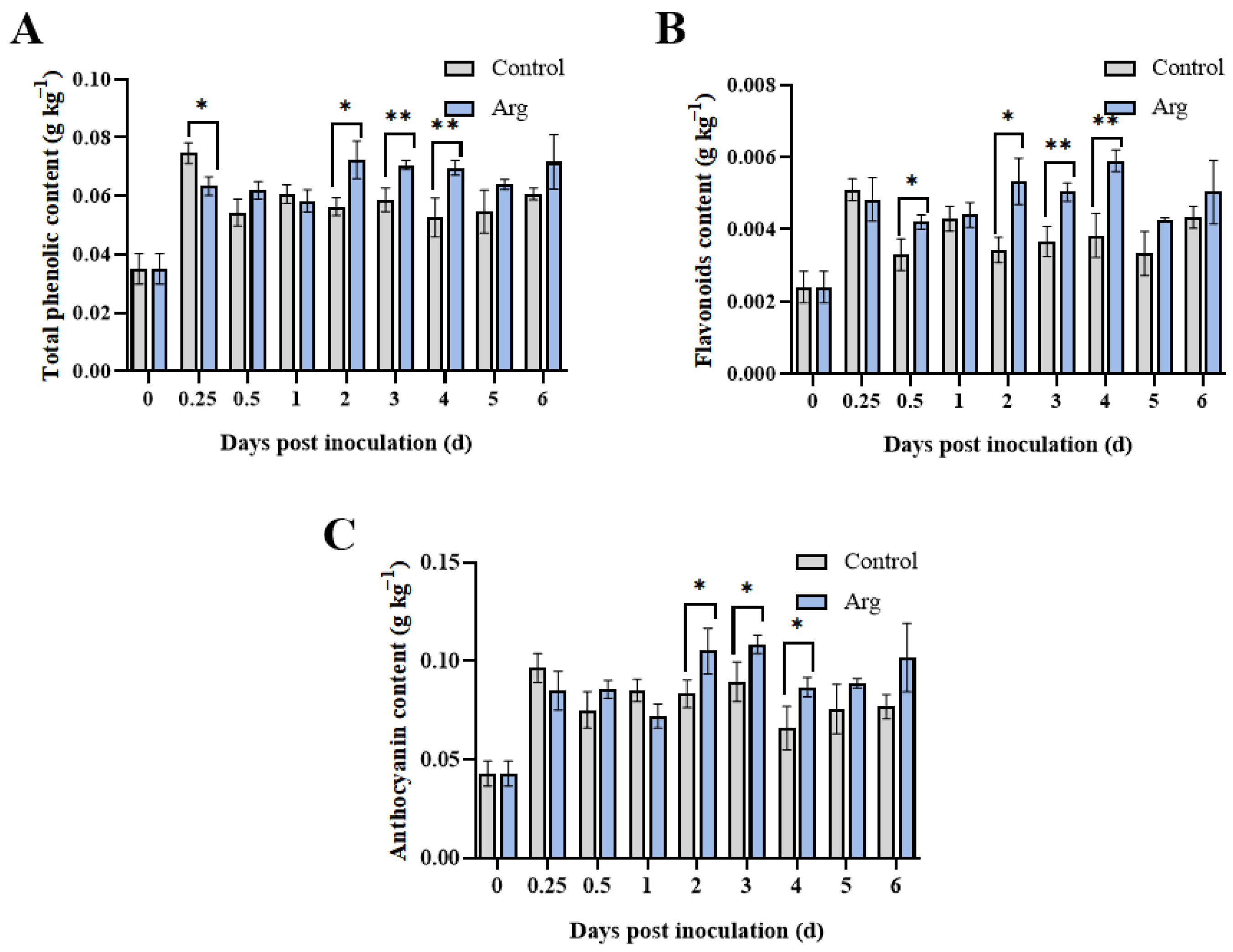
2.6. Effect of Arg Treatment on Total Phenolic, Flavonoid, and Anthocyanin Content in Inoculated Blueberries
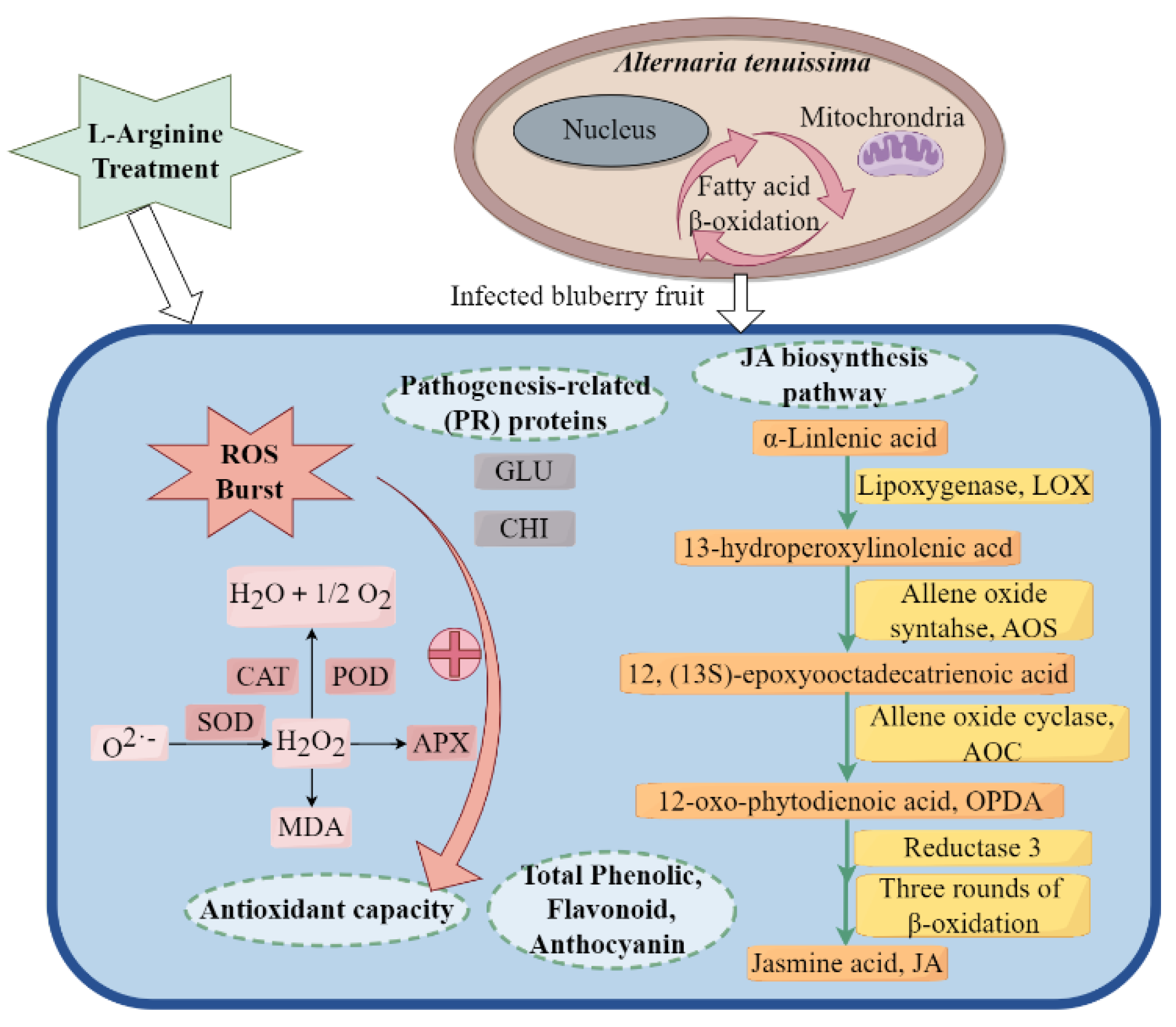
3. Discussion
4. Materials and Methods
4.1. Pathogen
4.2. Arg Treatment and Pathogen Inoculation
4.3. Assessment of Disease Index
4.4. Assessment of JA Content
4.5. Measurement of GLU and CHI Activity
4.6. Measurement of ROS Levels and Key Antioxidant Enzyme Activities
4.6.1. Determination of H2O2 and MDA Levels
4.6.2. Analysis of Antioxidant Enzyme Activity
4.7. Measurement of Total Phenolic, Flavonoid, and Anthocyanin Content
4.8. Real-Time Quantitative PCR (RT-qPCR)
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| GADPH VcPOD24 VcCAT2 | ACTACCATCCACTCTATCACCG AAGCAGCCTAAATGGGACG ATGGAGTATTTGCTGGGTCTG | AACACCTTACCAACAGCCTTG TGAGCACCTGATAACACTACAAGAGGATCTAGCGCATAGTATGGTAC |
| VcAPX1 | CTCCTGCTAGGGCTTTGGC | CCTCGCTCACAGTCGGGTA |
| VcSODCC.2 VcGLU2 VcCHI VcAOC VcAOC3 VcCOI1 VcAOS1 VcLOX1 VcOPR1 VcOPR3 VcMYC2 | AAGATGGGTACTGTGAAAGCG ATGCTTCTCGCCCTTGC ATCCGCTCATCGCTTACA TCTGACCGCCACCTCTACCG TTACGAGATGAACGAGGGAGA TGGGTGCCATAAACTCCG GGCTACCGAATCCTTTCTTACCT GGATCACCATGATGCGCTAA TTTTAGGTTTCAGGGACAGGA ACCAAGGAACAAGTGGAGGCA GGTAGGAAACCAGCAAACGG | GAATTGCAGCCGTTGGTG GTGATATTGGTGTCTGGGTAATG CACTCCAATGCACCGTTA AAGCTGGGCTGCCACGA CAGCTTGTTACTGAACGGGAC CAGAAGCATCCACCTCACG GTTGGCATCTCCGAAACTGG ATGGCTTCAGTGTTCCGTCA TTGGTCGGGCAGTGGTG TCGTGGATGAAATCGGAGC CGCAATCGCATCACCAAGT |
References
- Dai, H.; Li, B.; Song, Z.; Tian, J.; Wei, B.; Lang, X.; Zhou, Q. Anthocyanin treatment delays the senescence of blueberry fruit by regulating abscisic acid and anthocyanin synthesis processes. Food Qual. Saf. 2024, 8, fyad053. [Google Scholar] [CrossRef]
- Song, X.; Dai, H.; Wang, S.; Ji, S.; Zhou, X.; Li, J.; Zhou, Q. Putrescine Treatment Delayed the softening of postharvest blueberry fruit by inhibiting the expression of cell wall metabolism key gene VcPG1. Plants 2022, 11, 1356. [Google Scholar] [CrossRef]
- Pratap-Singh, A.; Shojaei, M.; Singh, A.; Ye, Y.; Mandal, R.; Yan, Y.; Pico, J.; Gerbrandt, E.M.; Castellarin, S.D. Effects of pulsed light on the post harvest quality and shelf-life of highbush blueberries (cv. Draper). Appl. Food Res. 2023, 3, 100273. [Google Scholar] [CrossRef]
- Bell, S.R.; Hernández Montiel, L.G.; González Estrada, R.R.; Gutiérrez Martínez, P. Main diseases in postharvest blueberries, conventional and eco-friendly control methods: A review. LWT Food Sci. Technol. 2021, 149, 112046. [Google Scholar] [CrossRef]
- Zhu, X.; Xiao, C. Phylogenetic, morphological, and pathogenic characterization of Alternaria species associated with fruit rot of blueberry in California. Phytopathology 2015, 105, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Munitz, M.S.; Garrido, C.E.; Gonzalez, H.H.L.; Resnik, S.L.; Montti, M.T. Mycoflora and potential mycotoxin production of freshly harvested blueberry in Concordia, Entre Ríos Province, Argentina. Int. J. Fruit. Sci. 2013, 13, 312–325. [Google Scholar] [CrossRef]
- Wang, F.; Saito, S.; Michailides, T.J.; Xiao, C.L. Postharvest use of natamycin to control Alternaria rot on blueberry fruit caused by Alternaria alternata and A. arborescens. Postharvest Biol. Technol. 2021, 172, 111383. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, W.; Liao, J.; Xiu, Z.; Jiang, A.; Yang, X.; Guan, Y.; Feng, K.; Saren, G. Effect of ethanol vapor treatment on the growth of Alternaria alternata and Botrytis cinerea and defense-related enzymes of fungi-inoculated blueberry during storage. Front. Microbiol. 2021, 12, 618252. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, O.; Wilson, K.; Mohamed, R.; Avis, T.J. Bacillus and Pseudomonas spp. provide antifungal activity against gray mold and Alternaria rot on blueberry fruit. Biol. Control 2018, 126, 136–141. [Google Scholar] [CrossRef]
- McNeal, C.J.; Meininger, C.J.; Reddy, D.; Wilborn, C.D.; Wu, G.Y. Safety and effectiveness of Arginine in adults. J. Nutr. 2016, 146, 2587–2593. [Google Scholar] [CrossRef]
- Zhang, X.H.; Min, D.D.; Li, F.J.; Ji, N.N.; Meng, D.M.; Li, L. Synergistic effects of L-Arginine and methyl salicylate on alleviating postharvest disease caused by Botrysis cinerea in tomato fruit. J. Agric. Food Chem. 2017, 65, 4890–4896. [Google Scholar] [CrossRef]
- Chang, L.; Yu, Y.; Zhang, L.; Wang, X.; Zhang, S. Arginine induces the resistance of postharvest jujube fruit against Alternaria rot. Food Qual. Saf. 2022, 6, fyac008. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Li, Y.; Yang, L.; Sun, B.; Zhang, Y.; Xu, Y.; Yan, X. L-Arginine treatment maintains postharvest quality in blueberry fruit by enhancing antioxidant capacity during storage. J. Food Sci. 2023, 88, 3666–3680. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, M.; Zhang, J.; Ge, Y.; Li, C.; Meng, K.; Li, J. Effects of methyl jasmonate on expression of genes involved in ethylene biosynthesis and signaling pathway during postharvest ripening of apple fruit. Sci. Hortic. 2018, 229, 157–166. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Melotto, M.; Yao, J.; He, S.Y. Jasmonate signaling and manipulation by pathogens and insects. J. Exp. Bot. 2017, 68, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Hause, B.; Wasternack, C.; Strack, D. Jasmonates in stress responses and development. Phytochemistry 2009, 70, 1483–1484. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhao, L.N.; Zhang, X.Y.; Dhanasekaran, S.; Abdelhai, M.H.; Yang, Q.Y.; Jiang, Z.H.; Zhang, H.Y. Study on biocontrol of postharvest decay of table grapes caused by Penicillium rubens and the possible resistance mechanisms by Yarrowia lipolytica. Biol. Control 2018, 130, 110–117. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Li, Y.; Yang, L.; Sun, B.; Zhang, Y.; Xu, F.; Yan, X. Controlling effect and mechanism of burdock fructooligosaccharide against Alternaria fruit rot in blueberry during postharvest. Postharvest Biol. Technol. 2023, 196, 112175. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Bi, Y.; Zhang, B.; Shen, S.; Jiang, T.; Zheng, X. Melatonin treatment inhibits gray mold and induces disease resistance in cherry tomato fruit during postharvest. Postharvest Biol. Technol. 2019, 157, 110962. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.; Chen, X.; Chen, Z.; Zhang, Z.; Li, T.; Qu, H.; Jiang, Y. Effects of hydrogen water treatment on antioxidant system of litchi fruit during the pericarp browning. Food Chem. 2021, 336, 127618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, X.; Hou, H.; Lin, Q.; Zhu, S.; Wang, G. 6-Benzyladenine treatment maintains storage quality of Chinese flowering cabbage by inhibiting chlorophyll degradation and enhancing antioxidant capacity. Plants 2023, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Et-tazy, L.; Lamiri, A.; Satia, L.; Essahli, M.; Krimi Bencheqroun, S. In vitro antioxidant and antifungal activities of four essential oils and their major compounds against post-harvest fungi associated with chickpea in storage. Plants 2023, 12, 3587. [Google Scholar] [CrossRef]
- Tan, X.; Zhao, Y.; Shan, W.; Kuang, J.; Lu, W.; Su, X.; Tao, N.; Lakshmanan, P.; Chen, J. Melatonin delays leaf senescence of postharvest Chinese flowering cabbage through ROS homeostasis. Food Res. Int. 2020, 138, 109790. [Google Scholar] [CrossRef]
- Sang, Y.; Liu, Y.; Tang, Y.; Yang, W.; Guo, M.; Chen, G. Transcriptome sequencing reveals mechanism of improved antioxidant capacity and maintained postharvest quality of winter jujube during cold storage after salicylic acid treatment. Postharvest Biol. Technol. 2022, 189, 111929. [Google Scholar] [CrossRef]
- Ding, J.; Liu, C.; Huang, P.; Zhang, Y.; Hu, X.; Li, H.; Liu, Y.; Chen, L.; Liu, Y.; Qin, W. Effects of thymol concentration on postharvest diseases and quality of blueberry fruit. Food Chem. 2023, 402, 134227. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, H.; Kou, X.; Wu, C.; Fan, G.; Li, T.; Zhou, D. Metabolomics analysis reveals that MeJA treatment induces postharvest blueberry resistance to Botrytis cinerea. Postharvest Biol. Technol. 2022, 194, 112075. [Google Scholar] [CrossRef]
- Sun, T.; Ouyang, H.; Sun, P.; Zhang, W.; Wang, Y.; Cheng, S.; Chen, G. Postharvest UV-C irradiation inhibits blackhead disease by inducing disease resistance and reducing mycotoxin production in ‘Korla’ fragrant pear (Pyrus sinkiangensis). Int. J. Food Microbiol. 2022, 362, 109485. [Google Scholar] [CrossRef]
- Huang, K.; Sui, Y.; Miao, C.; Chang, C.; Wang, L.; Cao, S.; Huang, X.; Li, W.; Zou, Y.; Sun, Z.; et al. Melatonin enhances the resistance of ginger rhizomes to postharvest fungal decay. Postharvest Biol. Technol. 2021, 182, 111706. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, L.; Zhang, L.; Guo, Y.; Qi, X.; He, L. Effects of quercetin on postharvest blue mold control in kiwifruit. Sci. Hortic. 2018, 228, 18–25. [Google Scholar] [CrossRef]
- Asghari, M.; Rezaei-Rad, R. 24-Epibrassinolide enhanced the quality parameters and phytochemical contents of table grape. J. Appl. Bot. Food. Qual. 2018, 91, 226–231. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2019, 46, 197–212. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Zhao, R.; Li, R.; Zhang, S.; Yu, W.; Sheng, J.; Shen, L. Melatonin induces disease resistance to Botrytis Cinerea in tomato fruit by activating jasmonic acid signaling pathway. J. Agric. Food Chem. 2019, 67, 6116–6124. [Google Scholar] [CrossRef]
- Ye, Y.; Xiao, Y.; Han, Y.; Shan, W.; Fan, Z.; Xu, Q.; Kuang, J.; Lu, W.; Lakshmanan, P.; Chen, J. Banana fruit VQ motif-containing protein5 represses cold-responsive transcription factor MaWRKY26 involved in the regulation of JA biosynthetic genes. Sci. Rep. 2016, 6, 23632. [Google Scholar] [CrossRef]
- Wang, B.; Bi, Y. The role of signal production and transduction in induced resistance of harvested fruits and vegetables. Food Qual. Saf. 2021, 5, fyab011. [Google Scholar] [CrossRef]
- Shi, J.; Liu, N.; Gu, R.; Zhu, L.; Zhang, C.; Wang, Q.; Lei, Z.; Liu, Y.; Ren, J. Signals induced by exogenous nitric oxide and their role in controlling brown rot disease caused by Monilinia fructicola in postharvest peach fruit. J. Gen. Plant Pathol. 2015, 81, 68–76. [Google Scholar] [CrossRef]
- Huan, C.; Yang, X.; Wang, L.; Kebbeh, M.; Wang, Y.; Dai, B.; Shen, S.; Zheng, X.; Zhou, H. Methyl jasmonate treatment regulates α-linolenic acid metabolism and jasmonate acid signaling pathway to improve chilling tolerance in both stony hard and melting flesh peaches. Postharvest Biol. Technol. 2022, 190, 111960. [Google Scholar] [CrossRef]
- Ji, N.; Wang, J.; Zuo, X.; Li, Y.; Li, M.; Wang, K.; Jin, P.; Zheng, Y. PpWRKY45 is involved in methyl jasmonate primed disease resistance by enhancing the expression of jasmonate acid biosynthetic and pathogenesis-related genes of peach fruit. Postharvest Biol. Technol. 2021, 172, 111390. [Google Scholar] [CrossRef]
- Pan, L.; Chen, X.; Xu, W.; Fan, S.; Wan, T.; Zhang, J.; Cai, Y. Methyl jasmonate induces postharvest disease resistance to decay caused by Alternaria alternata in sweet cherry fruit. Sci. Hortic. 2022, 292, 110624. [Google Scholar] [CrossRef]
- Qu, G.F.; Wu, W.N.; Ba, L.J.; Ma, C.; Ji, N.; Cao, S. Melatonin enhances the postharvest disease resistance of blueberries fruit by modulating the jasmonic acid signaling pathway and phenylpropanoid metabolites. Front. Chem. 2022, 10, 957581. [Google Scholar] [CrossRef]
- Wang, H.B.; Kou, X.H.; Wu, C.E.; Fan, G.J.; Li, T.T. Methyl jasmonate induces the resistance of postharvest blueberry against gray mold caused by Botrytis cinerea. J. Sci. Food Agric. 2020, 100, 4272–4281. [Google Scholar] [CrossRef]
- Shu, P.; Min, D.; Ai, W.; Li, J.; Guo, Y. L-arginine treatment attenuates postharvest decay and maintains quality of strawberry fruit by promoting nitric oxide synthase pathway. Postharvest Biol. Technol. 2020, 168, 111253. [Google Scholar] [CrossRef]
- Yan, H.; Chen, H.; Zhao, J.; Yao, T.; Ding, X. Postharvest H2O2 treatment affects flavor quality, texture quality and ROS metabolism of ‘Hongshi’ kiwifruit fruit kept at ambient conditions. Food Chem. 2023, 405, 134908. [Google Scholar] [CrossRef]
- Ma, W.; Li, J.; Murtaza, A.; Iqbal, A.; Zhang, J.; Zhu, L.; Xu, X.; Pan, S.; Hu, W. High-pressure carbon dioxide treatment alleviates browning development by regulating membrane lipid metabolism in fresh-cut lettuce. Food Control 2022, 134, 108749. [Google Scholar] [CrossRef]
- Ge, Y.; Tane, Q.; Li, C.; Duan, B.; Li, X.; Wei, M.; Li, J. Acibenzolar-S-methyl treatment enhances antioxidant ability and phenylpropanoid pathway of blueberries during low temperature storage. LWT Food Sci. Technol. 2019, 110, 48–53. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Zou, M.; Jiang, P.; Hu, W.; Li, J.; Zhi, Z.; Chen, J.; Li, S.; Ding, T.; et al. Effects of ultrasound on spoilage microorganisms, quality, and antioxidant capacity of postharvest cherry tomatoes. J. Food Sci. 2015, 80, C2117–C2126. [Google Scholar] [CrossRef]
- Ren, Y.L.; Wang, Y.F.; Bi, Y.; Ge, Y.H.; Wang, Y.; Fan, C.F.; Li, D.Q.; Deng, H.W. Postharvest BTH treatment induced disease resistance and enhanced reactive oxygen species metabolism in muskmelon (Cucumis melo L.) fruit. Eur. Food Res. Technol. 2012, 234, 963–971. [Google Scholar] [CrossRef]
- Alijani, Z.; Amini, J.; Karimi, K.; Pertot, I. Characterization of the mechanism of action of Serratia rubidaea Mar61-01 against Botrytis cinerea in strawberries. Plants 2023, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bi, Y.; Zhang, Z.; Zhang, H.; Ge, Y. Reduction of latent infection and enhancement of disease resistance in muskmelon by preharvest application of harpin. J. Agric. Food Chem. 2011, 59, 12527–12533. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, H.; Pei, J.; Dong, Y.; Wang, F.; Chen, H.; Sun, Y.; Wang, N.; Li, H.; Li, Y. De novo sequencing and comparative analysis of the blueberry transcriptome to discover putative genes related to antioxidants. Gene 2012, 511, 54–61. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhao, R.; Li, Y.; Rong, H.; Yang, L.; Gao, M.; Sun, B.; Zhang, Y.; Xu, Y.; Yan, X. Effect and Mechanism of L-Arginine against Alternaria Fruit Rot in Postharvest Blueberry Fruit. Plants 2024, 13, 1058. https://doi.org/10.3390/plants13081058
Wang J, Zhao R, Li Y, Rong H, Yang L, Gao M, Sun B, Zhang Y, Xu Y, Yan X. Effect and Mechanism of L-Arginine against Alternaria Fruit Rot in Postharvest Blueberry Fruit. Plants. 2024; 13(8):1058. https://doi.org/10.3390/plants13081058
Chicago/Turabian StyleWang, Jiaqi, Runan Zhao, Yuxuan Li, Haifeng Rong, Ling Yang, Ming Gao, Bingxin Sun, Yunhe Zhang, Yufeng Xu, and Xuerui Yan. 2024. "Effect and Mechanism of L-Arginine against Alternaria Fruit Rot in Postharvest Blueberry Fruit" Plants 13, no. 8: 1058. https://doi.org/10.3390/plants13081058




