Effect of Secondary Paper Sludge on Physiological Traits of Lactuca sativa L. under Heavy-Metal Stress
Abstract
:1. Introduction
2. Results
2.1. Soil Properties
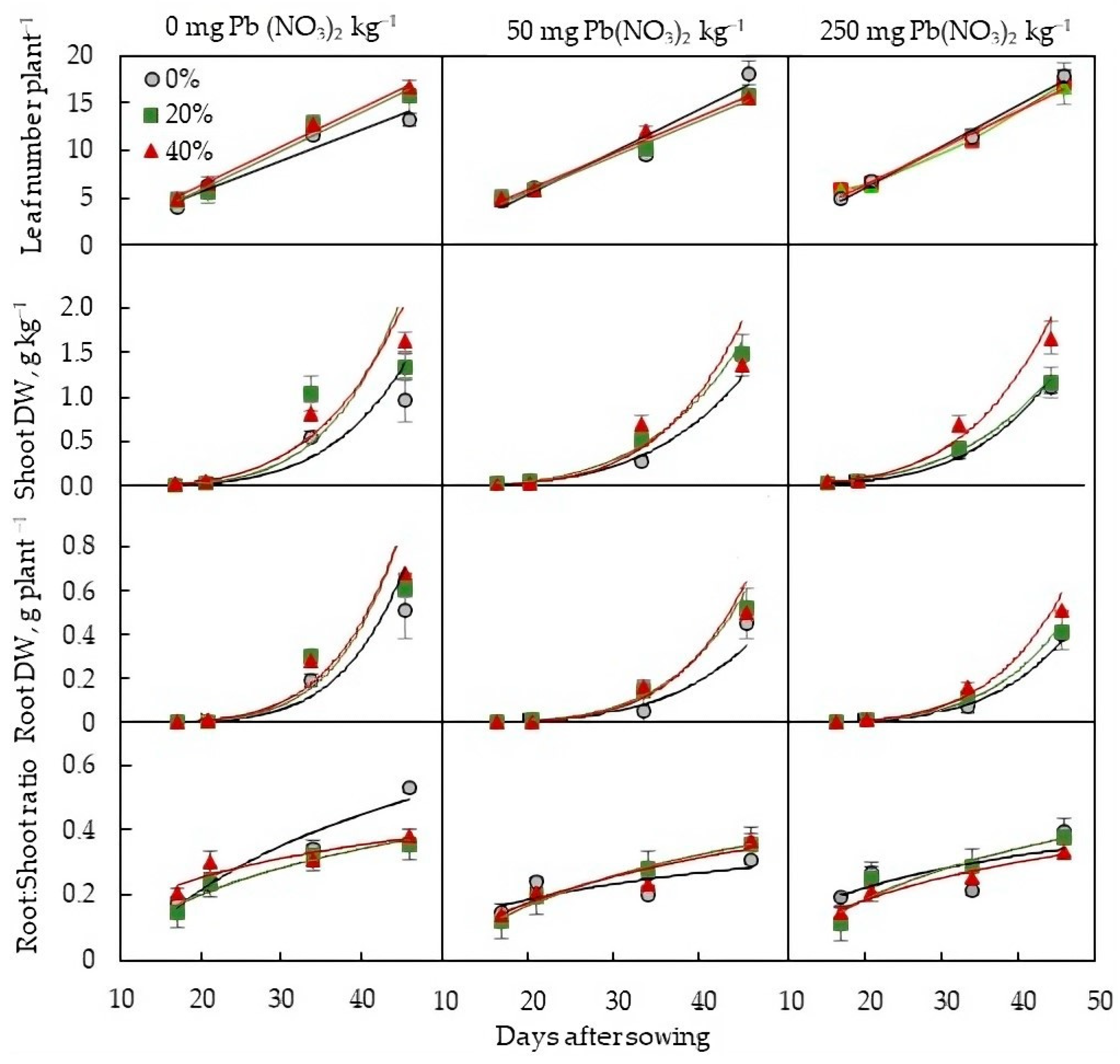
2.2. Plant-Growth Parameters
2.3. Plant Physiological Parameters
2.3.1. Photosynthesis
2.3.2. Leaf Respiration
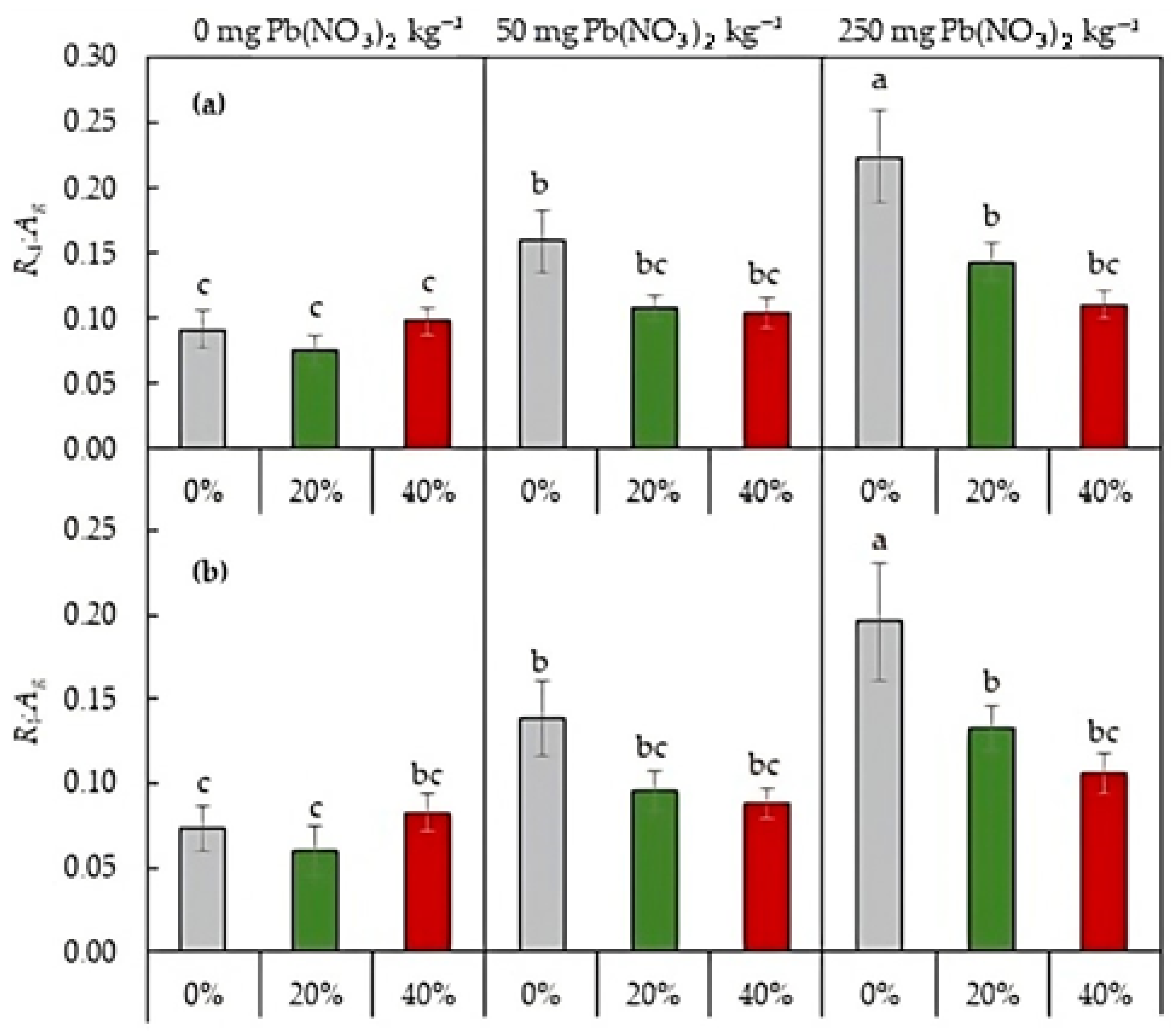
2.3.3. Respiration-to-Photosynthesis Ratio
2.3.4. Leaf H2O Exchange
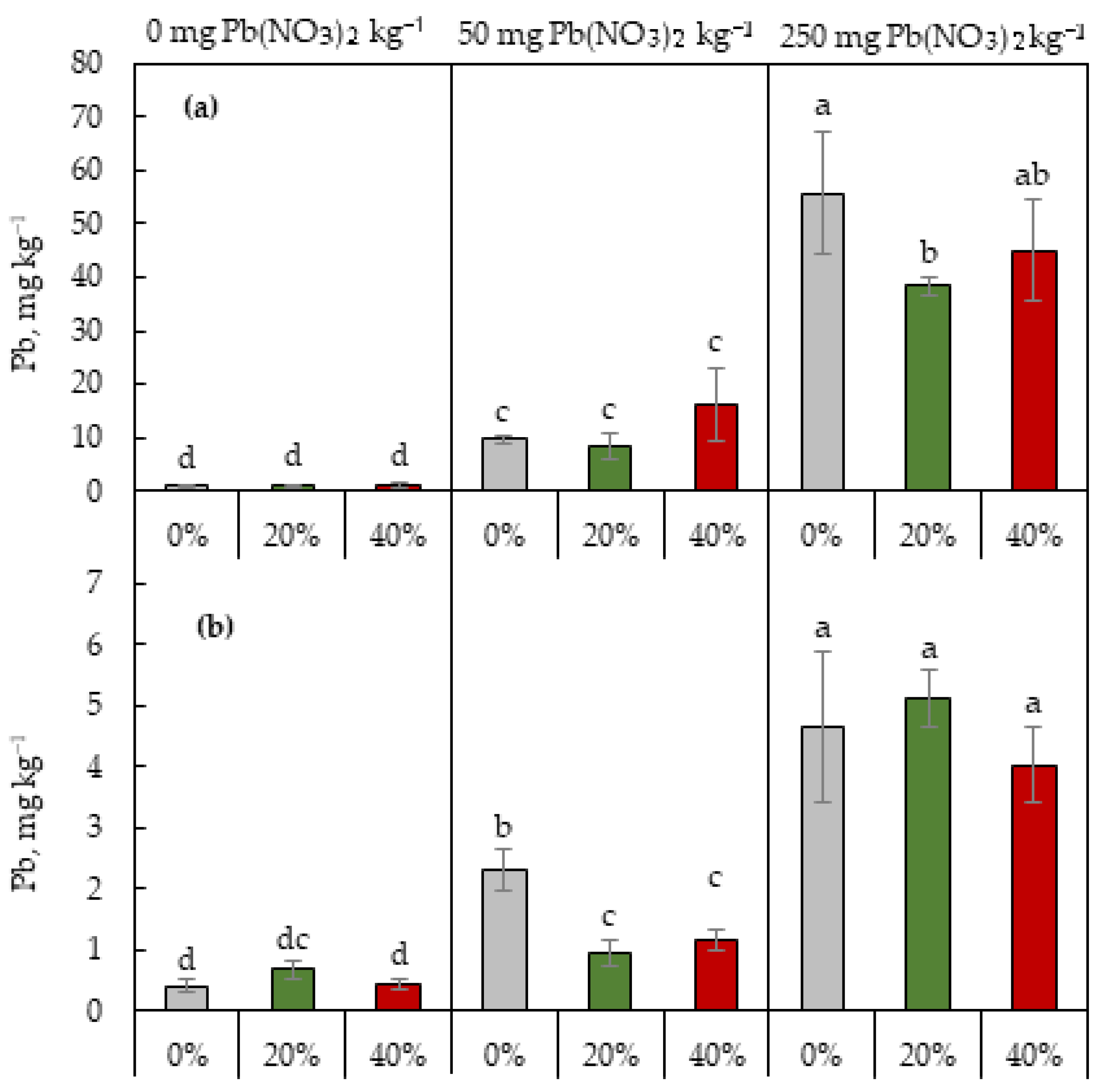
2.4. Pb Content in Shoots and Roots
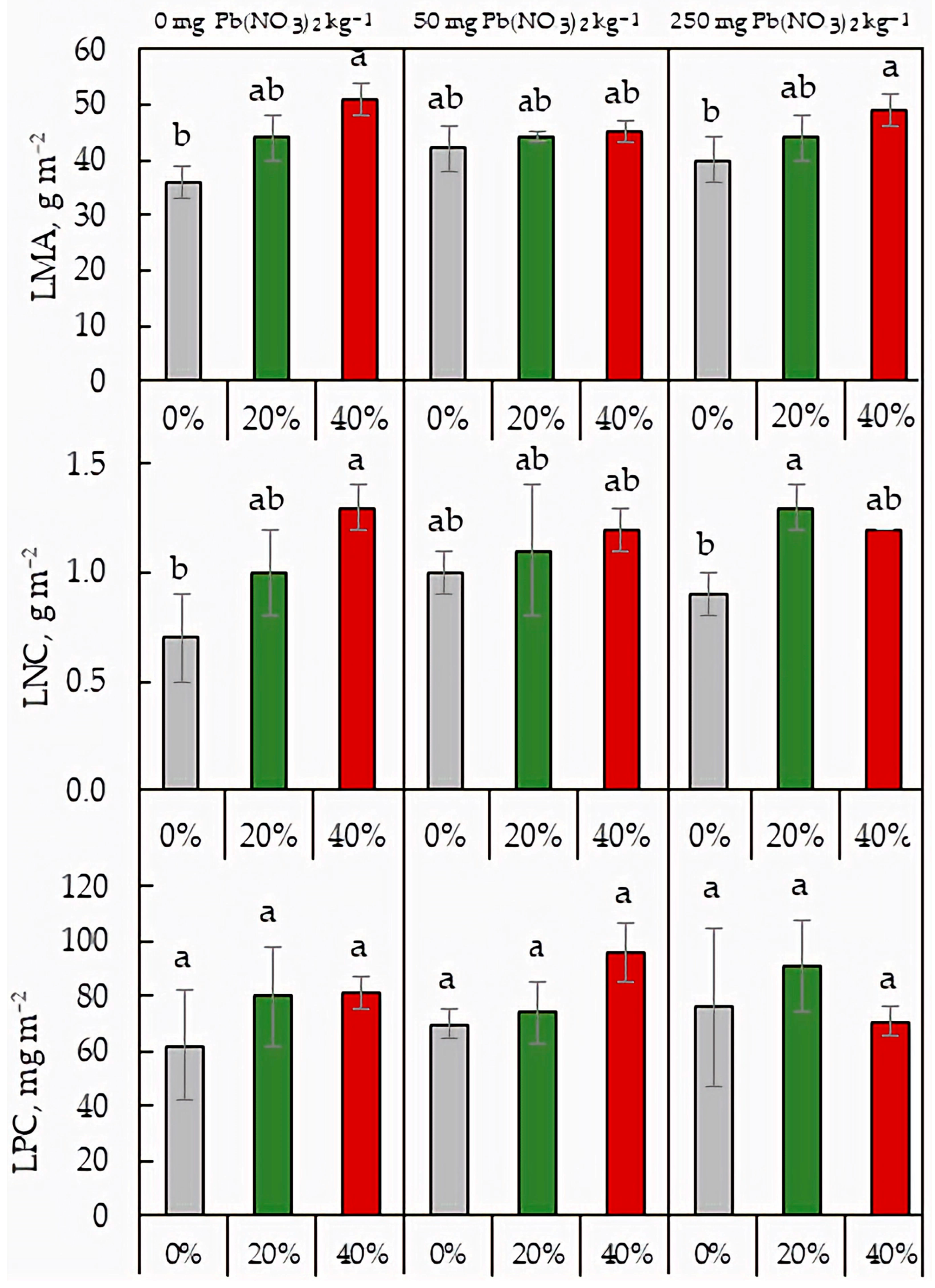
2.5. Leaf Mass per Area and Area-Based Leaf Nutrient Content
2.6. Photosynthetic Nutrient-Use Efficiency
3. Discussion
4. Materials and Methods
4.1. Plant Materials Preparation
4.2. Plant-Growth Parameters
4.3. Gas-Exchange Parameters
4.4. Chlorophyll Fluorescence and Content; Relative Water Content of Leaves
4.5. Chemical Analyses
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinnula, S.; Toivonen, M.; Soinne, H.; Joona, J.; Kivelä, J. Effects of mixed pulp mill sludges on crop yields and quality. Agric. Food Sci. 2020, 29, 276–286. [Google Scholar] [CrossRef]
- Turner, T.; Wheeler, R.; Oliver, I.W. Evaluating land application of pulp and paper mill sludge: A review. J. Environ. Manag. 2022, 317, 115439. [Google Scholar] [CrossRef] [PubMed]
- Camberato, J.J.; Gagnon, B.; Angers, D.A.; Chantigny, M.H.; Pan, W.L. Pulp and paper mill by-products as soil amendments and plant nutrient sources. Can. J. Soil Sci. 2006, 86, 641–653. [Google Scholar] [CrossRef]
- Butylkina, M.; Ikkonen, E. Physical Properties of Retisol under Secondary Pulp and Paper Sludge Application. Land 2023, 12, 2022. [Google Scholar] [CrossRef]
- Nunes, J.R.; Cabral, F.; López-Piñeiro, A. Short-term effects on soil properties and wheat production from secondary paper sludge application on two Mediterranean agricultural soils. Bioresour. Technol. 2008, 99, 4935–4942. [Google Scholar] [CrossRef] [PubMed]
- Fahim, S.; Nisar, N.; Ahmad, Z.; Asghar, Z.; Said, A.; Atif, S.; Ghani, N.; Qureshi, N.; Soomro, G.A.; Iqbal, M.; et al. Managing Paper and Pulp Industry By-Product Waste Utilizing Sludge as a Bio-Fertilizer. Pol. J. Environ. Stud. 2019, 28, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Faubert, P.; Barnabé, S.; Bouchard, S.; Côté, R.; Villeneuve, C. Pulp and paper mill sludge management practices: What are the challenges to assess the impacts on greenhouse gas emissions? Resour. Conserv. Recycl. 2016, 108, 107–133. [Google Scholar] [CrossRef]
- Mahmood, T.; Elliott, A. A review of secondary sludge reduction technologies for the pulp and paper industry. Water Res. 2006, 40, 2093–2112. [Google Scholar] [CrossRef]
- Bajpai, P. Management of Pulp and Paper Mill Waste, 1st ed.; Springer: Cham, Switzerland, 2015; p. 197. [Google Scholar]
- Curnoe, W.E.; Irving, D.C.; Dow, C.B.; Velema, G.; Unc, A. Effect of spring application of a paper mill soil conditioner on corn yield. Agron. J. 2006, 98, 423–429. [Google Scholar] [CrossRef]
- Kuokkanen, T.; Nurmesniemi, H.; Pöykiö, R.; Kujala, K.; Kaakinen, Y.; Kuokkanen, M. Chemical and leaching properties of paper mill sludge. Chem. Spec. Bioavailab. 2008, 20, 111–122. [Google Scholar] [CrossRef]
- Lister, S.K.; Line, M.A. Potential utilization of sewage sludge and paper mill waste for biosorption of metals from polluted waterways. Bioresour. Technol. 2021, 79, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska-Malina, J. Functions of organic matter in polluted soils: The effect of organic amendments on phytoavailability of heavy metals. Appl. Soil Ecol. 2018, 123, 542–545. [Google Scholar] [CrossRef]
- Campillo-Cora, C.; Conde-Cid, M.; Arias-Estévez, M.; Fernández-Calviño, D.; Alonso-Vega, F. Specific Adsorption of Heavy Metals in Soils: Individual and Competitive Experiments. Agronomy 2020, 10, 1113. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and Metalloid Toxicity in Plants: An Overview on Molecular Aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Flores, L.C. Plant mediated detoxification of mercury and lead. Arab. J. Chem. 2017, 10, 2335–2342. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, G.; Guo, H.; Yang, M.; Yang, Q. Accumulation and sub cellular distribution of lead (Pb) in industrial hemp grown in Pb contaminated soil. Ind. Crops Prod. 2021, 161, 113220. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils. Trace Metals and Metalloids in Soils and their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. [Google Scholar]
- Sharma, P.; Dubey, R. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K.; Rishi, V. Biochemical Adaptations in Zea mays Roots to Short-Term Pb2+ Exposure: ROS Generation and Metabolism. Bull. Environ. Contam. Toxicol. 2015, 95, 246–253. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Bali, S.; Khanna, K.; Arora, S.; Sharma, A.; Bhardwaj, R. Current Scenario of Pb Toxicity in Plants: Unraveling Plethora of Physiological Responses. Residue Rev. 2019, 249, 153–197. [Google Scholar]
- Madhu, P.M.; Sadagopan, R.S. Effect of Heavy Metals on Growth and Development of Cultivated Plants with Reference to Cadmium, Chromium and Lead—A Review. J. Stress Physiol. Biochem. 2020, 16, 84–102. [Google Scholar]
- Shu, X.; Yin, L.Y.; Zhang, Q.F.; Wang, W.B. Effect of Pb toxicity on leaf growth, antioxidant enzyme activities, and photosynthesis in cuttings and seedlings of Jatropha curcas L. Environ. Sci. Pollut. Res. 2012, 19, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Romanowska, E.; Igamberdiev, A.U.; Parys, E.; Gardeström, P. Stimulation of respiration by Pb2+ in detached leaves and mitochondria of C3 and C4 plants. Physiol. Plant. 2002, 116, 148–154. [Google Scholar] [CrossRef]
- Semikhatova, O.A. Evaluation of plant adaptation potential by assessing dark respiration. Russ. J. Plant Physiol. 1998, 45, 122–128. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kumar, A.; Prasad, M.N.V. Plant-lead interactions: Transport, toxicity, tolerance, and detoxification mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef]
- Collin, S.; Baskar, A.; Geevarghese, D.M.; Ali, M.N.V.S.; Bahubali, P.; Choudhary, R.; Lvov, V.; Tovar, G.I.; Senatov, F.; Koppala, S.; et al. Bioaccumulation of lead (Pb) and its effects in plants: A review. J. Hazard. Mater. 2022, 3, 100064. [Google Scholar] [CrossRef]
- Ikkonen, E.; Kaznina, N. Physiological Responses of Lettuce (Lactuca sativa L.) to Soil Contamination with Pb. Horticulturae 2022, 8, 951. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationship in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Poorter, H.; Evans, J.R. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 1998, 116, 26–37. [Google Scholar] [CrossRef]
- Hikosaka, K. Interspecific difference in the photosynthesis-nitrogen relationship: Patterns, physiological causes, and ecological importance. J. Plant Res. 2004, 117, 481–494. [Google Scholar] [CrossRef]
- Yamori, W.; Noguchi, K.; Hikosaka, K.; Terashima, I. Cold tolerant crop species have greater temperature homeostasis of leaf respiration and photosynthesis than cold-sensitive species. Plant Cell Physiol. 2009, 50, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Atkin, O.K.; Scheurwater, I.; Pons, T.L. High thermal acclimation potential of both photosynthesis and respiration in two lowland Plantago species in contrast to an alpine congeneric. Glob. Chang. Biol. 2006, 12, 500–515. [Google Scholar] [CrossRef]
- Passioura, J.B. Soil structure and plant growth. Soil Res. 1991, 29, 717–728. [Google Scholar] [CrossRef]
- Zibilske, L.; Clapham, W.; Rourke, R. Multiple applications of paper mill sludge in an agricultural system: Soil effects. J. Environ. Qual. 2000, 29, 1975–1981. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Yaseen, M.; Hussain, B.; Zehra, A.; Aziz, M.Z.; He, Z.; Yang, X. Comparative efficacy of organic and inorganic amendments for cadmium and lead immobilization in contaminated soil under rice-wheat cropping system. Chemosphere 2019, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Popescu, S.M.; Zheljazkov, V.D.; Astatkie, T.; Burducea, M.; Termeer, W.C. Immobilization of Pb in Contaminated Soils with the Combination Use of Diammonium Phosphate with Organic and Inorganic Amendments. Horticulturae 2023, 9, 278. [Google Scholar] [CrossRef]
- Cao, X.D.; Ammar, L.W.; Bing, M.; Yongliang, L.Y. Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J. Hazard. Mater. 2009, 164, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Ikkonen, E.; Chazhengina, S.; Jurkevich, M. Photosynthetic Nutrient and Water Use Efficiency of Cucumis sativus under Contrasting Soil Nutrient and Lignosulfonate Levels. Plants 2021, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Brien, C.J.; Sermarini, R.A.; Borges Demétrio, C. Exposing the confounding in experimental designs to understand and evaluate them, and formulating linear mixed models for analyzing the data from a designed experiment. Biom. J. 2023, 65, 2200284. [Google Scholar] [CrossRef]
- Garmash, E.V.; Golovko, T.K. CO2 gas exchange and growth in Rhaponticum carthamoides under the condition of middle taiga subzone of Northeastern Europe: Dependence of photosynthesis and respiration on environmental factors. Russ. J. Plant Physiol. 1997, 44, 737–745. [Google Scholar]
- Farquhar, G.D.; von Caemmerer, S. Modelling of photosynthetic response to environmental conditions. In Encyclopedia of Plant Physiology. V. 12B. Physiological Plant Ecology II. Water Relations and Carbon Assimilation; Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; Volume 12, pp. 549–587. [Google Scholar]
- Kok, B. A critical consideration of the quantum yield of Chlorella-photosynthesis. Enzymologia 1948, 13, 1–5. [Google Scholar]
- González, L.; González-Vilar, M. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Reigosa Roger, M.J., Ed.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 2001; pp. 207–212. [Google Scholar]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, chemistry of the procedure, and titrimetric finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]



| Parameter | 0 mg Pb(NO3)2 kg−1 | 50 mg Pb(NO3)2 kg−1 | 250 mg Pb(NO3)2 kg−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0% | 20% | 40% | 0% | 20% | 40% | 0% | 20% | 40% | |
| total C, % | 4.3 ± 0.1 abc | 4.4 ± 0.1 abc | 4.7 ± 0.1 a | 4.2 ± 0.2 b | 4.5 ± 0.2 ab | 4.4 ± 0.1 abc | 4.0 ± 0.2 c | 4.3 ± 0.1 abc | 4.7 ± 0.2 a |
| total N, % | 0.27 ± 0.00 c | 0.29 ± 0.00 b | 0.28 ± 0.00 b | 0.29 ± 0.01 b | 0.28 ± 0.00 b | 0.28 ± 0.01 b | 0.40 ± 0.00 a | 0.41 ± 0.00 a | 0.40 ± 0.00 a |
| available P, g kg−1 | 0.91 ± 0.04 c | 1.16 ± 0.08 a | 1.26 ± 0.02 a | 0.91 ± 0.06 c | 1.00 ± 0.09 bc | 1.14 ± 0.06 ab | 0.88 ± 0.03 c | 0.85 ± 0.02 c | 0.95 ± 0.04 c |
| available Mg, mg kg−1 | 196 ± 2 abc | 196 ± 9 abc | 198 ± 6 abc | 212 ± 8 a | 189 ± 10 c | 190 ± 1 bc | 193 ± 10 abc | 210 ± 4 ab | 202 ± 6 abc |
| available Ca, g kg−1 | 1.95 ± 0.05 bc | 2.07 ± 0.06 bc | 2.22 ± 0.06 ab | 1.93 ± 0.24 bc | 1.86 ± 0.10 c | 1.99 ± 0.06 bc | 2.15 ± 0.08 abc | 2.34 ± 0.03 a | 2.39 ± 0.05 a |
| available Pb, mg kg−1 | 0.1 ± 0.0 c | 0.1 ± 0.0 c | 0.1 ± 0.0 c | 4.0 ± 0.5 c | 2.8 ± 0.1 c | 3.0 ± 0.5 c | 41.6 ± 3.6 a | 45.4 ± 2.3 a | 36.6 ± 1.5 b |
| pH | 5.40 ± 0.03 a | 5.28 ± 0.04 b | 5.26 ± 0.03 bc | 5.25 ± 0.02 bc | 5.19 ± 0.02 c | 5.19 ± 0.01 c | 5.29 ± 0.02 b | 5.25 ± 0.01 bc | 5.28 ± 0.03 b |
| Parameter | 0 mg Pb(NO3)2 kg−1 | 50 mg Pb(NO3)2 kg−1 | 250 mg Pb(NO3)2 kg−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0% | 20% | 40% | 0% | 20% | 40% | 0% | 20% | 40% | |
| Ag, μmol m−2 s−1 | 7.6 ± 0.5 abc | 7.9 ± 0.6 ab | 8.9 ± 0.6 a | 6.6 ± 0.5 bc | 7.6 ± 0.5 abc | 7.5 ± 0.7 abc | 5.9 ± 0.4 c | 7.7 ± 0.5 abc | 8.7 ± 0.4 a |
| Ci:Ca | 0.59 ± 0.04 ab | 0.55 ± 0.05 ab | 0.59 ± 0.03 ab | 0.61 ± 0.02 ab | 0.56 ± 0.02 ab | 0.53 ± 0.04 b | 0.62 ± 0.07 ab | 0.63 ± 0.03 a | 0.60 ± 0.04 ab |
| J, µmol m−2 s−1 | 48 ± 4 b | 53 ± 3 ab | 58 ± 3 a | 46 ± 4 ab | 53 ± 4 ab | 48 ± 5 ab | 43 ± 6 b | 46 ± 3 b | 55 ± 4 a |
| νc, µmol m−2 s−1 | 8.9 ± 0.6 b | 9.7 ± 0.7 ab | 10.8 ± 0.6 a | 8.7 ± 0.7 ab | 9.7 ± 0.6 ab | 9.1 ± 0.9 ab | 8.1 ± 1.0 b | 8.7 ± 0.8 ab | 10.4 ± 0.7 a |
| νo, µmol m−2 s−1 | 1.9 ± 0.3 a | 1.7 ± 0.4 a | 2.2 ± 0.4 a | 2.1 ± 0.1 a | 1.7 ± 0.1 a | 2.1 ± 0.3 a | 1.9 ± 0.3 a | 2.2 ± 0.2 a | 2.4 ± 0.2 a |
| α, µmol µmol−1 | 0.018 ± 0.001 a | 0.020 ± 0.002 a | 0.018 ± 0.001 a | 0.017 ± 0.001 ab | 0.017 ± 0.001 a | 0.018 ± 0.001 a | 0.014 ± 0.002 b | 0.017 ± 0.001 ab | 0.018 ± 0.001 a |
| Fo | 449 ± 8 a | 427 ± 8 a | 417 ± 20 a | 449 ± 35 a | 413 ± 9 a | 427 ± 15 a | 396 ± 22 a | 419 ± 14 a | 437 ± 23 a |
| Fv:Fm | 0.844 ± 0.004 a | 0.846 ± 0.004 a | 0.834 ± 0.012 a | 0.827 ± 0.014 a | 0.839 ± 0.007 a | 0.843 ± 0.009 a | 0.842 ± 0.007 a | 0.833 ± 0.009 a | 0.826 ± 0.016 a |
| SPAD index | 15.3 ± 0.6 ab | 16.1 ± 0.9 a | 14.2 ± 0.5 ab | 14.1 ± 0.6 ab | 14.5 ± 0.6 ab | 15.8 ± 1.6 a | 14.0 ± 1.1 ab | 13.0 ± 0.5 b | 13.0 ± 0.8 b |
| LCP, µmol m−2 s−1 | 33 ± 5 cd | 24 ± 6 d | 42 ± 7 bcd | 54 ± 6 b | 43 ± 5 bcd | 37 ± 4 bcd | 78 ± 15 a | 59 ± 6 b | 52 ± 9 c |
| Rd, µmol m−2 s−1 | 0.71 ± 0.07 bc | 0.61 ± 0.13 c | 0.87 ± 0.11 ab | 0.99 ± 0.08 ab | 0.90 ± 0.07 bc | 0.74 ± 0.06 b | 1.14 ± 0.18 a | 1.07 ± 0.09 a | 0.97 ± 0.14 ab |
| Rl, µmol m−2 s−1 | 0.58 ± 0.08 bc | 0.49 ± 0.15 c | 0.74 ± 0.11 abc | 0.86 ± 0.08 ab | 0.73 ± 0.09 abc | 0.64 ± 0.07 bc | 1.00 ± 0.17 a | 0.99 ± 0.09 a | 0.93 ± 0.15 ab |
| Rl:Rd | 0.81 ± 0.04 b | 0.76 ± 0.09 b | 0.83 ± 0.04 ab | 0.87 ± 0.01 ab | 0.87 ± 0.03 ab | 0.86 ± 0.04 ab | 0.87 ± 0.01 ab | 0.93 ± 0.03 a | 0.95 ± 0.05 a |
| 1 − Rl:Rd, % | 19 ± 4 a | 24 ± 9 a | 17 ± 4 ab | 13 ± 1 ab | 13 ± 1 ab | 16 ± 3 ab | 13 ± 1 ab | 7 ± 3 a | 5 ± 2 a |
| Tr, mmol m−2 s−1 | 1.1 ± 0.1 a | 1.0 ± 0.2 a | 1.2 ± 0.1 a | 0.9 ± 0.1 a | 1.1 ± 0.1 a | 0.9 ± 0.1 a | 0.9 ± 0.1 a | 1.1 ± 0.1 a | 1.2 ± 0.1 a |
| qs, mmol m−2 s−1 | 81 ± 9 a | 73 ± 11 a | 94 ± 9 a | 69 ± 7 a | 78 ± 8 a | 69 ± 12 a | 70 ± 10 a | 82 ± 6 a | 89 ± 7 a |
| RWC, % | 71 ± 2 a | 69 ± 3 a | 70 ± 1 a | 69 ± 2 a | 73 ± 2 a | 72 ± 1 a | 76 ± 1 a | 70 ± 2 a | 70 ± 2 a |
| PWUE, µmol mmol−1 | 6.8 ± 0.7 a | 7.3 ± 0.7 a | 6.8 ± 0.2 a | 6.4 ± 0.6 a | 6.6 ± 0.3 a | 7.2 ± 0.7 a | 6.4 ± 0.9 a | 6.4 ± 0.7 a | 6.6 ± 0.7 a |
| C, % | pH | N, mg L−1 | P, mg L−1 | K, mg L−1 | Na, mg L−1 | Ca, mg L−1 | Mg, mg L−1 |
|---|---|---|---|---|---|---|---|
| 56.7 | 7.16 | 238 | 64 | 9 | 47 | 233 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurkevich, M.; Kurbatov, A.; Ikkonen, E. Effect of Secondary Paper Sludge on Physiological Traits of Lactuca sativa L. under Heavy-Metal Stress. Plants 2024, 13, 1098. https://doi.org/10.3390/plants13081098
Yurkevich M, Kurbatov A, Ikkonen E. Effect of Secondary Paper Sludge on Physiological Traits of Lactuca sativa L. under Heavy-Metal Stress. Plants. 2024; 13(8):1098. https://doi.org/10.3390/plants13081098
Chicago/Turabian StyleYurkevich, Marija, Arkadiy Kurbatov, and Elena Ikkonen. 2024. "Effect of Secondary Paper Sludge on Physiological Traits of Lactuca sativa L. under Heavy-Metal Stress" Plants 13, no. 8: 1098. https://doi.org/10.3390/plants13081098






