Effects of Short-Term Nitrogen Additions on Biomass and Soil Phytochemical Cycling in Alpine Grasslands of Tianshan, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Sample Collection and Sample Determination Methods
2.3.1. Sample Collection
2.3.2. Sample Determination Methods
2.4. Data Handling
3. Results
3.1. Plant Aboveground Biomass Response to Nitrogen Addition
3.2. Response of Plant Chemical Traits to Nitrogen Addition
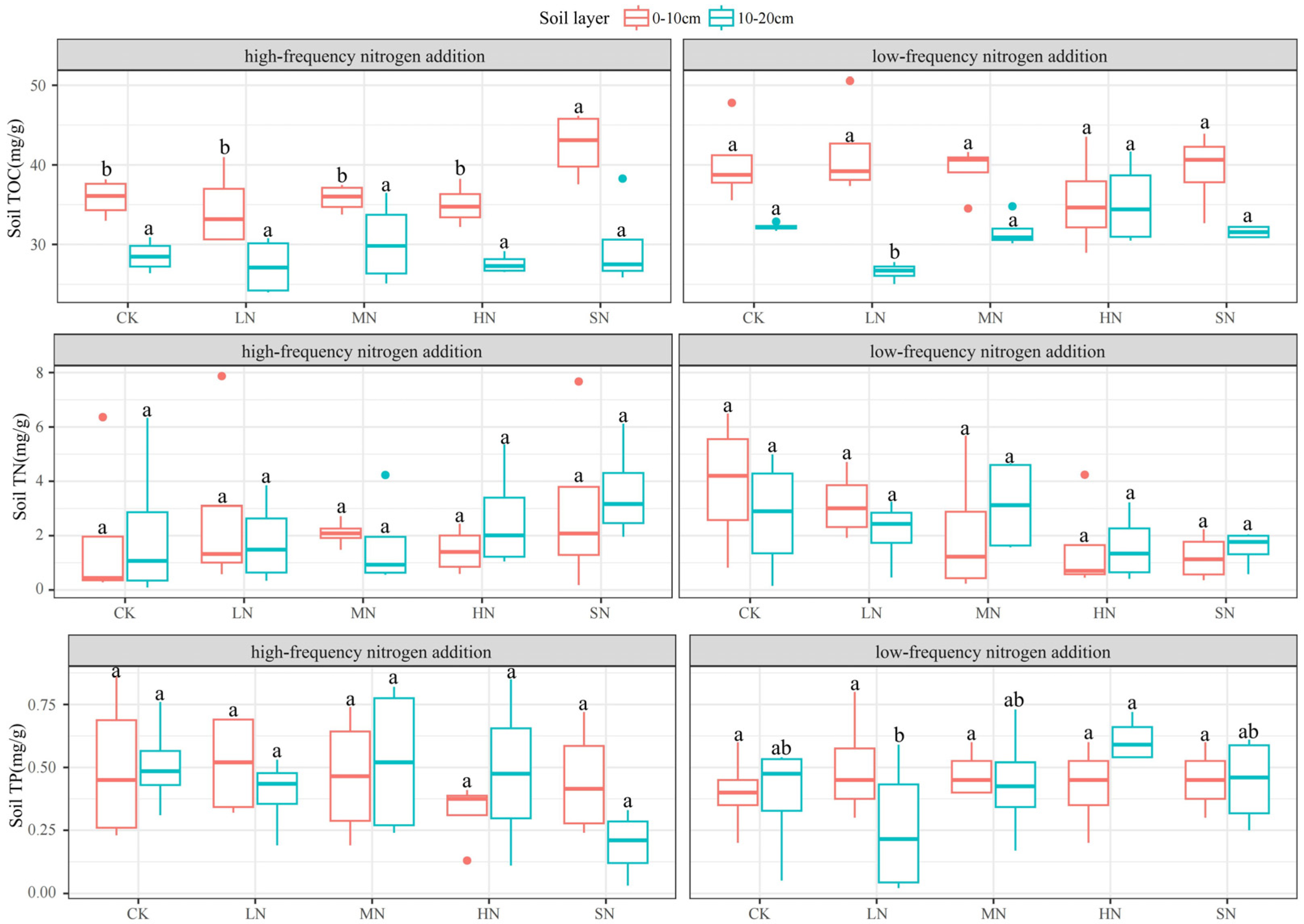
3.3. Response of Physicochemical Soil Properties to Nitrogen Addition
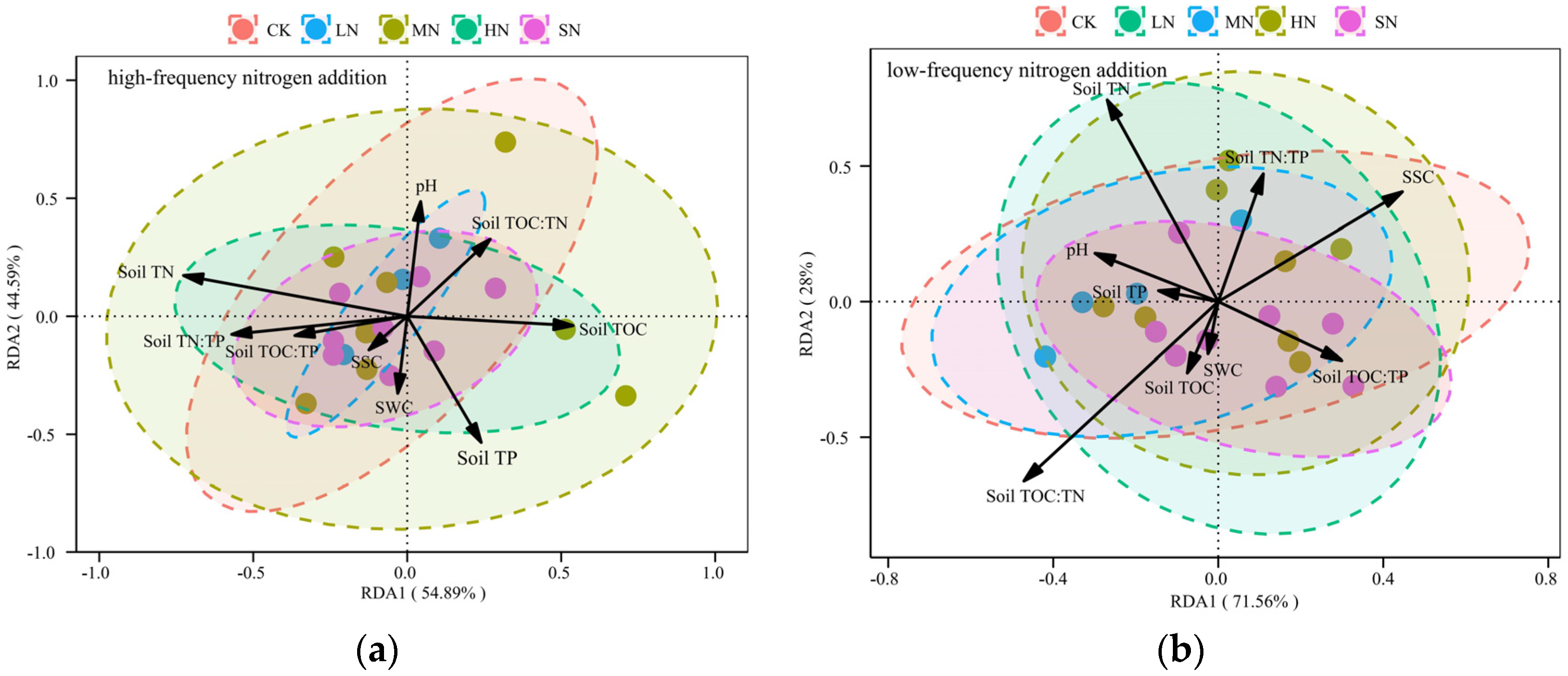
3.4. Redundant Analysis of Biomass Changes
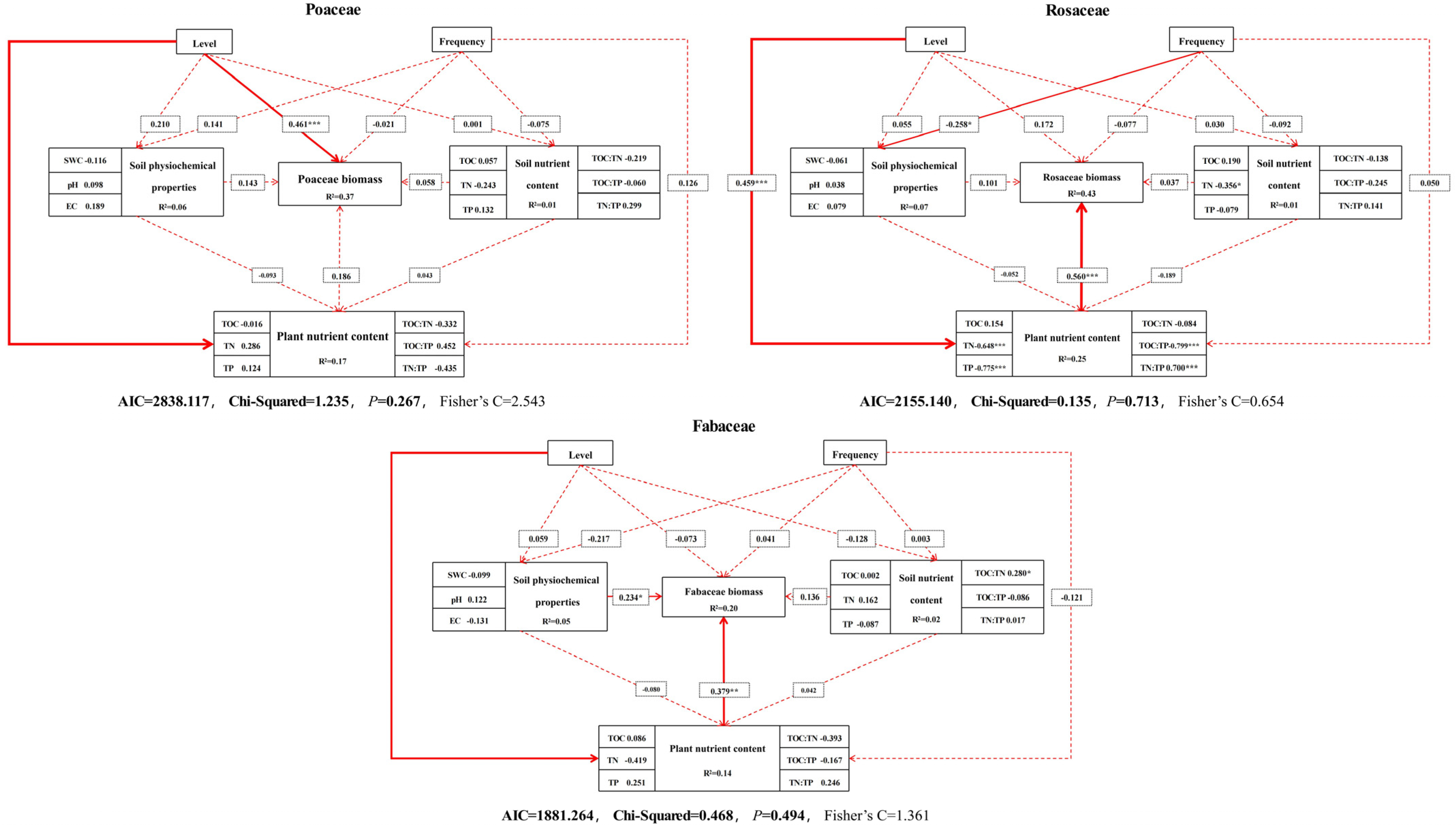
3.5. Impact Pathway Analysis of Biomass Changes in Different Functional Groups
4. Discussion
4.1. Nitrogen Addition Promotes Aboveground Biomass Growth
4.2. Nitrogen Addition Exacerbates P Limitation of Plant Growth
4.3. Nitrogen Addition Improves Plant Growth
4.4. Nitrogen Addition Level Key to Plant Productivity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, X.; Zheng, J.; An, Y.; Xin, X.; Xu, D.; Yan, R.; Xu, L.; Shen, B.; Hou, L. Grassland Ecosystem Progress: A Review and Bibliometric Analysis Based on Research Publication over the Last Three Decades. Agronomy 2023, 13, 614. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Wu, J. Grassland ecosystem services: A systematic review of research advances and future directions. Landsc. Ecol. 2020, 35, 793–814. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, J.; Yuan, X.; Zhu, B. Effects of warming on carbon and nitrogen cycling in alpine grassland ecosystems on the Tibetan Plateau: A meta-analysis. Geoderma 2020, 370, 114363. [Google Scholar] [CrossRef]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’connor, T.; O’farrell, P.; Smith, H.G.; Lindborg, R. Grasslands—More important for ecosystem services than you might think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- Fang, J.; Yang, Y.; Ma, W.; Mohammat, A.; Shen, H. Ecosystem carbon stocks and their changes in China’s grasslands. Sci. China Life Sci. 2010, 53, 757–765. [Google Scholar] [CrossRef]
- Zhang, C.H.; Guo, H.R.; Huang, H.; Ma, T.Y.; Song, W.; Chen, C.J.; Liu, X.Y. Atmospheric nitrogen deposition and its responses to anthropogenic emissions in a global hotspot region. Atmos. Res. 2021, 248, 105137. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Yu, G.; Jia, Y.; He, N.; Zhu, J.; Chen, Z.; Wang, Q.; Piao, S.; Liu, X.; He, H.; Guo, X.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Zhang, R.; Schellenberg, M.P.; Tian, D.; Ma, F.; Zhang, T.; Wang, H.; Wu, Q.; Bai, Y.; Han, G.; Niu, S. Shifting community composition determines the biodiversity–productivity relationship under increasing precipitation and N deposition. J. Veg. Sci. 2021, 32, e12998. [Google Scholar] [CrossRef]
- Verma, P.; Sagar, R. Responses of diversity, productivity, and stability to the nitrogen input in a tropical grassland. Ecol. Appl. 2020, 30, e02037. [Google Scholar] [CrossRef]
- Lu, P.; Hao, T.; Li, X.; Wang, H.; Zhai, X.; Tian, Q.; Bai, W.; Stevens, C.; Zhang, W.H. Ambient nitrogen deposition drives plant-diversity decline by nitrogen accumulation in a closed grassland ecosystem. J. Appl. Ecol. 2021, 58, 1888–1898. [Google Scholar] [CrossRef]
- Liu, S.; Yang, R.; Peng, X.; Hou, C.; Ma, J.; Guo, J. Contributions of Plant Litter Decomposition to Soil Nutrients in Ecological Tea Gardens. Agriculture 2022, 12, 957. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, H.; Yi, H. Impact of Fertilizer on Crop Yield and C:N:P Stoichiometry in Arid and Semi-Arid Soil. Int. J. Environ. Res. Public Health 2021, 18, 4341. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Xu, C.; Xu, X.; Ju, C.; Chen, H.Y.; Wilsey, B.J.; Luo, Y.; Fan, W. Long-term, amplified responses of soil organic carbon to nitrogen addition worldwide. Glob. Chang. Biol. 2021, 27, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Lu, X.; Mo, J.; Gilliam, F.S.; Fang, H.; Zhu, F.; Fang, Y.; Zhang, W.; Huang, J. Nitrogen Addition Shapes Soil Phosphorus Availability in Two Reforested Tropical Forests in Southern China. Biotropica 2012, 44, 302–311. [Google Scholar] [CrossRef]
- Schleuss, P.M.; Widdig, M.; Heintz-Buschart, A.; Guhr, A.; Martin, S.; Kirkman, K.; Spohn, M. Stoichiometric controls of soil carbon and nitrogen cycling after long-term nitrogen and phosphorus addition in a mesic grassland in South Africa. Soil Biol. Biochem. 2019, 135, 294–303. [Google Scholar] [CrossRef]
- Tian, J.; Dungait, J.A.; Lu, X.; Yang, Y.; Hartley, I.P.; Zhang, W.; Mo, J.; Yu, G.; Zhou, J.; Kuzyakov, Y. Long-term nitrogen addition modifies microbial composition and functions for slow carbon cycling and increased sequestration in tropical forest soil. Glob. Chang. Biol. 2019, 25, 3267–3281. [Google Scholar] [CrossRef]
- Chen, D.; Xing, W.; Lan, Z.; Saleem, M.; Wu, Y.; Hu, S.; Bai, Y. Direct and indirect effects of nitrogen enrichment on soil organisms and carbon and nitrogen mineralization in a semi-arid grassland. Funct. Ecol. 2019, 33, 175–187. [Google Scholar] [CrossRef]
- Aber, J.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen Saturation in Temperate Forest Ecosystems: Hypotheses revisited. BioScience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Elrys, A.S.; Wang, J.; Meng, L.; Zhu, Q.; El-Sawy, M.M.; Chen, Z.; Tu, X.; El-Saadony, M.T.; Zhang, Y.; Zhang, J.; et al. Integrative knowledge-based nitrogen management practices can provide positive effects on ecosystem nitrogen retention. Nat. Food 2023, 4, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Gurmesa, G.A.; Hobbie, E.A.; Zhang, S.; Wang, A.; Zhu, F.; Zhu, W.; Koba, K.; Yoh, M.; Wang, C.; Zhang, Q.; et al. Natural 15N abundance of ammonium and nitrate in soil profiles: New insights into forest ecosystem nitrogen saturation. Ecosphere 2022, 13, e3998. [Google Scholar] [CrossRef]
- Gilliam, F.S. Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J. Ecol. 2006, 94, 1176–1191. [Google Scholar] [CrossRef]
- Humbert, J.Y.; Dwyer, J.M.; Andrey, A.; Arlettaz, R. Impacts of nitrogen addition on plant biodiversity in mountain grasslands depend on dose, application duration and climate: A systematic review. Glob. Chang. Biol. 2016, 22, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yang, Y.; Luo, Y.; Fang, C.; Zhou, X.; Chen, J.; Yang, X.; Li, B. Responses of ecosystem nitrogen cycle to nitrogen addition: A meta-analysis. New Phytol. 2011, 189, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Loreau, M.; Lü, X.; He, N.; Zhang, G.; Han, X. Nitrogen enrichment weakens ecosystem stability through decreased species asynchrony and population stability in a temperate grassland. Glob. Chang. Biol. 2016, 22, 1445–1455. [Google Scholar] [CrossRef]
- Wang, W.; Tang, J.; Zhang, N.; Wang, Y.; Xu, X.; Zhang, A. Spatiotemporal Pattern of Invasive Pedicularis in the Bayinbuluke Land, China, during 2019–2021: An Analysis Based on PlanetScope and Sentinel-2 Data. Remote Sens. 2023, 15, 4383. [Google Scholar] [CrossRef]
- Li, K.; Liu, X.; Song, L.; Gong, Y.; Lu, C.; Yue, P.; Tian, C.; Zhang, F. Response of alpine grassland to elevated nitrogen deposition and water supply in China. Oecologia 2015, 177, 65–72. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Zhang, L.X.; Zhou, X.L.; Fan, L.L.; Mao, J.F.; Li, Y.M. Response of soil aggregate structure and nutrient content to simulated nitrogen and phosphorus deposition in alpine grassland of Tianshan Mountain. J. Ecol. 2023, 43, 4130–4141. (In Chinese) [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis; China Agricultural Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Zhang, J.; Zuo, X.; Lv, P. Effects of Grazing, Extreme Drought, Extreme Rainfall and Nitrogen Addition on Vegetation Characteristics and Productivity of Semiarid Grassland. Int. J. Environ. Res. Public Health 2023, 20, 960. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Charles, L.S.; Yang, Z. Differential Mechanisms Drive Species Loss under Artificial Shade and Fertilization in the Alpine Meadow of the Tibetan Plateau. Front. Plant Sci. 2022, 13, 832473. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gilliam, F.S.; Guo, J.; Hou, E.; Kuang, Y. Decrease in soil pH has greater effects than increase in above-ground carbon inputs on soil organic carbon in terrestrial ecosystems of China under nitrogen enrichment. J. Appl. Ecol. 2022, 59, 768–778. [Google Scholar] [CrossRef]
- Band, N.; Kadmon, R.; Mandel, M.; DeMalach, N. Assessing the roles of nitrogen, biomass, and niche dimensionality as drivers of species loss in grassland communities. Proc. Natl. Acad. Sci. USA 2022, 119, e2112010119. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wu, J.; Clark, C.M.; Naeem, S.; Pan, Q.; Huang, J.; Zhang, L.; Han, X. Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: Evidence from inner Mongolia Grasslands. Glob. Chang. Biol. 2010, 16, 358–372. [Google Scholar] [CrossRef]
- Van Sundert, K.; Arfin Khan, M.A.; Bharath, S.; Buckley, Y.M.; Caldeira, M.C.; Donohue, I.; Dubbert, M.; Ebeling, A.; Eisenhauer, N.; Eskelinen, A.; et al. Fertilized graminoids intensify negative drought effects on grassland productivity. Glob. Chang. Biol. 2021, 27, 2441–2457. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Huang, Y.; Qi, Y.; Sheng, Z.; Chen, H. Effects of nitrogen addition on vegetation and soil and its linkages to plant diversity and productivity in a semi-arid steppe. Sci. Total Environ. 2021, 778, 146299. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wen, S.; Hu, W.; Du, G. Root–shoot competition interactions cause diversity loss after fertilization: A field experiment in an alpine meadow on the Tibetan Plateau. J. Plant Ecol. 2011, 4, 138–146. [Google Scholar] [CrossRef]
- DeMalach, N.; Kadmon, R. Light competition explains diversity decline better than niche dimensionality. Funct. Ecol. 2017, 31, 1834–1838. [Google Scholar] [CrossRef]
- Yang, B.; Qiao, N.; Xu, X.; Ouyang, H. Symbiotic nitrogen fixation by legumes in two Chinese grasslands estimated with the 15N dilution technique. Nutr. Cycl. Agroecosystems 2011, 91, 91–98. [Google Scholar] [CrossRef]
- Mao, J.; Mao, Q.; Zheng, M.; Mo, J. Responses of Foliar Nutrient Status and Stoichiometry to Nitrogen Addition in Different Ecosystems: A Meta-analysis. J. Geophys. Res. Biogeosciences 2020, 125, e2019JG005347. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2003. [Google Scholar]
- Pang, Y.; Tian, J.; Wang, D. Response of multi-ecological component stoichiometry and tree nutrient resorption to medium-term whole-tree harvesting in secondary forests in the Qinling Mountains, China. For. Ecol. Manag. 2021, 498, 119573. [Google Scholar] [CrossRef]
- Zhang, Y.; Mai, H.; Qiu, Q.; Zhu, Y.; Long, J.; Chen, S.; Chen, Y. The Responses of C, N, P and Stoichiometric Ratios to Biochar and Vermicompost Additions Differ from Alfalfa and a Mine Soil. Agriculture 2023, 13, 1954. [Google Scholar] [CrossRef]
- Long, M.; Wu, H.H.; Smith, M.D.; La Pierre, K.J.; Lü, X.T.; Zhang, H.Y.; Han, X.G.; Yu, Q. Nitrogen deposition promotes phosphorus uptake of plants in a semi-arid temperate grassland. Plant Soil 2016, 408, 475–484. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The Vegetation N:P Ratio: A New Tool to Detect the Nature of Nutrient Limitation. J. Appl. Ecol. 1996, 33, 1441. [Google Scholar] [CrossRef]
- Güsewell, S. N : P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Xie, Y.; Qiu, K.; Xu, D.; Shi, X.; Qi, T.; Pott, R. Spatial heterogeneity of soil and vegetation characteristics and soil-vegetation relationships along an ecotone in Southern Mu Us Sandy Land, China. J. Soils Sediments 2015, 15, 1584–1601. [Google Scholar] [CrossRef]
- Deekshitha, D.K.D.; Rao, C.S.; Subbaiah, P.V.; Luther, M.M.; Rao, V.S. Direct and Residual Effect of Integrated Nitrogen Management on Physico-Chemical Properties of Soil. Int. J. Environ. Clim. Chang. 2021, 11, 84–99. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Guo, D.; Zhang, H.; Che, Y.; Li, Y.; Tian, B.; Wang, Z.; Sun, G.; Zhang, H. Physiological and comparative transcriptome analysis of leaf response and physiological adaption to saline alkali stress across pH values in alfalfa (Medicago sativa). Plant Physiol. Biochem. 2021, 167, 140–152. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, S.; Xing, F.; Mu, X.; Bai, Y. Nitrogen addition interacted with salinity-alkalinity to modify plant diversity, microbial PLFAs and soil coupled elements: A 5-year experiment. Appl. Soil Ecol. 2019, 137, 78–86. [Google Scholar] [CrossRef]
- Han, J.; Shi, J.; Zeng, L.; Xu, J.; Wu, L. Effects of nitrogen fertilization on the acidity and salinity of greenhouse soils. Environ. Sci. Pollut. Res. 2015, 22, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.N.; Lü, X.T.; Liu, Y.; Guo, J.X.; Zhang, N.Y.; Yang, J.Q.; Wang, R.Z. The Effects of Warming and Nitrogen Addition on Soil Nitrogen Cycling in a Temperate Grassland, Northeastern China. PLoS ONE 2011, 6, e27645. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.M.; Naqvi, S.S.M.; Ansari, R. Impact of soil pH on nutrient uptake by crop plants. In Handbook of Plant and Crop Stress; Marcel Dekker, Inc.: New York, NY, USA, 1999; pp. 51–60. [Google Scholar]
- Zhang, R.; Nie, L.; Huang, M.; Yang, H.; Shi, C.; Wei, Y.; Song, L.; Zhu, J.; Bo, H.; Wang, J.; et al. Effects of Irrigation and Nitrogen Application on Soil Nutrients in Triploid Populus tomentosa Stands. Forests 2022, 13, 1046. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, W.; Zheng, C.; Zhu, B. Nitrogen addition has contrasting effects on particulate and mineral-associated soil organic carbon in a subtropical forest. Soil Biol. Biochem. 2020, 142, 107708. [Google Scholar] [CrossRef]
- Fathi, A. Role of nitrogen (N) in plant growth, photosynthesis pigments, and N use efficiency: A review. Agrisost 2022, 28, 1–8. [Google Scholar]
- Gao, W.; Yang, H.; Kou, L.; Li, S. Effects of nitrogen deposition and fertilization on N transformations in forest soils: A review. J. Soils Sediments 2015, 15, 863–879. [Google Scholar] [CrossRef]
- Devau, N.; Le Cadre, E.; Hinsinger, P.; Jaillard, B.; Gérard, F. Soil pH controls the environmental availability of phosphorus: Experimental and mechanistic modelling approaches. Appl. Geochem. 2009, 24, 2163–2174. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef]
- Deng, Q.; Hui, D.; Dennis, S.; Reddy, K.C. Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition: A meta-analysis. Glob. Ecol. Biogeogr. 2017, 26, 713–728. [Google Scholar] [CrossRef]
- Huang, J.Y.; Lai, R.S.; Yu, H.L.; Chen, W.M. Effects of N addition on the ecological stoichiometric characteristics of plant and soil C:N:P in Ningxia desert grassland. J. Ecol. 2013, 32, 2850–2856. (In Chinese) [Google Scholar]
- Sun, X.; Yu, K.; Shugart, H.H.; Wang, G. Species richness loss after nutrient addition as affected by N:C ratios and phytohormone GA3 contents in an alpine meadow community. J. Plant Ecol. 2016, 9, 201–211. [Google Scholar] [CrossRef]
- Wang, N.; Xu, S.S.; Jia, X.; Gao, J.; Zhang, W.P.; Qiu, Y.P.; Wang, G.X. Variations in foliar stable carbon isotopes among functional groups and along environmental gradients in China—A meta-analysis. Plant Biol. 2013, 15, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Fayiah, M.; Dong, S.; Li, Y.; Xu, Y.; Gao, X.; Li, S.; Shen, H.; Xiao, J.; Yang, Y.; Wessell, K. The relationships between plant diversity, plant cover, plant biomass and soil fertility vary with grassland type on Qinghai-Tibetan Plateau. Agric. Ecosyst. Environ. 2019, 286, 106659. [Google Scholar] [CrossRef]


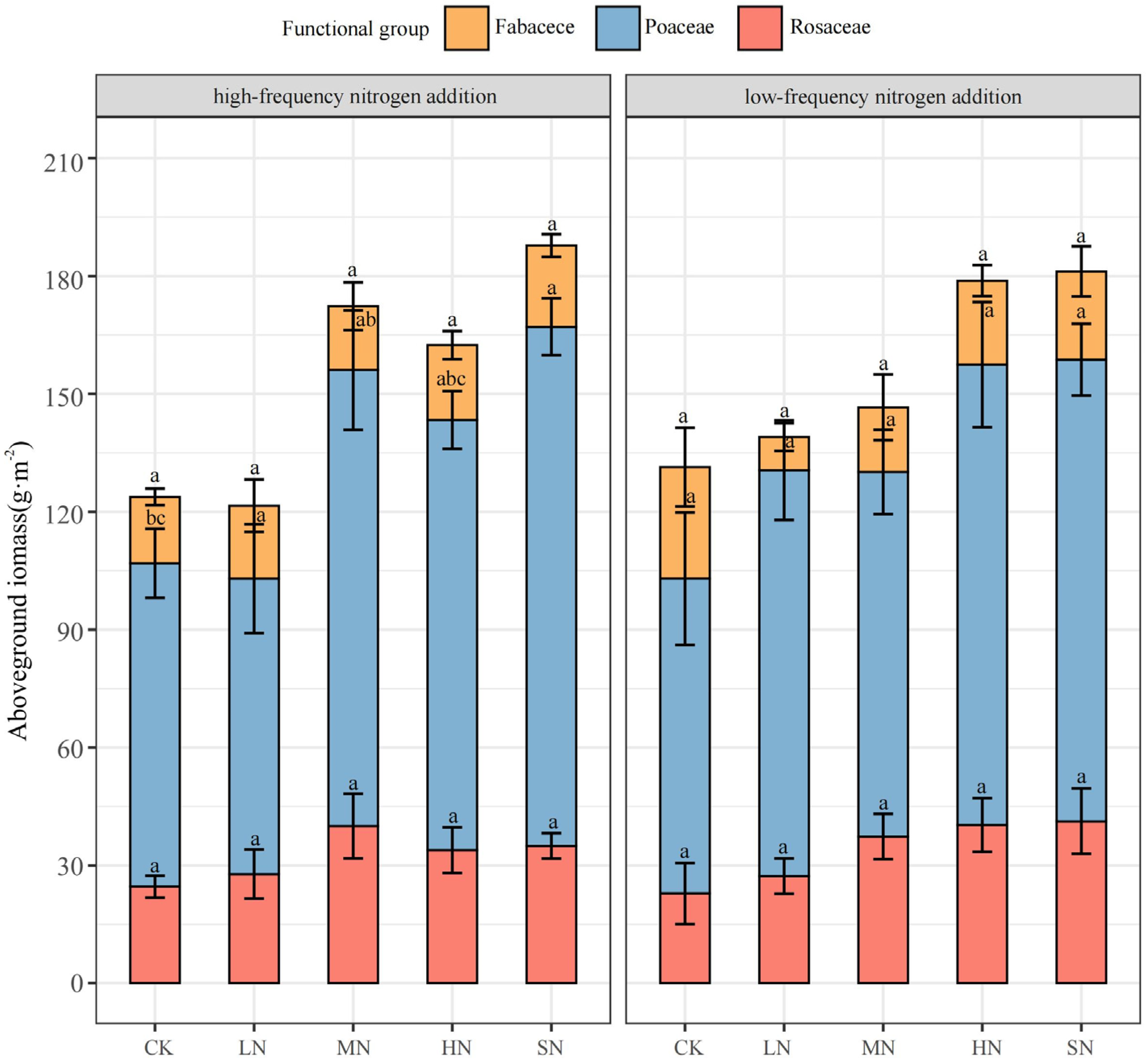
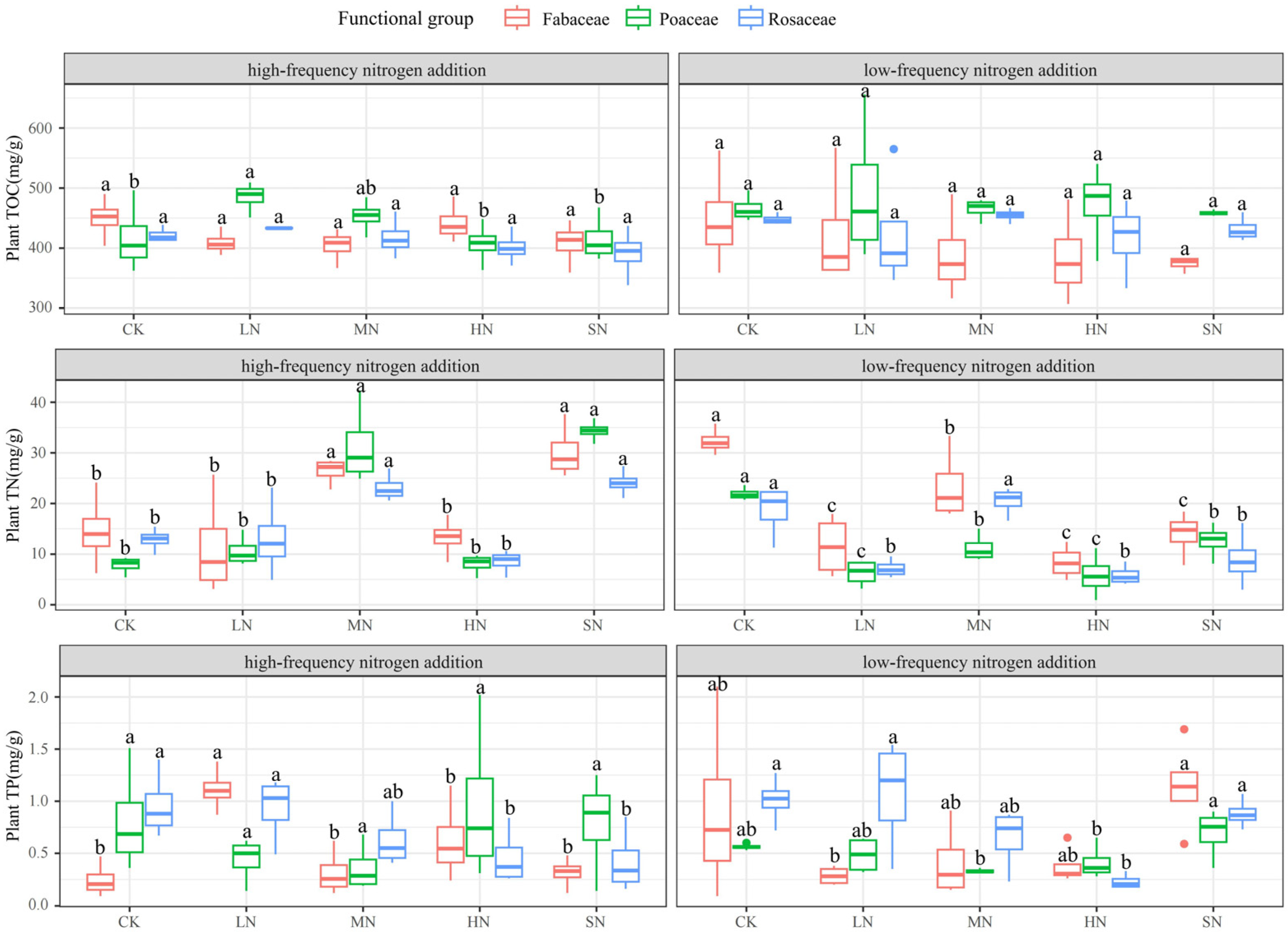
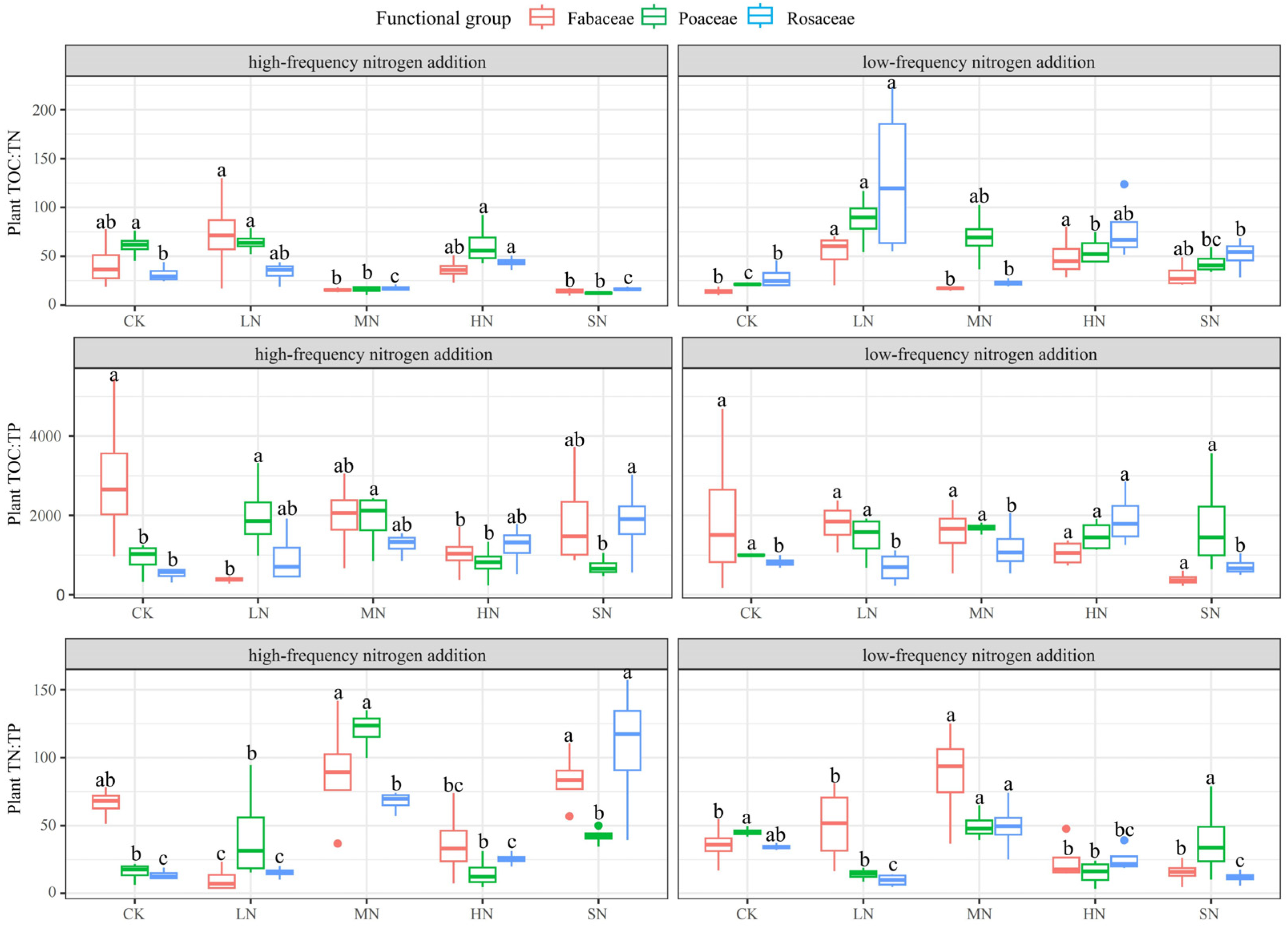
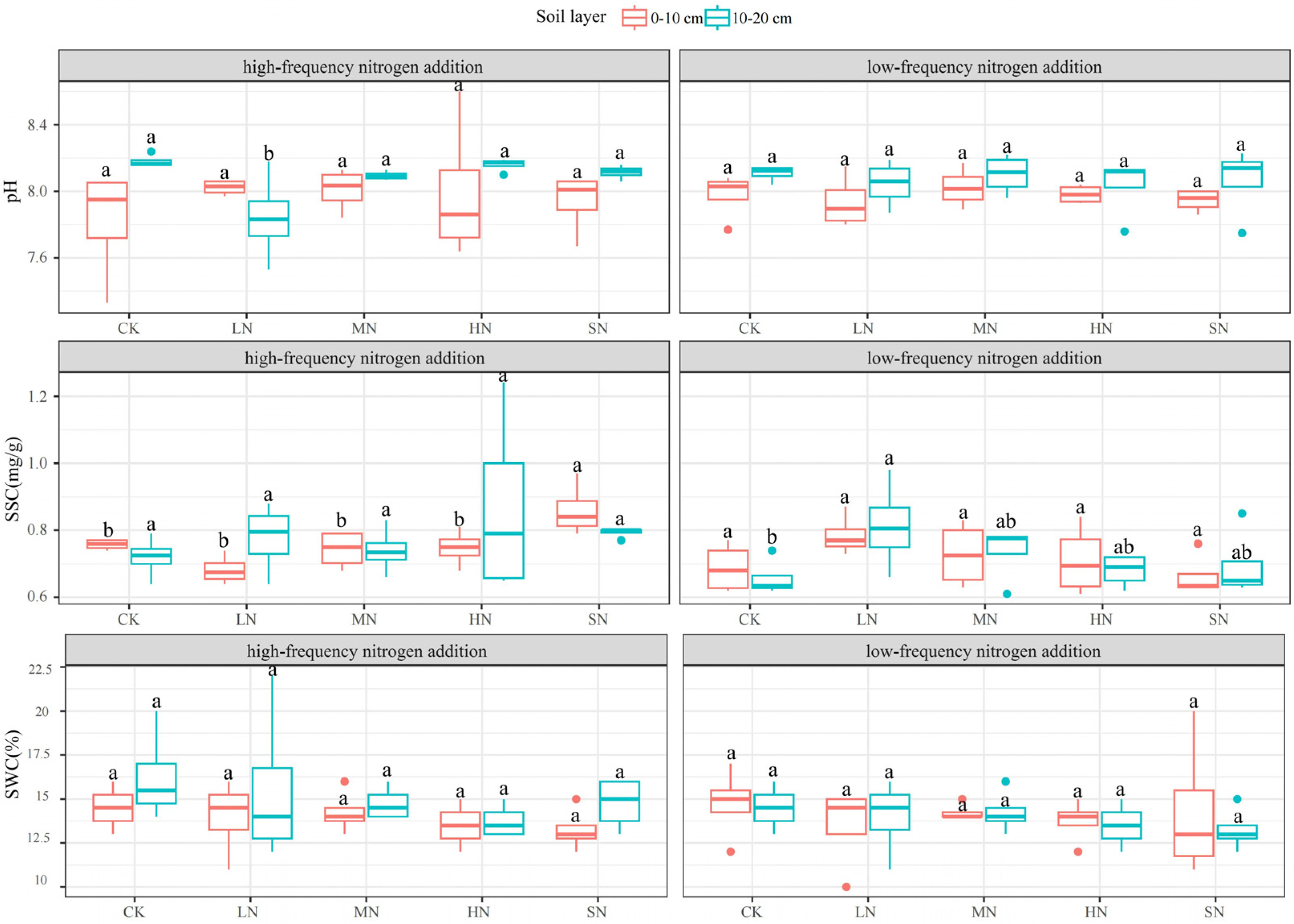

| AGB (g·m−2) | Nitrogen Addition Levels | ||||
|---|---|---|---|---|---|
| CK | LN | MN | HN | SN | |
| High-frequency nitrogen addition | 123.79 ± 8.13 b | 121.55 ± 9.94 b | 172.40 ± 16.14 a | 163.42 ± 4.36 a | 187.88 ± 3.48 a |
| Low-frequency nitrogen addition | 132.43 ± 9.77 b | 139.03 ± 12.62 b | 146.57 ± 4.18 ab | 178.82 ± 18.86 a | 181.17 ± 8.11 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Liu, J.; Wang, J.; Ding, X. Effects of Short-Term Nitrogen Additions on Biomass and Soil Phytochemical Cycling in Alpine Grasslands of Tianshan, China. Plants 2024, 13, 1103. https://doi.org/10.3390/plants13081103
Liu C, Liu J, Wang J, Ding X. Effects of Short-Term Nitrogen Additions on Biomass and Soil Phytochemical Cycling in Alpine Grasslands of Tianshan, China. Plants. 2024; 13(8):1103. https://doi.org/10.3390/plants13081103
Chicago/Turabian StyleLiu, Chao, Junjie Liu, Juan Wang, and Xiaoyu Ding. 2024. "Effects of Short-Term Nitrogen Additions on Biomass and Soil Phytochemical Cycling in Alpine Grasslands of Tianshan, China" Plants 13, no. 8: 1103. https://doi.org/10.3390/plants13081103






