GmMYB114 Facilitates the Synthesis of Anthocyanins in Soybean Sprouts under Blue Light
Abstract
:1. Introduction
2. Results
2.1. Blue Light Promotes the Synthesis of Anthocyanins in Soybean Sprouts
2.2. Identification of GmMYB114 as a Positive Regulator of Anthocyanins in Soybean Sprouts Response to Blue Light
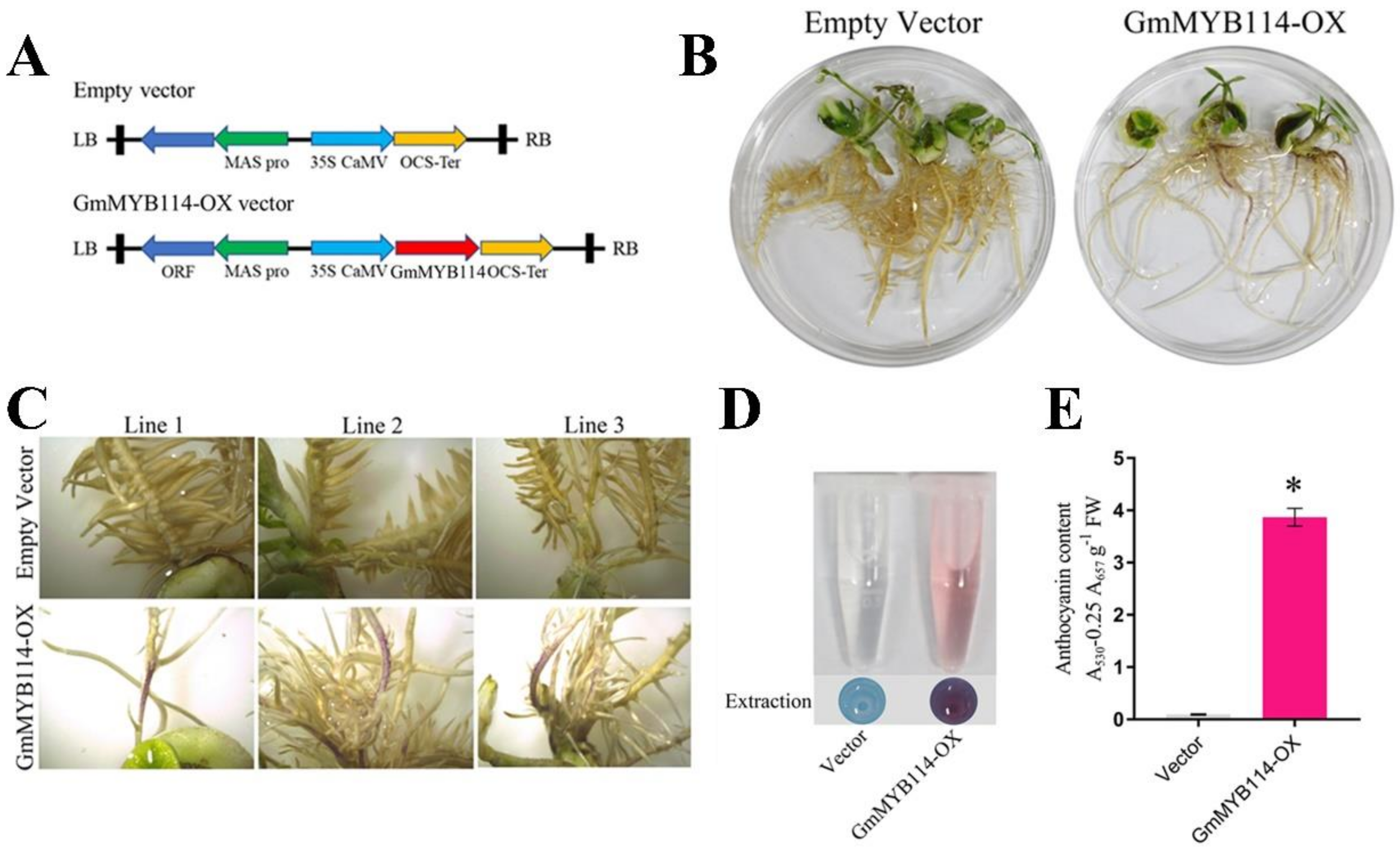
2.3. GmMYB114 Can Promote the Synthesis of Anthocyanins in Soybean Hairy Roots
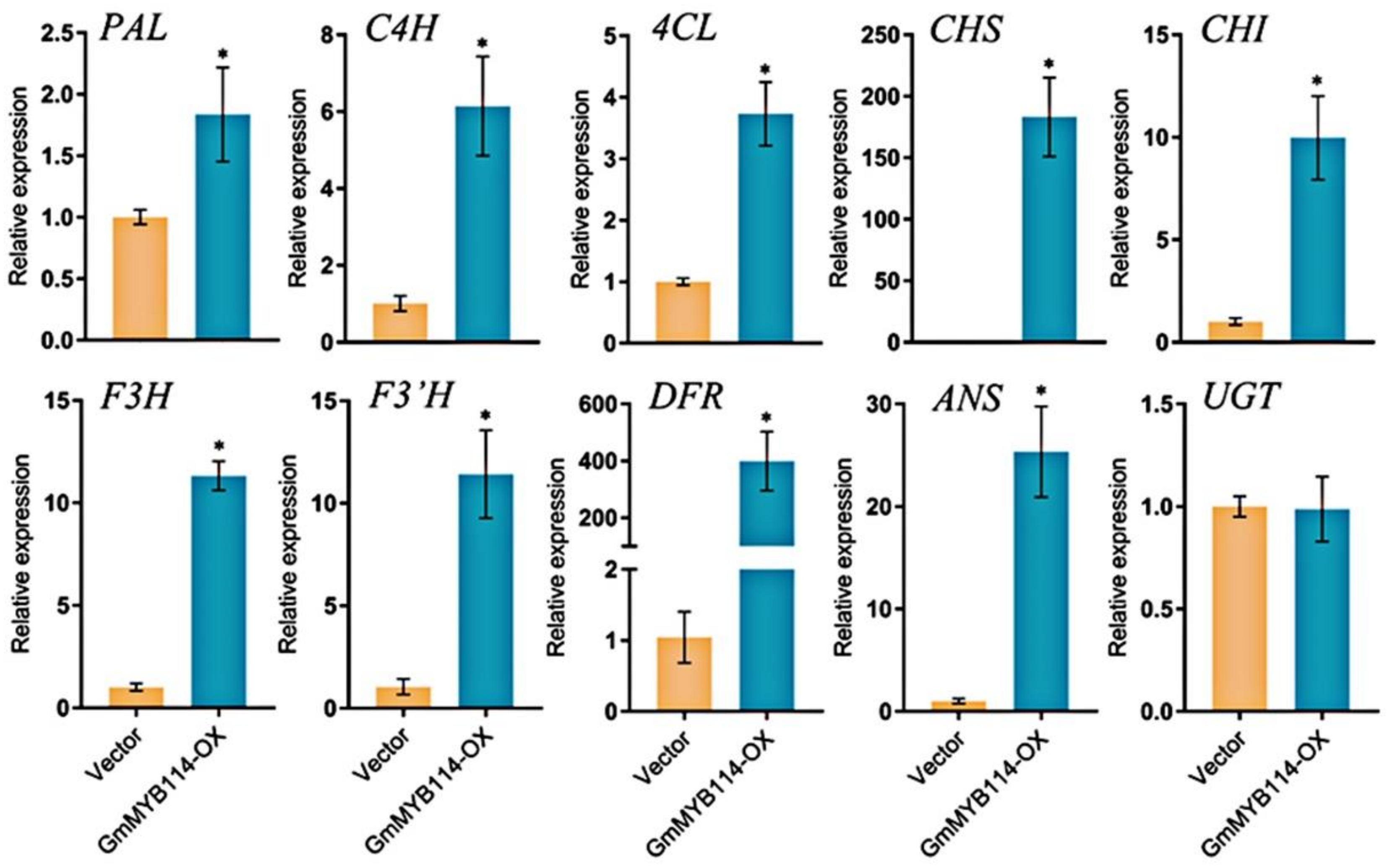
2.4. Overexpression of GmMYB114 Can Promote the Expression of Anthocyanin Synthesis Genes
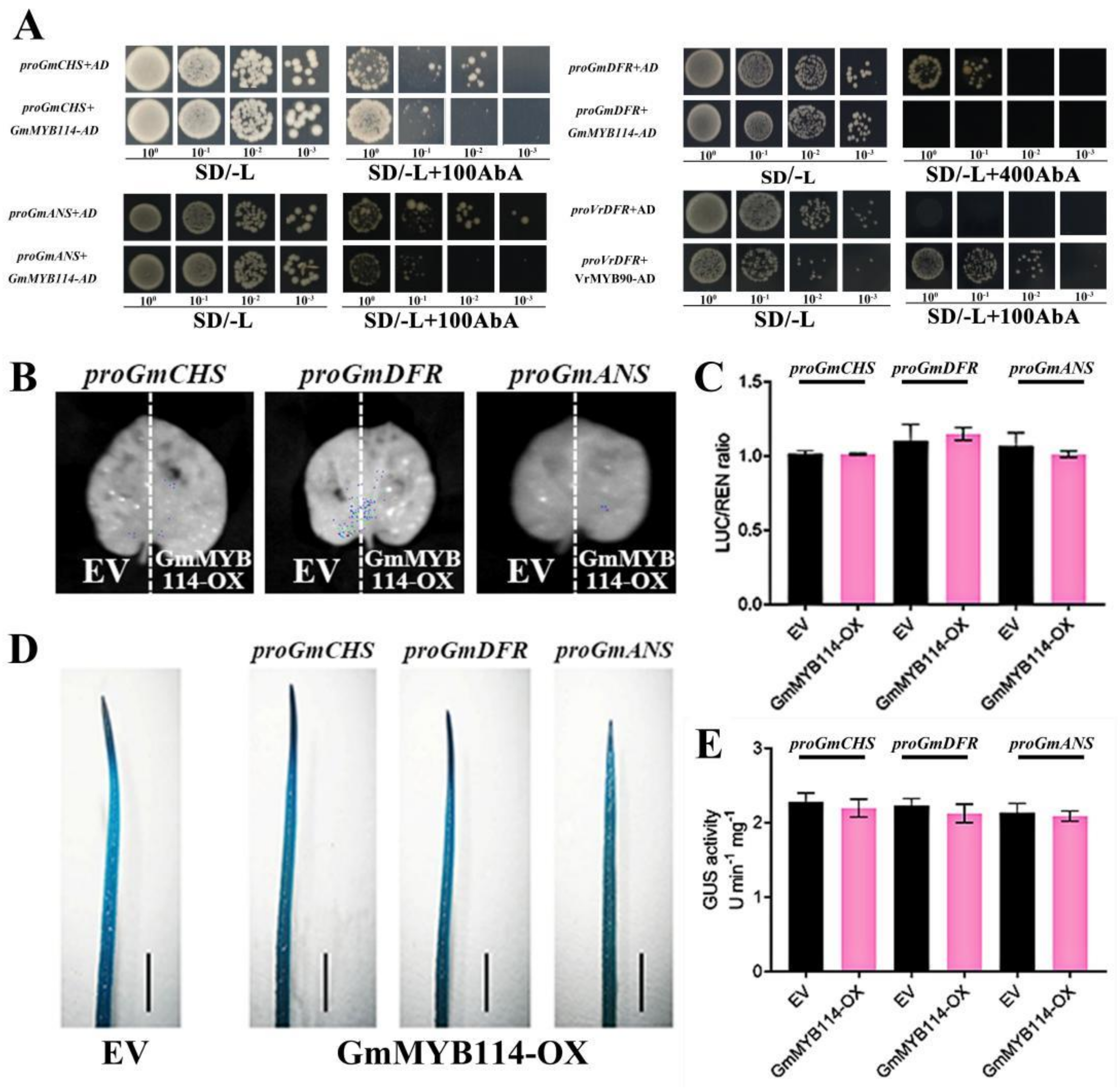
2.5. GmMYB114 Cannot Directly Regulate the Expression of GmCHS, GmDFR, and GmANS
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Extraction and Content Determination of Anthocyanins
4.3. Determination of Anthocyanin Monomers
4.4. RNA Extraction and Real-Time Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR) Analysis
4.5. Phylogenetic Tree and Amino Acid Sequence Analysis
4.6. Subcellular Localization Assay
4.7. Yeast One-Hybrid (Y1H) Assays
4.8. Dual Luciferase (LUC) Assays
4.9. β-Glucuronidase Activity (GUS) Assays
4.10. Transient Expression Assays in Soybean Hairy Roots
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khattak, A.B.; Zeb, A.; Bibi, N. Impact of germination time and type of illumination on carotenoid content, protein solubility and in vitro protein digestibility of chickpea (Cicer arietinum L.) sprouts. J. Agric. Food Chem. 2008, 109, 797–801. [Google Scholar]
- Guo, X.B.; Li, T.; Tang, K.X.; Liu, R.H. Effect of germination on phytochemical profiles and antioxidant activity of mung bean sprouts (Vigna radiata). J. Agric. Food Chem. 2021, 60, 11050–11055. [Google Scholar] [CrossRef]
- Li, X.; Thwe, A.A.; Park, N.I.; Suzuki, T.; Kim, S.J.; Park, S.U. Accumulation of phenylpropanoids and correlated gene expression during the development of Tartary Buckwheat sprouts. J. Agric. Food Chem. 2021, 60, 5629–5635. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, S.H.; Chung, J.I.; Chi, H.Y.; Kim, J.A.; Chung, I.M. Analysis of phenolic compounds and isoflavones in soybean seeds (Glycine max (L.) Merill) and sprouts grown under different conditions. Eur. Food Res. Technol. 2006, 222, 201–208. [Google Scholar]
- Chen, Y.M.; Chang, S.K.C. Macronutrients, phytochemicals, and antioxidant activity of soybean sprout germinated with or without light exposure. J Food Sci. 2015, 80, S1391–S1398. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.W.; Chang, C.H.; Yan, M.R.; Yang, R.Y. Nutritional composition of mungbean and soybean sprouts compared to their adult growth stage. J. Agric. Food Chem. 2017, 237, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.L.; Tian, S.; Li, S.P.; Guo, M.X.; Liu, H.X.; Li, Y.Y.; Wang, Q.J.; Zhao, X.S. A simple, rapid and efficient method for essential element supplementation based on seed germination. J. Agric. Food Chem. 2020, 325, 126827. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.W.; Zhao, G.X.; Zhou, Y.T.; Xia, X.Y.; Wang, J.S.; Wang, G.J.; Lu, S.W.; He, W.J.; Bi, T.F.; Li, J.R. Metabonomics analysis of flavonoids in seeds and sprouts of two Chinese soybean cultivars. Sci. Rep. 2022, 12, 5541. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Jia, X.J.; Ding, C.B.; Zeng, H.Q.; Du, L.; Yuan, S.; Zhang, Z.W.; Wu, Q.; Hu, C.; Liu, J. Effect of fluorescence light on phenolic compounds and antioxidant activities of soybeans (Glycine max L. Merrill) during germination. Food Sci. Biotechnol. 2015, 24, 1859–1865. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Lei, Y.; Zhang, J.; Tong, W.J.; Zhang, Y.S.; Du, L.H. Physiological characterization, antioxidant potential, and bacterial survival of soybean sprouts subjected to pre- and post-harvest low intensity ultrasound and exogenous ascorbic acid application. Postharvest Biol. Technol. 2023, 198, 112258. [Google Scholar] [CrossRef]
- Geng, J.Z.; Li, J.X.; Zhu, F.M.; Chen, X.N.; Du, B.; Tian, H.L.; Li, J. Plant sprout foods: Biological activities, health benefits, and bioavailability. J. Food Biochem. 2022, 46, 3. [Google Scholar] [CrossRef]
- Nolasco, E.; Krassovskaya, I.; Hong, K.L.; Hansen, K.; Alvarez, S.; Obata, T.; Majumder, K. Sprouting alters metabolite and peptide contents in the gastrointestinal digest of soybean and enhances in-vitro anti-inflammatory activity. J. Funct. Foods 2023, 109, 105780. [Google Scholar] [CrossRef]
- Su, N.N.; Wu, Q.; Qi, N.N.; Liu, Y.Y.; Li, N.; Cui, J. Effect of partial shading treatments on anthocyanin synthesis in the hypocotyls of soybean sprouts under UV-A irradiation. J. Plant Growth Regul. 2017, 36, 50–59. [Google Scholar] [CrossRef]
- Lim, Y.J.; Lyu, J.I.; Kwon, S.J.; Eom, S.H. Effects of UV-A radiation on organ-specific accumulation and gene expression of isoflavones and flavonols in soybean sprout. Food Chem. 2021, 339, 128080. [Google Scholar] [CrossRef]
- Qiu, G.; Wang, D.F.; Song, X.Y.; Deng, Y.; Zhao, Y.Y. Degradation kinetics and antioxidant capacity of anthocyanins in air-impingement jet dried purple potato slices. Food Res. Int. 2018, 105, 121–128. [Google Scholar] [CrossRef]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Agcam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic digestion, bioavailability, therapeutic effects, current pharmaceutical/industrial use, and innovation potential. Antioxidants 2023, 12, 48. [Google Scholar] [CrossRef]
- Skrede, G.; Wrolstad, R.E.; Lea, P.; Enersen, G. Color stability of strawberry and black-currant syrups. J. Food Sci. 1992, 57, 172–177. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2001, 18, 3. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Xie, T.; Zan, X.Y.; Chen, X.; Zhu, H.T.; Rong, H.; Wang, Y.P.; Jiang, J.J. An R3-MYB repressor, BnCPC forms a feedback regulation with MBW complex to modulate anthocyanin biosynthesis in Brassica napus. Biotechnol. Biofuels Bioprod. 2022, 15, 1. [Google Scholar] [CrossRef]
- Wu, H.Y.; Yang, K.M.; Chiang, P.Y. Roselle anthocyanins: Antioxidant properties and stability to heat and pH. Molecules 2018, 23, 1357. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Quecini, V.; Peres, L.E.P. Hormonal modulation of photomorphogenesis-controlled anthocyanin accumulation in tomato (Solanum lycopersicum L. cv Micro-Tom) hypocotyls: Physiological and genetic studies. Plant Sci. 2010, 178, 258–264. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Jiang, L.Y.; Li, Y.L.; Chen, Q.; Ye, Y.T.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.R.; Tang, H.R. Effect of red and blue light on anthocyanin accumulation and differential gene expression in strawberry (Fragaria × ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Yue, X.M.; Song, J.X.; Xing, G.P.; Chen, J.; Wang, H.X.; Su, N.; Cui, J. Calcium positively mediates blue light-induced anthocyanin accumulation in hypocotyl of soybean sprouts. Front. Plant Sci. 2021, 12, 662091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Bian, Z.H.; Li, S.; Chen, X.; Lu, C.G. Comparative analysis of phenolic compound profiles, antioxidant capacities, and expressions of phenolic biosynthesis-related genes in soybean microgreens grown under different light spectra. J. Agric. Food Chem. 2019, 67, 13577–13588. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Zhan, J.Y.; Wang, H.X.; Zhao, Y.D.; Zhang, D.R.; Chen, X.; Su, N.N.; Cui, J. VrMYB90 functions synergistically with VrBHLHA and VrMYB3 to regulate anthocyanin biosynthesis in mung bean. Plant Cell Physiol. 2022, 64, 221–233. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kim, W.W.; Park, C.H.; Cho, D.H. Effect of artificial LED light and far infrared irradiation on phenolic compound, isoflavones and antioxidant capacity in soybean (Glycine max L.) sprout. Foods 2018, 7, 174. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Dipierro, N.; Paciolla, C. Effects of darkness and light spectra on nutrients and pigments in radish, soybean, mung bean and pumpkin sprouts. Antioxidants 2020, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, D.L.; Gonzali, S.; Loreti, E.; Pucciariello, C.; Degl’Innocenti, E.; Guidi, L.; Alpi, A.; Perata, P. Arabidopsis thaliana MYB75/PAP1 transcription factor induces anthocyanin production in transgenic tomato plants. Funct. Plant Biol. 2008, 35, 606–618. [Google Scholar] [CrossRef]
- Maier, A.; Schrader, A.; Kokkelink, L.; Falke, C.; Welter, B.; Iniesto, E.; Rubio, V.; Uhrig, J.F.; Hülskamp, M.; Hoecker, U. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J. 2013, 74, 638–651. [Google Scholar] [CrossRef]
- Koo, Y.; Poethig, R.S. Expression pattern analysis of three R2R3-MYB transcription factors for the production of anthocyanin in different vegetative stages of Arabidopsis leaves. Appl. Biol. Chem. 2021, 64, 1. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 5. [Google Scholar] [CrossRef] [PubMed]
- Deluc, L.; Barrieu, F.; Marchive, C.; Lauvergeat, V.; Decendit, A.; Richard, T.; Carde, J.P.; Merillon, J.M.; Hamdi, S. Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol. 2006, 140, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Deluc, L.; Bogs, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.M.; Robinson, S.P.; Barrieu, F. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, L.; Sáez, V.; Hinrichsen, P.; Castro, M.H.; Vergara, C.; von Baer, D.; Mardones, C. Differences in VvUFGT and VvMYBA1 gene expression levels and phenolic composition in table grape (Vitis vinifera L.) ‘Red Globe’ and its somaclonal variant ‘Pink Globe’. J. Agric. Food Chem. 2017, 65, 2793–2804. [Google Scholar] [CrossRef] [PubMed]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 2007, 3, 49. [Google Scholar] [CrossRef]
- Ban, Y.; Honda, C.; Hatsuyama, Y.; Igarashi, M.; Bessho, H.; Moriguchi, T. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol. 2007, 48, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.Q.; Wang, Y.L.; Yang, S.; Xu, Y.T.; Chen, X.S. Anthocyanin biosynthesis in pears is regulated by a R2R3-MYB transcription factor PyMYB10. Planta 2010, 232, 245–255. [Google Scholar] [CrossRef]
- Yao, G.F.; Ming, M.L.; Allan, A.C.; Gu, C.; Li, L.T.; Wu, X.; Wang, R.Z.; Chang, Y.J.; Qi, K.J.; Zhang, S.L.; et al. Map-based cloning of the pear gene MYB114 identifies an interaction with other transcription factors to coordinately regulate fruit anthocyanin biosynthesis. Plant J. 2017, 92, 437–451. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, J.; Rhee, J.; Lee, J.Y.; Lim, S.H. Loss of the R2R3 MYB transcription factor RsMYB1 shapes anthocyanin biosynthesis and accumulation in Raphanus sativus. Int. J. Mol. Sci. 2021, 22, 10927. [Google Scholar] [CrossRef]
- Gao, R.F.; Han, T.T.; Xun, H.W.; Zeng, X.S.; Li, P.H.; Li, Y.Q.; Wang, Y.A.; Shao, Y.; Cheng, X.; Feng, X.Z.; et al. transcription factors GmMYBA2 and GmMYBR function in a feedback loop to control pigmentation of seed coat in soybean. J. Exp. Bot. 2021, 72, 4401–4418. [Google Scholar] [CrossRef]
- Baudry, A.; Caboche, M.; Lepiniec, L. TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana. Plant J. 2006, 46, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.C.; Strahle, J.T.; Selinger, D.A.; Chandler, V.L. Mutations in the pale aleurone color1 regulatory gene of the Zea mays anthocyanin pathway have distinct phenotypes relative to the functionally similar TRANSPARENT TESTA GLABRA1 gene in Arabidopsis thaliana. Plant Cell. 2004, 16, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Wu, Y.C.; Kuang, J.; Wang, H.Q.; Du, T.Z.; Huang, Y.Y.; Zhang, Y.; Cao, X.Y.; Wang, Z.Z. SmMYB111 is a key factor to phenolic acid biosynthesis and interacts with both SmTTG1 and SmbHLH51 in Salvia miltiorrhiza. J. Agric. Food Chem. 2018, 66, 8069–8078. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.Y.; Di, T.T.; Chen, X.; Zheng, T.R.; Sun, W.J.; Yang, M.; Zhou, M.; Shen, Z.G.; Chen, H.; Su, N.A. CbMYB108 integrates the regulation of diterpene biosynthesis and trichome development in Conyza blinii against UV-B. Plant Cell Environ. 2024, 47, 1300–1318. [Google Scholar] [CrossRef]
- Li, S.X.; Cong, Y.H.; Liu, Y.P.; Wang, T.T.; Shuai, Q.; Chen, N.N.; Gai, J.Y.; Li, Y. Optimization of Agrobacterium-mediated transformation in soybean. Front. Plant Sci. 2017, 8, 246. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Xu, H.; Xu, X.; Gao, K.; Zhao, K.; Dong, J.; Su, N. GmMYB114 Facilitates the Synthesis of Anthocyanins in Soybean Sprouts under Blue Light. Plants 2024, 13, 1107. https://doi.org/10.3390/plants13081107
Jia L, Xu H, Xu X, Gao K, Zhao K, Dong J, Su N. GmMYB114 Facilitates the Synthesis of Anthocyanins in Soybean Sprouts under Blue Light. Plants. 2024; 13(8):1107. https://doi.org/10.3390/plants13081107
Chicago/Turabian StyleJia, Li, Hong Xu, Xinxin Xu, Kai Gao, Keying Zhao, Jingran Dong, and Nana Su. 2024. "GmMYB114 Facilitates the Synthesis of Anthocyanins in Soybean Sprouts under Blue Light" Plants 13, no. 8: 1107. https://doi.org/10.3390/plants13081107



