Elevated Ozone Reduces the Quality of Tea Leaves but May Improve the Resistance of Tea Plants
Abstract
1. Introduction
2. Results
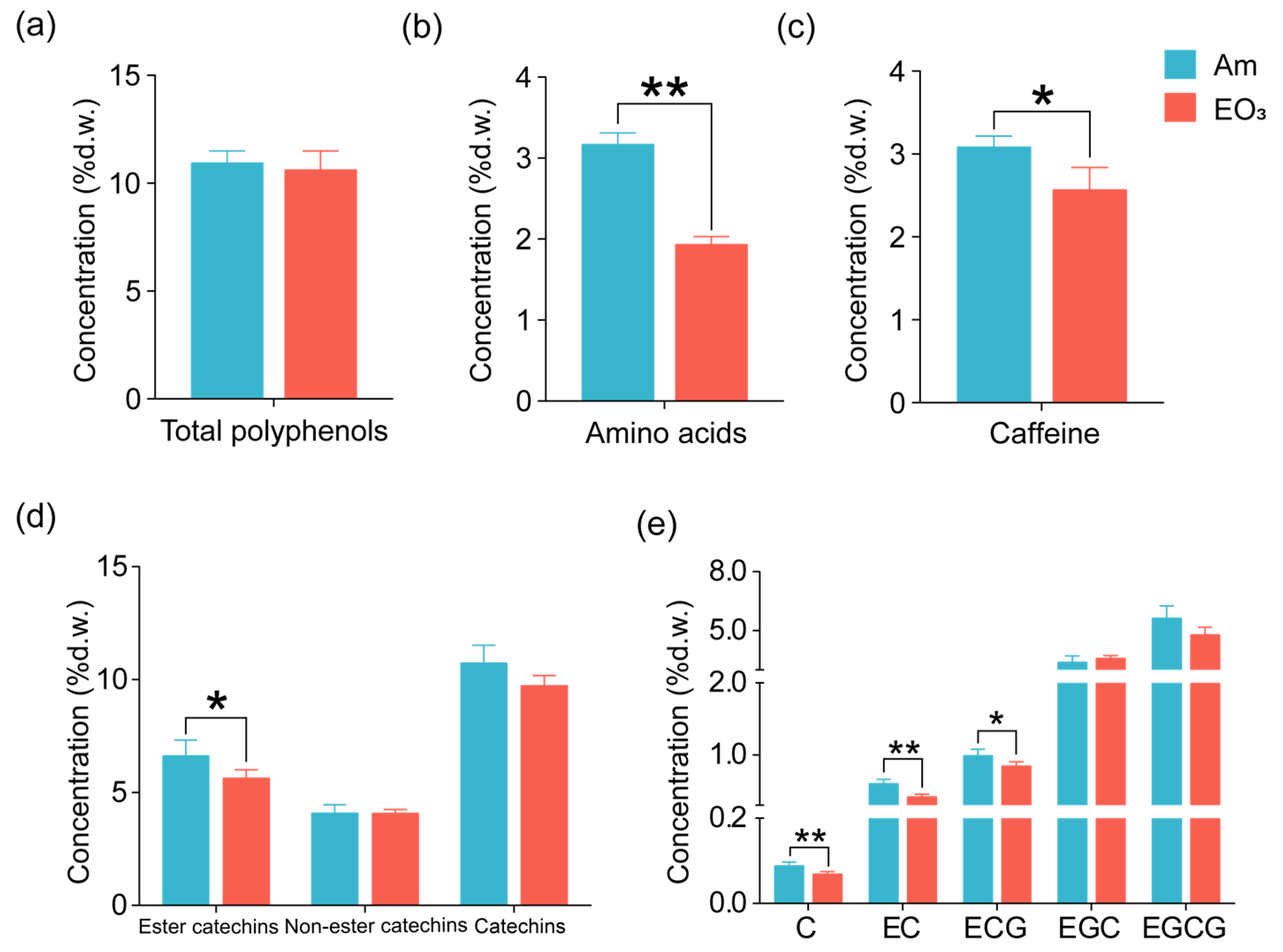
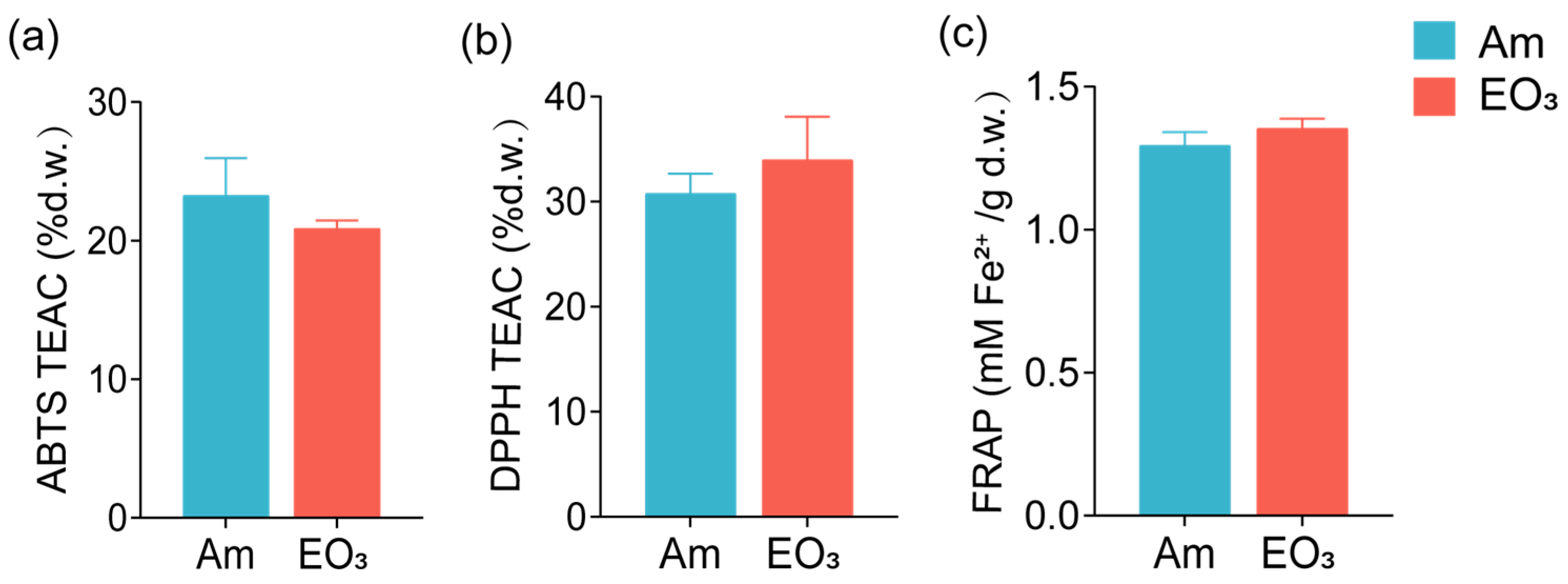
2.1. Effect of Elevated O3 on Main Quality Compounds
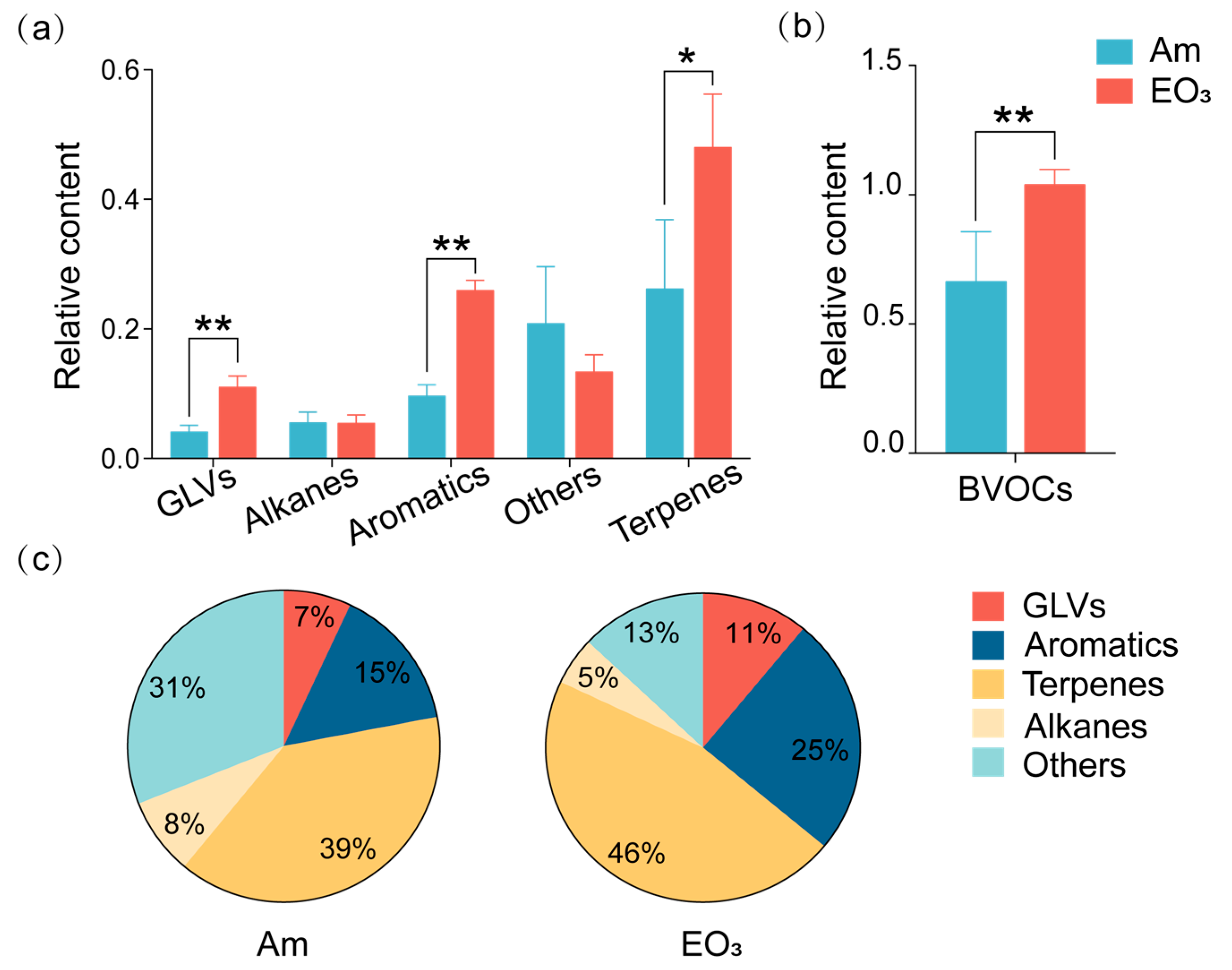
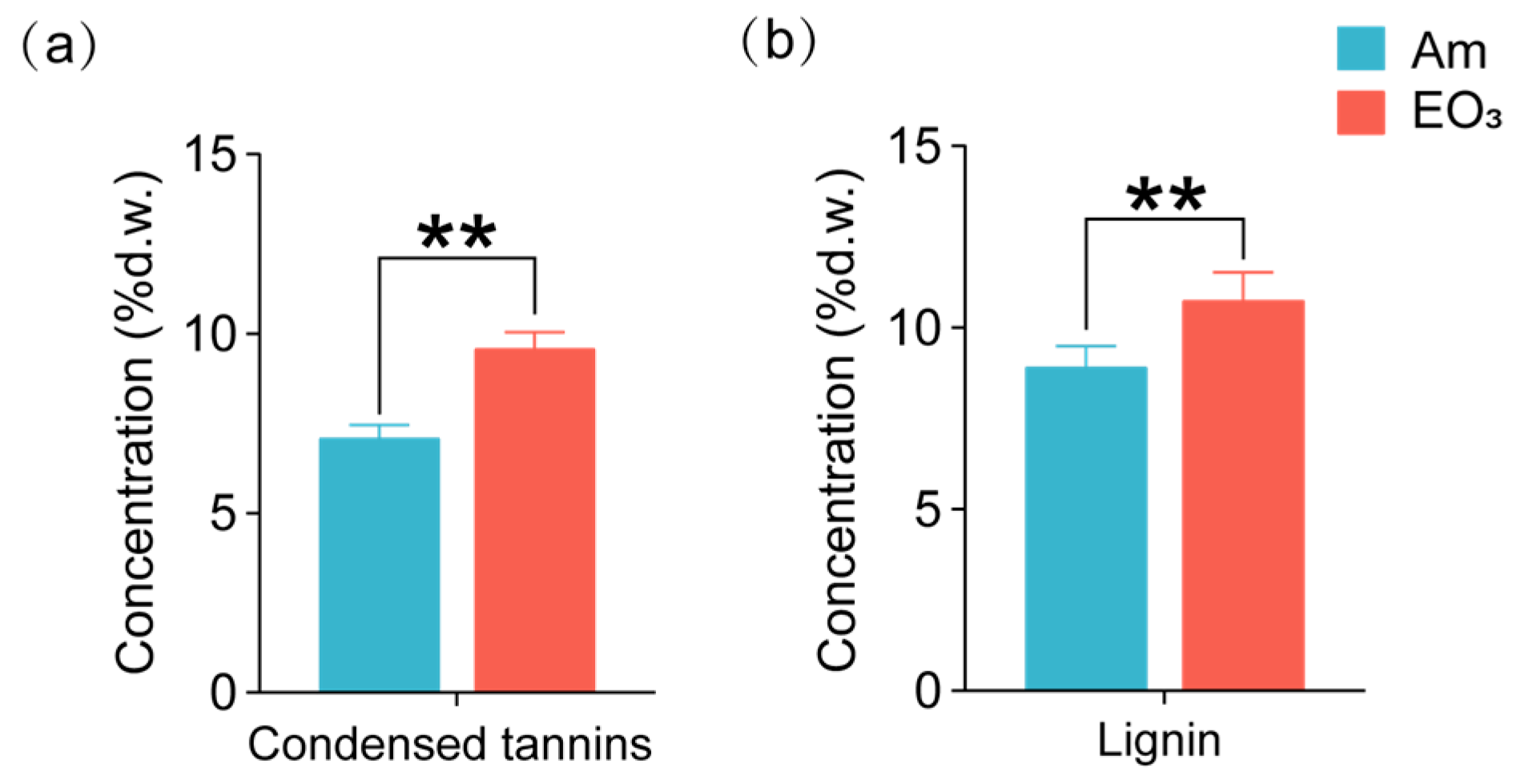
2.2. Effect of Elevated O3 on Chemicals with Defensive Functions
3. Discussion
3.1. O3 Impacts on Main Quality Compounds
3.2. O3 Impacts on Chemicals with Defensive Functions
4. Materials and Methods
4.1. Experimental Site and Plant Materials
4.2. Experimental Design
4.3. Sample Collection
4.4. Chemical Analyses
4.5. Antioxidant Activity
4.6. Assessment of Tea Quality Indexes
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, T.; Xue, L.; Feng, Z.; Dai, J.; Zhang, Y.; Tan, Y. Ground-level ozone pollution in China: A synthesis of recent findings on influencing factors and impacts. Environ. Res. Lett. 2022, 17, 063003. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, Z.; Jin, Z.; Li, M.; Sheridan, S.C.; Wang, T. Ozone variability driven by the synoptic patterns over China during 2014–2022 and its implications for crop yield and economy. Atmos. Pollut. Res. 2023, 14, 101843. [Google Scholar] [CrossRef]
- Li, K.; Jacob, D.J.; Liao, H.; Shen, L.; Zhang, Q.; Bates, K.H. Anthropogenic drivers of 2013–2017 trends in summer surface ozone in China. Proc. Natl. Acad. Sci. USA 2019, 116, 422–427. [Google Scholar] [CrossRef]
- Lu, X.; Hong, J.; Zhang, L.; Cooper, O.R.; Schultz, M.G.; Xu, X.; Wang, T.; Gao, M.; Zhao, Y.; Zhang, Y. Severe surface ozone pollution in China: A global perspective. Environ. Sci. Technol. Lett. 2018, 5, 487–494. [Google Scholar] [CrossRef]
- Chen, B.; Wang, Y.; Huang, J.; Zhao, L.; Chen, R.; Song, Z.; Hu, J. Estimation of near-surface ozone concentration and analysis of main weather situation in China based on machine learning model and Himawari-8 TOAR data. Sci. Total Environ. 2023, 864, 160928. [Google Scholar] [CrossRef]
- Feng, Z.; Xu, Y.; Kobayashi, K.; Dai, L.; Zhang, T.; Agathokleous, E.; Calatayud, V.; Paoletti, E.; Mukherjee, A.; Agrawal, M.; et al. Ozone pollution threatens the production of major staple crops in East Asia. Nat. Food 2022, 3, 47–56. [Google Scholar] [CrossRef]
- Mills, G.; Sharps, K.; Simpson, D.; Pleijel, H.; Broberg, M.; Uddling, J.; Jaramillo, F.; Davies, W.J.; Dentener, F.; Van den Berg, M.; et al. Ozone pollution will compromise efforts to increase global wheat production. Glob. Chang. Biol. 2018, 24, 3560–3574. [Google Scholar] [CrossRef]
- Li, S.; Leakey, A.D.B.; Moller, C.A.; Montes, C.M.; Sacks, E.J.; Lee, D.; Ainsworth, E.A. Similar photosynthetic but different yield responses of C3 and C4 crops to elevated O3. Proc. Natl. Acad. Sci. USA 2023, 120, e2313591120. [Google Scholar] [CrossRef]
- FAO; CAAS. Carbon Neutral Tea Production in China—Three Pilot Case Studies; FAO: Rome, Italy, 2021. [Google Scholar]
- National Bureau of Statistics. Available online: https://data.stats.gov.cn/easyquery.htm?cn=C01 (accessed on 20 December 2023).
- Zhang, L.; Cao, Q.-Q.; Granato, D.; Xu, Y.-Q.; Ho, C.-T. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Ahmed, S.; Unachukwu, U.; Stepp, J.R.; Peters, C.M.; Long, C.; Kennelly, E. Pu-erh tea tasting in Yunnan, China: Correlation of drinkers’ perceptions to phytochemistry. J. Ethnopharmacol. 2010, 132, 176–185. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Y.; Li, X. Spatiotemporal distribution of ground-level ozone in China at a city level. Sci. Rep. 2020, 10, 7229. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Conti, B.; Lorenzini, G.; Pellegrini, E.; Nali, C. In the tripartite combination ozone-poplar-Chrysomela populi, the pollutant alters the plant-insect interaction via primary metabolites of foliage. Environ. Res. 2021, 201, 111581. [Google Scholar] [CrossRef]
- Feng, Z.; Agathokleous, E.; Yue, X.; Oksanen, E.; Paoletti, E.; Sase, H.; Gandin, A.; Koike, T.; Calatayud, V.; Yuan, X.; et al. Emerging challenges of ozone impacts on asian plants: Actions are needed to protect ecosystem health. Ecosyst. Health Sustain. 2021, 7, 1911602. [Google Scholar] [CrossRef]
- da Rosa Santos, A.C.; Furlan, C.M. Levels of phenolic compounds in Tibouchina pulchra after fumigation with ozone. Atmos. Pollut. Res. 2013, 4, 250–256. [Google Scholar] [CrossRef]
- Singh, A.A.; Ghosh, A.; Agrawal, M.; Agrawal, S.B. Secondary metabolites responses of plants exposed to ozone: An update. Environ. Sci. Pollut. Res. 2023, 30, 88281–88312. [Google Scholar] [CrossRef]
- Shang, B.; Feng, Z.; Peng, J.; Xu, Y.; Paoletti, E. Effects of elevated ozone and nitrogen addition on leaf nitrogen metabolism in poplar. J. Plant Ecol. 2021, 14, 555–568. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Shang, B.; Xu, Y.; Couture, J.J.; Yuan, X.; Kobayashi, K.; Feng, Z. Water stress rather than N addition mitigates impacts of elevated O3 on foliar chemical profiles in poplar saplings. Sci. Total Environ. 2020, 707, 135935. [Google Scholar] [CrossRef]
- Couture, J.J.; Holeski, L.M.; Lindroth, R.L. Long-term exposure to elevated CO2 and O3 alters aspen foliar chemistry across developmental stages. Plant Cell Environ. 2014, 37, 758–765. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Shang, B.; Agathokleous, E.; Rubert-Nason, K.F.; Xu, Y.; Feng, Z. Nonlinear responses of foliar phenylpropanoids to increasing O3 exposure: Ecological implications in a Populus model system. Sci. Total Environ. 2021, 767, 144358. [Google Scholar] [CrossRef]
- Cui, H.; Su, J.; Wei, J.; Hu, Y.; Ge, F. Elevated O3 enhances the attraction of whitefly-infested tomato plants to Encarsia formosa. Sci. Rep. 2014, 4, 5350. [Google Scholar] [CrossRef]
- Yuan, X.; Calatayud, V.; Gao, F.; Fares, S.; Paoletti, E.; Tian, Y.; Feng, Z. Interaction of drought and ozone exposure on isoprene emission from extensively cultivated poplar. Plant Cell Environ. 2016, 39, 2276–2287. [Google Scholar] [CrossRef]
- Feng, Z.; Yuan, X.; Fares, S.; Loreto, F.; Li, P.; Hoshika, Y.; Paoletti, E. Isoprene is more affected by climate drivers than monoterpenes: A meta-analytic review on plant isoprenoid emissions. Plant Cell Environ. 2019, 42, 1939–1949. [Google Scholar] [CrossRef]
- Dumont, J.; Keski-Saari, S.; Keinänen, M.; Cohen, D.; Ningre, N.; Kontunen-Soppela, S.; Baldet, P.; Gibon, Y.; Dizengremel, P.; Vaultier, M.-N. Ozone affects ascorbate and glutathione biosynthesis as well as amino acid contents in three Euramerican poplar genotypes. Tree Physiol. 2014, 34, 253–266. [Google Scholar] [CrossRef]
- Munivenkatappa, N.; Sarikonda, S.; Rajagopal, R.; Balakrishnan, R.; Nagarathana, C.K. Variations in quality constituents of green tea leaves in response to drought stress under south Indian condition. Sci. Hortic. 2018, 233, 359–369. [Google Scholar] [CrossRef]
- Saviranta, N.M.; Julkunen-Tiitto, R.; Oksanen, E.; Karjalainen, R.O. Leaf phenolic compounds in red clover (Trifolium pratense L.) induced by exposure to moderately elevated ozone. Environ. Pollut. 2010, 158, 440–446. [Google Scholar] [CrossRef]
- He, X.; Huang, W.; Chen, W.; Dong, T.; Liu, C.; Chen, Z.; Xu, S.; Ruan, Y. Changes of main secondary metabolites in leaves of Ginkgo biloba in response to ozone fumigation. J. Environ. Sci. 2009, 21, 199–203. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Hormesis can enhance agricultural sustainability in a changing world. Glob. Food Secur. 2019, 20, 150–155. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Y.; Zhang, L.; Hasan, M.K.; Zhang, L.; Yan, P.; Fu, J.; Han, W.; Li, X. A chemical explanation for variations in antioxidant capacity across Camellia sinensis L. cultivars. Forests 2023, 14, 249. [Google Scholar] [CrossRef]
- Tounekti, T.; Joubert, E.; Hernández, I.; Munné-Bosch, S. Improving the polyphenol content of tea. Crit. Rev. Plant Sci. 2013, 32, 192–215. [Google Scholar] [CrossRef]
- JIANG, C.-K.; NI, D.-J.; YAO, M.-Z. Metabolic and transcriptome analysis reveals metabolite variation and flavonoid regulatory networks in fresh shoots of tea (Camellia sinensis) over three seasons. Front. Agric. Sci. Eng. 2021, 8, 215–230. [Google Scholar]
- Xu, Y.-Q.; Zhang, Y.-N.; Chen, J.-X.; Wang, F.; Du, Q.-Z.; Yin, J.-F. Quantitative analyses of the bitterness and astringency of catechins from green tea. Food Chem. 2018, 258, 16–24. [Google Scholar] [CrossRef]
- Masui, N.; Agathokleous, E.; Tani, A.; Matsuura, H.; Koike, T. Plant-insect communication in urban forests: Similarities of plant volatile compositions among tree species (host vs. non-host trees) for alder leaf beetle Agelastica coerulea. Environ. Res. 2022, 204, 111996. [Google Scholar] [CrossRef]
- Li, T.; Blande, J.D. Associational susceptibility in broccoli: Mediated by plant volatiles, impeded by ozone. Glob. Chang. Biol. 2015, 21, 1993–2004. [Google Scholar] [CrossRef]
- Sugai, T.; Okamoto, S.; Agathokleous, E.; Masui, N.; Satoh, F.; Koike, T. Leaf defense capacity of Japanese elm (Ulmus davidiana var. japonica) seedlings subjected to a nitrogen loading and insect herbivore dynamics in a free air ozone-enriched environment. Environ. Sci. Pollut. Res. 2020, 27, 3350–3360. [Google Scholar]
- Acton, W.J.F.; Jud, W.; Ghirardo, A.; Wohlfahrt, G.; Hewitt, C.; Taylor, J.E.; Hansel, A. The effect of ozone fumigation on the biogenic volatile organic compounds (BVOCs) emitted from Brassica napus above-and below-ground. PLoS ONE 2018, 13, e0208825. [Google Scholar] [CrossRef]
- Shiojiri, K.; Kishimoto, K.; Ozawa, R.; Kugimiya, S.; Urashimo, S.; Arimura, G.; Horiuchi, J.; Nishioka, T.; Matsui, K.; Takabayashi, J. Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance against both herbivores and pathogens. Proc. Natl. Acad. Sci. USA 2006, 103, 16672–16676. [Google Scholar] [CrossRef]
- Matsui, K.; Kurishita, S.; Hisamitsu, A.; Kajiwara, T. A lipid-hydrolysing activity involved in hexenal formation. Biochem. Soc. Trans. 2000, 28, 857–860. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, M.; Jing, T.; Zhang, M.; Lu, M.; Yu, G.; Wang, J.; Guo, D.; Pan, Y.; Hoffmann, T.D. Volatile compound-mediated plant–plant interactions under stress with the tea plant as a model. Hortic. Res. 2023, 10, uhad143. [Google Scholar] [CrossRef]
- Allmann, S.; Baldwin, I.T. Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science 2010, 329, 1075–1078. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef]
- Mu, D.; Cui, L.; Ge, J.; Wang, M.X.; Liu, L.F.; Yu, X.P.; Zhang, Q.H.; Han, B.Y. Behavioral responses for evaluating the attractiveness of specific tea shoot volatiles to the tea green leafhopper, Empoaca vitis. Insect Sci. 2012, 19, 229–238. [Google Scholar] [CrossRef]
- Han, B.Y.; Chen, Z.M. Composition of the volatiles from intact and mechanically pierced Tea Aphid—Tea shoot complexes and their attraction to natural enemies of the Tea Aphid. J. Agric. Food Chem. 2002, 50, 2571–2575. [Google Scholar] [CrossRef]
- Lindroth, R.L. Impacts of elevated atmospheric CO2 and O3 on forests: Phytochemistry, trophic interactions, and ecosystem dynamics. J. Chem. Ecol. 2010, 36, 2–21. [Google Scholar] [CrossRef]
- Sakikawa, T.; Shi, C.; Nakamura, M.; Watanabe, M.; Oikawa, M.; Satoh, F.; Koike, T. Leaf phenology and insect grazing of Japanese white birch saplings grown under free-air ozone exposure. J. Agric. Meteorol. 2016, 72, 80–84. [Google Scholar] [CrossRef]
- Duque, L.; Poelman, E.H.; Steffan-Dewenter, I. Plant-mediated effects of ozone on herbivores depend on exposure duration and temperature. Sci. Rep. 2019, 9, 19891. [Google Scholar] [CrossRef]
- Miranda, M.; Ralph, S.G.; Mellway, R.; White, R.; Heath, M.C.; Bohlmann, J.; Constabel, C.P. The transcriptional response of hybrid poplar (Populus trichocarpa × P. deltoids) to infection by Melampsora medusae leaf rust involves induction of flavonoid pathway genes leading to the accumulation of proanthocyanidins. Mol. Plant Microbe Interact. 2007, 20, 816–831. [Google Scholar] [CrossRef]
- Couture, J.J.; Lindroth, R.L. Atmospheric change alters performance of an invasive forest insect. Glob. Chang. Biol. 2012, 18, 3543–3557. [Google Scholar] [CrossRef]
- GB/T 8314-2013; Tea-Determination of Free Amino Acids Concentration. Standardization Administration of China: Beijing, China, 2013.
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef]
- Suzuki, S.; Suzuki, Y.; Yamamoto, N.; Hattori, T.; Sakamoto, M.; Umezawa, T. High-throughput determination of thioglycolic acid lignin from rice. Plant Biotechnol. 2009, 26, 337–340. [Google Scholar] [CrossRef]
- Liu, G.; Yang, M.; Yang, X.; Ma, X.; Fu, J. Five TPSs are responsible for volatile terpenoid biosynthesis in Albizia julibrissin. J. Plant Physiol. 2021, 258–259, 153358. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Li, Z.-X.; Zhang, L.; Wei, J.-P.; Shen, C.; Yan, P.; Zhang, L.-P.; Han, W.-Y. Brassinosteroids improve quality of summer tea (Camellia sinensis L.) by balancing biosynthesis of polyphenols and amino acids. Front. Plant Sci. 2016, 7, 1304. [Google Scholar] [CrossRef]
- Yuan, Y.; Cheng, C. The relation between the components of tea catechins and the quality of green tea. Acta Hortic. Sin. 1964, 3, 287–300. [Google Scholar]
| Tea Quality Index | Am | EO3 |
|---|---|---|
| TP/AA | 3.45 ± 0.10 b | 5.50 ± 0.45 a |
| CQI | 1.96 ± 0.23 a | 1.57 ± 0.07 b |
| Volatile Class | Individual Compounds | Retention Time (min) | Am | EO3 |
|---|---|---|---|---|
| Green leaf volatiles | E-2-hexenal | 6.632 | 0.025 ± 0.010 b | 0.106 ± 0.014 a |
| 2-Ethyl-1-hexanol | 11.901 | 0.016 ± 0.008 a | 0.005 ± 0.001 b | |
| Aromatics | Toluene | 5.303 | 0.050 ± 0.007 b | 0.228 ± 0.008 a |
| Benzyl alcohol | 12.156 | 0.005 ± 0.003 a | 0.003 ± 0.001 a | |
| 2,4-Di-tert-butylphenol | 25.103 | 0.042 ± 0.006 a | 0.029 ± 0.009 a | |
| Terpenes | Trans-Furanic linalool oxid | 13.591 | 0.005 ± 0.003 a | 0.005 ± 0.001 a |
| Geraniol | 18.901 | 0.003 ± 0.001 a | 0.003 ± 0.001 a | |
| Neophytadiene | 32.261 | 0.172 ± 0.064 b | 0.362 ± 0.059 a | |
| Phytol | 37.536 | 0.078 ± 0.024 a | 0.091 ± 0.020 a | |
| 6-epi-shyobunol | 28.565 | 0.005 ± 0.002 b | 0.021 ± 0.004 a | |
| Alkanes | Undecane | 11.312 | 0.002 ± 0.001 a | 0.003 ± 0.001 a |
| Dodecane | 12.635 | 0.010 ± 0.005 b | 0.015 ± 0.003 a | |
| Tridecane | 17.246 | 0.009 ± 0.001 a | 0.007 ± 0.001 a | |
| Pentadecane | 19.088 | 0.032 ± 0.009 a | 0.027 ± 0.007 a | |
| 1-Methoxyadamantane | 16.628 | 0.003 ± 0.001 a | 0.004 ± 0.001 a | |
| Others | 2,4-Dimethyl-1-heptanol | 13.976 | 0.007 ± 0.002 a | 0.006 ± 0.001 a |
| Hexyl octyl ether | 14.151 | 0.002 ± 0.001 a | 0.001 ± 0.000 a | |
| Cyclohexanol | 7.442 | 0.201 ± 0.076 a | 0.128 ± 0.021 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Wang, Y.; Zhang, X.; Wu, Y.; Zhang, L.; Liu, G.; Fu, J.; Li, X.; Mu, D.; Li, Z. Elevated Ozone Reduces the Quality of Tea Leaves but May Improve the Resistance of Tea Plants. Plants 2024, 13, 1108. https://doi.org/10.3390/plants13081108
Wang N, Wang Y, Zhang X, Wu Y, Zhang L, Liu G, Fu J, Li X, Mu D, Li Z. Elevated Ozone Reduces the Quality of Tea Leaves but May Improve the Resistance of Tea Plants. Plants. 2024; 13(8):1108. https://doi.org/10.3390/plants13081108
Chicago/Turabian StyleWang, Nuo, Yuxi Wang, Xinyang Zhang, Yiqi Wu, Lan Zhang, Guanhua Liu, Jianyu Fu, Xin Li, Dan Mu, and Zhengzhen Li. 2024. "Elevated Ozone Reduces the Quality of Tea Leaves but May Improve the Resistance of Tea Plants" Plants 13, no. 8: 1108. https://doi.org/10.3390/plants13081108
APA StyleWang, N., Wang, Y., Zhang, X., Wu, Y., Zhang, L., Liu, G., Fu, J., Li, X., Mu, D., & Li, Z. (2024). Elevated Ozone Reduces the Quality of Tea Leaves but May Improve the Resistance of Tea Plants. Plants, 13(8), 1108. https://doi.org/10.3390/plants13081108







