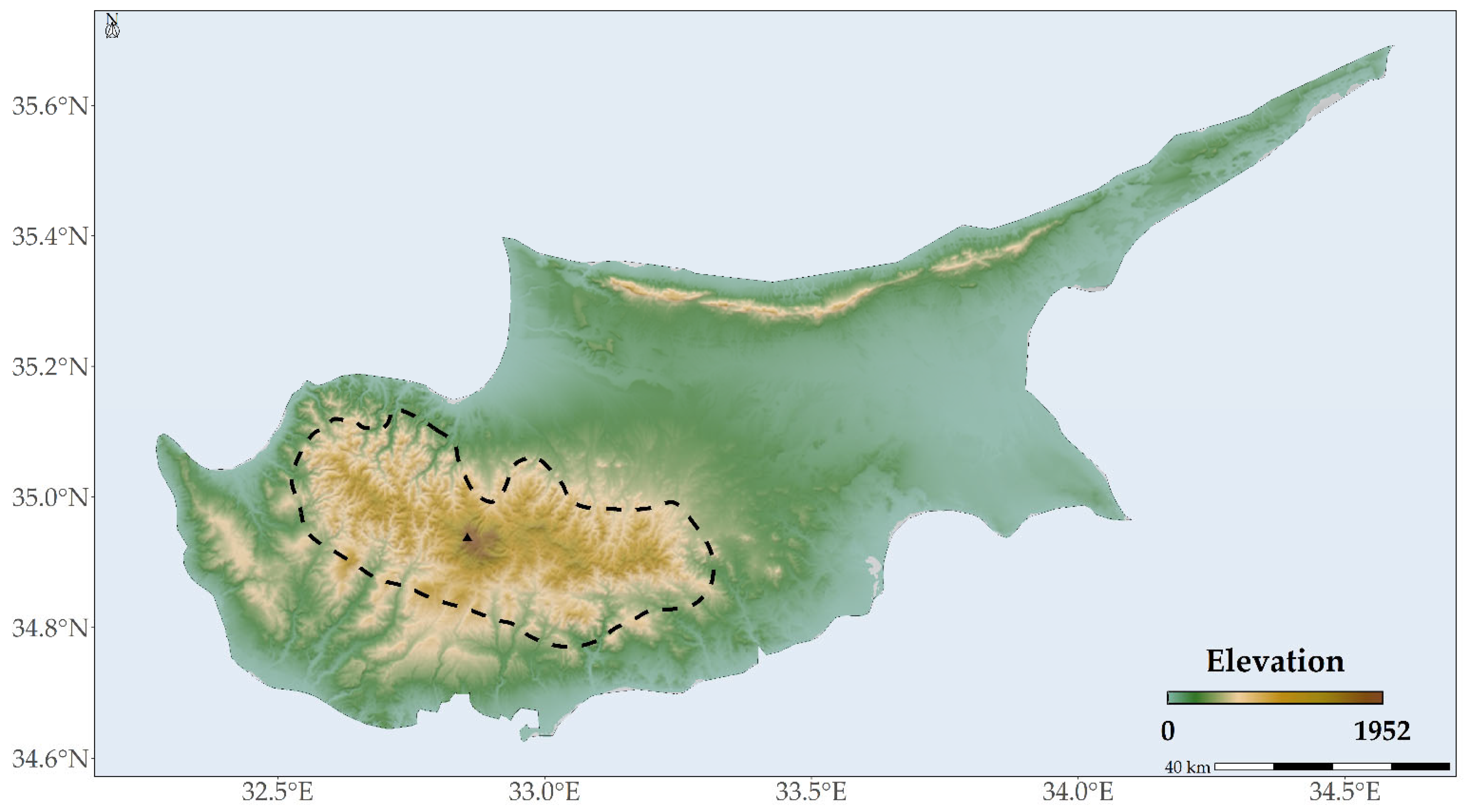
Rising Temperatures, Falling Leaves: Predicting the Fate of Cyprus’s Endemic Oak under Climate and Land Use Change
Abstract
:1. Introduction
- (a)
- map the future potential distribution of Quercus alnifolia under climate and land-use change scenarios,
- (b)
- critically evaluate Quercus alnifolia’s sensitivity, exposure, and vulnerability to these changes, and
- (c)
- conduct a preliminary IUCN extinction risk assessment at a global scale under Criteria A and B, thereby offering actionable insights for conservation strategies and policy-making.
2. Results
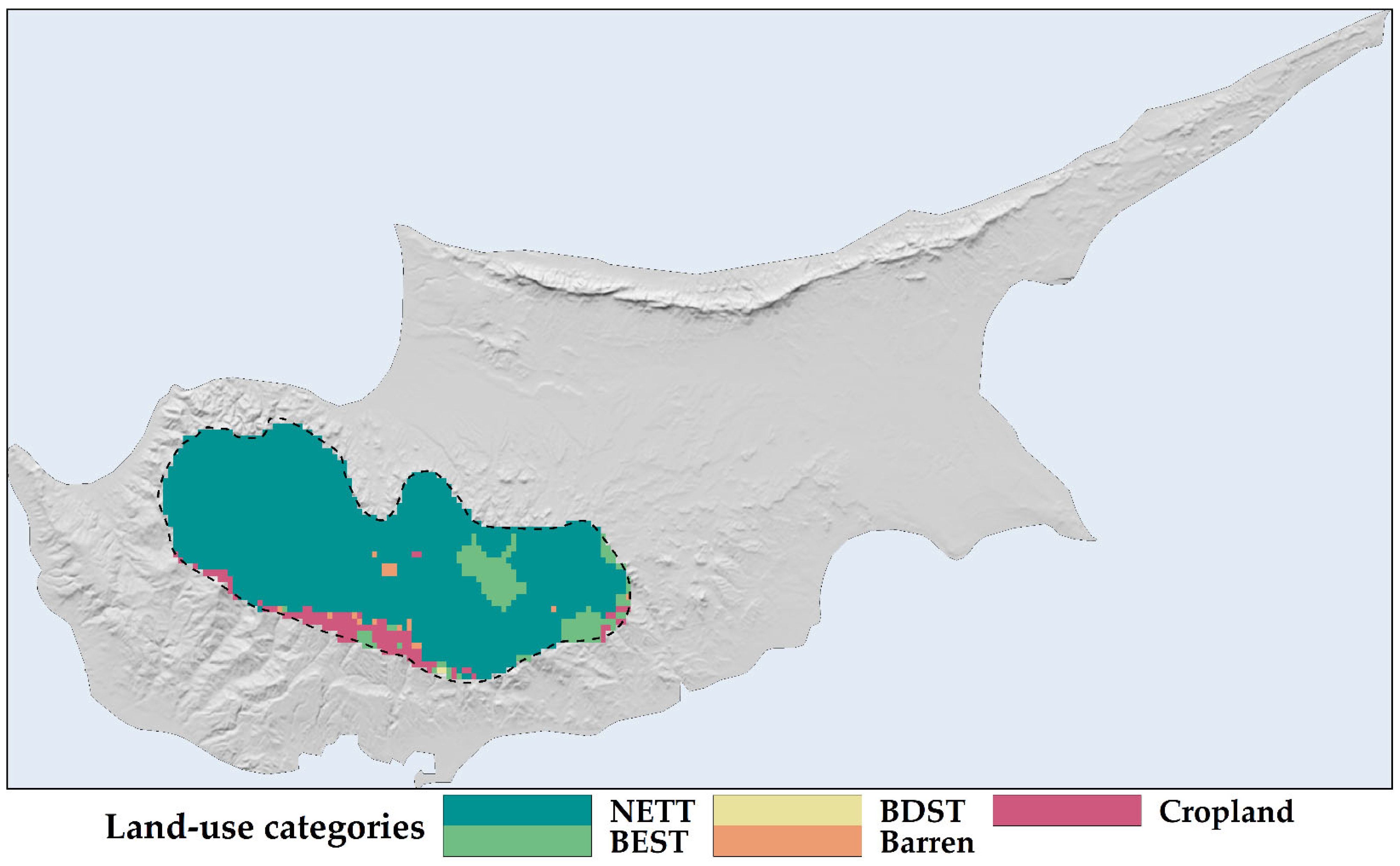
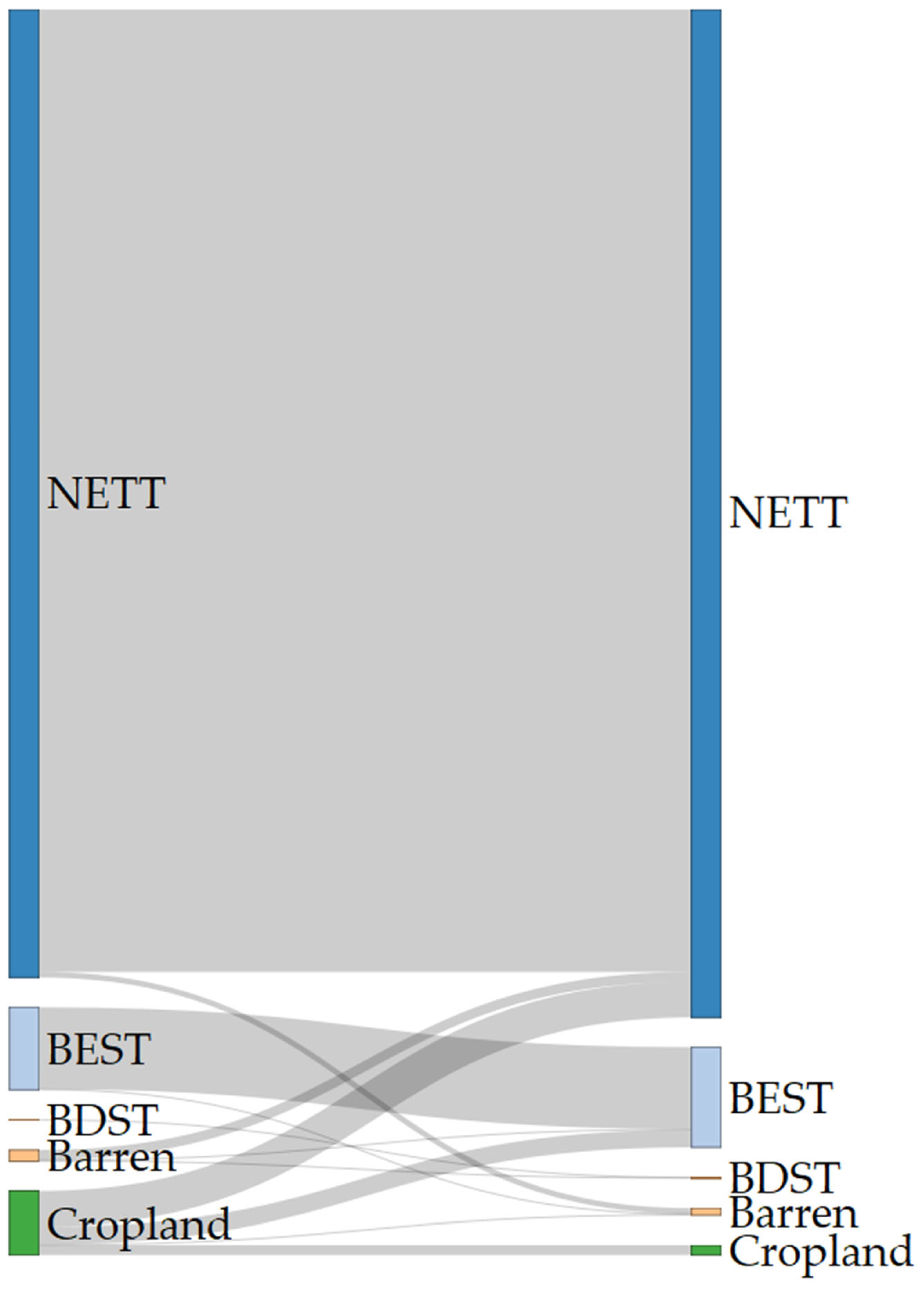
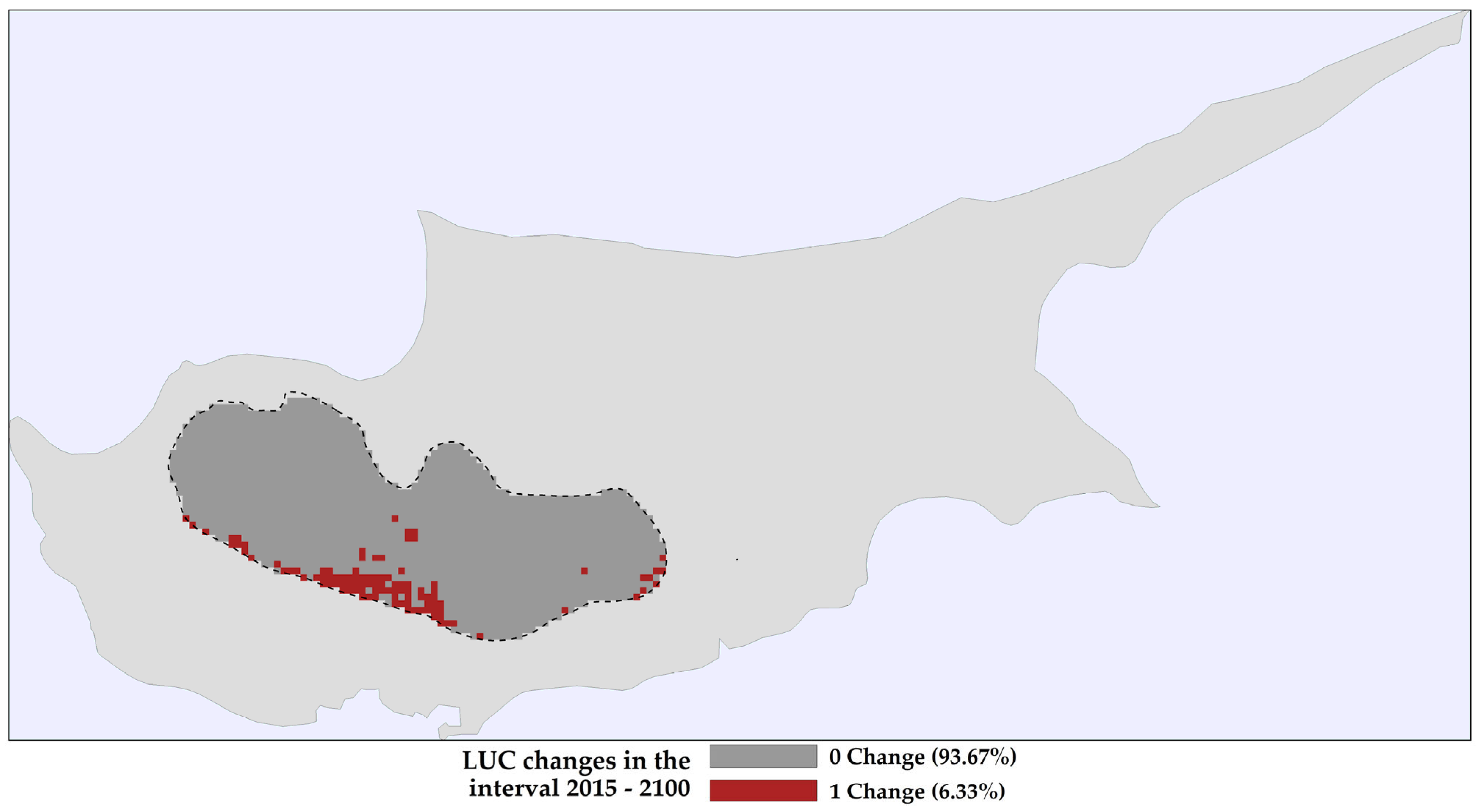
2.1. Land Use and Land Cover Changes
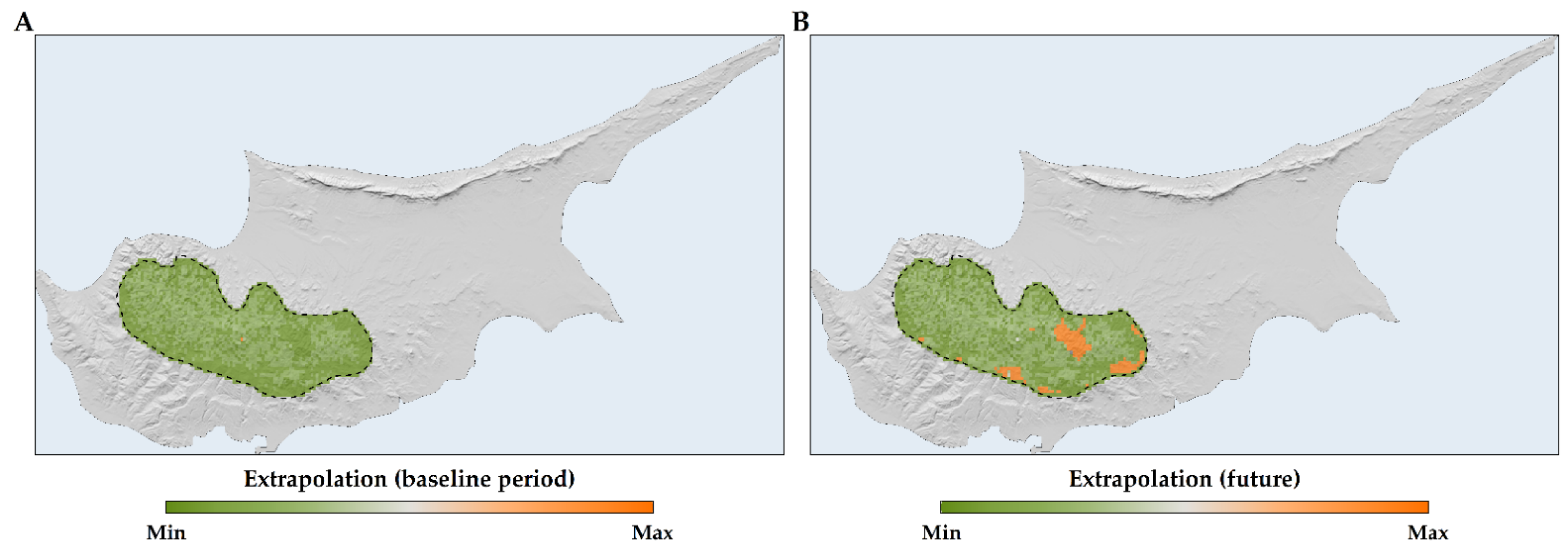
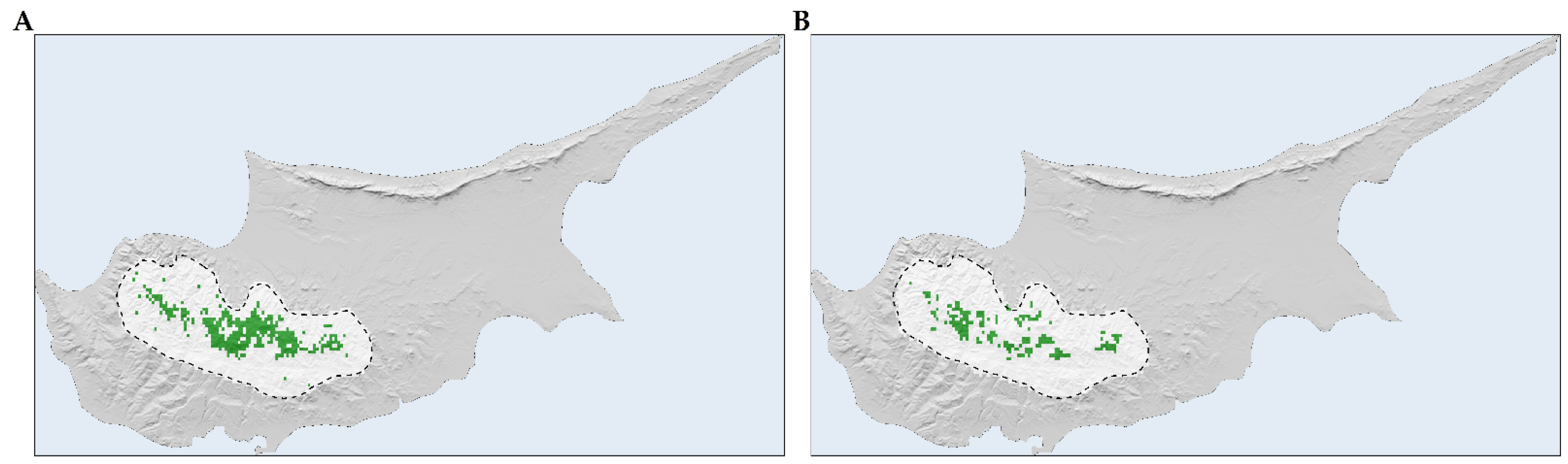
2.2. Species Distribution Models
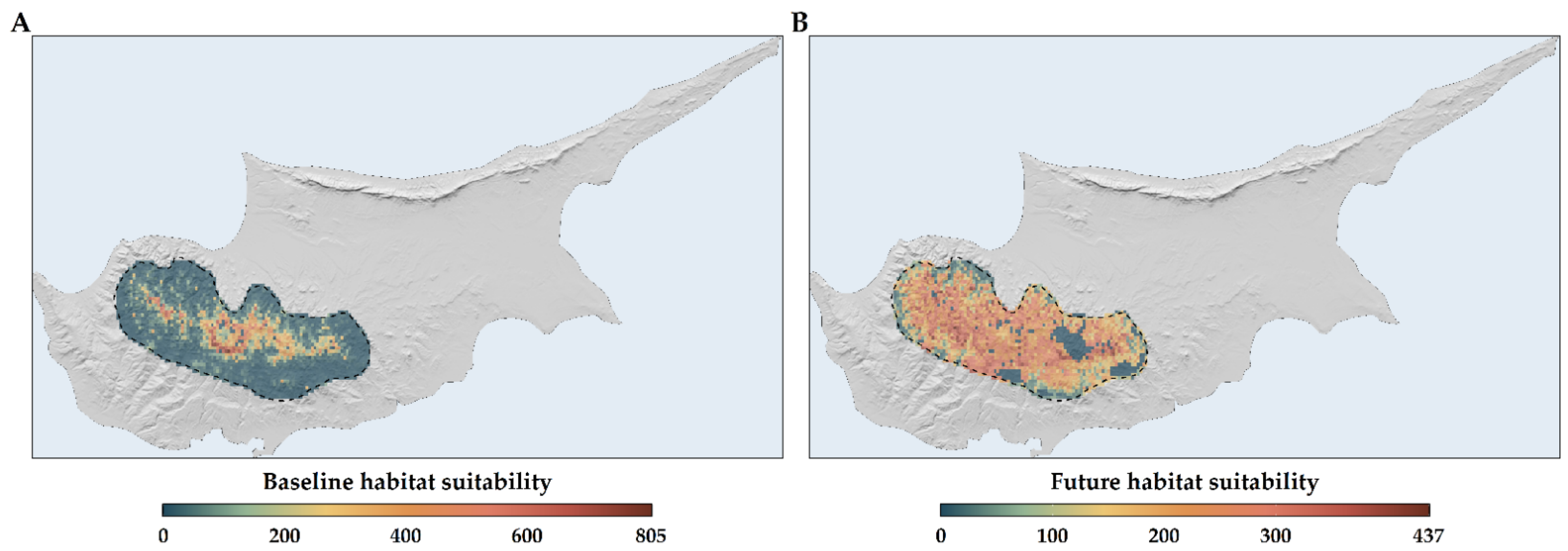
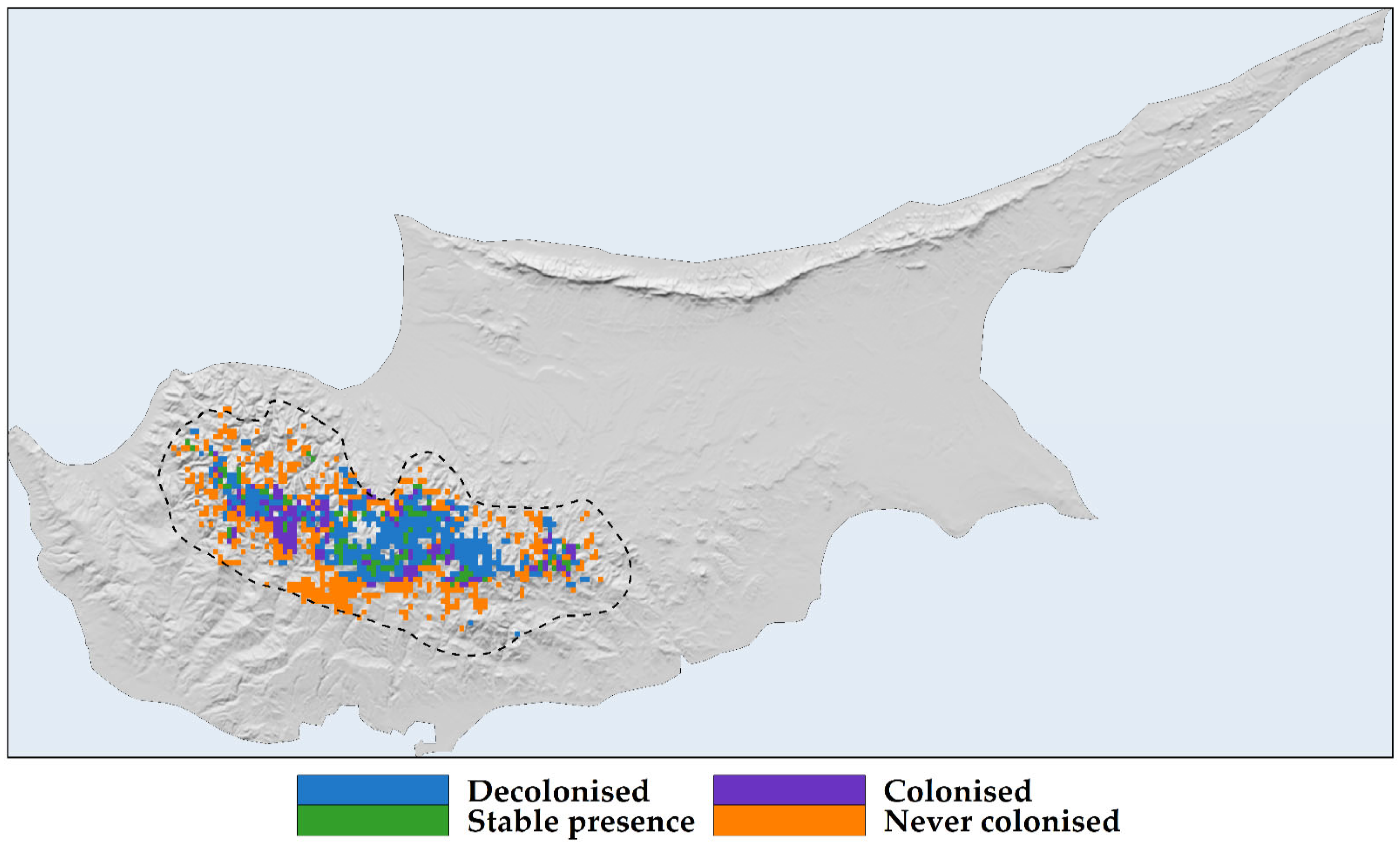
2.3. Habitat Suitability Range Change
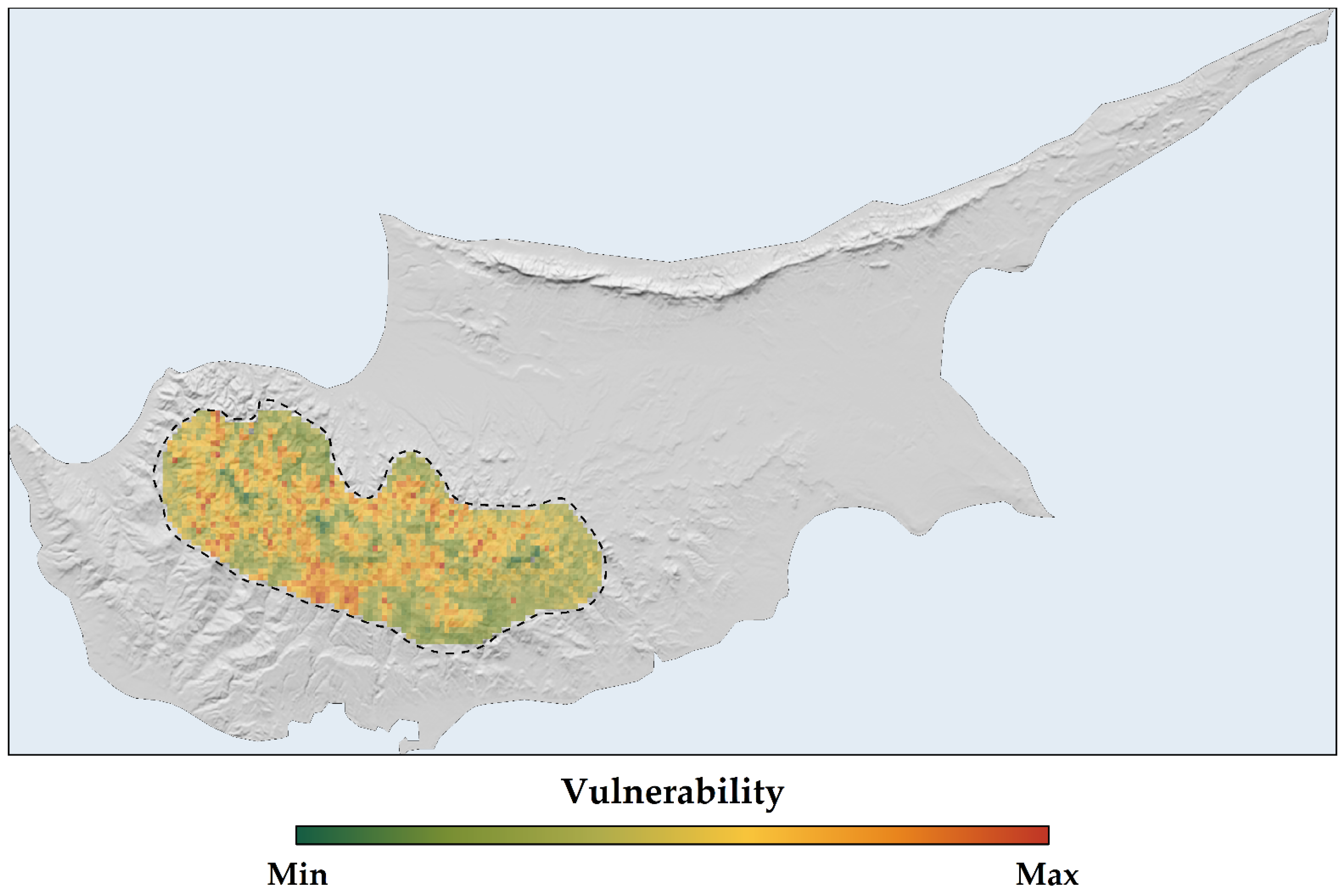
2.4. Sensitivity, Exposure, and Vulnerability to Climate and Land-Use Change
2.5. IUCN Extinction Risk Assessment
- Critically Endangered, according to the IUCN Criterion A assessment,
- Least Concern or Near Threatened in most scenarios, with the exception of the CCSM4 4.5 GCM/RCP combination in the 2070s and the CCSM4 4.5 SSP3 GCM/RCP/SSP combination in the 2050s (Vulnerable), according to the IUCN Criterion B assessment and
- Critically Endangered, when considering the combined IUCN Criteria A and B assessment.
3. Discussion
3.1. Abiotic Variables Affecting the Distribution of Quercus alnifolia
3.2. Habitat Suitability Range Change and IUCN Extinction Risk Assessment
3.3. Sensitivity, Exposure, and Vulnerability to Climate and Land-Use Change
3.4. Conservation Implications and Measures
4. Materials and Methods
4.1. Species Occurrence Data
- The Global Biodiversity Information Facility database documenting 92 occurrences [144];
- Research conducted by Constantinou & Panitsa (2023), detailing 36 occurrences [57];
- Data extracted from the EUForest database, indicating 8 occurrences [145];
- The WOODIV database, which provided occurrence data in 10 × 10 km grids, albeit with significantly less precision [146].
4.2. Environmental Data
4.3. Land Use and Land Cover Changes
4.4. Species Distribution Models
4.5. Sensitivity Analysis
- Environmental outcomes:
- Climate-only: 19 bioclimatic WorldClim variables and 16 environmental variables from ENVIREM;
- Climate + Topography: Climate-only data supplemented by topographical variables;
- Climate + Topography + Soil + Hydrology: Addition of soil and hydrological data to a prior combination;
- Climate + Topography + Soil + Hydrology + LULC: The comprehensive suite with land-use and land-cover data.
- Dispersal scenarios:
- No Dispersal: Assumption of static range without species movement to suitable habitat;
- Unlimited Dispersal: A hypothetical scenario of unhindered range shifts towards ideal conditions;
- Specific Dispersal Constraints: Implemented only for the full model (incorporating all environmental variables) to evaluate dispersal limitations based on Quercus alnifolia specific characteristics.
4.6. Sensitivity, Exposure, and Vulnerability to Climate and Land-Use Change
4.7. Future IUCN Extinction Risk Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPBES. Global Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Brondízio, E., Settele, J., Díaz, S., Ngo, H., Eds.; IPBES: Bonn, Germany, 2019. [Google Scholar]
- Vogiatzakis, I.N.; Mannion, A.M.; Sarris, D. Mediterranean island biodiversity and climate change: The last 10,000 years and the future. Biodivers. Conserv. 2016, 25, 2597–2627. [Google Scholar] [CrossRef]
- Wiens, J.J.; Zelinka, J. How many species will earth lose to climate change? Glob. Chang. Biol. 2024, 30, e17125. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, E.M.; Fenu, G.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within hotspots: Endemic plant richness, environmental drivers, and implications for conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- MedECC. Climate and Environmental Change in the Mediterranean Basin—Current Situation and Risks for the Future; First Mediterranean Assessment Report; MedECC: Marseille, France, 2020. [Google Scholar]
- Zittis, G.; Almazroui, M.; Alpert, P.; Ciais, P.; Cramer, W.; Dahdal, Y.; Fnais, M.; Francis, D.; Hadjinicolaou, P.; Howari, F.; et al. Climate change and weather extremes in the Eastern Mediterranean and Middle East. Rev. Geophys. 2022, 60, e2021RG000762. [Google Scholar] [CrossRef]
- Gordo, O.; Sanz, J.J. Impact of climate change on plant phenology in Mediterranean ecosystems. Glob. Chang. Biol. 2010, 16, 1082–1106. [Google Scholar] [CrossRef]
- Morán-Ordóñez, A.; Ramsauer, J.; Coll, L.; Brotons, L.; Ameztegui, A. Ecosystem services provision by Mediterranean forests will be compromised above 2 °C warming. Glob. Chang. Biol. 2021, 27, 4210–4222. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction risk assessment of the Greek endemic flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Trigas, P.; Tsakiri, M.; Kokkoris, I.P.; Koumoutsou, E.; Dimopoulos, P.; Tzanoudakis, D.; Iatrou, G.; Panitsa, M. Climate and land-cover change impacts and extinction risk assessment of rare and threatened endemic taxa of Chelmos-Vouraikos National Park (Peloponnese, Greece). Plants 2022, 11, 3548. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, I.P.; Kougioumoutzis, K.; Charalampopoulos, I.; Apostolidis, E.; Apostolidis, I.; Strid, A.; Dimopoulos, P. Conservation responsibility for priority habitats under future climate conditions: A case study on Juniperus drupacea forests in Greece. Land 2023, 12, 1976. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Tsakiri, M.; Kokkoris, I.P.; Trigas, P.; Iatrou, G.; Lamari, F.N.; Tzanoudakis, D.; Koumoutsou, E.; Dimopoulos, P.; Strid, A. Assessing the vulnerability of medicinal and aromatic plants to climate and land-use changes in a Mediterranean biodiversity hotspot. Land 2024, 13, 133. [Google Scholar] [CrossRef]
- Al-Qaddi, N.; Vessella, F.; Stephan, J.; Al-Eisawi, D.; Schirone, B. Current and future suitability areas of kermes oak (Quercus coccifera L.) in the Levant under climate change. Reg. Environ. Chang. 2017, 17, 143–156. [Google Scholar] [CrossRef]
- Fyllas, N.M.; Koufaki, T.; Sazeides, C.I.; Spyroglou, G.; Theodorou, K. Potential impacts of climate change on the habitat suitability of the dominant tree species in Greece. Plants 2022, 11, 1616. [Google Scholar] [CrossRef] [PubMed]
- Bede-Fazekas, Á.; Horváth, L.; Kocsis, M. Impact of climate change on the potential distribution of Mediterranean pines. Idojaras 2014, 118, 41–52. [Google Scholar]
- Ruiz-Labourdette, D.; Nogués-Bravo, D.; Ollero, H.S.; Schmitz, M.F.; Pineda, F.D. Forest composition in Mediterranean Mountains is projected to shift along the entire elevational gradient under climate change. J. Biogeogr. 2012, 39, 162–176. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Hui, C.; Castillo, M.L.; Iriondo, J.M.; Keet, J.H.; Khapugin, A.A.; Médail, F.; Rejmánek, M.; Theron, G.; Yannelli, F.A.; et al. Recent anthropogenic plant extinctions differ in biodiversity hotspots and coldspots. Curr. Biol. 2019, 29, 2912–2918.e2. [Google Scholar] [CrossRef] [PubMed]
- Lehsten, V.; Sykes, M.T.; Scott, A.V.; Tzanopoulos, J.; Kallimanis, A.; Mazaris, A.; Verburg, P.H.; Schulp, C.J.E.; Potts, S.G.; Vogiatzakis, I. Disentangling the effects of land-use change, climate and CO2 on projected future European habitat types. Glob. Ecol. Biogeogr. 2015, 24, 653–663. [Google Scholar] [CrossRef]
- Edwards, S.; Hudson-Edwards, K.; Cann, J.; Malpas, J.; Xenophontos, C. Classic Geology in Europe 7: Cyprus; Terra Publishing: Rhinebeck, NY, USA, 2010. [Google Scholar]
- Médail, F.; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Médail, F. The specific vulnerability of plant biodiversity and vegetation on Mediterranean Islands in the face of global change. Reg. Environ. Chang. 2017, 17, 1775–1790. [Google Scholar] [CrossRef]
- Varotsos, K.V.; Karali, A.; Lemesios, G.; Kitsara, G.; Moriondo, M.; Dibari, C.; Leolini, L.; Giannakopoulos, C. Near future climate change projections with implications for the agricultural sector of three major Mediterranean islands. Reg. Environ. Chang. 2021, 21, 16. [Google Scholar] [CrossRef]
- Papadopoulou, M.P.; Charchousi, D.; Spanoudaki, K.; Karali, A.; Varotsos, K.V.; Giannakopoulos, C.; Markou, M.; Loizidou, M. Agricultural water vulnerability under climate change in Cyprus. Atmosphere 2020, 11, 648. [Google Scholar] [CrossRef]
- Delipetrou, P.; Makhzoumi, J.; Dimopoulos, P.; Georghiou, K. Cyprus. In Mediterranean Island Landscapes: Natural and Cultural Approaches; Springer: Dordrecht, The Netherlands, 2008; pp. 170–203. [Google Scholar]
- Tsintides, T.; Christodoulou, C.S.; Delipetrou, P.; Georghiou, K. The Red Data Book of the Flora of Cyprus; Cyprus Forestry Association: Lefkosia, Cyprus, 2007; Volume 465. [Google Scholar]
- Hand, R.; Hadjikyriakou, G.; Christodoulou, C.S. Flora of Cyprus—A Dynamic Checklist. Available online: http://www.flora-of-cyprus.eu/ (accessed on 10 January 2024).
- Panitsa, M.; Kagiampaki, A.; Kougioumoutzis, K. Plant diversity and biogeography of the Aegean archipelago: A New Synthesis. In Biogeography and Biodiversity of the Aegean. In Honour of Prof. Moysis Mylonas; Spyros, S., Panayiotis, P., Aristeidis, P., Nikos, P., Triantis, K., Eds.; Broken Hill Publishers Ltd.: Nicosia, Cyprus, 2018; pp. 223–244. ISBN 9789925563784. [Google Scholar]
- Christodoulou, C.S.; Griffiths, G.H.; Vogiatzakis, I.N. Using threatened plant species to identify conservation gaps and opportunities on the island of Cyprus. Biodivers. Conserv. 2018, 27, 2837–2858. [Google Scholar] [CrossRef]
- Kounnamas, C.; Andreou, M. Mapping and assessment of ecosystem services at Troodos National Forest Park in Cyprus. One Ecosyst. 2022, 7, e77584. [Google Scholar] [CrossRef]
- Christodoulou, C.S.; Griffiths, G.H.; Vogiatzakis, I.N. Systematic conservation planning in a Mediterranean island context: The example of Cyprus. Glob. Ecol. Conserv. 2021, 32, e01907. [Google Scholar] [CrossRef]
- Vogiatzakis, I.N.; Litskas, V.D.; Koumpis, T.; Kassinis, N.; Constantinou, E.; Leontiou, S. The past, present and future of nature conservation in Crete and Cyprus: So close and yet so far. Environ. Sustain. Indic. 2020, 8, 100070. [Google Scholar] [CrossRef]
- Kolios, S.; Mitrakos, S.; Stylios, C. Detection of areas susceptible to land degradation in Cyprus using remote sensed data and environmental quality indices. Land Degrad. Dev. 2018, 29, 2338–2350. [Google Scholar] [CrossRef]
- Foden, W.; Young, B.; Baker, D.J.; Bickford, D.; Butchart, S.; Carr, J.; Garcia, R.A.; Hoffmann, A.; Hole, D.; Kovacs, K.M.; et al. Guidelines for Assessing Species’ Vulnerability to Climate Change; IUCN Species Survival Commission: Gland, Switzerland, 2016. [Google Scholar]
- IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria; Version 15.1, Prepared by the Standards and Petitions Committee; IUCN: Gland, Switzerland, 2022. [Google Scholar]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Kelley, C.P.; Mohtadi, S.; Cane, M.A.; Seager, R.; Kushnir, Y. Climate change in the fertile crescent and implications of the recent Syrian drought. Proc. Natl. Acad. Sci. USA 2015, 112, 3241–3246. [Google Scholar] [CrossRef]
- López-Tirado, J.; Vessella, F.; Schirone, B.; Hidalgo, P.J. Trends in evergreen oak suitability from assembled species distribution models: Assessing climate change in South-Western Europe. New For. 2018, 49, 471–487. [Google Scholar] [CrossRef]
- Hama, A.A.; Khwarahm, N.R. Predictive mapping of two endemic oak tree species under climate change scenarios in a semiarid region: Range overlap and implications for conservation. Ecol. Inform. 2023, 73, 101930. [Google Scholar] [CrossRef]
- Valencia, A.S. Diversidad Del Género Quercus (Fagaceae) En México. Bot. Sci. 2004, 75, 33–53. [Google Scholar] [CrossRef]
- Taib, M.; Rezzak, Y.; Bouyazza, L.; Lyoussi, B. Medicinal uses, phytochemistry, and pharmacological activities of Quercus species. Evid. Based Complement. Altern. Med. 2020, 2020, 1920683. [Google Scholar] [CrossRef] [PubMed]
- Rathore, P.; Roy, A.; Karnatak, H. Assessing the vulnerability of oak (Quercus) forest ecosystems under projected climate and land use land cover changes in Western Himalaya. Biodivers. Conserv. 2019, 28, 2275–2294. [Google Scholar] [CrossRef]
- Carrero, C.; Jerome, D.; Beckman, E.; Byrne, A.; Coombes, A.J.; Deng, M.; González-Rodríguez, A.; Hoang, V.S.; Khoo, E.; Nguyen, N. The Red List of Oaks 2020; The Morton Arboretum: Lisle, IL, USA, 2020; p. 5. [Google Scholar]
- Fortini, P.; Di Pietro, R.; Proietti, E.; Cardoni, S.; Quaranta, L.; Simeone, M.C. Dissecting the continuum and unravelling the phylogeographic knot of plastid DNA in European white oaks (Quercus Sect. Quercus): Ancient signatures and multiple diversity reservoirs. Eur. J. For. Res. 2023, 143, 107–127. [Google Scholar] [CrossRef]
- Hipp, A.L.; Manos, P.S.; Hahn, M.; Avishai, M.; Bodénès, C.; Cavender-Bares, J.; Crowl, A.A.; Deng, M.; Denk, T.; Fitz-Gibbon, S. Genomic landscape of the global oak phylogeny. New Phytol. 2020, 226, 1198–1212. [Google Scholar] [CrossRef] [PubMed]
- Benito Garzón, M.; Sánchez de Dios, R.; Sainz Ollero, H. Effects of climate change on the distribution of Iberian tree species. Appl. Veg. Sci. 2008, 11, 169–178. [Google Scholar] [CrossRef]
- Alaoui, A.; Laaribya, S.; Ayan, S.; Ghallab, A.; López-Tirado, J. Modelling spatial distribution of endemic Moroccan fir (Abies marocana Trabut) in Talassemtane National Park, Morocco. Austrian J. For. Sci. 2021, 138, 73–94. [Google Scholar]
- López-Tirado, J.; Moreno-García, M.; Romera-Romera, D.; Zarco, V.; Hidalgo, P.J. Forecasting the circum-Mediterranean firs (Abies spp., Pinaceae) distribution: An assessment of a threatened conifers’ group facing climate change in the twenty-first century. New For. 2023, 55, 143–156. [Google Scholar] [CrossRef]
- Arar, A.; Nouidjem, Y.; Bounar, R.; Tabet, S.; Kouba, Y. Modeling of the current and future potential distribution of atlas cedar (Cedrus atlantica) forests revealed shifts in the latitudinal, longitudinal and altitudinal range towards more humid conditions. Ecol. Quest. 2020, 31, 49–62. [Google Scholar] [CrossRef]
- Ben-Said, M. Upward shifts of species range in Mediterranean high-mountain forests under current climate change: A review. Biol. Environ. 2022, 122B, 39–52. [Google Scholar] [CrossRef]
- Appiagyei, B.D.; Belhoucine-Guezouguli, L.; Bessah, E.; Morsli, B.; Fernandes, P.A.M. A Review on climate change impacts on forest ecosystem services in the Mediterranean Basin. J. Landsc. Ecol. 2022, 15, 1–26. [Google Scholar] [CrossRef]
- Moukrim, S.; Lahssini, S.; Rhazi, M.; Menzou, K.; El Madihi, M.; Rifai, N.; Bouziani, Y.; Azedou, A.; Boukhris, I.; Rhazi, L. Climate change impact on potential distribution of an endemic species Abies marocana Trabut. Ekológia 2022, 41, 329–339. [Google Scholar] [CrossRef]
- Dorado-Liñán, I.; Piovesan, G.; Martínez-Sancho, E.; Gea-Izquierdo, G.; Zang, C.; Cañellas, I.; Castagneri, D.; Di Filippo, A.; Gutiérrez, E.; Ewald, J.; et al. Geographical adaptation prevails over species-specific determinism in trees’ vulnerability to climate change at Mediterranean rear-edge forests. Glob. Chang. Biol. 2019, 25, 1296–1314. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Plant diversity patterns and conservation implications under climate-change scenarios in the Mediterranean: The case of Crete (Aegean, Greece). Diversity 2020, 12, 270. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Spatial phylogenetics, biogeographical patterns and conservation implications of the endemic flora of Crete (Aegean, Greece) under climate change scenarios. Biology 2020, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Mousikos, A.; Manolaki, P.; Knez, N.; Vogiatzakis, I.N. Can distribution modeling inform rare and endangered species monitoring in Mediterranean islands? Ecol. Inform. 2021, 66, 101434. [Google Scholar] [CrossRef]
- Louca, M.; Vogiatzakis, I.N.; Moustakas, A. Modelling the combined effects of land use and climatic changes: Coupling bioclimatic modelling with Markov-Chain cellular automata in a case study in Cyprus. Ecol. Inform. 2015, 30, 241–249. [Google Scholar] [CrossRef]
- Constantinou, I.; Panitsa, M. Contribution to the study of the plant diversity in communities with Quercus alnifolia in Cyprus. Flora Mediterr. 2022, 32, 291–304. [Google Scholar] [CrossRef]
- Sotiriou, A.; Gerasimidis, A. Contribution to the phytosociological research of Pinus nigra forests of the Troodos Mountain of Cyprus. In Proceedings of the Plant, Fungal and Habitat Diversity Investigation and Conservation—Proceedings of IV Balkan Botanical Congress, Sofia, Bulgaria, 20–26 June 2006; pp. 308–313. [Google Scholar]
- Sotiriou, A. Phytosociological Research of the National Forest Park of Mountain Troodos of Cyprus. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2010. (In Greek). [Google Scholar]
- Milios, E.; Petrou, P.; Pytharidis, K.; Christou, A.; Eliades, N.-G. Assessment of how natural stand structure for narrow endemic Cedrus brevifolia Henry supports silvicultural treatments for its sustainable management. South-East Eur. For. 2021, 12, 21–34. [Google Scholar] [CrossRef]
- Neophytou, C.; Palli, G.; Dounavi, A.; Aravanopoulos, F.A. Morphological differentiation and hybridization between Quercus alnifolia Poech and Quercus coccifera L. (Fagaceae) in Cyprus. Silvae Genet. 2007, 56, 271–277. [Google Scholar] [CrossRef]
- Neophytou, C. A Study of Genetic Differentiation and Hybridization among Oak Species with Divergent Ecological and Evolutionary Profiles. Ph.D. Thesis, Albert-Ludwigs-Universität Freiburg, Breisgau, Germany, 2010. [Google Scholar]
- Knopf, H.E. Quercus alnifolia. In Enzyklopädie der Holzgewächse: Handbuch und Atlas der Dendrologie; Wiley: Hoboken, NJ, USA, 2004; pp. 1–8. [Google Scholar]
- Meikle, R.D. Flora of Cyprus; Royal Botanic Gardens: London, UK, 1977; Volume 1. [Google Scholar]
- Tsintides, T.; Hadjikyriakou, G.; Christodoulou, C. Trees and Shrubs of Cyprus; Foundation, A.G. Leventis: Nicosia, Cyprus, 2002. [Google Scholar]
- Loizides, M. Quercus Alnifolia: The indigenous golden oak of Cyprus and its fungi. Field Mycol. 2011, 12, 81–88. [Google Scholar] [CrossRef]
- Gorener, V.; Beech, E. Quercus alnifolia; IUCN Red List: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Oldfield, S.; Eastwood, A. The Red List of Oaks; IUCN: Gland, Switzerland, 2007. [Google Scholar]
- Neophytou, C.; Jansen, S.; Hand, R.; Chrysostomou, G.; Iosif, K.; Christodoulou, C. Exploring the gene pools of Cypriot oaks: No evidence of intersectional hybridization. Silvae Genet. 2023, 72, 11–24. [Google Scholar] [CrossRef]
- Vitelli, M.; Vessella, F.; Cardoni, S.; Pollegioni, P.; Denk, T.; Grimm, G.W.; Simeone, M.C. Phylogeographic structuring of plastome diversity in Mediterranean oaks (Quercus Group Ilex, Fagaceae). Tree Genet. Genomes 2017, 13, 3. [Google Scholar] [CrossRef]
- Toumi, L.; Lumaret, R. Allozyme Characterisation of four Mediterranean evergreen oak species. Biochem. Syst. Ecol. 2001, 29, 799–817. [Google Scholar] [CrossRef] [PubMed]
- Médail, F.; Baumel, A. Using Phylogeography to define conservation priorities: The case of narrow endemic plants in the Mediterranean basin hotspot. Biol. Conserv. 2018, 224, 258–266. [Google Scholar] [CrossRef]
- Anagiotos, G.; Tsakaldimi, M.; Ganatsas, P. Variation in acorn traits among natural populations of Quercus alnifolia, an endangered species in Cyprus. Dendrobiology 2012, 68, 3–10. [Google Scholar]
- Kadis, C.; Pantazi, C.; Tsintides, T.; Christodoulou, C.; Papadopoulos, M.; Thanos, C.A.; Georghiou, K.; Kounnamas, C.; Constantinou, C.; Andreou, M.; et al. Establishment of a Plant Micro-Reserve network in Cyprus for the conservation of priority species and habitats. In Proceedings of the TOP Biodiversity Threats, Opportunities and Paces–Cyprus 2010, Larnaca, Cyprus, 14 July 2010; Conference Proceedings. Intercollege-Larnaca: Larnaca, Cyprus, 2010; pp. 113–120. [Google Scholar]
- Koo, K.A.; Park, S.U.; Seo, C. Effects of climate change on the climatic niches of warm-adapted evergreen plants: Expansion or contraction? Forests 2017, 8, 500. [Google Scholar] [CrossRef]
- Boisvert-Marsh, L.; Pedlar, J.H.; de Blois, S.; Le Squin, A.; Lawrence, K.; McKenney, D.W.; Williams, C.; Aubin, I. Migration-based simulations for Canadian trees show limited tracking of suitable climate under climate change. Divers. Distrib. 2022, 28, 2330–2348. [Google Scholar] [CrossRef]
- Tamme, R.; Götzenberger, L.; Zobel, M.; Bullock, J.M.; Hooftman, D.A.P.; Kaasik, A.; Pärtel, M. Predicting species’ maximum dispersal distances from simple plant traits. Ecology 2014, 95, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Mckenney, D.W.; Pedlar, J.H.; Rood, R.B.; Price, D. Revisiting projected shifts in the climate envelopes of North American trees using updated General Circulation Models. Glob. Chang. Biol. 2011, 17, 2720–2730. [Google Scholar] [CrossRef]
- Prasad, A.M.; Gardiner, J.D.; Iverson, L.R.; Matthews, S.N.; Peters, M. Exploring tree species colonization potentials using a spatially explicit simulation model: Implications for four oaks under climate change. Glob. Chang. Biol. 2013, 19, 2196–2208. [Google Scholar] [CrossRef]
- Lososová, Z.; Axmanová, I.; Chytrý, M.; Midolo, G.; Abdulhak, S.; Karger, D.N.; Renaud, J.; Van Es, J.; Vittoz, P.; Thuiller, W. Seed dispersal distance classes and dispersal modes for the European flora. Glob. Ecol. Biogeogr. 2023, 32, 1485–1494. [Google Scholar] [CrossRef]
- Regos, A.; Gagne, L.; Alcaraz-Segura, D.; Honrado, J.P.; Domínguez, J. Effects of species traits and environmental predictors on performance and transferability of ecological niche models. Sci. Rep. 2019, 9, 4221. [Google Scholar] [CrossRef] [PubMed]
- Sirami, C.; Caplat, P.; Popy, S.; Clamens, A.; Arlettaz, R.; Jiguet, F.; Brotons, L.; Martin, J.L. Impacts of global change on species distributions: Obstacles and solutions to integrate climate and land use. Glob. Ecol. Biogeogr. 2017, 26, 385–394. [Google Scholar] [CrossRef]
- Martin, Y.; Van Dyck, H.; Dendoncker, N.; Titeux, N. Testing instead of assuming the importance of land use change scenarios to model species distributions under climate change. Glob. Ecol. Biogeogr. 2013, 22, 1204–1216. [Google Scholar] [CrossRef]
- Hengl, T.; de Jesus, J.M.; Heuvelink, G.B.M.; Gonzalez, M.R.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef]
- Buri, A.; Grand, S.; Yashiro, E.; Adatte, T.; Spangenberg, J.E.; Pinto-Figueroa, E.; Verrecchia, E.; Guisan, A. What are the most crucial soil variables for predicting the distribution of mountain plant species? A comprehensive study in the Swiss Alps. J. Biogeogr. 2020, 47, 1143–1153. [Google Scholar] [CrossRef]
- Title, P.O.; Bemmels, J.B. ENVIREM: An expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography 2018, 41, 291–307. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Booth, T.H. The need for a global tree trial database. New For. 2023, 54, 1–7. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. Bioclim: The first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Liu, X. Global land projection based on plant functional types with a 1-km resolution under socio-climatic scenarios. Sci. Data 2022, 9, 125. [Google Scholar] [CrossRef]
- Panagos, P.; Hengl, T.; Wheeler, I.; Marcinkowski, P.; Rukeza, M.B.; Yu, B.; Yang, J.E.; Miao, C.; Chattopadhyay, N.; Sadeghi, S.H. Global rainfall erosivity database (GloREDa) and monthly R-factor data at 1 km spatial resolution. Data Brief 2023, 50, 109482. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.; Weynants, M.; Pásztor, L.; Hengl, T. 3D Soil hydraulic database of Europe at 250 m resolution. Hydrol. Process. 2017, 31, 2662–2666. [Google Scholar] [CrossRef]
- Austin, M.P.; Van Niel, K.P. Improving species distribution models for climate change studies: Variable selection and scale. J. Biogeogr. 2011, 38, 1–8. [Google Scholar] [CrossRef]
- Scherrer, D.; Guisan, A. Ecological indicator values reveal missing predictors of species distributions. Sci. Rep. 2019, 9, 3061. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Barbosa, A.M.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef] [PubMed]
- Mod, H.K.; Scherrer, D.; Luoto, M.; Guisan, A. What we use is not what we know: Environmental predictors in plant distribution models. J. Veg. Sci. 2016, 27, 1308–1322. [Google Scholar] [CrossRef]
- Araújo, M.B.; Peterson, A.T. Uses and misuses of bioclimatic envelope modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.J.; Smith, A.B.; Dekker, S.C.; Eppinga, M.B.; Leitão, P.J.; Moreno-Mateos, D.; Morueta-Holme, N.; Ruggeri, M. The role of land use and land cover change in climate change vulnerability assessments of biodiversity: A systematic review. Landsc. Ecol. 2021, 36, 3367–3382. [Google Scholar] [CrossRef]
- Vicente, J.; Randin, C.F.; Gonçalves, J.; Metzger, M.J.; Lomba, Â.; Honrado, J.; Guisan, A. Where will conflicts between alien and rare species occur after climate and land-use change? A test with a novel combined modelling approach. Biol. Invasions 2011, 13, 1209–1227. [Google Scholar] [CrossRef]
- Ramírez-Preciado, R.P.; Gasca-Pineda, J.; Arteaga, M.C. Effects of global warming on the potential distribution ranges of six Quercus species (Fagaceae). Flora Morphol. Distrib. Funct. Ecol. Plants 2019, 251, 32–38. [Google Scholar] [CrossRef]
- Milbau, A.; Stout, J.C. Factors associated with alien plants transitioning from casual, to naturalized, to invasive. Conserv. Biol. 2008, 22, 308–317. [Google Scholar] [CrossRef]
- Collart, F.; Broennimann, O.; Guisan, A.; Vanderpoorten, A. Ecological and biological indicators of the accuracy of species distribution models: Lessons from European bryophytes. Ecography 2023, 8, e06721. [Google Scholar] [CrossRef]
- Qiao, H.; Feng, X.; Escobar, L.E.; Peterson, A.T.; Soberón, J.; Zhu, G.; Papeş, M. An evaluation of transferability of ecological niche models. Ecography 2019, 42, 521–534. [Google Scholar] [CrossRef]
- Brown, J.L.; Carnaval, A.C. A tale of two niches: Methods, concepts, and evolution. Front. Biogeogr. 2019, 11, e44158. [Google Scholar] [CrossRef]
- Pasta, S.; Perez-Graber, A.; Fazan, L.; de Montmollin, B. The Top 50 Mediterranean Island Plants UPDATE 2017; IUCN/SSC/Mediterranean Plant Specialist Group: Neuchâtel, Switzerland, 2017. [Google Scholar]
- Woodward, F.I.; Williams, B.G. Climate and plant distribution at global and local scales. Vegetatio 1987, 69, 189–197. [Google Scholar] [CrossRef]
- Körner, C.; Basler, D.; Hoch, G.; Kollas, C.; Lenz, A.; Randin, C.F.; Vitasse, Y.; Zimmermann, N.E. Where, why and how? Explaining the low-temperature range limits of temperate tree species. J. Ecol. 2016, 104, 1076–1088. [Google Scholar] [CrossRef]
- Kenar, N.; Kikvidze, Z. Modelling the distribution of the caucasian oak (Quercus macranthera) in Western Asia under future climate change scenarios. Bot. Serbica 2023, 47, 215–226. [Google Scholar] [CrossRef]
- Testolin, R.; Attorre, F.; Jiménez-Alfaro, B. Global distribution and bioclimatic characterization of alpine biomes. Ecography 2020, 43, 779–788. [Google Scholar] [CrossRef]
- Sancho-Knapik, D.; Escudero, A.; Mediavilla, S.; Scoffoni, C.; Zailaa, J.; Cavender-Bares, J.; Álvarez-Arenas, T.G.; Molins, A.; Alonso-Forn, D.; Ferrio, J.P.; et al. Deciduous and evergreen oaks show contrasting adaptive responses in leaf mass per area across environments. New Phytol. 2021, 230, 521–534. [Google Scholar] [CrossRef]
- Sarris, D.; Christodoulakis, D.; Körner, C. Impact of recent climatic change on growth of low elevation Eastern Mediterranean forest trees. Clim. Chang. 2011, 106, 203–223. [Google Scholar] [CrossRef]
- Joët, T.; Ourcival, J.M.; Capelli, M.; Dussert, S.; Morin, X. Explanatory ecological factors for the persistence of desiccation-sensitive seeds in transient soil seed banks: Quercus ilex as a case study. Ann. Bot. 2016, 117, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.M.; Orrock, J.L. Climatic variation and seed persistence: Freeze–thaw cycles lower survival via the joint action of abiotic stress and fungal pathogens. Oecologia 2015, 179, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Ogaya, R.; Peñuelas, J. Comparative field study of Quercus Ilex and Phillyrea latifolia: Photosynthetic response to experimental drought conditions. Environ. Exp. Bot. 2003, 50, 137–148. [Google Scholar] [CrossRef]
- Altman, J.; Fibich, P.; Trotsiuk, V.; Altmanova, N. Global pattern of forest disturbances and its shift under climate change. Sci. Total Environ. 2024, 915, 170117. [Google Scholar] [CrossRef] [PubMed]
- Boonman, C.C.F.; Serra-Diaz, J.M.; Hoeks, S.; Guo, W.-Y.; Enquist, B.J.; Maitner, B.; Malhi, Y.; Merow, C.; Buitenwerf, R.; Svenning, J.-C. More than 17,000 tree species are at risk from rapid global change. Nat. Commun. 2024, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Buras, A.; Menzel, A. Projecting tree species composition changes of European forests for 2061–2090 under RCP 4.5 and RCP 8.5 Scenarios. Front. Plant Sci. 2019, 9, 1986. [Google Scholar] [CrossRef]
- Pecchi, M.; Marchi, M.; Moriondo, M.; Forzieri, G.; Ammoniaci, M.; Bernetti, I.; Bindi, M.; Chirici, G. Potential impact of climate change on the forest coverage and the spatial distribution of 19 key forest tree species in Italy under RCP4.5 IPCC trajectory for 2050s. Forests 2020, 11, 934. [Google Scholar] [CrossRef]
- Nobis, M.P.; Normand, S. KISSMig—A simple mModel for R to aAccount for limited migration in analyses of species distributions. Ecography 2014, 37, 1282–1287. [Google Scholar] [CrossRef]
- Ah Koo, K.; Uk Park, S. The effect of interplays among climate change, land-use change, and dispersal capacity on plant redistribution. Ecol. Indic. 2022, 142, 109192. [Google Scholar] [CrossRef]
- Holloway, P.; Miller, J.A.; Gillings, S. Incorporating movement in species distribution models: How do simulations of dispersal affect the accuracy and uncertainty of projections? Int. J. Geogr. Inf. Sci. 2016, 30, 2050–2074. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; Ferreira De Siqueira, M.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Vittoz, P.; Engler, R. Seed dispersal distances: A typology based on dispersal modes and plant traits. Bot. Helv. 2007, 117, 109–124. [Google Scholar] [CrossRef]
- Collingham, Y.C.; Huntley, B. Impacts of habitat fragmentation and patch size upon migration rates. Ecol. Appl. 2000, 10, 131–144. [Google Scholar] [CrossRef]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kokkoris, I.P.; Strid, A.; Raus, T.; Dimopoulos, P. Climate-change impacts on the southernmost Mediterranean arctic-alpine plant populations. Sustainability 2021, 13, 13778. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Papanikolaou, A.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P.; Panitsa, M. Climate change impacts and extinction risk assessment of Nepeta representatives (Lamiaceae) in Greece. Sustainability 2022, 14, 4269. [Google Scholar] [CrossRef]
- Bisht, V.K.; Kuniyal, C.P. Climate change matters because the oaks cannot move upward. Curr. Sci. 2013, 10, 689–690. [Google Scholar]
- Noce, S.; Cipriano, C.; Santini, M. Altitudinal shifting of major forest tree species in Italian Mountains under climate change. Front. For. Glob. Chang. 2023, 6, 1250651. [Google Scholar] [CrossRef]
- Cudlín, P.; Klopčič, M.; Tognetti, R.; Mališ, F.; Alados, C.L.; Bebi, P.; Grunewald, K.; Zhiyanski, M.; Andonowski, V.; La Porta, N.; et al. Drivers of treeline shift in different European Mountains. Clim. Res. 2017, 73, 135–150. [Google Scholar] [CrossRef]
- Harsch, M.A.; Hulme, P.E.; McGlone, M.S.; Duncan, R.P. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol. Lett. 2009, 12, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Garamvölgyi, Á.; Hufnagel, L. Impacts of climate change on vegetation distribution No. 1: Climate change induced vegetation shifts in the palearctic region. Appl. Ecol. Environ. Res. 2013, 11, 79–122. [Google Scholar] [CrossRef]
- García-Valdés, R.; Zavala, M.A.; Araújo, M.B.; Purves, D.W. Chasing a moving target: Projecting climate change-induced shifts in non-equilibrial tree species distributions. J. Ecol. 2013, 101, 441–453. [Google Scholar] [CrossRef]
- Greenwood, S.; Jump, A.S. Consequences of tree line shifts for the diversity and function of high-altitude ecosystems. Arct. Antarct. Alp. Res. 2014, 46, 829–840. [Google Scholar] [CrossRef]
- Suzette Lorilla, R.; Kefalas, G.; Christou, A.K.; Poirazidis, K.; Homer Eliades, N.G. Enhancing the conservation status and resilience of a narrowly distributed forest: A challenge to effectively support ecosystem services in practice. J. Nat. Conserv. 2023, 73, 126414. [Google Scholar] [CrossRef]
- López-Tirado, J.; Vessella, F.; Stephan, J.; Ayan, S.; Schirone, B.; Hidalgo, P.J. Effect of climate change on potential distribution of Cedrus libani A. Rich in the twenty-first century: An ecological niche modeling assessment. New For. 2021, 52, 363–376. [Google Scholar] [CrossRef]
- Wang, W.T.; Guo, W.Y.; Jarvie, S.; Serra-Diaz, J.M.; Svenning, J.C. Anthropogenic climate change increases vulnerability of Magnolia species more in Asia than in the Americas. Biol. Conserv. 2022, 265, 109425. [Google Scholar] [CrossRef]
- Kyratzis, A.C.; Kourtellarides, D.; Chrysostomou, G.; Iosif, C.K.; Papachristophorou, T.; Kounnamas, C.; Nikiforou, C.; Christodoulou, C.S. Ex situ conservation of threatened species of the flora of Cyprus: Current status and future priorities with respect to the global strategy for plant conservation target 8. Kew Bull. 2024, 79, 115–129. [Google Scholar] [CrossRef]
- Lemesios, G.; Karali, A.; Papadaskalopoulou, C.; Pitsari, S.; Malamis, D.; Ioannou, K.; Zachariou-Dodou, M.; Giannakopoulos, C.; Petrakis, M.; Loizidou, M. Future vulnerability assessment of forest fire sector to climate change impacts in Cyprus. In Proceedings of the AdaptToClimate Conference, Lefkosia, Cyprus, 27–28 March 2014. [Google Scholar]
- Zachariadis, T. Climate change in Cyprus: Impacts and adaptation policies. Cyprus Econ. Policy Rev. 2012, 6, 21–37. [Google Scholar]
- Brauneck, J.; Lange, M. The Applicability of black carbon for tracing soil erosion: Fire impacts on landscape dynamics in Cyprus. In Proceedings of the IAHS-AISH Conference, Banff, AB, Canada, 11–14 June 2012. [Google Scholar]
- Rivers, M.; Beech, E.; Bazos, I.; Bogunić, F.; Buira, A.; Caković, D.; Carapeto, A.; Carta, A.; Cornier, B.; Fenu, G. European Red List of Trees; International Union for Conservation of Nature and Natural Resources (IUCN): Gland, Switzerland, 2019. [Google Scholar]
- IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria; Version 14; IUCN: Gland, Switzerland, 2019; p. 113. [Google Scholar]
- GBIF.org. Occurrence Download—Quercus alnifolia. 2023. Available online: https://www.gbif.org/ (accessed on 10 December 2023).
- Mauri, A.; Strona, G.; San-Miguel-Ayanz, J. EU-Forest, a high-resolution tree occurrence dataset for Europe. Sci. Data 2017, 4, 160123. [Google Scholar] [CrossRef]
- Monnet, A.-C.; Cilleros, K.; Médail, F.; Albassatneh, M.C.; Arroyo, J.; Bacchetta, G.; Bagnoli, F.; Barina, Z.; Cartereau, M.; Casajus, N.; et al. WOODIV, a database of occurrences, functional traits, and phylogenetic data for all Euro-Mediterranean trees. Sci. Data 2021, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Moudrý, V.; Bazzichetto, M.; Remelgado, R.; Devillers, R.; Lenoir, J.; Mateo, R.G.; Sillero, N.; Lecours, V.; Cord, A.F.; Barták, V. Optimising Species Distribution Models: Sample size, positional error, and sampling bias matter. EcoEvoRxiv 2023. [Google Scholar] [CrossRef]
- Smith, A.B.; Murphy, S.J.; Henderson, D.; Erickson, K.D. Including imprecisely georeferenced specimens improves accuracy of species distribution models and estimates of niche breadth. Glob. Ecol. Biogeogr. 2023, 32, 342–355. [Google Scholar] [CrossRef]
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Duarte Ritter, C.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R. CoordinateCleaner: Standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Smith, A.B. EnmSdm: Tools for Modeling Species Niches and Distributions R Package. Version 0.5.1.5. 2020. Available online: http://github.com/adamlilith/enmSdm (accessed on 10 December 2023).
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. SpThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Robertson, M.P.; Visser, V.; Hui, C. Biogeo: An R package for assessing and improving data quality of occurrence record datasets. Ecography 2016, 39, 394–401. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Márcia Barbosa, A.; Ghosh, A.; Mandel, A. Geodata: Download Geographic Data R Package, version 0.5-8; R Core Team: Cary, CA, USA, 2023. [Google Scholar]
- Marchi, M.; Castellanos-Acuña, D.; Hamann, A.; Wang, T.; Ray, D.; Menzel, A. ClimateEU, scale-free climate normals, historical time series, and future projections for Europe. Sci. Data 2020, 7, 428. [Google Scholar] [CrossRef]
- Hijmans, R.; Philipps, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling R Package. Version 1.1-4. 2017. Available online: https://www.rdocumentation.org/packages/dismo/versions/1.1-4 (accessed on 10 December 2023).
- Hamann, A.; Wang, T.; Spittlehouse, D.L.; Murdock, T.Q. A comprehensive, high-resolution database of historical and projected climate surfaces for Western North America. Bull. Am. Meteorol. Soc. 2013, 94, 1307–1309. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Spittlehouse, D.L.; Murdock, T.Q. ClimateWNA—High-resolution spatial climate data for Western North America. J. Appl. Meteorol. Climatol. 2012, 51, 16–29. [Google Scholar] [CrossRef]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. Version 3.6-26. 2018. Available online: https://cran.r-project.org/web/packages/raster/raster.pdf (accessed on 10 December 2023).
- Hijmans, R. Terra: Spatial Data Analysis. R Package Version 1.7-46. 2023. Available online: https://rspatial.org/spatial/ (accessed on 10 December 2023).
- Evans, J.S. SpatialEco—R Package. Version 1.2-0. 2019. Available online: https://www.rdocumentation.org/packages/spatialEco/versions/1.2-0 (accessed on 10 December 2023).
- Panagos, P.; Van Liedekerke, M.; Borrelli, P.; Köninger, J.; Ballabio, C.; Orgiazzi, A.; Lugato, E.; Liakos, L.; Hervas, J.; Jones, A. European soil data centre 2.0: Soil data and knowledge in support of the EU Policies. Eur. J. Soil Sci. 2022, 73, e13315. [Google Scholar] [CrossRef]
- Panagos, P.; Van Liedekerke, M.; Jones, A.; Montanarella, L. European soil data centre: Response to European policy support and public data requirements. Land Use Policy 2012, 29, 329–338. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, F.; Tseng, T.-H.; Carver, S.; Chen, X.; Zhao, J.; Yu, L.; Li, F.; Zhao, Z.; Yang, R. Identifying ecosystem service value and potential loss of wilderness areas in China to support post-2020 global biodiversity conservation. Sci. Total Environ. 2022, 846, 157348. [Google Scholar] [CrossRef] [PubMed]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Benito, B. Collinear: R Package for Seamless Multicollinearity Management. 2023. Available online: https://cran.r-project.org/web/packages/collinear/collinear.pdf (accessed on 10 December 2023).
- Exavier, R.; Zeilhofer, P. OpenLand: Software for quantitative analysis and visualization of land use and cover change. R J. 2020, 12, 359. [Google Scholar] [CrossRef]
- Breiner, F.T.; Nobis, M.P.; Bergamini, A.; Guisan, A. Optimizing ensembles of small models for predicting the distribution of species with few occurrences. Methods Ecol. Evol. 2018, 9, 802–808. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Broennimann, O.; Di Cola, V.; Guisan, A. Ecospat: Spatial Ecology Miscellaneous Methods; R Package Version 3.2; R Core Team: Cary, CA, USA, 2021. [Google Scholar]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Modelling species presence-only data with random forests. Ecography 2021, 44, 1731–1742. [Google Scholar] [CrossRef]
- Valavi, R.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Predictive performance of presence-only species distribution models: A Benchmark study with reproducible code. Ecol. Monogr. 2022, 92, e1486. [Google Scholar] [CrossRef]
- Velazco, S.J.E.; Rose, M.B.; de Andrade, A.F.A.; Minoli, I.; Franklin, J. Flexsdm: An R package for supporting a comprehensive and flexible Species Distribution Modelling workflow. Methods Ecol. Evol. 2022, 13, 1661–1669. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Roberts, D.R.; Bahn, V.; Ciuti, S.; Boyce, M.S.; Elith, J.; Guillera-Arroita, G.; Hauenstein, S.; Lahoz-Monfort, J.J.; Schröder, B.; Thuiller, W.; et al. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography 2017, 40, 913–929. [Google Scholar] [CrossRef]
- Santini, L.; Benítez-López, A.; Maiorano, L.; Čengić, M.; Huijbregts, M.A.J. Assessing the reliability of species distribution projections in climate change research. Divers. Distrib. 2021, 27, 1035–1050. [Google Scholar] [CrossRef]
- Raes, N.; Ter Steege, H. A null-model for significance testing of presence-only species distribution models. Ecography 2007, 30, 727–736. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Sofaer, H.R.; Hoeting, J.A.; Jarnevich, C.S. The area under the precision-recall curve as a performance metric for rare binary events. Methods Ecol. Evol. 2019, 10, 565–577. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Measuring and comparing the accuracy of species distribution models with presence-absence data. Ecography 2011, 34, 232–243. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Regos, A.; Martins, I.; Honrado, J.; Alonso, J. Effects of input data sources on species distribution model predictions across species with different distributional ranges. J. Biogeogr. 2022, 49, 1299–1312. [Google Scholar] [CrossRef]
- Hammer, B.; Frasco, M. Metrics: Evaluation Metrics for Machine Learning. R Package Version 0.1.4. 2018. Available online: https://cran.r-project.org/web/packages/Metrics/Metrics.pdf (accessed on 10 December 2023).
- Márcia Barbosa, A.; Real, R.; Muñoz, A.R.; Brown, J.A. New measures for assessing model equilibrium and prediction mismatch in species distribution models. Divers. Distrib. 2013, 19, 1333–1338. [Google Scholar] [CrossRef]
- Schwarz, J.; Heider, D. GUESS: Projecting machine learning Scores to well-calibrated probability estimates for clinical decision-making. Bioinformatics 2019, 35, 2458–2465. [Google Scholar] [CrossRef]
- Signorell, A.; Aho, K.; Anderegg, N.; Aragon, T.; Arppe, A.; Baddeley, A.; Bolker, B.; Caeiro, F.; Champely, S.; Chessel, D. DescTools: Tools for Descriptive Statistics; R Package Version 0.99-40; R Core Team: Cary, CA, USA, 2021. [Google Scholar]
- Collart, F.; Hedenäs, L.; Broennimann, O.; Guisan, A.; Vanderpoorten, A. Intraspecific differentiation: Implications for niche and distribution modelling. J. Biogeogr. 2021, 48, 415–426. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef]
- Velazco, S.J.E.; Rose, M.B.; De Marco, P., Jr.; Regan, H.M.; Franklin, J. How far can I extrapolate my species distribution model? exploring shape, a novel method. Ecography 2024, 2024, e06992. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Biomod2: Ensemble Platform for Species Distribution Modeling. Ecography 2016, 32, 369–373. [Google Scholar] [CrossRef]
- Engler, R.; Hordijk, W.; Guisan, A. The MIGCLIM R package—Seamless integration of dispersal constraints into projections of species distribution models. Ecography 2012, 35, 872–878. [Google Scholar] [CrossRef]
- Engler, R.; Guisan, A. MigClim: Predicting plant distribution and dispersal in a changing climate. Divers. Distrib. 2009, 15, 590–601. [Google Scholar] [CrossRef]
- Mauri, A.; Girardello, M.; Strona, G.; Beck, P.S.A.; Forzieri, G.; Caudullo, G.; Manca, F.; Cescatti, A. EU-Trees4F, a dataset on the future distribution of European tree species. Sci. Data 2022, 9, 37. [Google Scholar] [CrossRef]
- Thomson, F.J.; Letten, A.D.; Tamme, R.; Edwards, W.; Moles, A.T. Can dispersal investment eExplain why tall plant species achieve longer dispersal distances than short plant species? New Phytol. 2018, 217, 407–415. [Google Scholar] [CrossRef]
- Jablonski, E.J. Oaks of Cyprus. J. Int. Oak Soc. 2013, 24, 27–34. [Google Scholar]
- McGarigal, K. FRAGSTATS: Spatial Pattern Analysis Program for Categorical Maps. Computer Software Program Produced by the Authors at the University of Massachusets, Amherst. 2002. Available online: www.umass.edu/landeco/research/fragstats/fragstats.html (accessed on 10 December 2023).
- Hesselbarth, M.H.K.; Sciaini, M.; With, K.A.; Wiegand, K.; Nowosad, J. Landscapemetrics: An open-source R tool to calculate landscape metrics. Ecography 2019, 42, 1648–1657. [Google Scholar] [CrossRef]
- Rinnan, D.S.; Lawler, J. Climate-niche factor analysis: A spatial approach to quantifying species vulnerability to climate change. Ecography 2019, 42, 1494–1503. [Google Scholar] [CrossRef]
- Rinnan, D.S. CENFA: Climate and Ecological Nich Factor Analysis, R Package version 1.0.0; R Core Team: Cary, CA, USA, 2018. [Google Scholar]
- Hirzel, A.H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-niche factor analysis: How to compute habitat-suitability maps without absence data? Ecology 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Dauby, G.; Stévart, T.; Droissart, V.; Cosiaux, A.; Deblauwe, V.; Simo-Droissart, M.; Sosef, M.S.M.; Lowry, P.P.; Schatz, G.E.; Gereau, R.E.; et al. ConR: An R package to assist large-scale multispecies preliminary conservation assessments using distribution data. Ecol. Evol. 2017, 7, 11292–11303. [Google Scholar] [CrossRef]
- Stévart, T.; Dauby, G.; Lowry, P.P.; Blach-Overgaard, A.; Droissart, V.; Harris, D.J.; Mackinder, B.A.; Schatz, G.E.; Sonké, B.; Sosef, M.S.M.; et al. A third of the tropical african flora is potentially threatened with extinction. Sci. Adv. 2019, 5, eaax9444. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kougioumoutzis, K.; Constantinou, I.; Panitsa, M. Rising Temperatures, Falling Leaves: Predicting the Fate of Cyprus’s Endemic Oak under Climate and Land Use Change. Plants 2024, 13, 1109. https://doi.org/10.3390/plants13081109
Kougioumoutzis K, Constantinou I, Panitsa M. Rising Temperatures, Falling Leaves: Predicting the Fate of Cyprus’s Endemic Oak under Climate and Land Use Change. Plants. 2024; 13(8):1109. https://doi.org/10.3390/plants13081109
Chicago/Turabian StyleKougioumoutzis, Konstantinos, Ioannis Constantinou, and Maria Panitsa. 2024. "Rising Temperatures, Falling Leaves: Predicting the Fate of Cyprus’s Endemic Oak under Climate and Land Use Change" Plants 13, no. 8: 1109. https://doi.org/10.3390/plants13081109




