Chemical Profile and Bioactivity of Rubus idaeus L. Fruits Grown in Conventional and Aeroponic Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Extraction Procedure
2.4. Bioactive Compounds Analysis of Raspberry Fruits
2.4.1. Determination of Total Phytochemicals (Phenols, Flavonoids, and Anthocyanins)
2.4.2. High-Performance Liquid Chromatography—Diode Array Detection (HPLC-DAD) Analyses
2.5. In Vitro Antioxidant Activity
2.6. Anti-Inflammatory Activity
2.6.1. Cell Cultures
2.6.2. Inhibition of NO Production in LPS-Stimulated RAW 264.7 Cells
2.7. Cell Viability Assay
2.8. α-Glucosidase Inhibitory Activity Assay
2.9. Pancreatic Lipase Inhibitory Activity Test
2.10. Statistical Analysis
3. Results and Discussion
3.1. Bioactive Compound Content in Raspberry Fruit
3.2. Antioxidant Activity
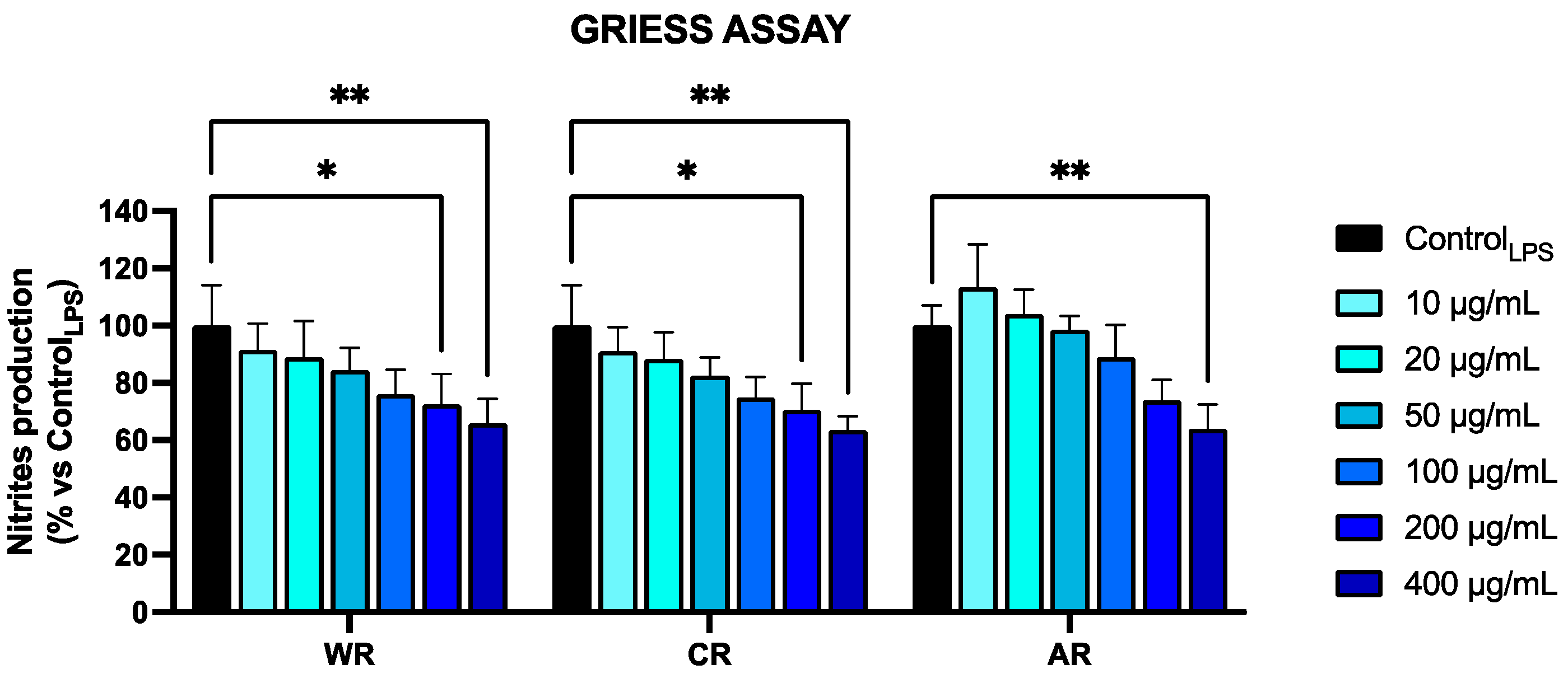
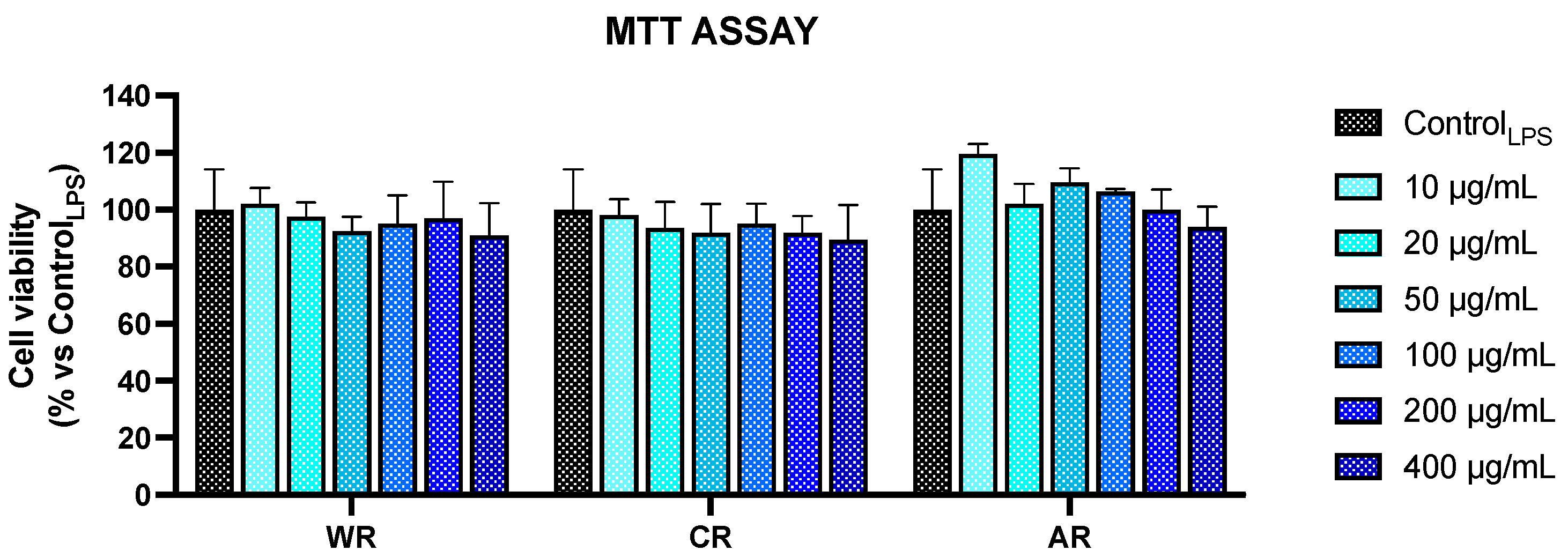
3.3. Anti-Inflammatory Activity
3.4. Enzymes Inhibition Assay of Raspberry Fruits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.G.; Di Mascio, P.; Wagner, J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; del Nobile, M.A. Antioxidant compounds from vegetable matrices: Biosynthesis, occurrence, and extraction systems. Crit. Rev. Food Sci. Nutr. 2015, 56, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Cosme, F.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Gonçalves, B. Red fruits composition and their health benefits—A review. Foods 2022, 11, 644. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Synder, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Del Bo, C.; Martini, D.; Porrini, M.; Klimis-Zacas, D.; Riso, P. Berries and oxidative stress markers: An overview of human intervention studies. Food Funct. 2015, 6, 2890–2917. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, J.; Chen, Z.; Jiang, J.; Jackson, A. Characterization of carotenoids and phenolics during fruit ripening of Chinese raspberry (Rubus chingii Hu). RSC Adv. 2021, 11, 10804–10813. [Google Scholar] [CrossRef] [PubMed]
- Hendawy, O.; Gomaa, H.A.; Hussein, S.; Alzarea, S.I.; Qasim, S.; Rahman, F.E.Z.S.A.; Ali, A.T.; Ahmed, S.R. Cold-pressed raspberry seeds oil ameliorates high-fat diet triggered non-alcoholic fatty liver disease. Saudi Pharm. J. 2021, 29, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Maríc, B.; Pavlic Colovíc, D.; Abramovíc, B.; Zekovíc, Z.; Bodroža-Solarov, M.; Ilíc, N.; Teslíc, N. Recovery of high-content ω–3 fatty acid oil from raspberry (Rubus idaeus L.) seeds: Chemical composition and functional quality. LWT-Food Sci. Technol. 2020, 130, 109627. [Google Scholar]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, anti-inflammatory and cytotoxic activity of phenolic compound family extracted from raspberries (Rubus idaeus): A general review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, Y.; Zhang, S.; Yang, H.; Wu, W.; Lyu, L.; Li, W. Changes in antioxidant substances and antioxidant enzyme activities in raspberry fruits at different developmental stages. Sci. Hortic. 2023, 321, 112314. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Szwajgier, D.; Olkowicz, M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS ONE 2017, 12, e0188583. [Google Scholar] [CrossRef] [PubMed]
- Mazur, S.P.; Nes, A.; Wold, A.B.; Remberg, S.F.; Aaby, K. Quality and chemical composition of ten red raspberry (Rubus idaeus L.) genotypes during three harvest seasons. Food Chem. 2014, 160, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.; Grez, J.; Alcalde Furber, J.A.; Neri, D.; Gambardella, M. Effect of low temperature in the first development stage for five red raspberry genotypes. Hortic. Sci. 2019, 46, 9–16. [Google Scholar] [CrossRef]
- Woznicki, T.L.; Heide, O.M.; Remberg, S.F.; Sonsteby, A. Effects of controlled nutrient feeding and different temperatures during floral initiation on yield, berry size and drupelet numbers in red raspberry (Rubus idaeus L.). Sci. Hortic. 2016, 212, 148–154. [Google Scholar] [CrossRef]
- Devanathan, R. Ion sieving and desalination: Energy penalty for excess baggage. Nat. Nanotechnol. 2017, 12, 500–501. [Google Scholar] [CrossRef] [PubMed]
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Chandio, F.A.; Buttar, N.A. Modern plant cultivation technologies in agriculture under controlled environment: A review on aeroponics. J. Plant Interact. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Wimmerova, L.; Keken, Z.; Solcova, O.; Bartos, L.; Spacilova, M. A Comparative LCA of aeroponic, hydroponic, and soil cultivations of bioactive substance producing plants. Sustainability 2022, 14, 2421. [Google Scholar] [CrossRef]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.M.; Remais, J.; Fung, M.C.; Xu, L.; Sun, S.S.M. Food supply and food safety issues in China. Lancet 2013, 381, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sampath, B.; Kumar, S.; Babu, B.; Ahalya, N. Hydroponics, aeroponics, and aquaponics technologies in modern agricultural cultivation. In Trends, Paradigms, and Advances in Mechatronics Engineering; IGI Global: Hershey, PA, USA, 2023; pp. 223–241. [Google Scholar]
- Gruda, N. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Treftz, C.; Omaye, S.T. Nutrient analysis of soil and soilless strawberries and raspberries grown in a greenhouse. Food Nutr. Sci. 2015, 6, 805–815. [Google Scholar] [CrossRef]
- Chow, K.K.; Price, T.V.; Hanger, B.C. Effects of nitrogen, potassium, calcium concentrations and solution temperatures on the growth and yield of strawberry cv. Red gauntlet in a nutrient film (NFT) hydroponic system. In Proceedings of the XXVI International Horticultural Congress: Protected Cultivation 2002: In Search of Structures, Systems and Plant Materials for Sustainable Greenhouse Production, Toronto, ON, Canada, 11–17 August 2002; Volume 633, pp. 315–327. [Google Scholar]
- Pascual, C.S.; Agulto, I.C.; Espino Jr, A.N.; Malamug, V.U. Effect of ground heat exchanger for root-zone cooling on the growth and yield of aeroponically-grown strawberry plant under tropical greenhouse condition. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 301, p. 012006. [Google Scholar]
- Neocleous, D.; Papadopoulos, I.; Vasilakakis, M. Growing red raspberry in soilless culture under different chilling treatments for early summer production. Small Fruits Rev. 2005, 4, 37–48. [Google Scholar] [CrossRef]
- Balawejder, M.; Matłok, N.; Piechowiak, T.; Szostek, M.; Kapusta, I.; Niemiec, M.; Komorowska, M.; Wróbel, M.; Mudryk, K.; Szeląg-Sikora, A.; et al. The modification of substrate in the soilless cultivation of raspberries (Rubus idaeus L.) as a factor stimulating the biosynthesis of selected bioactive compounds in fruits. Molecules 2022, 28, 118. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Dugay, A.; Acquaviva, R.; Malfa, G.A.; Bonesi, M.; Bouzidi, C.; Tundis, R. Contribution of flavonoids and iridoids to the hypoglycaemic, antioxidant, and nitric oxide (NO) inhibitory activities of Arbutus unedo L. Antioxidants 2020, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Unit F1.2. In Current Protocols in Food Analytical Chemistry; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Donno, D.; Cavanna, M.; Beccaro, G.L.; Mellano, M.G.; Torello Marinoni, D.; Cerutti, A.K.; Bounous, G. Currants and strawberries as bioactive compound sources: Determination of antioxidant profiles with HPLC-DAD/MS. J. Appl. Botany Food Qual. 2013, 86, 1–10. [Google Scholar]
- Loizzo, M.R.; Pacetti, D.; Lucci, P.; Núñez, O.; Menichini, F.; Frega, N.G.; Tundis, R. Prunus persica var. platycarpa (Tabacchiera Peach): Bioactive compounds and antioxidant activity of pulp, peel and seed ethanolic extracts. Plant Foods Hum. Nutr. 2015, 70, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Brindisi, M.; Fiorillo, M.; Frattaruolo, L.; Sotgia, F.; Lisanti, M.P.; Cappello, A.R. Cholesterol and mevalonate: Two metabolites involved in breast cancer progression and drug resistance through the ERRα Pathway. Cells 2020, 9, 1819. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Frattaruolo, L.; Carullo, G.; Armentano, B.; Badolato, M.; Loizzo, M.R.; Aiello, F.; Cappello, A.R. An ancient remedial repurposing: Synthesis of new pinocembrin fatty acid acyl derivatives as potential antimicrobial/ anti-inflammatory agents. Nat. Prod. Res. 2019, 33, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Deguin, B.; Cappello, A.R.; Cappello, M.S. Chemical profile, antioxidant, anti-inflammatory, and anti-cancer effects of Italian Salvia rosmarinus Spenn. methanol leaves extracts. Antioxidants 2020, 9, 826. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, M.C.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Bouzidi, C.; Marie, A.; Acquaviva, R.; Cappello, A.R.; Deguin, B. Iridoid- and flavonoid-enriched fractions of Cornus sanguinea and Cornus mas exert antioxidant and anti-inflammatory effects and inhibit key enzymes in the treatment of metabolic disorders. Food Funct. 2023, 14, 8838. [Google Scholar] [CrossRef] [PubMed]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, Y.; Qin, Y.; Wang, L.; Wu, Z. HPLC-ESI-qTOF-MS/MS Characterization, Antioxidant activities and inhibitory ability of digestive enzymes with molecular docking analysis of various parts of raspberry (Rubus ideaus L.). Antioxidants 2019, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Katarzyna, R.; Paweł, P.; Joanna, R.; Aneta, K.; Audrius, M.; Monika, N.; Boguslaw, B. Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chem. 2019, 289, 16–25. [Google Scholar]
- Bortolini, D.G.; Maciel, G.M.; de Andrade Arruda Fernandes, I.; Rossetto, R.; Brugnari, T.; Rampazzo Ribeiro, V.; Haminiuk, C.W.I. Biological potential and technological applications of red fruits: An overview. Food Chem. Adv. 2022, 1, 100014. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- de Souza, V.R.; Pereira, P.A.P.; da Silva, T.L.T.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Veljković, B.; Jakovljević, V.; Stanković, M.; Dajić-Stevanović, Z. Phytochemical and antioxidant properties of fresh fruits and some traditional products of wild grown raspberry (Rubus idaeus L.). Not. Bot. Horti. Agrobot. Cluj-Na, 2019; 47, 565–573. [Google Scholar]
- Veličković, I.; Živković, J.; Stojković, D.; Sokovic, M.D.; Marin, P.D.; Grujić, S. Evaluation of antioxidant, antimicrobial and potential food preserving properties of Rubus discolor (Rosaceae) fruit extracts. Nat. Prod. Comm. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Chwil, M.; Matraszek-Gawron, R.; Kostryco, M.; Rózanska-Boczula, M. Nutritionally important pro-health active ingredients and antioxidant properties of fruits and fruit juice of selected biennial fruiting Rubus idaeus L. cultivars. Pharmaceuticals 2023, 16, 1698. [Google Scholar] [CrossRef] [PubMed]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Fredes, C.; Montenegro, G.; Zoffoli, J.P.; Santander, F.; Robert, P. Comparison of the total phenolic content, total anthocyanin content and antioxidant activity of polyphenol-rich fruits grown in Chile. Cienc. Investig. Agric. 2014, 41, 49–60. [Google Scholar] [CrossRef]
- Zheng, C.D.; Li, G.; Li, H.Q.; Xu, X.J.; Gao, J.M.; Zhang, A.L. DPPH-scavenging activities and structure-activity relationships of phenolic compounds. Nat. Prod. Commun. 2010, 5, 1759–1765. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT—Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Rusmana, D.; Wahyudianingsih, R.; Elisabeth, M.; Balqis, B.; Maesaroh, M.; Wahyu, W. Antioxidant activity of Phyllanthus niruri extract, rutin and quercetin. Ind. Biomed. J. 2017, 9, 84–90. [Google Scholar] [CrossRef]
- Brighente, I.M.C.; Dias, M.; Verdi, L.G.; Pizzolatti, M.G. Antioxidant activity and total phenolic content of some Brazilian species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.; Vreeburg, R.A.; Savelkoul, H.F.; Wichers, H.J. Transcription profiles of LPS-stimulated THP 1 monocytes and macrophages: A tool to study inflammation modulating effects of food-derived compounds. Food Funct. 2010, 1, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Frattaruolo, L.; Durante, M.; Cappello, M.S.; Montefusco, A.; Mita, G.; Cappello, A.R.; Lenucci, M.S. The ability of supercritical CO2 carrot and pumpkin extracts to counteract inflammation and oxidative stress in RAW 264.7 macrophages stimulated with LPS or MDA-MB-231 cell-conditioned media. Food Funct. 2023, 14, 10083–10096. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, 6234. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, S.; Frattaruolo, L.; Brindisi, M.; Ulivieri, C.; Vanni, F.; Brizzi, A.; Carullo, G.; Cappello, A.R.; Aiello, F. 3-Amino-alkylated indoles: Unexplored green products acting as anti-inflammatory agents. Fut. Med. Chem. 2020, 12, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Weiskirchen, R. The role of obesity in type 2 diabetes mellitus—An overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P. α-Glucosidase inhibitors and their use in clinical practice. Arch. Med. Sci. 2012, 8, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Bae, S.H.; Park, Y.; Choi, H.S.; Suh, H.J. Lipase-mediated lipid removal from propolis extract and its antiradical and antimicrobial activity. J. Sci. Food. Agric. 2015, 95, 1697–1705. [Google Scholar] [CrossRef]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Romeo, F.V.; Rapisarda, P. Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals. J. Sci. Food Agric. 2016, 96, 4713–4723. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Pinto, J.; Llorent-Martínez, E.J.; Tomás, H.; Castilho, P.C. Evaluation of Rubus grandifolius L. (wild blackberries) activities targeting management of type-2 diabetes and obesity using in vitro models. Food Chem. Toxicol. 2019, 123, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Perri, V.; Menichini, F.; Marrelli, M.; Uzunov, D.; Statti, G.A.; Menichini, F. Wild Mediterranean dietary plants as inhibitors of pancreatic lipase. Phytother. Res. 2012, 26, 600–604. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Leporini, M.; D’Urso, G.; Gagliano Candela, R.; Falco, T.; Piacente, S.; Bruno, M.; Sottile, F. Almond (Prunus dulcis cv. Casteltermini) skin confectionery by-products: New opportunity for the development of a functional blackberry (Rubus ulmifolius Schott) jam. Antioxidants 2021, 10, 1218. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Dwikat, M.; Amer, J.; Hawash, M.; Hussein, F.; Qneibi, M.; Issa, L.; Asab, J.A.; Hallak, H.; Arar, D.N.; et al. Anticancer, free radicals, and digestive enzyme inhibitory activities of Rubus sanctus Schreb Root four solvent fractions. Evid. Based Complement Altern. Med. 2021, 2021, 6690646. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Chen, Y.T.; Ruan, J.C.; Wang, W.J.; Chen, J.C.; Zhang, Q.F. Structure-activity relationship of dietary flavonoids on pancreatic lipase. Curr. Res. Food Sci. 2023, 6, 100424. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, A.I.; Alvarez-Parrilla, E.; Díaz-Sánchez, A.G.; de la Rosa, L.A.; Núñez-Gastélum, J.A.; Vazquez-Flores, A.A.; Gonzalez-Aguilar, G.A. In vitro inhibition of pancreatic lipase by polyphenols: A kinetic, fluorescence spectroscopy and molecular docking study. Food Technol. Biotechnol. 2017, 55, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, T.; Melzig, M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef]
- Karamać, M.; Amarowicz, R. Inhibition of pancreatic lipase by phenolic acids—Examination in vitro. Z. Naturforsch. 1996, 51, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Dzydzan, O.; Brodyak, I.; Strugała-Danak, P.; Strach, A.; Kucharska, A.Z.; Gabrielska, J.; Sybirna, N. Biological activity of extracts of red and yellow fruits of Cornus mas L.—An in vitro evaluation of antioxidant activity, inhibitory activity against α-glucosidase, acetylcholinesterase, and binding capacity to human serum albumin. Molecules 2022, 27, 2244. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic effects of simple phenolic acids: A comprehensive review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademosun, A.O.; Ayeni, P.O.; Omojokun, O.S.; Bello, F. Comparative effect of quercetin and rutin on α-amylase, α-glucosidase, and some pro-oxidant-induced lipid peroxidation in rat pancreas. Comp. Clin. Pathol. 2015, 24, 1103–1110. [Google Scholar] [CrossRef]
| Extract | TPC | TFC | TAC |
|---|---|---|---|
| (mg CGAE/100 g) | (mg QE/100 g) | (mg CyE/100 g) | |
| Wild | 430.5 ± 3.6 c | 19.5 ± 0.9 b | 85.5 ± 2.2 b |
| Conventionally cultivated | 501.1 ± 4.4 a | 26.3 ± 1.0 a | 91.6 ± 2.4 a |
| Aeroponic system | 482.7 ± 3.7 b | 23.6 ± 1.4 a | 86.1 ± 3.4 b |
| Sign. | ** | ** | ** |
| Compound | Raspberries | Sign. | ||
|---|---|---|---|---|
| Wild | Conventionally Cultivated | Aeroponic System | ||
| Caffeic acid | 35.8 ± 0.1 a | 36.1 ± 1.1 a | 35.8 ± 0.1 a | ns |
| Catechin | 39.6 ± 0.9 a | 40.0 ± 0.1 a | 50.5 ± 0.5 b | ** |
| Chlorogenic acid | 65.4 ± 0.2 a | 65.7 ± 0.6 a | 66.0 ± 0.7 a | ns |
| p-Coumaric acid | 32.8 ± 0.1 a | 32.8 ± 0.1 a | 33.2 ± 0.1 a | ns |
| Ferulic acid | 36.9 ± 0.4 a | 37.14 ± 0.7 a | nd | ns |
| Gallic acid | 58.2 ± 1.3 a | 59.3 ± 1.2 a | 56.6 ± 1.5 a | ns |
| Quercetin-3-O-rutinoside | 67.4 ± 0.4 a | 68.1 ± 0.2 a | 76.5 ± 0.1 b | ** |
| Extract | DPPH Test (IC50 μg/mL) | ABTS Test (IC50 μg/mL) | β-Carotene Bleaching ^ Test (IC50 μg/mL) | FRAP Test § (μM Fe(II)/g) | |
|---|---|---|---|---|---|
| 30 min | 60 min | ||||
| Wild | 28.3 ± 1.3 c | 3.4 ± 0.3 c | 5.6 ± 1.8 a | 5.4 ± 0.7 a | 24.6 ± 1.1 b |
| Conventionally cultivated | 8.9 ± 0.8 a | 1.6 ± 0.1 a | 5.1 ± 1.4 a | 5.2 ± 0.2 a | 44.9 ± 1.7 a |
| Aeroponic system | 11.5 ± 1.7 b | 2.9 ± 0.3 b | 21.1 ± 1.2 b | 42.6% ^ | 42.4 ± 1.6 a |
| Sign. | ** | ** | ** | ns | ** |
| Positive control | |||||
| Ascorbic acid | 5.2 ± 0.9 | 1.1 ± 0.8 | |||
| Propyl gallate | 1.3 ± 0.5 | 1.1 ± 0.3 | |||
| BHT | 32.9 ± 1.8 | ||||
| Raspberry Extract | Pancreatic Lipase (IC50 μg/mL) | α-Glucosidase (IC50 μg/mL) |
|---|---|---|
| Wild | 5.4 ± 0.8 a | 128.44 ± 3.70 a |
| Conventionally cultivated | 5.1 ± 0.9 a | 152.65 ± 3.97 b |
| Aeroponic system | 6.8 ± 0.9 b | 166.09 ± 4.08 c |
| Sign. | ** | ** |
| Positive control | ||
| Orlistat | 37.1 ± 1.6 | |
| Acarbose | 36.2 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Torre, C.; Loizzo, M.R.; Frattaruolo, L.; Plastina, P.; Grisolia, A.; Armentano, B.; Cappello, M.S.; Cappello, A.R.; Tundis, R. Chemical Profile and Bioactivity of Rubus idaeus L. Fruits Grown in Conventional and Aeroponic Systems. Plants 2024, 13, 1115. https://doi.org/10.3390/plants13081115
La Torre C, Loizzo MR, Frattaruolo L, Plastina P, Grisolia A, Armentano B, Cappello MS, Cappello AR, Tundis R. Chemical Profile and Bioactivity of Rubus idaeus L. Fruits Grown in Conventional and Aeroponic Systems. Plants. 2024; 13(8):1115. https://doi.org/10.3390/plants13081115
Chicago/Turabian StyleLa Torre, Chiara, Monica Rosa Loizzo, Luca Frattaruolo, Pierluigi Plastina, Antonio Grisolia, Biagio Armentano, Maria Stella Cappello, Anna Rita Cappello, and Rosa Tundis. 2024. "Chemical Profile and Bioactivity of Rubus idaeus L. Fruits Grown in Conventional and Aeroponic Systems" Plants 13, no. 8: 1115. https://doi.org/10.3390/plants13081115





