Combined Application of Biochar and Plant Growth-Promoting Rhizobacteria Improves Heavy Metal and Drought Stress Tolerance in Zea mays
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Groundnut Shell Biochar (GS-BC)
2.2. Characterization of Strain ARN7
2.3. Effect of ARN7 and GS-BC on Z. mays under HM and Drought Stress Condition
2.3.1. Effect on Growth Parameters
2.3.2. Effect on Physiological Parameters
2.3.3. Effect on Stress-Related Metabolites and Antioxidant Activity
2.3.4. Effect on HM Accumulation
2.4. Effect of ARN7 and GS-BC on Rhizospheric Soil under HM and Drought Condition
Effect on Soil Enzymes
3. Materials and Methods
3.1. Preparation and Characterization of Groundnut Shell Biochar
3.2. Isolation, Characterization, and Identification of PGPR
3.3. Pot Experiment
3.4. Analyses of Z. mays Growth Parameters
3.5. Analyses of Z. mays Physiological Parameters
3.6. Effect on Stress-Related Metabolites and Antioxidant Activity
3.7. Analysis of Zn and Ni Accumulation
3.8. Soil Enzymatic Activity and ARN7 Colonization
3.9. Statistical Analyzes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, D.; Wang, R.; Sun, X.; Liu, L.; Liu, P.; Tang, J.; Zhang, C.; Liu, H. Heavy Metal Stress in Plants: Ways to Alleviate with Exogenous Substances. Sci. Total Environ. 2023, 897, 165397. [Google Scholar] [CrossRef] [PubMed]
- Stolpe, C.; Kramer, U.; Mullera, C. Heavy Metal (Hyper) Accumulation in Leaves of Arabidopsis halleri is Accompanied by a Reduced Performance of Herbivores and Shifts in Leaf Glucosinolate and Element Concentrations. Environ. Exp. Bot. 2017, 133, 78–86. [Google Scholar] [CrossRef]
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S.; et al. Drought-tolerant Bacillus megaterium Isolated from Semi-Arid Conditions Induces Systemic Tolerance of Wheat under Drought Conditions. Plant Cell Rep. 2022, 41, 549–569. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Sandhi, A. Heavy Metal and Drought Stress in Plants: The Role of Microbes—A Review. Gesunde Pflanz. 2023, 75, 695–708. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; Zhou, J.; Yang, W.; Gu, Q.; Yu, X. Biochar-Bacteria-Plant Partnerships: Eco-Solutions for Tackling Heavy Metal Pollution. Ecotoxicol. Environ. Saf. 2020, 204, 111020. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Shi, Y.; Qiu, Y.; Che, L.; Xue, C. Performance and Mechanisms of Emerging Animal-Derived Biochars for Immobilization of Heavy Metals. Sci. Total Environ. 2019, 646, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Anbuganesan, V.; Vishnupradeep, R.; Mehnaz, N.; Kumar, A.; Freitas, H.; Rajkumar, M. Synergistic Effect of Biochar and Plant Growth Promoting Bacteria Improve the Growth and Phytostabilization Potential of Sorghum bicolor in Cd and Zn Contaminated Soils. Rhizosphere 2024, 29, 100844. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Lv, X.; Zhang, Y.; Wang, W. Effects of Biochar and Biofertilizer on Cadmium-Contaminated Cotton Growth and the Antioxidative Defense System. Sci. Rep. 2020, 10, 20112. [Google Scholar] [CrossRef]
- Kamran, M.; Malik, Z.; Parveen, A.; Zong, Y.; Abbasi, G.H.; Rafiq, M.T.; Shaabane, M.; Mustafa, A.; Bashir, S.; Rafay, M.; et al. Biochar Alleviates Cd Phytotoxicity by Minimizing Bioavailability and Oxidative Stress in Pakchoi (Brassica chinensis L.) Cultivated in Cd-Polluted Soil. J. Environ. Manag. 2019, 250, 109500. [Google Scholar] [CrossRef]
- Haroun, M.; Xie, S.; Awadelkareem, W.; Wang, J.; Qian, X. Influence of Biofertilizer on Heavy Metal Bioremediation and Enzyme Activities in the Soil to Revealing the Potential for Sustainable Soil Restoration. Sci. Rep. 2023, 13, 20684. [Google Scholar] [CrossRef]
- Guo, X.; Li, H. Effects of Iron-Modified Biochar and AMF Inoculation on the Growth and Heavy Metal Uptake of Senna occidentalis in Heavy Metal-Contaminated Soil. Pol. J. Environ. Stud. 2019, 28, 2611–2621. [Google Scholar] [CrossRef]
- Lalarukh, I.; Amjad, S.F.; Mansoora, N.; Al-Dhumri, S.A.; Alshahri, A.H.; Almutari, M.M.; Alhusayni, F.S.; Al-Shammari, W.B.; Poczai, P.; Abbas, M.H.H.; et al. Integral Effects of Brassinosteroids and Timber Waste Biochar Enhances the Drought Tolerance Capacity of Wheat Plant. Sci. Rep. 2022, 12, 12842. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, B.; Raza, M.A.S.; Saleem, M.F.; Aslam, M.U.; Iqbal, R.; Muhammad, F.; Amin, J.; Ibrahim, M.A.; Khan, I.H. Biochar Enhances Wheat Crop Productivity by Mitigating the Effects of Drought: Insights into Physiological and Antioxidant Defense Mechanisms. PLoS ONE 2022, 17, 0267819. [Google Scholar] [CrossRef] [PubMed]
- Waqar, A.; Bano, A.; Ajmal, M. Effects of PGPR Bioinoculants, Hydrogel and Biochar on Growth and Physiology of Soybean under Drought Stress. Commun. Soil Sci. Plant Anal. 2022, 53, 827–847. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Ali, H.H.; Soufan, W.; Iqbal, R.; Habib-ur-Rahman, M.; Iqbal, J.; Israr, M.; El Sabagh, A. Potential Effects of Biochar Application for Improving Wheat (Triticum aestivum L.) Growth and Soil Biochemical Properties under Drought Stress Conditions. Land 2021, 10, 1125. [Google Scholar] [CrossRef]
- Tripti; Kumar, A.; Maleva, M.; Borisova, G.; Rajkumar, M. Amaranthus Biochar-Based Microbial Cell Composites for Alleviation of Drought and Cadmium Stress: A Novel Bioremediation Approach. Plants 2023, 12, 1973. [Google Scholar] [CrossRef]
- Vishnupradeep, R.; Bruno, L.B.; Taj, Z.; Karthik, C.; Challabathula, D.; Tripti; Kumar, A.; Freitas, H.; Rajkumar, M. Plant Growth Promoting Bacteria Improve Growth and Phytostabilization Potential of Zea mays under Chromium and Drought Stress by Altering Photosynthetic and Antioxidant Responses. Environ. Technol. Innov. 2022, 25, 102154. [Google Scholar] [CrossRef]
- Bruno, L.B.; Anbuganesan, V.; Karthik, C.; Tripti; Kumar, A.; Banu, J.R.; Rajkumar, M. Enhanced Phytoextraction of Multi-Metal Contaminated Soils under Increased Atmospheric Temperature by Bioaugmentation with Plant Growth Promoting Bacillus cereus. J. Environ. Manag. 2021, 289, 112553. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Arikan, B.; Alp-Turgut, F.N.; Gulenturk, C. The Regulatory Effects of Biochar on PSII Photochemistry, Antioxidant System and Nitrogen Assimilation in Lemna minor Exposed to Inorganic Pollutants, Arsenic and Fluoride. J. Environ. Chem. Eng. 2023, 11, 110713. [Google Scholar] [CrossRef]
- Bruno, L.B.; Karthik, C.; Ma, Y.; Kadirvelu, K.; Freitas, H.; Rajkumar, M. Amelioration of Chromium and Heat Stresses in Sorghum bicolor by Cr6+ Reducing Thermotolerant Plant Growth Promoting Bacteria. Chemosphere 2020, 244, 125521. [Google Scholar] [CrossRef]
- Gondek, K.; Mierzwa-Hersztek, M.; Kopec, M.; Sikora, J.; Głąb, T.; Szczurowska, K. Influence of Biochar Application on Reduced Acidification of Sandy Soil, Increased Cation Exchange Capacity, and the Content of Available Forms of K, Mg, and P. Pol. J. Environ. Stud. 2019, 28, 1–9. [Google Scholar] [CrossRef]
- Tao, Q.; Li, B.; Li, Q.; Han, X.; Jiang, Y.; Jupa, R.; Wang, C.; Li, T. Simultaneous Remediation of Sediments Contaminated with Sulfamethoxazole and Cadmium using Magnesium-Modified Biochar Derived from Thalia dealbata. Sci. Total Environ. 2019, 659, 1448–1456. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of Biochar Amendment on Sorption and Leaching of Nitrate, Ammonium, and Phosphate in a Sandy Soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 2014, 9, 113888. [Google Scholar] [CrossRef]
- Yang, Y.; Piao, Y.; Wang, R.; Su, Y.; Liu, N.; Lei, Y. Nonmetal Function Groups of Biochar for Pollutants Removal: A Review. J. Hazard. Mater. Adv. 2022, 8, 100171. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; Skz, A. Enhancement of Drought Stress Tolerance in Crops by Plant Growth Promoting Rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Tripti; Kumar, A.; Kumar, V.; Anshumali; Bruno, L.B.; Rajkumar, M. Synergism of Industrial and Agricultural Waste as a Suitable Carrier Material for Developing Potential Biofertilizer for Sustainable Agricultural Production of Eggplant. Horticulturae 2022, 8, 444. [Google Scholar] [CrossRef]
- Ren, H.; Li, Z.; Chen, H.; Zhou, J.; Lv, C. Effects of Biochar and Plant Growth-Promoting Rhizobacteria on Plant Performance and Soil Environmental Stability. Sustainability 2022, 14, 10922. [Google Scholar] [CrossRef]
- Malik, L.; Sanaullah, M.; Mahmood, F.; Hussain, S.; Siddique, M.H.; Anwar, F.; Shahzad, T. Unlocking the Potential of Co-Applied Biochar and Plant Growth-Promoting Rhizobacteria (PGPR) for Sustainable Agriculture under Stress Conditions. Chem. Biol. Technol. Agric. 2022, 9, 58. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Du, B.; Li, H. Effect of Biochar Applied with Plant Growth-Promoting Rhizobacteria (PGPR) on Soil Microbial Community Composition and Nitrogen Utilization in Tomato. Pedosphere 2021, 31, 872–881. [Google Scholar] [CrossRef]
- Ashrafi, M.; Azimi-Moqadam, M.; Moradi, P.; Fard, E.M.; Shekari, F.; Kompany-Zareh, M. Effect of Drought Stress on Metabolite Adjustments in Drought Tolerant and Sensitive Thyme. Plant Physiol. Biochem. 2018, 132, 391–399. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ali, S.; Hameed, A.; Bharwana, S.A.; Rizwan, M.; Ishaque, W.; Farid, M.; Mahmood, K.; Iqbal, Z. Cadmium Stress in Cotton Seedlings: Physiological, Photosynthesis and Oxidative Damages Alleviated by Glycinebetaine. S. Afr. J. Bot. 2016, 104, 61–68. [Google Scholar] [CrossRef]
- Naveed, M.; Mustafa, A.; Azhar, S.Q.; Kamran, M.; Zahir, Z.A.; Nunez-Delgado, A. Burkholderia phytofirmans PsJN and Tree Twigs Derived Biochar Together Tetrieved Pb-Induced Growth, Physiological and Biochemical Disturbances by Minimizing its Uptake and Translocation in Mung Bean (Vigna radiata L.). J. Environ. Manag. 2020, 257, 109974. [Google Scholar] [CrossRef]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of Drought Stress in Pulse Crops with ACC Deaminase Producing Rhizobacteria Isolated from Acidic Soil of Northeast India. Sci. Rep. 2018, 8, 3560. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M. Co-Application of ACC-Deaminase Producing PGPR and Timber-Waste Biochar Improves Pigments Formation, Growth and Yield of Wheat under Drought Stress. Sci. Rep. 2019, 9, 5999. [Google Scholar] [CrossRef]
- Ahmad, M.; Wang, X.; Hilger, T.H.; Luqman, M.; Nazli, F.; Hussain, A.; Zahir, Z.A.; Latif, M.; Saeed, Q.; Malik, H.A.; et al. Evaluating Biochar-Microbe Synergies for Improved Growth, Yield of Maize, and Post-Harvest Soil Characteristics in a Semi-Arid Climate. Agronomy 2020, 10, 1055. [Google Scholar] [CrossRef]
- Ma, H.; Wei, M.; Wang, Z.; Hou, S.; Li, X.; Xu, H. Bioremediation of Cadmium Polluted Soil Using a Novel Cadmium Immobilizing Plant Growth Promotion Strain Bacillus sp. TZ5 Loaded on Biochar. J. Hazard. Mater. 2020, 388, 122065. [Google Scholar] [CrossRef]
- Alidou-Arzika, I.; Lebrun, M.; Miard, F.; Nandillon, R.; Baycu, G.; Bourgerie, S.; Morabito, D. Assessment of Compost and Three Biochars Associated with Ailanthus altissima (Miller) Swingle for Lead and Arsenic Stabilization in a Post-Mining Technosol. Pedosphere 2021, 31, 944–953. [Google Scholar] [CrossRef]
- Karer, J.; Zehetner, F.; Dunst, G.; Fessl, J.; Wagner, M.; Puschenreiter, M.; Stapkevica, M.; Friesl-Hanl, W.; Soja, G. Immobilisation of Metals in a Contaminated Soil with Biochar-Compost Mixtures and Inorganic Additives: 2-year Greenhouse and Field Experiments. Environ. Sci. Pollut. Res. Int. 2018, 25, 2506–2516. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, Y.; Zhang, L.; Huang, Q. Study of the Mechanism of Remediation of Cd-Contaminated Soil by Novel Biochars. Environ. Sci. Pollut. Res. Int. 2017, 24, 24844–24855. [Google Scholar] [CrossRef]
- Rojjanateeranaj, P.; Sangthong, C.; Prapagdee, B. Enhanced Cadmium Phytoremediation of Glycine max L. Through Bioaugmentation of Cadmium-Resistant Bacteria Assisted by Biostimulation. Chemosphere 2017, 185, 764–771. [Google Scholar] [CrossRef]
- Qu, Q.; Wang, Z.; Gan, Q.; Liu, R.; Xu, H. Impact of Drought on Soil Microbial Biomass and Extracellular Enzyme Activity. Front. Plant Sci. 2023, 14, 1221288. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Xu, H.; Song, F.; Ge, H.; Yue, S. Effects of Heavy Metals on Microorganisms and Enzymes in Soils of Lead-Zinc Tailing Ponds. Environ. Res. 2022, 207, 112174. [Google Scholar] [CrossRef] [PubMed]
- Jabborova, D.; Kannepalli, A.; Davranov, K.; Narimanov, A.; Enakiev, Y.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Wirth, S.; Sayyed, R.Z. Co-Inoculation of Rhizobacteria Promotes Growth, Yield, and Nutrient Contents in Soybean and Improves Soil Enzymes and Nutrients under Drought Conditions. Sci. Rep. 2021, 11, 22081. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Rahman, M.K.U.; Ma, C.; Zheng, X.; Wu, F.; Zhou, X. Silicon Modification Improves Biochar’s Ability to Mitigate Cadmium Toxicity in Tomato by Enhancing Root Colonization of Plant-Beneficial Bacteria. Ecotoxicol. Environ. Saf. 2023, 249, 114407. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Xiao, Z.; Weinmann, M.; Li, Z. Phosphate Uptake is Correlated with the Root Length of Celery Plants following the Association between Arbuscular Mycorrhizal Fungi, Pseudomonas sp. and Biochar with Different Phosphate Fertilization Levels. Agronomy 2019, 9, 824. [Google Scholar] [CrossRef]
- Liang, C.; Zhu, X.; Fu, S.; Mendez, A.; Gasco, G.; Paz-Ferreiro, J. Biochar Alters the Resistance and Resilience to Drought in a Tropical Soil. Environ. Res. Lett. 2014, 9, 3–10. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and Characterization of Biochar Produced from Slow Pyrolysis of Pigeon Pea Stalk and Bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P. Analysis of layer charge, cation and anion exchange capacities and synthesis of reduced charge clays. In Methods of Soil Analysis: Mineralogical Methods; Ulery, A.L., Drees, R.L., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 2008; pp. 499–501. [Google Scholar]
- ASTM D 2216-90; Standard Method for Laboratory Determination of Water (Moisture) Content of Soil and Rock. Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 1990.
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis, Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumnner, M.E., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Sadaka, S.; Sharara, M.A.; Ashworth, A.; Keyser, P.; Allen, F.; Wright, A. Characterization of Biochar from Switchgrass Carbonization. Energies 2014, 7, 548–567. [Google Scholar] [CrossRef]
- Sheng, X.; Xia, J.; Jiang, C.; He, L.; Qian, M. Characterization of Heavy Metal-Resistant Endophytic Bacteria from Rape (Brassica napus) Roots and their Potential in Promoting the Growth and Lead Accumulation of Rape. Environ. Pollut. 2008, 156, 1164–1170. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Luo, Y.M.; Freitas, H. Inoculation of Endophytic Bacteria on Host and Non-Host Plants-Effects on Plant Growth and Ni Uptake. J. Hazard. Mater. 2011, 195, 230–237. [Google Scholar] [CrossRef]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid In Situ Assay for Indole Acetic Acid Production by Bacteria Immobilized on a Nitrocellulose Membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Sen, S.K.; Maiti, T.K. Production and Metabolism of IAA by Enterobacter spp. (Gamma proteobacteria) Isolated from Root Nodules of a Legume Abrus precatorius L. Biocatal. Agric. Biotechnol. 2015, 4, 296–303. [Google Scholar] [CrossRef]
- Atkin, C.L.; Neilands, J.B.; Phaff, H.J. Rhodotorulic Acid from Species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a New Alanine-Containing Ferrichrome from Cryptococcus Melibiosum. J. Bacteriol. 1970, 103, 722–733. [Google Scholar] [CrossRef]
- Arnow, E. Colorimetric Determination of the Components of 3,4- Dihydroxyphenylalanine Tyrosine Mixtures. J. Biol. Chem. 1937, 118, 531–537. [Google Scholar] [CrossRef]
- Pikovskaya, R. Mobilization of Phosphorus in Soil in Connection with Vital Activity of Some Microbial Species. Mikrobiologiya 1948, 17, 370. [Google Scholar]
- Rao, B.P.; Sudharsan, K.; Sekaran, R.C.H.; Mandal, A.B. Characterization of Exopolysaccharide from Bacillus amyloliquefaciens BPRGS for its Bioflocculant Activity. Int. J. Eng. Res. 2013, 4, 1696–1704. [Google Scholar]
- Ni, Z.; Kim, E.; Chen, E.Z. Chlorophyll and starch assays. Protoc. Exch. Nat. 2009. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 24, 519–570. [Google Scholar] [CrossRef]
- Campos, P.S.; Quartin, V.; Ramalho, J.C.; Nunes, M.A. Electrolyte Leakage and Lipid Degradation Account for Cold Sensitivity in Leaves of Coffea sp. Plants. J. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and other Oxidation Substrates and Antioxidants by Means of Folin- Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–177. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kono, Y. Generation of Superoxide Radical during Auto Oxidation of Hydroxyl Amine and an Assay for Superoxide Dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Chen, G.; Asada, K. Ascorbate Peroxidase in Tea Leaves: Occurrence of Two Isoenzymes and the Differences in their Enzymatic and Molecular Properties. Plant Cell Physiol. 1989, 30, 987–998. [Google Scholar] [CrossRef]
- Lata, C.; Jha, S.; Dixit, V.; Prasad, M. Differential Antioxidative Responses to Dehydrationinduced Oxidative Stress in Core Set of Foxtail Millet Cultivars [Setaria italica (L.)]. Protoplasma 2011, 248, 817–828. [Google Scholar] [CrossRef]
- Wang, S.; Wu, W.; Liu, F.; Liao, R.; Hu, Y. Accumulation of Heavy Metals in Soil Crop Systems: A Review for Wheat and Corn. Environ. Sci. Pollut. Res. 2017, 24, 15209–15225. [Google Scholar] [CrossRef]
- Małachowska-Jutsz, A.; Matyja, K. Discussion on methods of soil dehydrogenase determination. Int. J. Environ. Sci. Technol. 2019, 16, 7777–7790. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in Soils. Soil Boil. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Li, Y.; Feng, H.; Chen, J.; Lu, J.; Wu, W.; Liu, X.; Li, C.; Dong, Q.; Siddique, K.H.M. Biochar Incorporation Increases Winter Wheat (Triticum aestivum L.) Production with Significantly Improving Soil Enzyme Activities at Jointing Stage. Catena 2022, 211, 105979. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and Galactosidases in Soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
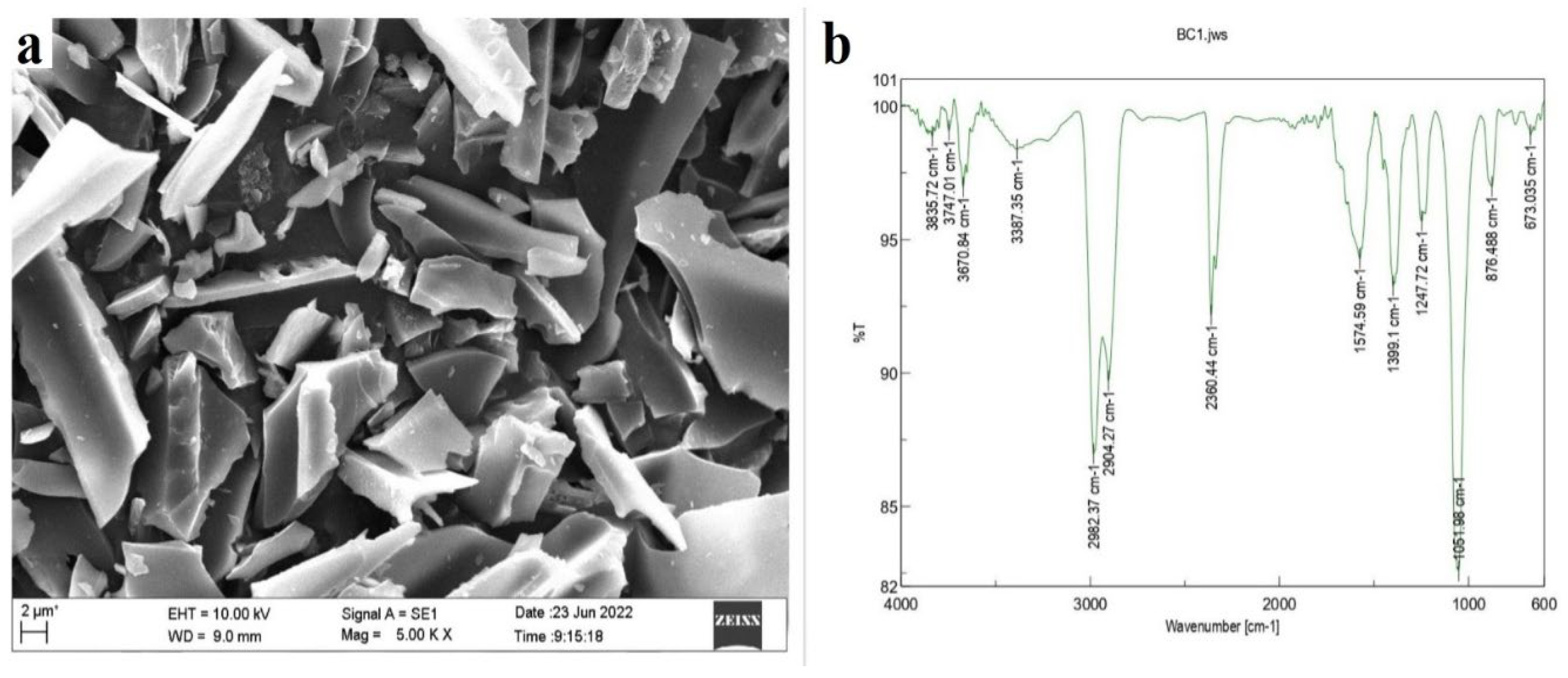
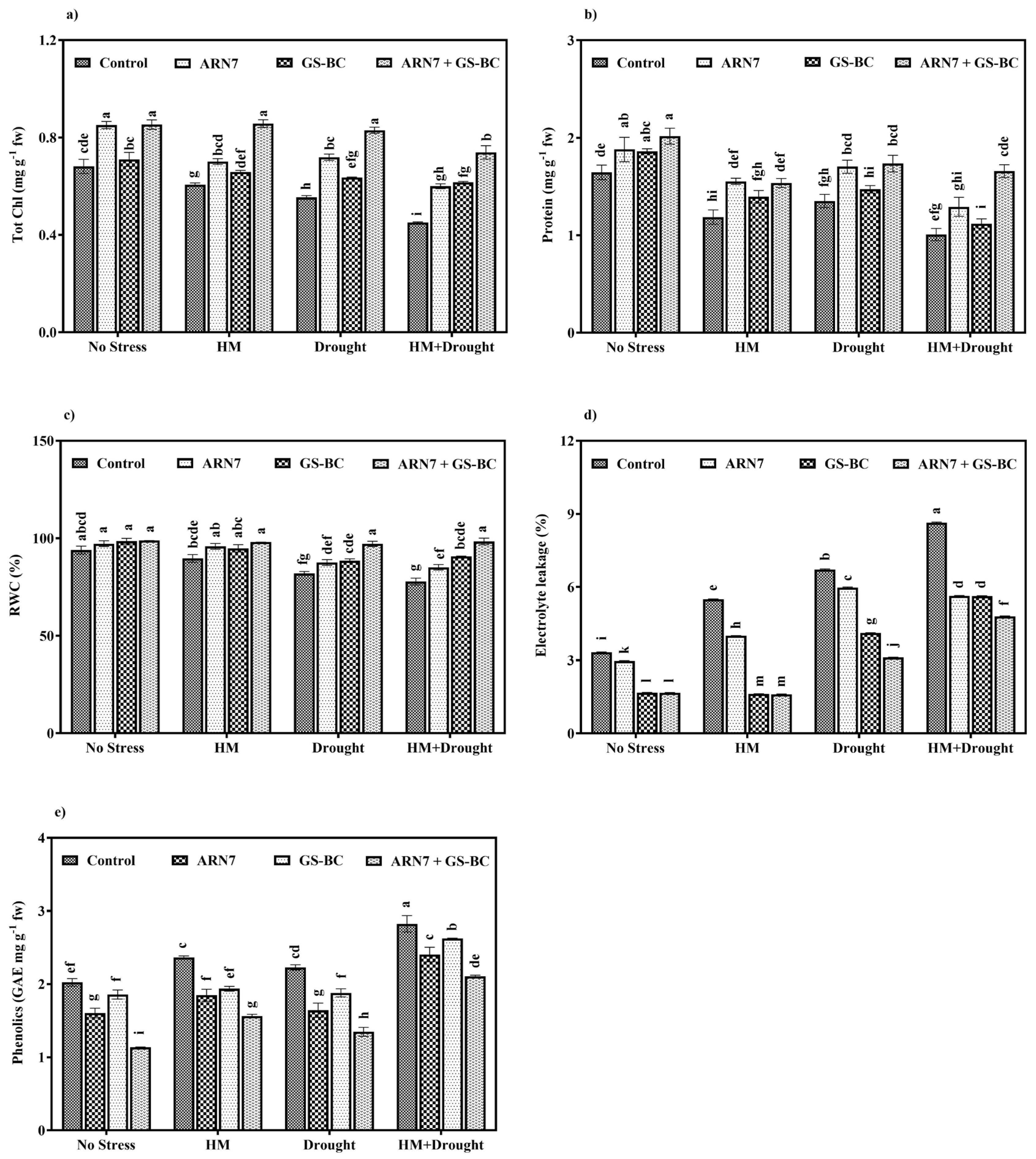

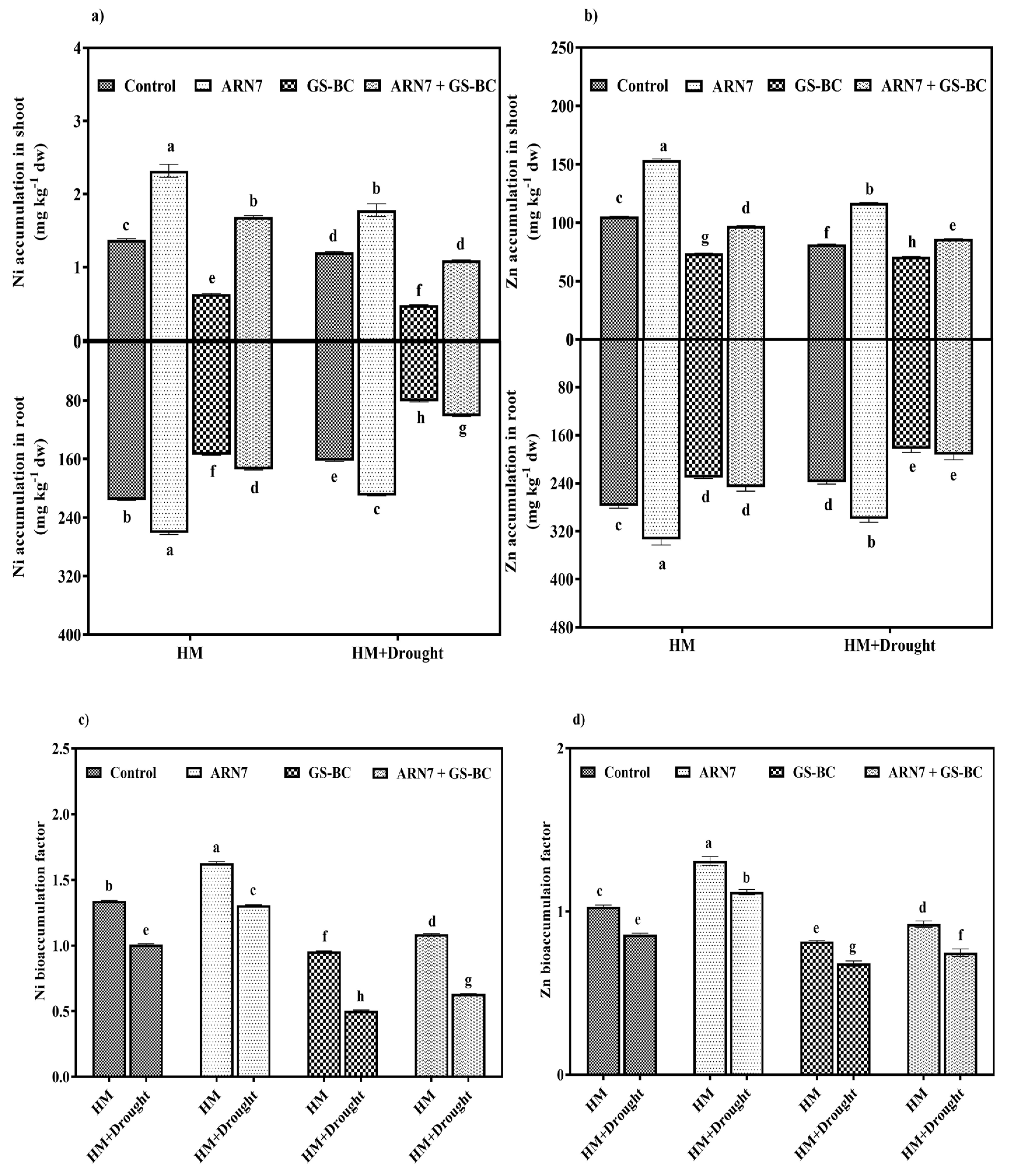

| Treatment Abbreviation | Shoot Length (cm) | Root Length (cm) | Fresh Weight (g/plant) | Dry Weight (g/plant) | |
|---|---|---|---|---|---|
| No Stress | Control | 18.2 ± 0.96 cdef | 26.0 ± 1.46 fg | 3.25 ± 0.19 bcd | 0.48 ± 0.08 de |
| ARN7 | 23.5 ± 2.13 ab | 43.1 ± 1.25 b | 4.16 ± 0.41 a | 0.82 ± 0.05 ab | |
| GS-BC | 19.5 ± 0.31 abcd | 39.0 ± 2.16 bc | 3.44 ± 0.17 abc | 0.79 ± 0.02 ab | |
| ARN7 + GS-BC | 21.9 ± 2.05 abc | 55.9 ± 2.05 a | 4.20 ± 0.22 a | 0.89 ± 0.08 a | |
| HM | Control | 14.6 ± 1.08 ef | 20.9 ± 0.66 gh | 2.25 ± 0.40 f | 0.47 ± 0.05 de |
| ARN7 | 21.2 ± 2.71 abc | 38.8 ± 2.59 bc | 3.53 ± 0.38 abc | 0.79 ± 0.06 ab | |
| GS-BC | 20.7 ± 0.87 abcd | 34.1 ± 2.01 cd | 2.95 ± 0.07 cdef | 0.71 ± 0.05 bc | |
| ARN7 + GS-BC | 24.0 ± 2.80 a | 40.4 ± 2.35 b | 3.95 ± 0.18 ab | 0.79 ± 0.05 ab | |
| Drought | Control | 16.2 ± 1.03 def | 20.5 ± 0.72 gh | 2.51 ± 0.07 def | 0.41 ± 0.04 de |
| ARN7 | 20.5 ± 1.86 abcd | 32.5 ± 2.08 de | 3.80 ± 0.38 ab | 0.77 ± 0.09 ab | |
| GS-BC | 19.0 ± 1.15 bcde | 27.4 ± 3.59 ef | 3.2 ± 0.05 bcde | 0.66 ± 0.02 bc | |
| ARN7 + GS-BC | 21.2 ± 1.20 abc | 39.5 ± 1.31 bc | 3.46 ± 0.23 abc | 0.72 ± 0.05 abc | |
| HM+ Drought | Control | 13.8 ± 1.11 f | 19.5 ± 0.55 h | 2.45 ± 0.04 ef | 0.40 ± 0.06 e |
| ARN7 | 18.1 ± 0.82 cdef | 31.3 ± 1.53 def | 3.54 ± 0.21 abc | 0.49 ± 0.08 de | |
| GS-BC | 17.4 ± 0.67 cdef | 27.3 ± 1.80 ef | 2.63 ± 0.38 def | 0.35 ± 0.02 e | |
| ARN7 + GS-BC | 20.4 ± 0.79 abcd | 38.7 ± 0.91 bc | 3.74 ± 0.14 ab | 0.58 ± 0.06 cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anbuganesan, V.; Vishnupradeep, R.; Bruno, L.B.; Sharmila, K.; Freitas, H.; Rajkumar, M. Combined Application of Biochar and Plant Growth-Promoting Rhizobacteria Improves Heavy Metal and Drought Stress Tolerance in Zea mays. Plants 2024, 13, 1143. https://doi.org/10.3390/plants13081143
Anbuganesan V, Vishnupradeep R, Bruno LB, Sharmila K, Freitas H, Rajkumar M. Combined Application of Biochar and Plant Growth-Promoting Rhizobacteria Improves Heavy Metal and Drought Stress Tolerance in Zea mays. Plants. 2024; 13(8):1143. https://doi.org/10.3390/plants13081143
Chicago/Turabian StyleAnbuganesan, Vadivel, Ramasamy Vishnupradeep, L. Benedict Bruno, Krishnan Sharmila, Helena Freitas, and Mani Rajkumar. 2024. "Combined Application of Biochar and Plant Growth-Promoting Rhizobacteria Improves Heavy Metal and Drought Stress Tolerance in Zea mays" Plants 13, no. 8: 1143. https://doi.org/10.3390/plants13081143
APA StyleAnbuganesan, V., Vishnupradeep, R., Bruno, L. B., Sharmila, K., Freitas, H., & Rajkumar, M. (2024). Combined Application of Biochar and Plant Growth-Promoting Rhizobacteria Improves Heavy Metal and Drought Stress Tolerance in Zea mays. Plants, 13(8), 1143. https://doi.org/10.3390/plants13081143










