Abstract
Carotenoid, chlorophyll and tocochromanol biosynthesis and accumulation are interrelated and age-dependent in plants. Model plants produce tocopherols, but do not produce significant amounts of tocotrienols; consequently, the regulation of tocotrienol biosynthesis in plants has been scarcely studied. The Hypericum genus produces a variety of prenyllipids naturally in all parts of the plant, allowing for a glimpse into the relationship between them without genetic or other interference. Consequently, five Hypericum species’ leaves of different ages were investigated—H. androsaemum, H. pseudohenryi, H. hookerianum, H. patulum and one hybrid H. × inodorum (H. androsaemum × H. hircinum). The leaves contained predominantly α-tocopherol, γ-tocotrienol and δ-tocotrienol (30.9–212.8, 8.13–22.43 and 1.87–20.8 mg 100 g−1, respectively). Higher quantities of tocochromanols, a lower chlorophyll content and a higher a/b ratio were observed in the bottom (older) leaves. The predominant carotenoids were lutein (semi-quantitative) and β-carotene (7.60–28.63 and 2.33–12.43 mg 100 g−1, respectively). Carotenoid contents were lower in bottom leaves than in middle or top leaves, and the highest carotenoid content was observed in H. hookerianum and H. patulum. Leaf tocopherol, tocotrienol, chlorophyll and carotenoid accumulation were section and leaf age-dependent, and distinct relationships can be observed between the accumulation of some prenyl lipids, but not others.
1. Introduction
Now recognized as a separate plant family, Hypericaceae used to be classified as a subfamily of the Clusiaceae family until 2003, when the Angiosperm Phylogeny Group APG II system classified it as a separate family [1]. The Hypericum genus is one of six genera in the Hypericaceae family. It is collectively referred to as St. John’s wort or goatweed, and some members are referred to as tutsan. The genus includes mostly perennial flowering shrubs and herbs. The aerial, flowering parts of several species in the Hypericum genus are collected and used for phytomedicine. Although exact data are not available, the number and array of Hypericum products on the market demonstrates the economic viability and established agricultural practices of the genus. Extraction techniques include water extracts (tea), hydroalcoholic extracts [2,3] and oil macerations [4]. The primary compounds of concern are naphthodianthrones (hypericin, pseudohypericin) and prenylated phloroglucinols (hyperforin, adhyperforin), specific to the genus, and other phenolic compounds such as flavonoids [2]. Naphthodianthrones and acylphloroglucinols are considered characteristic compounds in the Hypericum genus, although the presence and content can vary significantly between species, with H. perforatum being the richest in both, while H. hircinum contains neither or trace amounts [5]. Other species contain varying amounts of flavonoids, catechins and phenolic acids, but their contents do not necessarily directly correlate with the antioxidant activity of plant extracts, indicating the presence of alternate antioxidant molecules [5].
Tocochromanols and carotenoids are lipophilic antioxidants present in all plant parts. Tocopherols and tocotrienols protect the plant cellular membrane and reserve lipids against peroxidation and act as signaling compounds [6]. The biosynthesis of both uses the shikimate and MEP pathway. Tocochromanols are made up of a chromane ring with specific methyl group placement and a lipophilic isoprenoid tail. The lipophilic tail determines the tocochromanol type—tocopherols have a saturated tail, while tocotrienols, tocodienols and tocomonoenols have three, two and one saturated bond in the tail, respectively. The placement of methyl groups on the chromane ring determines the tocochromanol homologue. Tocopherols and tocotrienols have common biosynthesis pathways, but use different precursor compounds—tocotrienol biosynthesis require homogentisate to produce geranylgeranyl pyrophosphate [7], the direct tocotrienol precursor, while phytyl pyrophosphate (PPP), used for the tocopherol tail, can be produced from either homogentisate or phytol from degraded chlorophyll [8]. Tocopherols are more common and their presence in plants is far more studied than that of tocotrienols, the next most recognized group of tocochromanols. There is a preference for accumulating α-T in the leaves, while other parts of plants tend to be richer in γ-T [9]. The literature generally indicates that higher levels of tocotrienols are found in the seeds and oils of certain plant species [10,11]. Initially, it was believed that tocotrienols were mainly associated with monocotyledonous plants, particularly those belonging to the Poaceae [6,9] and Arecaceae [12] families. However, more recent research has revealed that dicotyledonous plants, including seeds from families such as Apiaceae [13,14], Ericaceae [10,15] and Vitaceae [10] also contain significant quantities of tocotrienols. Additionally, emerging evidence highlights that the leaves of certain genera, such as Clusia and Hypericum, may serve as promising and underexplored sources of these bioactive compounds [16,17,18]; therefore, it is worth taking a closer look at these genera, especially Hypericum. While several reports have recently been published on the presence of tocopherols and tocotrienols in Hypericum leaves and several plant organs of Hypericum perforatum [17,19,20], these did not investigate the relationship between tocochromanols and other common minor lipids—chlorophyll, which shares chemical precursors with tocochromanols, and carotenoids, which share precursors and physiological functions.
While tocochromanols contain an isoprenoid chain, carotenoids are wholly isoprenoid compounds and contain several unsaturated bonds; all are tetraterpene derivatives. The isoprenoid chain is produced by condensing two GGPP molecules into phytoene, from which carotenes and xanthophylls are produced through successive desaturation, cyclization and epoxidation [21]. More total carotenoids are accumulated in leaves under high-light conditions than in the shade, and their composition is lighting-dependent—some xanthophylls may only be produced under sufficient light [22]. Carotenoids are health-promoting compounds that contribute to reduce the risk of cancer, cardiovascular disease and eye, bone and skin conditions. They are also associated with neurological and metabolic benefits [23]. Apart from providing health benefits and (some of them) being precursors of vitamin A, carotenoids are colorants and can protect foods from oxidation and provide cosmetic benefits, hence their versatility in a variety of products for the agri-food, pharma and cosmetic industries [24].
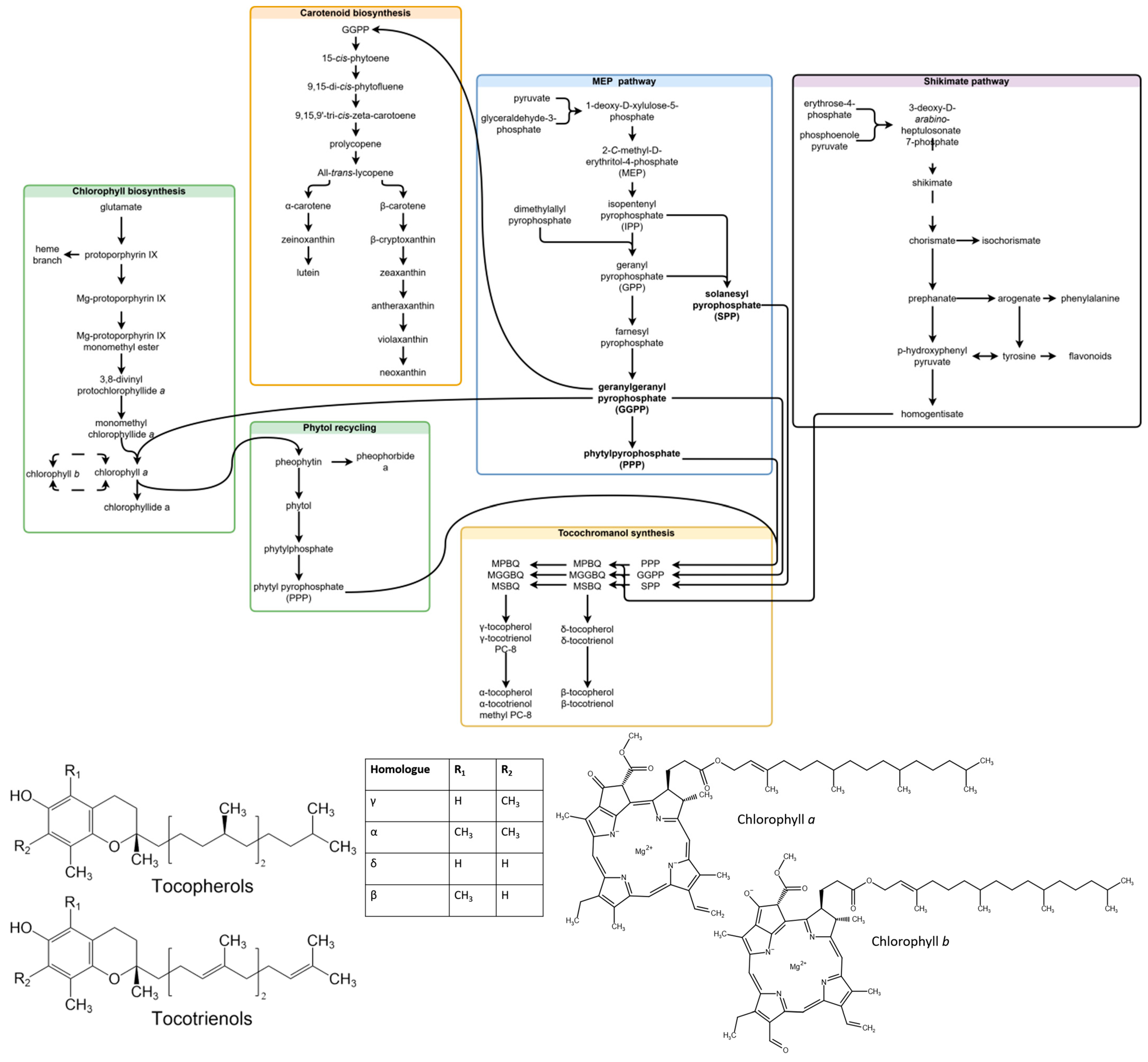
Chlorophylls are a type of green tetrapyrrole pigment involved in photosynthesis. Two types of chlorophyll are produced in plants—chlorophyll a (Chl a) and chlorophyll b (Chl b). Both consist of a tetrapyrrole macrocycle with Mg+2 at its core, a phytol chain and an additional fifth ring structure. Chlorophylls are differentiated by the chemical substituent at C7—a methyl group in Chl a, and a formyl group in Chl b. Chl a and Chl b can be converted into each other directly or indirectly (through chlorophyllides) in the chlorophyll cycle, and the indirect route is considered more common [25]. Chlorophylls (a + b) are accumulated under high-light conditions, and the a/b ratio is increased [22]. The basic common precursors and steps in chlorophyll, tocochromanol and carotenoid biosynthesis, as well as the compounds analyzed in the study are provided in Figure 1.
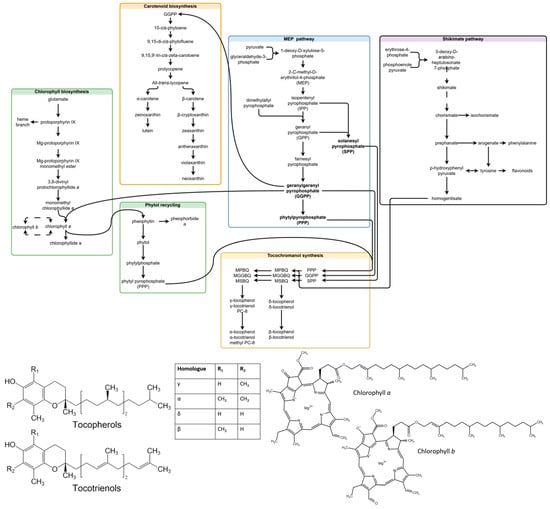
Figure 1.
Related steps in chlorophyll, carotenoid and tocochromanol biosynthesis, and molecular structures of the analyzed compounds. Some unrelated steps are shown, and the simplified transformation of contents (missing steps) is represented by dashed lines. The scheme is a combination of biosynthesis pathways published in existing articles [7,25,26,27,28,29]. Abbreviations not deciphered in chart: MPBQ, 2-methyl-6-phytyl-1,4-benzoquinol; MGGBQ, 2-methyl-6-geranylgeranyl-1,4-benzoquino; MSBQ, 2-methyl-6-solanesyl-1,4-benzoquinol; DMPBQ, 2,3-dimethyl-6-phytyl-1,4-benzoquinol; DMGGBQ, 2,3-dimethyl-6-geranylgeranyl-1,4-benzoquinol; DMSBQ, 2,3-dimethyl-6-solanesyl-1,4-benzoquinol.
Besides H. perforatum, a number of Hypericum species are cultivated as ornamental plants, including hardy shrubs, which produce a significant amount of biomass each year. The branches and leaves of the shrubs are typically cut each year for wintering. Considering recent reports on diverse tocochromanol profiles in Hypericum leaves [16,17,18], the investigation of the cut material presents opportunities for the study of leaf tocochromanol metabolism in a plant that produces tocotrienols naturally, as well as a potential raw material for tocopherol and tocotrienol extraction. For these reasons, the present study firstly investigates the tocochromanol, chlorophyll and carotenoid contents of Hypericum leaves from branches cut for wintering and secondly aims to provide initial reports on the relationship between prenyllipid accumulation in Hypericum leaves, since existing literature on other plant species has demonstrated that both leaf age and position influence the biosynthesis and accumulation of tocochromanols [30,31].
2. Materials and Methods
2.1. Reagents
Ethanol, methanol, ethyl acetate, n-hexane (HPLC grade), pyrogallol, sodium chloride and potassium hydroxide (reagent grade) were received from Sigma-Aldrich (Steinheim, Germany). Ethanol (96.2%), for the lab-scale extraction of bioactive compounds from H. perforatum, was purchased from SIA Kalsnavas Elevators (Jaunkalsnava, Latvia) and was used for the preparation of different hydroethanolic solutions. Standards of tocopherol homologues (α, β, γ and δ) (≥98%, HPLC) were purchased from Extrasynthese (Genay, France), and tocotrienol homologues (α, β, γ and δ) (≥98%, HPLC) and β-carotene (≥95%, HPLC) standards were purchased from Cayman Chemical (Ann Arbor, MI, USA), respectively.
2.2. Plant Material
The leaves of five Hypericum genus species were harvested in the garden of the Institute of Horticulture, Dobele, Latvia (GPS location: N: 56°36′39″ E: 23°17′50″) from plants planted three years prior. The plants were grown from seedlings in an open field using seeds received from botanical gardens—Hypericum androsaemum and Hypericum × inodorum (Plant World, St. Marychurch Rd, UK), Hypericum pseudohenryi (Botanical Garden of Marie Curie-Sklodowska University in Lublin, Poland), Hypericum hookerianum (Botanical Garden of the Faculty of Science, Masaryk University, Brno, Czech Republic) and Hypericum patulum (Tallinn Botanic Garden, Estonia). In the middle of October 2024, three plants from each species (biological replicates, n = 3) were randomly selected and cut off close to the soil line. Leaves from each plant were analyzed individually and treated as biological replicates in the experiment. Each plant had a minimum of 20 branches, from which 5 to 6 branches were randomly selected for sampling. For each plant, leaves were collected by separating 2–3 leaf pairs from each stem section—bottom, middle and top, as shown in the Supplementary Materials (Figures S1–S5). The plant branches were collected during the morning hours (9:00–10:00 local time) and transported to the lab in dark polyethylene bags within 15 min. All leaves were separated within 30–60 min after delivery to the lab. Separate leaves were immediately frozen in the freezer at −80 ± 2 °C for 2 h and then transferred for freeze-drying (Labconco, Kansas City, MO, USA) at a temperature of −51 ± 1 °C under a vacuum of 0.055–0.065 mbar for 72 h. On the same day (morning hours), after freeze-drying, leaves were milled using a MM400 ball mill (Retsch, Haan, Germany) to obtain a 5 µm final particle size. Moisture was determined gravimetrically. Sample preparation for tocochromanol, carotenoid and chlorophyll analysis were performed on the same day directly after milling the samples. The determination of phytochemicals was performed in the second part of the same day (chlorophyll by spectrometric method), and carotenoid and tocochromanol analyses were performed in the evening hours. All extractions were performed under limited light, low temperature and limited oxygen exposure.
2.3. Tocochromanol and Carotenoid Extraction
For tocopherols, tocotrienols and carotenoids, a single extraction procedure was proposed for the simultaneous isolation of phytosterols, tocopherols and lutein from soybeans [32]. The method reported by Slavin and Yu [32] was adapted and validated for the recovery of tocochromanols from apple seeds [33] and applied in the present study. The method consists of a semi-micro-saponification protocol followed by several extractions with a mixture of n-hexane:ethyl acetate (9:1, v/v), organic solvent evaporation, re-dissolving in 1 mL of ethanol and transfer to 2 mL glass vials for future LC analysis.
2.4. Tocopherol and Tocotrienol Analysis with RP-HPLC-FLD
A Shimadzu Nexera 40 Series HPLC system (Kyoto, Japan), with an RF-20Axs fluorescence detector (FLD) operated at the excitation wavelength of 295 nm and emission wavelength of 330 nm, was used for tocochromanol determination. Tocopherols and tocotrienols were separated using the Epic PFP-LB column (3 μm, 150 × 4.6 mm) (PerkinElmer, Waltham, MA, USA) secured with a PFP guard column (4 × 3 mm) (Phenomenex, Torrance, CA, USA). Analyses were performed under the following isocratic conditions: mobile phase, methanol:water in ratio of 91:9 (v/v); flow rate, 1.0 mL/min; column oven set at 40 °C; and total analysis run time of 13.5 min. Identification and quantification were performed based on tocopherol and tocotrienol standards and the obtained calibration curves. The tocopherol and tocotrienol stock solutions were appropriately diluted in ethanol to obtain an absorbance within the range of 0.2 to 0.5, ensuring linearity according to the Beer–Lambert law. Obtained standard solutions were determined spectrophotometrically using extinction coefficients for individual tocochromanols and the Beer–Lambert equation [34]. Details of the method of HPLC validation were provided earlier [3].
2.5. Carotenoid Content Semi-Quantitative Analysis
A Shimadzu Nexera 40 Series HPLC system (Kyoto, Japan) with an SPD-M40 diode-array detector (DAD) operated at a wavelength of 200–700 nm was used for carotenoid determination. Carotenoids were separated using the Kinetex PFP (pentafluorophenyl) column (5 μm, 250 × 4.6 mm) secured with a PFP guard column (4 × 3 mm) (Phenomenex, Torrance, CA, USA). The LC conditions for carotenoid determination were as follows: mobile phase, water (A) and methanol (B) with the following gradient: 0.01–3.5 min 87% B, 8.5 min 100% B, 10.5 min 100% B, 11.0 min 87% B; 15.0 min 87% B; flow rate, 1.0 mL/min; column oven temperature, 40 °C; and total analysis run time, 15 min. Due to budget limitations and the focus of the project on the presence of tocotrienols in the Hypericum genus, commercially available standards of carotenoids were not purchased. Instead, β-carotene was used for semi-quantification because it was in stock at the laboratory. Carotenoid identification was performed using isolated standards from various sources according to classical methodologies [35]: neoxanthin, violaxanthin and lutein from spinach; β-cryptoxanthin from mandarin flesh; zeaxanthin from goji berries; α-carotene from carrots; and a commercial β-carotene standard. UV spectra of the isolated standards and a representative chromatogram are provided in the Supplementary Materials. Due to the presence of some impurities in the isolated standards, quantifications were based on a calibration curve constructed for β-carotene at a detection wavelength of 450 nm. β-Carotene was dissolved in chloroform and subsequently diluted with hexane, 2-propanol and finally ethanol. The calibration curve was established within the range of 2–52 ng of β-carotene per injection. The application of a PFP column for carotenoid separation was based on a previous study, dictated, among other reasons, by the employment of less toxic solvents as a mobile phase [36]. The developed method was characterized by a short run time, but also lacked the separation of α-carotene and β-carotene. The separation of α-carotene and β-carotene was performed on the basis of their UV spectra. An example of an obtained chromatogram can be found in the Supplementary Materials (Figure S7).
2.6. Chlorophyll Extraction and Content Analysis
The chlorophyll was extracted from the leaf powder (0.100 ± 0.001g) using 96.2% ethanol (10 mL) and ultrasonic treatment. Briefly, the ultrasonic treatment was performed at 60 °C for 15 min in an ultrasonic bath Bandelin Sonorex RK 510 H (Bandelin electronic, Berlin, Germany) at a frequency of 35 kHz and nominal ultrasonic power of 160 W. The sample was mixed for 1 min on a vortex mixer REAX top (Heidolph, Schwabach, Germany) before and after the ultrasonic bath. Afterward, the sample was centrifuged at 11,000× g for 10 min at 21 °C to separate the supernatant from the plant material. The chlorophyll in the supernatant was measured at wavelengths of 665 nm and 649 nm using a UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan), with the software UVProbe Spectrum (Shimadzu, Kyoto, Japan) for data processing. The content of chlorophyll a and b was calculated according to the following formula (µg mL−1) [37]:
Chlorophyll a = 13.70 × A665 − 5.76 × A649
Chlorophyll b = −7.60 × A665 + 25.8 × A649
2.7. Statistical Analysis
Two-way analysis of variance (ANOVA) was used to identify statistically significant differences between groups separated by qualitative variables (species and leaf age interaction). Tukey’s pairwise test was used to determine which qualitative variable groups were different from each other and to identify homogenous groups. Differences were considered statistically significant at p < 0.05. Spearman correlation coefficients (ρ) were used to determine the relationships between quantitative factors, as predicted by species, and chosen quantitative variables. Data exploration and statistical analysis were performed using R base and the open-source libraries stats, agricolae and GGally. The data were modified and visualized using the R packages tidyr, stringr, forcats, dplyr, ggplot2, ggthemes and patchwork, using RStudio version 2025.05.0+496 “Mariposa Orchid” Release (f0b76cc00df96fe7f0ee687d4bed0423bc3de1f8, 2025-05-04) for windows.
3. Results and Discussion
The leaves of five Hypericum species (including one hybrid) were collected and analyzed for tocochromanol, chlorophyll and carotenoid contents. Two of the species, H. androsaemum and H. × inodorum, belong to the Androsaemum section, while the rest belong to the Ascyreia section: H. pseudohenryi, H. patulum and H. hookerianum. The leaves of the Androsaemum-section species had started to senesce, while the rest were still green. This is unlikely to be a result of the plant type, since all of the species used are shrubby plants. Information about quantitative differences among the different secondary metabolites in the samples collected is summarized in the sections below. At this point, it is important to note that changes in phytochemical contents are subject to the age of the plant [38], age of the leaf [39,40] and climatic conditions [41,42,43,44].
3.1. Tocochromanols
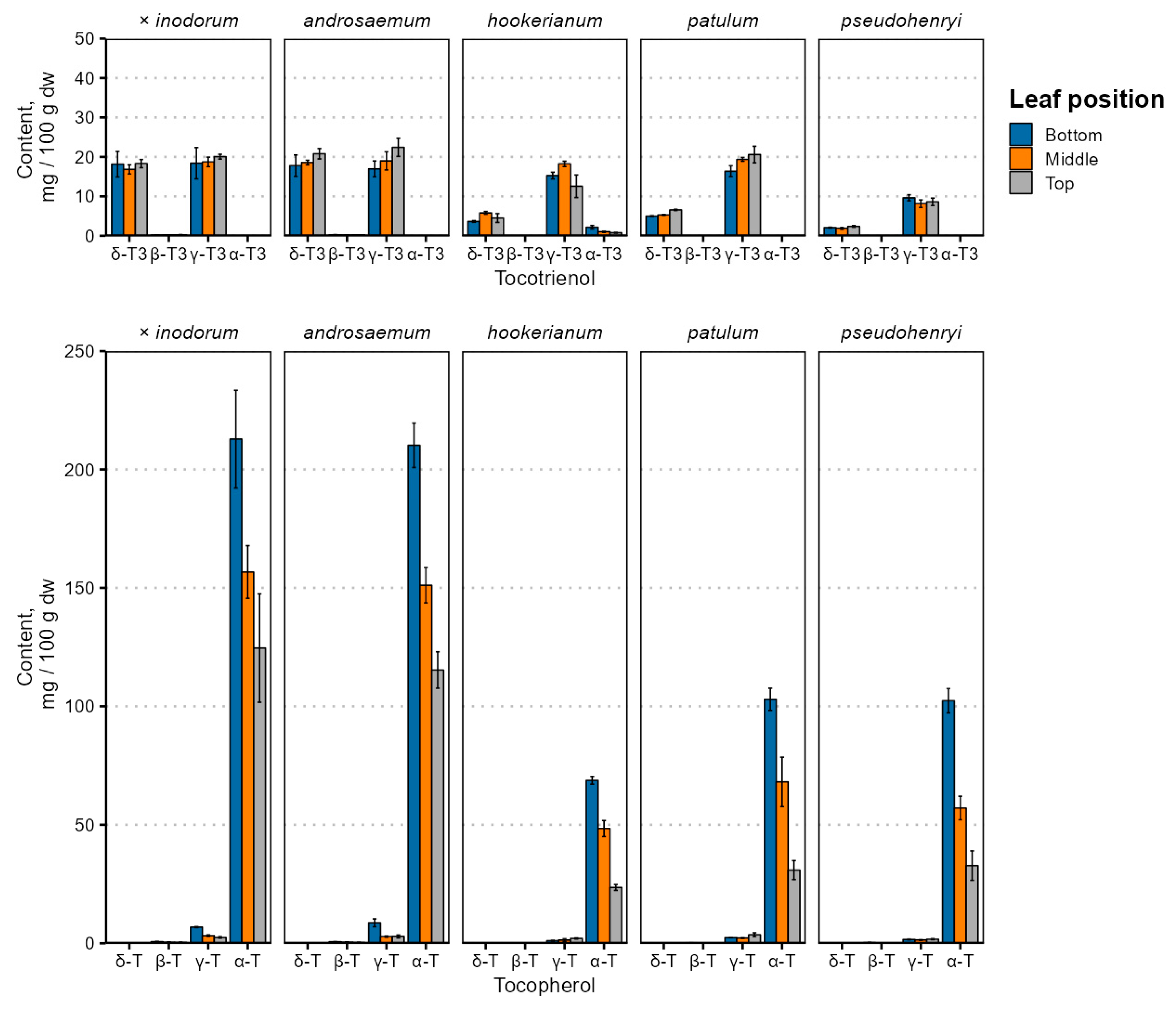
Leaf age and species-dependent individual tocochromanol content changes are depicted in Figure 2, and the tocochromanol content is provided in Table S1.
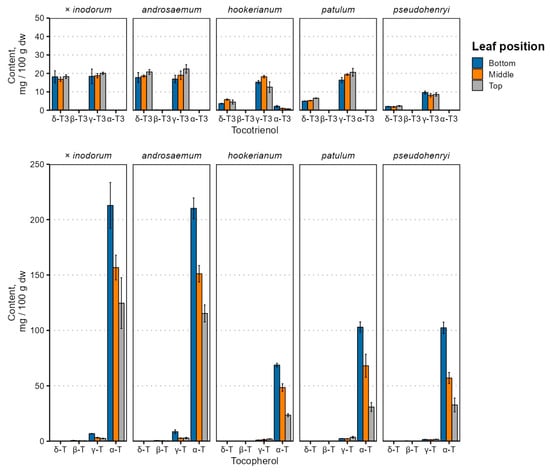
Figure 2.
Tocochromanol content variation in Hypericum species’ leaves depending on leaf position. Data are presented as means ± standard deviations (n = 3).
Tocochromanol contents were highest in early (bottom) leaves in all species, and tocopherol content differed significantly between leaves of different positions (p < 0.001), while the tocotrienol content was not significantly affected by the leaf position (p = 0.72) and was relatively constant. Species and age interactions were strong for tocopherol (p = 0.0017) and tocotrienol (p = 0.0068) content. There were similarities with the tocopherol accumulation reported in Phaseolus coccineus (runner bean), which showed that the α-T content increased in leaves over a 50-day period [31]—leaves grown earlier in the vegetative period would have a higher tocopherol content.
By far, the major tocochromanol in Hypericum leaves was α-T, followed by γ- and δ-T3 in varying proportions, and some γ-T. While tocochromanol profiles are not widely studied in the Hypericum genus, a previous investigation of H. perforatum leaf methanol extracts observed α-T and smaller amounts of δ-T3 using ESI(+)-LC-MS [45]. The tocochromanol composition of the Hypericum leaves and the relatively high tocotrienol content are not entirely unprecedented. A relatively high tocotrienol proportion, specifically δ-T3, has been observed and reported in perilla (Perilla frutescens, Lamiaceae family) leaves, confirmed through LC-MS [46].
Tocochromanol contents, especially δ-T3 and α-T, were much higher in H. × inodorum and H. androsaeumum, which are closely related—H. × inodorum is a hybrid of H. androsaemum and H. hircinum. While H. hircinum leaves were not investigated in the present study, their leaf tocochromanols are composed almost entirely of α-T, and the total content was much lower—around 45 mg 100 g−1 dw [17]. The tocochromanol profiles of H. × inodorum and H. androsaemum are similar to those observed previously [18,47]. Individual tocochromanol contents within species did not appear to be strongly correlated with each other. Regarding major tocochromanols, there were statistically significant (p < 0.05), mild positive correlations between δ-T3 and γ-T3 contents in H. androsaemum (ρ = 0.71), H. × inodorum (ρ = 0.84) and H. hookerianum (ρ = 0.72). Statistically significant, strong correlations were observed between γ-T and α-T in H. androsaemum (ρ = 0.84), H. × inodorum (ρ = 0.92), H. patulum (ρ = 0.67) and H. hookerianum (ρ = −0.74). The tocotrienol and tocopherol content was negatively associated in H. androsaemum (ρ = 0.74) and H. patulum (ρ = −0.87). Negative correlations between tocotrienol and tocopherol contents may be explained by the upregulation of enzymes converting GGPP into MGGBQ for tocotrienol synthesis or converting GGPP into PPP for chlorophyll or tocopherol synthesis [48]. Distinct differences between the tocochromanol content and profile are rather common—the palm oil (Elaeis guineensis) tocochromanol content is significantly affected by both the population and geographic region [49], and the same is true for H. perforatum [50,51]. Like the geographic region, the phylogeny and species are major tocochromanol profile and content determinants in Hypericum [16,17] and other genera, like the Prunus genus [52]. Even the plant variety has a significant effect on the tocochromanol content and biosynthesis [53]. However, metabolic regulation in the tocochromanol biosynthetic pathway has not been studied in the Hypericum genus and can be recommended in future studies.
Tocopherols (α-Ts) and plastoquinone-9 accumulate in fig and beech leaves during the vegetative period (increased 5.2 and 3.0 times, respectively) and their concentration is higher in older leaves (increased 18 and 20 times, compared to young leaves) [22]. In plants that accumulate tocotrienols in the leaves, such as Vellozia gigantea (Velloziaceae family), tocopherol and tocotrienol content varies significantly across the growing seasons [54]. In herbaceous plants such as Phaseolus coccineus (runner bean, Fabaceae family), the α-T, but not γ- or δ-T, content increases under unaltered conditions. In plant leaves grown in the shade, the α-T content decreased significantly by day 47 of 53 [31]. An increased tocochromanol content may be a protective mechanism in older leaves against increased oxidative stress regardless of senescence [55]. In Manihot esculenta (cassava, Euphorbiaceae family) leaves, the total tocopherol content increased over a 15-month period after planting [56].
3.2. Chlorophylls
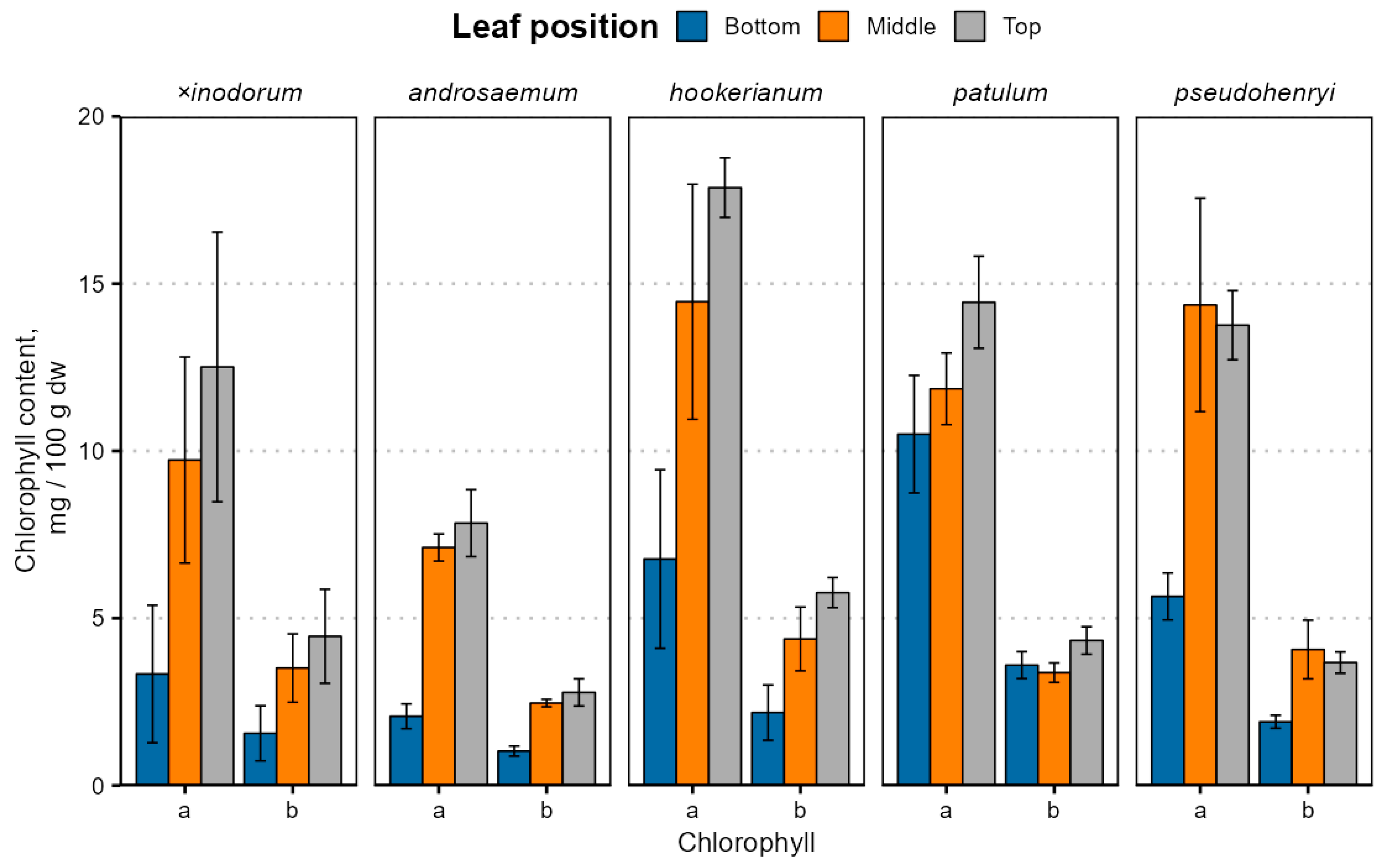
The leaf age- and species-dependent chlorophyll content variation is depicted in Figure 3; exact values are provided in Table S2.
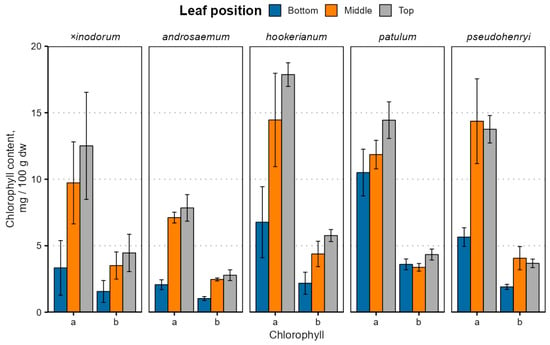
Figure 3.
Chlorophyll content variation in Hypericum species’ leaves depending on the leaf position. Data are presented as means ± standard deviations (n = 3).
Chlorophyll a and b contents were significantly different between leaves of different ages (p < 0.001) and species (p < 0.001) with a near-significant species–age interaction for chlorophyll a (p = 0.075) and a significant interaction for chlorophyll b (p = 0.016). The chlorophyll a and b content were similar in H. patulum, H. pseudohenryi and H. hookerianum (p > 0.05). The chlorophyll b, but not a, content was similar in H. × inodorum, H. hookerianum, H. patulum and H. pseudohenryi (p > 0.05). Only the chlorophyll a content was similar in H. androsaemum (p > 0.05), but, like chlorophyll b, it was also similar in H. × inodorum and H. pseudohenryi (p > 0.05). While early leaves were statistically significantly different from young and mature leaves (p < 0.001), the chlorophyll a content was similar in young and mature leaves (p = 0.076), but not chlorophyll b (p = 0.038). The chlorophyll content was relatively lower in H. × inodorum and H. androsaemum, largely due to distinct leaf senescence in the two species. The chlorophyll a and b content correlated positively in all species (ρ = 0.89 to 0.99), and a/b ratios were slightly lower (p < 0.001) in bottom leaves in most species, except H. hookerianum, but not significantly different between middle and top leaves (p = 0.69). The high standard deviation of chlorophyll observed in most of the leaf samples can be attributed to differences between sampled ages of branches. Among the various classes of prenyllipids, chlorophylls—and subsequently carotenoids—exhibit the lowest stability; as a result, their degradation is most pronounced during the later phases of plant maturation. Marked variations in chlorophyll content among the biological replicates were clearly observable via visual inspection, indicating noticeable differences in pigmentation between individual samples (Supplementary Materials, Figure S6).
The chlorophyll content was much lower than previously reported in H. perforatum leaves at 4 weeks old (212 mg 100 g−1 fw), grown hydroponically under white light [57], but the ratio of chlorophyll a and chlorophyll b was typical for most plants, including Hypericum. Decreased chlorophyll content in older (bottom) leaves is well-documented—tobacco leaf chlorophyll content decreases during natural leaf senescence [58], and basal (bottom) grapevine leaves have a lower chlorophyll content [59]. The chlorophyll content, especially chlorophyll a, decreases with stand age in the desert species Haloxylon ammodendron (Amaranthaceae or amaranth family), which negatively affects light use efficiency in older plants [60]. A slightly different trend has been observed in cassava leaves—chlorophyll a and b contents were slightly lower in the top leaves than middle and bottom [40]. It is important to note that different senescence-related genes are expressed in plant and leaves of different ages even at the same leaf senescence stage [61]. Besides natural senescence, cold may also cause chlorophyll contents to decrease [42]. Lower a/b ratios can be caused by low light reaching the bottom leaves or leaf aging, since lower a/b ratios have been observed in basal (bottom) grapevine leaves [59], as well as H. perforatum grown under red/blue light, compared to white light [57,62].
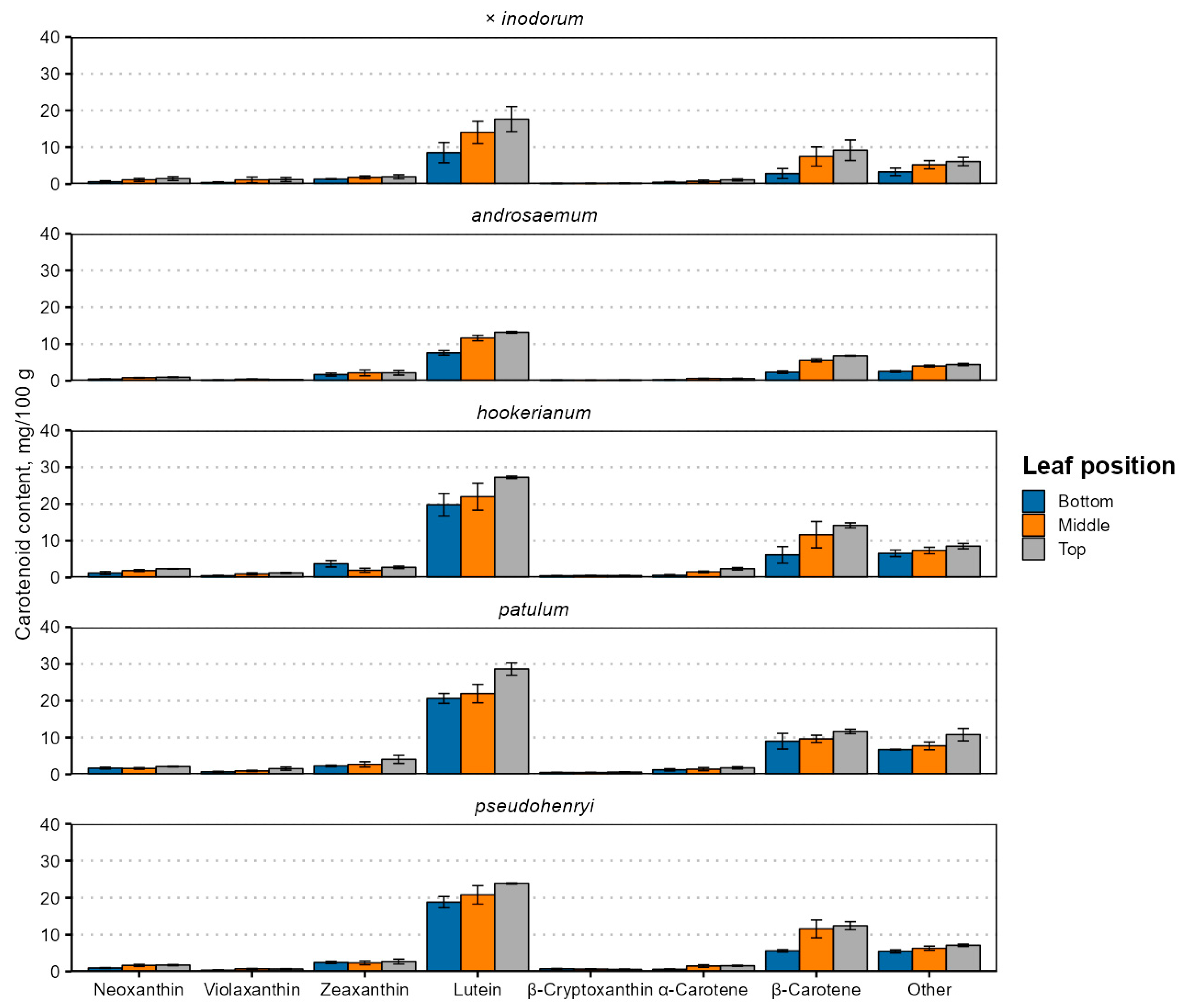
3.3. Carotenoids
Carotenoid contents were higher in younger leaves (Figure 4 and Table S3), and their content was generally strongly positively correlated, except for zeaxanthin and neoxanthin in H. hookerianum (ρ = −0.68) and α-carotene and β-cryptoxanthin in H. pseudohenryi (ρ = −0.68).
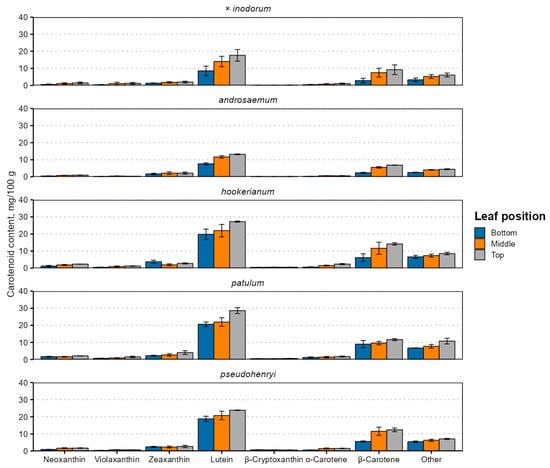
Figure 4.
Carotenoid contents in senescent Hypericum species’ leaves of different position. Data are presented as means ± standard deviations of three replicates (n = 3).
This is likely explained by the simultaneous upregulation of genes and enzymes involved in carotenoid biosynthesis [48]. The carotenoid content was higher in younger leaves. The main identified carotenoids in the leaves were lutein and β-carotene, in this order, regardless of their position. In total, up to seven carotenoids were identified. Apart from lutein and β-carotene, neoxanthin, violaxanthin, zeaxanthin (usually co-eluting with lutein), β-cryptoxanthin and α-carotene were present (Figure 4). The total carotenoid content ranged from 15.33 mg 100 g−1 in early (bottom) H. androsaemum leaves to 61.13 mg 100 g−1 in top (young) H. patulum leaves. The total carotenoid content was higher in Ascyreia species leaves than in Androsaemum section species leaves.
The results differ significantly from those observed in H. perforatum chloroform extracts, where the xanthophyll content was similar to the carotene content, and the α- and β-carotene content was much more similar, with the β-carotene content slightly higher than α-carotene [63]. However, the cited study investigated a different species, used the whole plant for extract preparation, as opposed to just the leaves, and used a different solvent than the present study, in addition to very roughly estimating individual carotenoid contents just from the absorbance at different wavelengths. The total carotenoid content in H. perforatum plants grown hydroponically under white light was 109 mg 100 g−1 in fresh leaves at 4 weeks old [57], much higher than in the present study. Unfortunately, detailed carotenoid profiles for Hypericum leaves or other organs are not available for comparison, and their investigations can be recommended in future studies on lipophilic Hypericum metabolites.
Lutein is typically accumulated in the highest concentration in plant leaves across a variety of species, families and plant types and is near-ubiquitous. In wide-scale screenings, its concentration can range from 16.4 mg 100 g−1 in Tamarindus indica (tamarind) leaves to 679 mg 100 g−1 dw in Clitoria terneata (butterfly pea) leaves [64]. Carotenoid contents in plant tissues are dependent on many variables, including the genotype, agronomic, climatic or physiological factors [65] and phylogeny. In the Citrus genus, the carotenoid profile is shaped mainly by the species and variety [66], and carotenoid profiles, related gene expression and deposit plastid structures differ significantly between different Prunus species and varieties [67]. A combination of these factors likely explains differences between Hypericum carotenoid profiles and contents and is a subject for future studies.
A small proportion of the other unidentified carotenoid sum may be antheraxanthin, which is an intermediary product between zeaxanthin and violaxanthin [21]. Alternatively, they may be products of carotenoid degradation, either as a result of normal plant metabolism or during sample preparation [68], or carotenoid derivatives, such as apocarotenoids [69], some of which act as chromophores.
The reduced carotenoid content in bottom leaves may be related to lower carotenoid biosynthesis and accumulation during the early months of growth or reduced light conditions resulting in low carotenoid and chlorophyll biosynthesis. In Arabidopsis, a model plant for studying lipid metabolism in plants, carotenoid accumulation decreases steadily with leaf age [40]. The same is true in tobacco leaves during natural senescence—older or earlier leaves at the bottom of the plant have lower carotenoid contents [39]. All investigated carotenoid contents were lower in the low-light stress group of Festuca arundinacea (tall fescue) leaves [41]. Existing research reports decreased chlorophyll a, chlorophyll b and carotenoid contents under red and blue lighting, an increased carotenoid content under blue lighting, compared to white light, and an increased net photosynthesis rate under blue light in hydroponically and foam-grown H. perforatum plants [57,62].
In Manihot esculenta (cassava, Euphorbiaceae family) leaves, carotenoid contents followed a slightly different trend—the carotenoid and chlorophyll content was lower in older plants (6 versus 12 months after planting), but was not distinctly more concentrated in higher leaves [38]. H. androsaemum and H. × inodorum, but not the other species, had senescent leaves. Like chlorophylls, the carotenoid content can decrease as a result of cold stress and acclimation [42], and their synthesis and accumulation cease upon leaf senescence [43]. Apart from leaf senescence, carotenoid biosynthesis is also affected by the temperature [44]. If the two Androsaemum-section species are less cold-hardy, carotenoid biosynthesis may have been downregulated regardless of leaf senescence.
3.4. Relationship Between Tocochromanol, Carotenoid and Chlorophyll Contents
While tocochromanols, carotenoids and chlorophylls serve different functions in plants, they have common biosynthetic pathways and precursor compounds. The biosynthesis upregulation of one group of compounds may necessitate the downregulation of others. To indirectly characterize the relationship among tocochromanol, carotenoid and chlorophyll biosynthesis, a correlation analysis was performed. Pair plots and Spearman corrrelation coefficients (ρ), along with the correlation significance, are provided in Figures S8–S10. A statistically significant correlation between tocotrienols and chlorophyll was only observed in H. patulum (ρ = 0.76) with chlorophyll a for δ-T3 (ρ = 0.78) and γ-T3 (ρ = 0.68). Tocopherol contents correlated negatively with the chlorophyll content in all species, especially chlorophyll a (ρ = −0.96 to −0.82), while chlorophyll b had a less pronounced correlation (ρ = −0.78 to −0.96). The negative correlation was most pronounced between α-T and chlorophyll a because α-T contents were the most significantly different between leaves of different ages. Other tocochromanol contents were relatively similar in leaves of different ages, resulting in a less significant correlation. Tocotrienols only had a significant correlation with carotenoids in H. androsaemum (ρ = 0.76) and H. patulum (ρ = 0.68). Tocopherols had a strong negative correlation with carotenoids in all species (ρ = −0.77 to −0.98). The ratios between analyzed metabolites are presented in Table 1.

Table 1.
Lipophilic metabolite ratios in Hypericum leaves.
Tocotrienol, tocopherol and the total tocochromanol ratio to total chlorophyll were lower in bottom leaves (p > 0.05), but similar in middle and top leaves (p < 0.001). The tocopherol and total tocochromanol ratio to total carotenoids was different between leaves of different ages (p < 0.05), but the tocotrienol ratio to total carotenoids was only different in bottom leaves (p < 0.01). Similarly, the carotenoid ratio to chlorophylls was similar in top and middle leaves (p = 0.85), but was generally higher in bottom leaves, except for H. patulum, in which the ratio did not differ significantly between leaves of different ages (p > 0.5), and the carotenoid content was diminished at the same rate as the chlorophyll content, whereas in other species, the carotenoid content was reduced more than chlorophyll.
It is important to note that while the plants were grown in the same soil and climatic conditions, they have different abilities to acclimate to those conditions, including frost, heat and sun exposure tolerance. While the effect of cold stress at 4 °C on H. androsaemum secondary metabolites (phenolic, anthraquinone and phloroglucinols compounds) was not pronounced, especially compared to tropical species like H. canariense, it did have an effect on the carotenoid content and antioxidant activity [70]. There is no available information on the effect of factors like cold stress on the tocochromanol profile in Hypericum plants at this time, and additional studies are necessary to discern between genetic and environmental effects on the tocochromanol profile. Of the investigated species, H. androsaemum is the only deciduous species, while the rest are semi-evergreen, depending on the climate, but this is only reflected in the lower chlorophyll content of the leaves.
4. Conclusions
Tocochromanol, carotenoid and chlorophyll accumulation in Hypericum leaves is species and leaf age-dependent. While tocopherol, carotenoid and chlorophyll contents are lower in older leaves, the tocotrienol content is lower in younger leaves. The present study provides insight into the content of and relationship between lipophilic metabolites in a species that has relatively high leaf tocotrienol contents and offers one of the first reports of the Hypericum leaf carotenoid profile. The results showed increased tocopherol contents in all species and increased tocotrienol contents in some species in the bottom leaves, while the chlorophyll and carotenoid contents were lower in older leaves. Considering the relatively high tocopherol and tocotrienol contents in the leaves, the Hypericum genus offers a unique opportunity for the study of the natural metabolic pathway of lipophilic antioxidants and pigments, which is only possible through genetic interference in current plant lipid metabolism model species.
Since the activity of the enzymes involved in the biosynthesis of the studied phytochemicals was not determined, it is not possible to make conclusions about their upregulation or downregulation depending on leaf age in Hypericum plants, and this study serves as an initial demonstration of the relationship between prenyl-lipid accumulation. Per general wintering advise, the plants were cut at the end of the vegetative season, but not during the vegetative season. As a result, the data reflect only phytochemical contents at one point in time in leaves of different heights and ages. As commented earlier, changes in phytochemical contents are subject to many variables besides the genotype, such as the age of the plant and the leaf, and their further study in the Hypericum genus is warranted. Changes in lipophilic secondary metabolite contents remain to be studied before, during and after blooming, as well as between growing seasons.
As a source of the analyzed prenyl-lipids, Hypericum leaves are not limited by biomass production and the concentration of these compounds in the biomass as it is by the presence of other compounds, which are co-extractable: phloroglucinols and naphtodianthrones, which will affect the biological properties of plant extracts and their applicability. However, some species produce little to no phloroglucinols and naphtodianthrones, like H. hircinum, which was investigated in the present study, while other Hypericum species produce no naphtodianthrones (H. androsaemum and H. calycinum) or no acylphloroglucinols (H. montanum) [5].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14142239/s1, Figure S1: Hypericum androsaemum and its top, middle, bottom leaves; Figure S2: Hypericum × inodorum and its top, middle, bottom leaves; Figure S3: Hypericum pseudohendryi; Figure S4: Hypericum patulum; Figure S5: Hypericum hookerianum; Figure S6: The powdered samples of top, middle, and bottom leaves of Hypericum × inodorum; Figure S7: Chromatogram of carotenoids separation of extracts from saponified samples of H. patulum top leaves by PFP column, together with the UV spectra of identified carotenoids and their retention times (top left corners); Figure S8: Spearman correlation between individual tocochromanols in Hypericum species’ leaves and tocochromanol content density plots; Figure S9: Spearman correlation between individual carotenoids in Hypericum species’ leaves and tocochromanol content density plots; Figure S10. Spearman correlation between total tocotrienols, total tocopherols, total tocochromanols, chlorophyll a and b, and total carotenoids in Hypericum species’ leaves and tocochromanol content density plots; Table S1: Tocochromanol content in Hypericum species’ leaves, mg 100 g−1 dw; Table S2: Chlorophyll content in Hypericum species’ leaves, mg 100 g−1 dw; Table S3: Carotenoid content in Hypericum species’ leaves, mg 100 g−1 dw.
Author Contributions
Conceptualization, P.G.; Software, K.D.; Validation, K.D.; Formal analysis, I.M. (Inga Mišina), A.M.B.-G. and C.M.S.; Investigation, D.L., I.M. (Ieva Miķelsone), A.M.B.-G., C.M.S. and P.G.; Resources, D.L., I.M. (Ieva Miķelsone), I.M. (Inga Mišina), K.D., A.M.B.-G. and C.M.S.; Data curation, D.L. and K.D.; Writing—original draft, D.L., I.M. (Ieva Miķelsone) and P.G.; Writing—review & editing, D.L., I.M. (Ieva Miķelsone), A.M.B.-G., C.M.S., A.J.M.-M. and P.G.; Visualization, D.L. and I.M. (Ieva Miķelsone); Supervision, A.J.M.-M. and P.G.; Funding acquisition, P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Latvian Council of Science, project “Genus Hypericum as a new valuable source of tocotrienols and tocochromanol-related molecules—from ornamental crop to industrial applications”, No. lzp-2021/1-0651. This article is based upon work from COST Action EUROCAROTEN (European network to advance carotenoid research and applications in agro-food and health, CA15136), supported by COST (European Cooperation in Science and Technology). Thanks to the Botanical Garden of Marie Curie-Sklodowska University in Lublin, Poland; the Botanical Garden of the Faculty of Science, Masaryk University, Brno, Czech Republic; and the Tallinn Botanic Garden, Estonia, for providing seeds.
Data Availability Statement
The data used to support the findings of this study are available in the Supplementary Materials.
Conflicts of Interest
A.J.M.-M. occasionally carries out consultancy work for companies. All other authors declare no conflicts of interest.
References
- Group, T.A.P. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot. J. Linn. Soc. 2003, 141, 399–436. [Google Scholar] [CrossRef]
- Ion, V.; Ielciu, I.; Cârje, A.-G.; Muntean, D.L.; Crişan, G.; Păltinean, R. Hypericum spp.—An Overview of the Extraction Methods and Analysis of Compounds. Separations 2022, 9, 17. [Google Scholar] [CrossRef]
- Mišina, I.; Perkons, I.; Siger, A.; Soliven, A.; Górnaś, P. Residues of St. John’s wort (Hypericum perforatum) tea infusions/water extracts as a valuable source of tocotrienols: An extraction study. Appl. Sci. 2025, 15, 2047. [Google Scholar] [CrossRef]
- Heinrich, M.; Vikuk, V.; Daniels, R.; Stintzing, F.C.; Kammerer, D.R. Characterization of Hypericum perforatum L. (St. John’s wort) macerates prepared with different fatty oils upon processing and storage. Phytochem. Lett. 2017, 20, 470–480. [Google Scholar] [CrossRef]
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species–A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Mène-Saffrané, L.; DellaPenna, D. Biosynthesis, regulation and functions of tocochromanols in plants. Plant Physiol. Biochem. 2010, 48, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Mène-Saffrané, L. Vitamin E biosynthesis and its regulation in plants. Antioxidants 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.; Zacarias, L.; Rodrigo, M.J. Regulation of tocopherol biosynthesis during fruit maturation of different Citrus species. Front. Plant Sci. 2021, 12, 743993. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Wessjohann, L.; Bigirimana, J.; Jansen, M.; Guisez, Y.; Caubergs, R.; Horemans, N. Differential distribution of tocopherols and tocotrienols in photosynthetic and non-photosynthetic tissues. Phytochemistry 2006, 67, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Siger, A.; Górnaś, P. Free tocopherols and tocotrienols in 82 plant species’ oil: Chemotaxonomic relation as demonstrated by PCA and HCA. Food Res. Int. 2023, 164, 112386. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Baškirovs, G.; Siger, A. Free and esterified tocopherols, tocotrienols and other extractable and non-extractable tocochromanol-related molecules: Compendium of knowledge, future perspectives and recommendations for chromatographic techniques, tools, and approaches used for tocochromanol determination. Molecules 2022, 27, 6560. [Google Scholar] [PubMed]
- Siles, L.; Cela, J.; Munné-Bosch, S. Vitamin E analyses in seeds reveal a dominant presence of tocotrienols over tocopherols in the Arecaceae family. Phytochemistry 2013, 95, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Bagci, E. Fatty acids and tocochromanol patterns of some Turkish Apiaceae (Umbelliferae) plants; a chemotaxonomic approach. Acta Bot. Gallica 2007, 154, 143–151. [Google Scholar] [CrossRef]
- Ivanov, S.A.; Aitzetmüller, K. Untersuchungen über die tocopherol-und tocotrienolzusammensetzung der samenöle einiger vertreter der familie Apiaceae. Lipid/Fett 1995, 97, 24–29. [Google Scholar] [CrossRef]
- Yang, B.; Ahotupa, M.; Määttä, P.; Kallio, H. Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Res. Int. 2011, 44, 2009–2017. [Google Scholar] [CrossRef]
- Mišina, I.; Lazdiņa, D.; Górnaś, P. Tocochromanols in the leaves of plants in the Hypericum and Clusia genera. Molecules 2025, 30, 709. [Google Scholar] [CrossRef] [PubMed]
- Lazdiņa, D.; Mišina, I.; Górnaś, P. Tocotrienols in eleven species of Hypericum genus leaves. Molecules 2025, 30, 662. [Google Scholar] [CrossRef] [PubMed]
- Miķelsone, I.; Sipeniece, E.; Mišina, I.; Bondarenko, E.; Segliņa, D.; Górnaś, P. Impact of soil on biomass yield and accumulation of lipophilic secondary metabolites in four Hypericum species. Agriculture 2025, 15, 526. [Google Scholar] [CrossRef]
- Miķelsone, I.; Sipeniece, E.; Segliņa, D.; Górnaś, P. Tocopherol and tocotrienol content in the leaves of the genus Hypericum: Impact of species and drying technique. Plants 2025, 14, 1079. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Mišina, I.; Soliven, A.; Segliņa, D. Tocopherol and tocotrienol profile in wild St. John’s wort populations in Latvia: Impact of the plant’s aerial parts. Nat. Prod. Res. 2025, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Biosynthesis, accumulation and emission of carotenoids, α-tocopherol, plastoquinone, and isoprene in leaves under high photosynthetic irradiance. Photosynth. Res. 2007, 92, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J. An overview of carotenoids, apocarotenoids, and vitamin A in agro-food, nutrition, health, and disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Böhm, V.; Borge, G.I.A.; Cano, M.P.; Fikselová, M.; Gruskiene, R.; Lavelli, V.; Loizzo, M.R.; Mandić, A.I.; Brahm, P.M. Carotenoids: Considerations for their use in functional foods, nutraceuticals, nutricosmetics, supplements, botanicals, and novel foods in the context of sustainability, circular economy, and climate change. Annu. Rev. Food Sci. Technol. 2021, 12, 433–460. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Kobayashi, K.; Masuda, T. Tetrapyrrole metabolism in Arabidopsis thaliana. Arab. Book/Am. Soc. Plant Biol. 2011, 9, e0145. [Google Scholar]
- Lushchak, V.I.; Semchuk, N.M. Tocopherol biosynthesis: Chemistry, regulation and effects of environmental factors. Acta Physiol. Plant. 2012, 34, 1607–1628. [Google Scholar] [CrossRef]
- Sun, T.; Tadmor, Y.; Li, L. Pathways for carotenoid biosynthesis, degradation, and storage. In Plant and Food Carotenoids; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–23. [Google Scholar]
- Ohmiya, A.; Hirashima, M.; Yagi, M.; Tanase, K.; Yamamizo, C. Identification of genes associated with chlorophyll accumulation in flower petals. PLoS ONE 2014, 9, e113738. [Google Scholar] [CrossRef] [PubMed]
- Durrett, T.P.; Welti, R. The tail of chlorophyll: Fates for phytol. J. Biol. Chem. 2021, 296, 100802. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, R.; Kruk, J. Tocopherol content and isomers’ composition in selected plant species. Plant Physiol. Biochem. 2008, 46, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, R.; Kruk, J. γ-Tocopherol dominates in young leaves of runner bean (Phaseolus coccineus) under a variety of growing conditions: The possible functions of γ-tocopherol. Phytochemistry 2008, 69, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- Slavin, M.; Yu, L.L. A single extraction and HPLC procedure for simultaneous analysis of phytosterols, tocopherols and lutein in soybeans. Food Chem. 2012, 135, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Segliņa, D.; Lācis, G.; Pugajeva, I. Dessert and crab apple seeds as a promising and rich source of all four homologues of tocopherol (a, b, g and d). LWT-Food Sci. Technol. 2014, 59, 211–214. [Google Scholar] [CrossRef]
- Kofler, M.; Sommer, P.F.; Bolliger, H.R.; Schmidli, B.; Vecchi, M. Physicochemical properties and assay of the tocopherols. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 1962; Volume 20, pp. 407–440. [Google Scholar]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001. [Google Scholar]
- Sanz, N.; García-Blanco, A.; Gavalás-Olea, A.; Loures, P.; Garrido, J.L. Phytoplankton pigment biomarkers: HPLC separation using a pentafluorophenyloctadecyl silica column. Methods Ecol. Evol. 2015, 6, 1199–1209. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Chaiareekitwat, S.; Latif, S.; Mahayothee, B.; Khuwijitjaru, P.; Nagle, M.; Amawan, S.; Müller, J. Protein composition, chlorophyll, carotenoids, and cyanide content of cassava leaves (Manihot esculenta Crantz) as influenced by cultivar, plant age, and leaf position. Food Chem. 2022, 372, 131173. [Google Scholar] [CrossRef] [PubMed]
- Uzelac, B.; Janošević, D.; Simonović, A.; Motyka, V.; Dobrev, P.I.; Budimir, S. Characterization of natural leaf senescence in tobacco (Nicotiana tabacum) plants grown in vitro. Protoplasma 2016, 253, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.; Tissue, D.T.; Cazzonelli, C.I. Leaf-age dependent response of carotenoid accumulation to elevated CO2 in Arabidopsis. Arch. Biochem. Biophys. 2018, 647, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, X.; You, X.; Li, Y.; Long, S.; Wen, S.; Liu, Q.; Liu, T.; Guo, H.; Xu, Y. Hydrogen sulfide improves tall fescue photosynthesis response to low-light stress by regulating chlorophyll and carotenoid metabolisms. Plant Physiol. Biochem. 2022, 170, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Doğru, A.; Çakirlar, H. Effects of leaf age on chlorophyll fluorescence and antioxidant enzymes activity in winter rapeseed leaves under cold acclimation conditions. Rev. Bras. Bot. 2020, 43, 11–20. [Google Scholar] [CrossRef]
- Dhami, N.; Cazzonelli, C.I. Environmental impacts on carotenoid metabolism in leaves. Plant Growth Regul. 2020, 92, 455–477. [Google Scholar] [CrossRef]
- Xu, P.; Yu, J.; Ma, R.; Ji, Y.; Hu, Q.; Mao, Y.; Ding, C.; Li, Z.; Ge, S.; Deng, W.-W. Chlorophyll and carotenoid metabolism varies with growth temperatures among tea genotypes with different leaf colors in Camellia sinensis. Int. J. Mol. Sci. 2024, 25, 10772. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tatemori, S.; Muranaka, N.; Hirahara, Y.; Homma, S.; Nakane, T.; Takano, A.; Nomi, Y.; Otsuka, Y. The Identification of Vitamin E Homologues in Medicinal Plant Samples Using ESI (+)-LC-MS3. J. Agric. Food Chem. 2012, 60, 9581–9588. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S.; Rengasamy, K.R. Profiling of nutritionally important metabolites in green/red and green perilla (Perilla frutescens Britt.) cultivars: A comparative study. Ind. Crops Prod. 2020, 151, 112441. [Google Scholar] [CrossRef]
- Miķelsone, I.; Sipeniece, E.; Mišina, I.; Bondarenko, E.; Górnaś, P. Cultivated St. John’s wort flower heads accumulate tocotrienols over tocopherols, regardless of the year of the plant. Plants 2025, 14, 852. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Baysal, C.; Drapal, M.; Sheng, Y.; Huang, X.; He, W.; Shi, L.; Capell, T.; Fraser, P.D.; Christou, P. The Coordinated upregulated expression of genes involved in MEP, chlorophyll, carotenoid and tocopherol pathways, mirrored the corresponding metabolite contents in rice leaves during de-etiolation. Plants 2021, 10, 1456. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, F.; Vaissayre, V.; Serret, J.; Avallone, S.; Domonhédo, H.; Jacob, F.; Dussert, S. Natural diversity in the carotene, tocochromanol and fatty acid composition of crude palm oil. Food Chem. 2021, 365, 130638. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Mišina, I.; Perkons, I.; Segliņa, D.; Czlapka-Matyasik, M. Characterization of tocochromanols in wild Hypericum perforatum populations in Latvia. Horticulturae 2025, 11, 205. [Google Scholar] [CrossRef]
- Górnaś, P.; Symoniuk, E. Tocotrienols in different parts of wild Hypericum perforatum L. populations in Poland. Molecules 2025, 30, 1137. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Symoniuk, E.; Soliven, A. Reversed phase HPLC with UHPLC benefits for the determination of tocochromanols in the seeds of edible fruits in the Rosaceae family. Food Chem. 2024, 460, 140789. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J.; Huang, S.; Li, H.; Liu, Y.; Gu, Q.; Guo, X.; Hu, Y. Tocochromanols and chlorophylls accumulation in young pomelo (Citrus maxima) during early fruit development. Foods 2021, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Garcia, Q.S.; Siqueira-Silva, A.I.; Silva, M.C.; Munné-Bosch, S. Tocotrienols in Vellozia gigantea leaves: Occurrence and modulation by seasonal and plant size effects. Planta 2014, 240, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. Plant aging increases oxidative stress in chloroplasts. Planta 2002, 214, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Laya, A.; Koubala, B.B. Changes in vitamin e and β-carotene contents in various edible cassava leaves (Manihot esculenta Crantz) of different ages across multiple seasons. Int. J. Agron. 2020, 2020, 4671018. [Google Scholar] [CrossRef]
- Karimi, M.; Ahmadi, N.; Ebrahimi, M. Red LED light promotes biomass, flowering and secondary metabolites accumulation in hydroponically grown Hypericum perforatum L.(cv. Topas). Ind. Crops Prod. 2022, 175, 114239. [Google Scholar] [CrossRef]
- Procházková, D.; Haisel, D.; Wilhelmová, N. Content of carotenoids during ageing and senescence of tobacco leaves with genetically modulated life-span. Photosynthetica 2009, 47, 409–414. [Google Scholar] [CrossRef]
- Bertamini, M.; Nedunchezhian, N. Leaf age effects on chlorophyll, Rubisco, photosynthetic electron transport activities and thylakoid membrane protein in field grown grapevine leaves. J. Plant Physiol. 2002, 159, 799–803. [Google Scholar] [CrossRef]
- He, X.-h.; Si, J.-h.; Zhou, D.-m.; Wang, C.-l.; Zhao, C.-y.; Jia, B.; Qin, J.; Zhu, X.-l. Leaf chlorophyll parameters and photosynthetic characteristic variations with stand age in a typical desert species (Haloxylon ammodendron). Front. Plant Sci. 2022, 13, 967849. [Google Scholar] [CrossRef] [PubMed]
- Zentgraf, U.; Jobst, J.; Kolb, D.; Rentsch, D. Senescence-related gene expression profiles of rosette leaves of Arabidopsis thaliana: Leaf age versus plant age. Plant Biol. 2004, 6, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Paponov, M.; Antonyan, M.; Slimestad, R.; Paponov, I.A. Decoupling of plant growth and accumulation of biologically active compounds in leaves, roots, and root exudates of Hypericum perforatum l. by the combination of jasmonate and far-red lighting. Biomolecules 2021, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Omarova, M.A.; Artamonova, N.A. Liposoluble pigments from the herb: Hypericum perforatum. Chem. Nat. Compd. 1997, 33, 691–692. [Google Scholar] [CrossRef]
- Sangeetha, R.K.; Baskaran, V. Carotenoid composition and retinol equivalent in plants of nutritional and medicinal importance: Efficacy of β-carotene from Chenopodium album in retinol-deficient rats. Food Chem. 2010, 119, 1584–1590. [Google Scholar] [CrossRef]
- Dias, M.G.; Borge, G.I.A.; Kljak, K.; Mandić, A.I.; Mapelli-Brahm, P.; Olmedilla-Alonso, B.; Pintea, A.M.; Ravasco, F.; Šaponjac, V.T.; Sereikaitė, J. European database of carotenoid levels in foods. Factors Affect. Carotenoid Content. Foods 2021, 10, 912. [Google Scholar] [PubMed]
- Fanciullino, A.-L.; Dhuique-Mayer, C.; Luro, F.; Morillon, R.; Ollitrault, P. Carotenoid biosynthetic pathway in the Citrus genus: Number of copies and phylogenetic diversity of seven genes. J. Agric. Food Chem. 2007, 55, 7405–7417. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Pengfei, W.; Brennan, H.; Ping, Q.; Bingxiang, L.; Feiyan, Z.; Hongbo, C.; Haijiang, C. Diversity of carotenoid composition, sequestering structures and gene transcription in mature fruits of four Prunus species. Plant Physiol. Biochem. 2020, 151, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the colour and the chemical structure of carotenoid pigments. Food Chem. 2007, 101, 1145–1150. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef] [PubMed]
- Bruňáková, K.; Bálintová, M.; Petijová, L.; Čellárová, E. Does phenotyping of Hypericum secondary metabolism reveal a tolerance to biotic/abiotic stressors? Front. Plant Sci. 2022, 13, 1042375. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).