Therapeutic Potential of Erythrina Genus: Bioactive Phytoconstituents with Potent Antiviral and Antimicrobial Activities
Abstract
1. Introduction
2. Results
2.1. Antiviral Properties of Erythrina
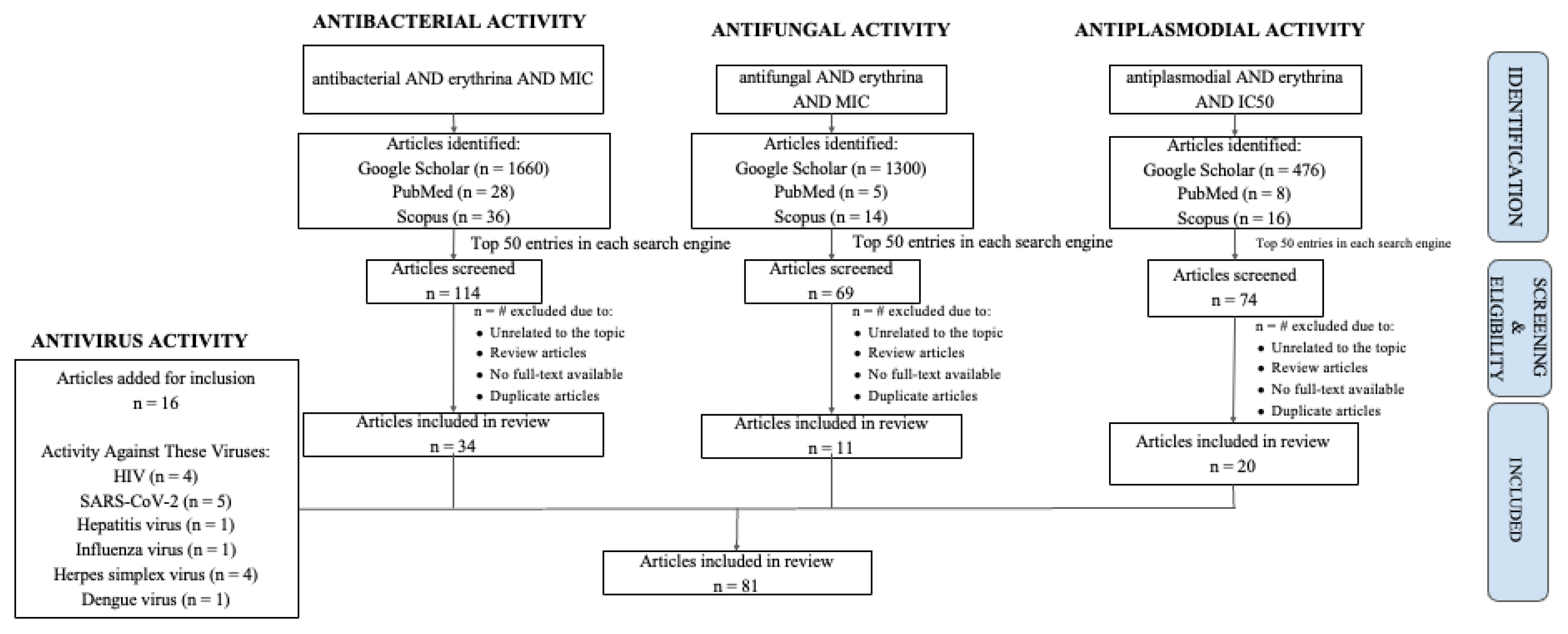
2.2. Antibacterial Activity of Erythrina
2.3. Antifungal Activity of Erythrina
2.4. Antiplasmodial Activity of Erythrina
3. Discussion
4. Methodology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Infectious Diseases. Available online: https://www.emro.who.int/health-topics/infectious-diseases/index.html (accessed on 11 September 2025).
- WHO. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 11 September 2025).
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Enoma, S.; Adewole, T.S.; Agunbiade, T.O.; Kuku, A. Antimicrobial Activities and Phylogenetic Study of Erythrina senegalensis DC (Fabaceae) Seed lectin. BioTechnologia 2023, 104, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, N.M.; Al-Sayed, E.; El-Shazly, M.; Singab, A.N. Comprehensive review on flavonoids biological activities of Erythrina plant species. Ind. Crops Prod. 2018, 123, 500–538. [Google Scholar] [CrossRef]
- Herlina, T.; Rizaldi Akili, A.W.; Nishinarizki, V.; Hardianto, A.; Latip, J.B. Review on antibacterial flavonoids from genus Erythrina: Structure-activity relationship and mode of action. Heliyon 2025, 11, e41395. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Na, M.; Dao, T.T.; Ndinteh, D.T.; Mbafor, J.T.; Park, J.; Cheong, H.; Oh, W.K. New stilbenoid with inhibitory activity on viral neuraminidases from Erythrina addisoniae. Bioorganic Med. Chem. Lett. 2010, 20, 6430–6434. [Google Scholar] [CrossRef]
- Kaushal, A.; Sharma, M.; Navneet; Sharma, M. Ethnomedicinal, phytochemical, therapeutic and pharmacological review of the genus Erythrina. Int. J. Bot. Stud. 2020, 5, 642–648. [Google Scholar]
- Lee, J.; Oh, W.; Ahn, J.; Kim, Y.; Mbafor, J.; Wandji, J.; Fomum, Z. Prenylisoflavonoids from Erythrina senegalensis as Novel HIV-1 Protease Inhibitors. Planta Medica 2008, 75, 268–270. [Google Scholar] [CrossRef] [PubMed]
- McKee, T.C.; Bokesch, H.R.; McCormick, J.L.; Rashid, M.A.; Spielvogel, D.; Gustafson, K.R.; Alavanja, M.M.; Cardellina, J.H.; Boyd, M.R. Isolation and Characterization of New Anti-HIV and Cytotoxic Leads from Plants, Marine, and Microbial Organisms. J. Nat. Prod. 1997, 60, 431–438. [Google Scholar] [CrossRef]
- Mohammed, M.M.D.; Ibrahim, N.A.; Awad, N.E.; Matloub, A.A.; Mohamed-Ali, A.G.; Barakat, E.E.; Mohamed, A.E.; Colla, P.L. Anti-HIV-1 and cytotoxicity of the alkaloids of Erythrina abyssinica Lam. growing in Sudan. Nat. Prod. Res. 2012, 26, 1565–1575. [Google Scholar] [CrossRef]
- Nkengfack, A.E.; Azebaze, A.G.B.; Waffo, A.K.; Fomum, Z.T.; Meyer, M.; van Heerden, F.R. Cytotoxic isoflavones from Erythrina indica. Phytochemistry 2001, 58, 1113–1120. [Google Scholar] [CrossRef]
- Phukhatmuen, P.; Meesakul, P.; Suthiphasilp, V.; Charoensup, R.; Maneerat, T.; Cheenpracha, S.; Limtharakul, T.; Pyne, S.G.; Laphookhieo, S. Antidiabetic and antimicrobial flavonoids from the twigs and roots of Erythrina subumbrans (Hassk.) Merr. Heliyon 2021, 7, e06904. [Google Scholar] [CrossRef]
- Tanaka, H.; Sudo, M.; Kawamura, T.; Sato, M.; Yamaguchi, R.; Fukai, T.; Sakai, E.; Tanaka, N. Antibacterial Constituents from the Roots of Erythrina herbacea against Methicillin-resistant Staphylococcus aureus. Planta Medica 2010, 76, 916–919. [Google Scholar] [CrossRef]
- Togola, A.; Hedding, B.; Theis, A.; Wangensteen, H.; Rise, F.; Smestad Paulsen, B.; Diallo, D.; Egil Malterud, K. 15-Lipoxygenase Inhibitory Effects of Prenylated Flavonoids from Erythrina senegalensis. Planta Medica 2009, 75, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- Wardani, A.K.; Mun’im, A.; Yanuar, A. Inhibition of HIV-1 Reverse Transcriptase of Selected Indonesia Medicinal Plants and Isolation of the Inhibitor from Erythrina variegata L. Leaves. J. Young Pharm. 2018, 10, 169–172. [Google Scholar] [CrossRef]
- Desta, Z.Y.; Sewald, N.; Majinda, R.R.T. New flavonoids from the stem bark of Erythrina caffra Thunb. Nat. Prod. Res. 2014, 28, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, N.M.; Al-Sayed, E.; Moghannem, S.; Azam, F.; El-Shazly, M.; Singab, A.N. Breaking Down the Barriers to a Natural Antiviral Agent: Antiviral Activity and Molecular Docking of Erythrina speciosa Extract, Fractions, and the Major Compound. Chem. Biodivers. 2020, 17, e1900511. [Google Scholar] [CrossRef]
- Hubert, D.J.; Dawe, A.; Florence, N.T.; Gilbert, K.D.W.F.; Angele, T.N.; Buonocore, D.; Finzi, P.V.; Vidari, G.; Bonaventure, N.T.; Marzatico, F.; et al. In vitro hepatoprotective and antioxidant activities of crude extract and isolated compounds from Ficus gnaphalocarpa. Inflammopharmacology 2010, 19, 35–43. [Google Scholar] [CrossRef]
- Mollel, J.T.; Said, J.S.; Masalu, R.J.; Hannoun, C.; Mbunde, M.V.N.; Nondo, R.S.O.; Bergström, T.; Trybala, E. Anti-respiratory syncytial virus and anti-herpes simplex virus activity of six Tanzanian medicinal plants with extended studies of Erythrina abyssinica stem bark. J. Ethnopharmacol. 2022, 292, 115204. [Google Scholar] [CrossRef]
- González-Lavaut, J.A.; Prieto-González, S.; Garrido-Garrido, G.; García, M.; González-Guevara, J.L.; González-García, K.L. Antiviral activity of Cuban vegetable species. Pharmacologyonline 2006, 3, 527–530. [Google Scholar]
- Akter, K.; Barnes, E.C.; Loa-Kum-Cheung, W.L.; Yin, P.; Kichu, M.; Brophy, J.J.; Barrow, R.A.; Imchen, I.; Vemulpad, S.R.; Jamie, J.F. Antimicrobial and antioxidant activity and chemical characterisation of Erythrina stricta Roxb. (Fabaceae). J. Ethnopharmacol. 2016, 185, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Chukwujekwu, J.C.; Van Heerden, F.R.; Van Staden, J. Antibacterial activity of flavonoids from the stem bark of Erythrina caffra thunb. Phytother. Res. 2010, 25, 46–48. [Google Scholar] [CrossRef]
- Djeussi, D.E.; Sandjo, L.P.; Noumedem, J.A.K.; Omosa, L.K.; T. Ngadjui, B.; Kuete, V. Antibacterial activities of the methanol extracts and compounds from Erythrina sigmoidea against Gram-negative multi-drug resistant phenotypes. BMC Complement. Altern. Med. 2015, 15, 453. [Google Scholar] [CrossRef] [PubMed]
- Ilodigwe, E. Wound Healing Activity of Ethanol Leaf Extract of Erythrina senegalensis. Br. J. Pharm. Res. 2014, 4, 531–540. [Google Scholar] [CrossRef]
- Innok, P.; Rukachaisirikul, T.; Phongpaichit, S.; Suksamrarn, A. Fuscacarpans A–C, new pterocarpans from the stems of Erythrina fusca. Fitoterapia 2010, 81, 518–523. [Google Scholar] [CrossRef]
- Jambwa, P.; Nkadimeng, S.M.; Mudimba, T.N.; Matope, G.; McGaw, L.J. Antibacterial and anti-inflammatory activity of plant species used in traditional poultry ethnomedicine in Zimbabwe: A first step to developing alternatives to antibiotic poultry feed additives. J. Ethnopharmacol. 2023, 300, 115687. [Google Scholar] [CrossRef]
- Kwesiga, G.; Kelling, A.; Kersting, S.; Sperlich, E.; von Nickisch-Rosenegk, M.; Schmidt, B. Total Syntheses of Prenylated Isoflavones from Erythrina sacleuxii and Their Antibacterial Activity: 5-Deoxy-3′-prenylbiochanin A and Erysubin F. J. Nat. Prod. 2020, 83, 3445–3453. [Google Scholar] [CrossRef]
- Olufunmiso, O.; Afolayan, A. In Vitro Antibacterial and Time-Kill Evaluation of the Erythrina caffra Thunb. Extract against Bacteria Associated with Diarrhoea. Sci. World J. 2012, 2012, 738314. [Google Scholar] [CrossRef]
- Ombito, J.O.; Bojase, G.; Majinda, R.; B. Masesane, I.; Schüffler, A.; Pusch, S.; Weber, C.; Opatz, T. Chemical constituents of the root wood of Erythrina sacleuxii and determination of the absolute configuration of suberectin. Bull. Chem. Soc. Ethiop. 2020, 34, 135–140. [Google Scholar] [CrossRef]
- Peleyeju, G.B.; Emmanuel, T.; Tata, C.M.; Djuidje Fotsing, M.C.; Niemann, N.; Rhyman, L.; Arderne, C.; Ndinteh, D.T.; Ramasami, P. Crystal structure and antibacterial activity of scandenone (warangalone) from Erythrina plants. J. Mol. Struct. 2019, 1191, 43–51. [Google Scholar] [CrossRef]
- Roumy, V.; Gutierrez-Choquevilca, A.-L.; Lopez Mesia, J.; Ruiz, L.; Ruiz Macedo, J.; Abedini, A.; Landoulsi, A.; Samaillie, J.; Hennebelle, T.; Rivière, C.; et al. In vitro antimicrobial activity of traditional plant used in mestizo shamanism from the Peruvian amazon in case of infectious diseases. Pharmacogn. Mag. 2015, 11, 625. [Google Scholar] [CrossRef]
- Rukachaisirikul, T.; Innok, P.; Suksamrarn, A. Erythrina Alkaloids and a Pterocarpan from the Bark of Erythrina subumbrans. J. Nat. Prod. 2008, 71, 156–158. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Oliveira, T.B.; Khumalo, G.P.; Vuuren, S.F.V.; van Wyk, B.E. Antimicrobial Isoflavones and Derivatives from Erythrina (Fabaceae): Structure Activity Perspective (Sar & Qsar) on Experimental and Mined Values Against Staphylococcus aureus. Antibiotics 2020, 9, 223. [Google Scholar] [CrossRef]
- Sato, M.; Tanaka, H.; Oh-Uchi, T.; Fukai, T.; Etoh, H.; Yamaguchi, R. Antibacterial activity of phytochemicals isolated from Erythrina zeyheri against vancomycin-resistant enterococci and their combinations with vancomycin. Phytother. Res. 2004, 18, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Tanaka, H.; Yamaguchi, R.; Oh-Uchi, T.; Etoh, H. Erythrina poeppigiana-derived phytochemical exhibiting antimicrobial activity against Candida albicans and methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2003, 37, 81–85. [Google Scholar] [CrossRef]
- Schultz, F.; Osuji, O.F.; Wack, B.; Anywar, G.; Garbe, L.-A. Antiinflammatory Medicinal Plants from the Ugandan Greater Mpigi Region Act as Potent Inhibitors in the COX-2/PGH2 Pathway. Plants 2021, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Atsumi, I.; Shirota, O.; Sekita, S.; Sakai, E.; Sato, M.; Murata, J.; Murata, H.; Darnaedi, D.; Chen, I.S. Three New Constituents from the Roots of Erythrina variegata and Their Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus. Chem. Biodivers. 2011, 8, 476–482. [Google Scholar] [CrossRef]
- Tanaka, H.; Sato, M.; Oh-Uchi, T.; Yamaguchi, R.; Etoh, H.; Shimizu, H.; Sako, M.; Takeuchi, H. Antibacterial properties of a new isoflavonoid from Erythrina poeppigiana against methicillin-resistant Staphylococcus aureus. Phytomedicine 2004, 11, 331–337. [Google Scholar] [CrossRef]
- Wintola, O.; Olufunmiso, O.; Afolayan, A. Brine Shrimps Toxicity and In vitro Antibacterial Potentials of the Crude Methanolic Stem Bark Extract of Erythrina caffra thunb. against Bacteria in Infections. 2015, 8, 4325–4334. [Google Scholar]
- Harley, B.K.; Quagraine, A.M.; Neglo, D.; Aggrey, M.O.; Orman, E.; Mireku-Gyimah, N.A.; Amengor, C.D.; Jato, J.; Saaka, Y.; Fleischer, T.C. Metabolite profiling, antifungal, biofilm formation prevention and disruption of mature biofilm activities of Erythrina senegalensis stem bark extract against Candida albicans and Candida glabrata. PLoS ONE 2022, 17, e0278096. [Google Scholar] [CrossRef]
- Sivalingam, A.M.; Pandian, A. Identification and characterization of silver nanoparticles from Erythrina indica and its antioxidant and Uropathogenic antimicrobial properties. Microb. Pathog. 2024, 190, 106635. [Google Scholar] [CrossRef]
- de Ávila, J.M.; Dalcol, I.I.; Pereira, A.O.; Santos, E.W.; Ferraz, A.; Santos, M.Z.; Mostardeiro, M.A.; Morel, A.F. Antimicrobial Evaluation of Erythrinan Alkaloids from Erythrina cristagalli L. Med. Chem. 2018, 14, 784–790. [Google Scholar] [CrossRef]
- Motsei, M.L.; Lindsey, K.L.; van Staden, J.; Jäger, A.K. Screening of traditionally used South African plants for antifungal activity against Candida albicans. J. Ethnopharmacol. 2003, 86, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ousmane, C.; Cyrille, G.K.R.; Lassina, S.P.; Karamoko, O. Antifungal Activities of Erythrina senegalensis Leaves Partitioned Extracts on the Germs Responsible for Opportunistic Cryptococcosis of HIV/AIDS. Sch. Acad. J. Biosci. 2023, 11, 455–460. [Google Scholar] [CrossRef]
- Surapuram, V.; Setzer, W.N.; McFeeters, R.L.; McFeeters, H. Antifungal Activity of Plant Extracts against Aspergillus niger and Rhizopus stolonifer. Nat. Product. Commun. 2014, 9, 1603–1605. [Google Scholar] [CrossRef]
- Begum, S.; Munissi, J.J.E.; Buriyo, A.S.; Makangara, J.J.; Lucantoni, L.; Avery, V.M.; Erdelyi, M.; Nyandoro, S.S. Antiplasmodial, Antimicrobial and Cytotoxic Activities of Extracts from Selected Medicinal Plants Growing in Tanzania. J. Biol. Act. Prod. Nat. 2020, 10, 165–176. [Google Scholar] [CrossRef]
- Waiganjo, B.; Moriasi, G.; Onyancha, J.; Elias, N.; Muregi, F. Antiplasmodial and Cytotoxic Activities of Extracts of Selected Medicinal Plants Used to Treat Malaria in Embu County, Kenya. J. Parasitol. Res. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Zarev, Y.; Foubert, K.; Cos, P.; Maes, L.; Elgorashi, E.; Apers, S.; Ionkova, I.; Pieters, L. HPLC-DAD-SPE-NMR isolation of tetracyclic spiro-alkaloids with antiplasmodial activity from the seeds of Erythrina latissima. Nat. Prod. Res. 2019, 34, 1037–1040. [Google Scholar] [CrossRef]
- Andayi, A.W.; Yenesew, A.; Derese, S.; Midiwo, J.O.; Gitu, P.M.; Jondiko, O.J.; Akala, H.; Liyala, P.; Wangui, J.; Waters, N.C.; et al. Antiplasmodial Flavonoids from Erythrina sacleuxii. Planta Medica 2006, 72, 187–189. [Google Scholar] [CrossRef]
- Tjahjandarie, T.S.; Tanjung, M.; Rahmania, D.F.; Rhidoma, C.I.; Saputri, R.D. Calodioscurins A and B, two new isoprenylated xanthones from the stem bark of Calophyllum dioscurii P.F. Stevens. Nat. Prod. Res. 2019, 35, 1153–1158. [Google Scholar] [CrossRef]
- Jemimah Sandra, T.N.; Christelle Nadia, N.A.; Cedric, Y.; Guy-Armand, G.N.; Azizi, M.A.; Aboubakar Sidiki, N.N.; Alex Kevin, T.D.; Payne, V.K.; Hu, H. In vitro and in vivo antimalarial activities of the ethanol extract of Erythrina sigmoidea stem bark used for the treatment of malaria in the Western Region of Cameroon. Front. Parasitol. 2024, 3, 1359442. [Google Scholar] [CrossRef]
- Kamanzi Atindehou, K.; Schmid, C.; Brun, R.; Koné, M.W.; Traore, D. Antitrypanosomal and antiplasmodial activity of medicinal plants from Côte d’Ivoire. J. Ethnopharmacol. 2004, 90, 221–227. [Google Scholar] [CrossRef]
- Sazed, S.A.; Islam, O.; Bliese, S.L.; Hossainey, M.R.H.; Soma, M.A.; Rashid, M.A.; Rahman, M.S.; Alam, M.S. Phytochemical, Biological and Computational Investigations of Erythrina fusca Lour. to Assess Antimalarial Property against Plasmodium falciparum. Preprints 2020, 2020100576. [Google Scholar] [CrossRef]
- Yenesew, A.; Akala, H.M.; Twinomuhwezi, H.; Chepkirui, C.; Irungu, B.N.; Eyase, F.L.; Kamatenesi-Mugisha, M.; Kiremire, B.T.; Johnson, J.D.; Waters, N.C. The antiplasmodial and radical scavenging activities of flavonoids of Erythrina burttii. Acta Trop. 2012, 123, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Aboelmagd, m.; Said, A.; Ross, S.; Haggag, E. A Novel Compound and Biological Evaluation of Phytoconstituents Isolated from Erythrina corallodendron L. Flowers. J. Adv. Pharm. Res. 2018, 2, 247–255. [Google Scholar] [CrossRef]
- Christopher, R.; Msonga, A.; Hoppe, H.C.; Boyom, F.F. Ethanol Extracts from Selected Tanzanian Medicinal Plants Selectively Inhibit Plasmodium falciparum Growth In Vitro. Tanzan. J. Sci. 2023, 49, 41–47. [Google Scholar] [CrossRef]
- Tati, H.; Euis, J.; Nurlelasari, N.; Dikdik, K.; Unang, S. Potential of Dadap Ayam (Erythrina variegata) Plant as Herbal Medicine. J. Med. Planta 2011, 1, 245902. [Google Scholar]
- Pino, S.; González, J.; González, J.; Garrido, G.; García, M.; Carballo, M.; Echemendía, O.; Urquiola, A.; Rastrelli, L.; Molina-Torres, J.; et al. Preliminary phytochemical screening and in vitro antiherpetic activity of Erythrina fusca Lour. Am. J. Pharm. 2004, 23, 453–458. [Google Scholar]
- Rasool, N.; Ashraf, A.; Waseem, M.; Hussain, W.; Mahmood, S. Computational exploration of antiviral activity of phytochemicals against NS2B/NS3 proteases from dengue virus. Turk. J. Biochem. 2018, 44, 261–277. [Google Scholar] [CrossRef]
- Tanaka, H.; Atsumi, I.; Hasegawa, M.; Hirata, M.; Sakai, T.; Sato, M.; Yamaguchi, R.; Tateishi, Y.; Tanaka, T.; Fukai, T. Two New Isoflavanones from the Roots of Erythrina variegata. Nat. Prod. Commun. 2015, 10, 499–501. [Google Scholar] [CrossRef]
- Herlina, T.; Nishinarizki, V.; Akili, A.W.R.; Hardianto, A.; Gaffar, S.; Muchtaridi, M.; Latip, J. Exploring Erythrina flavonoids as potential SARS-CoV-2 RdRp inhibitors through virtual screening, in silico ADMET evaluation, and molecular dynamics simulation studies. Sci. Rep. 2025, 15, 14259. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Mao, C.; Luan, X.; Shen, D.D.; Shen, Q.; Su, H.; Wang, X.; Zhou, F.; Zhao, W.; Gao, M.; et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 2020, 368, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, T.; Saekee, A.; Tharibun, C.; Watkuolham, S.; Suksamrarn, A. Biological Activities of the Chemical Constituents of Erythrina stricta and Erythrina subumbrans. Arch. Pharmacal Res. 2007, 30, 1398–1403. [Google Scholar] [CrossRef]
- Sato, M.; Tanaka, H.; Tani, N.; Nagayama, M.; Yamaguchi, R. Different antibacterial actions of isoflavones isolated from Erythrina poeppigiana against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2006, 43, 243–248. [Google Scholar] [CrossRef]
- Sato, M.; Tanaka, H.; Yamaguchi, R.; Kato, K.; Etoh, H. Synergistic effects of mupirocin and an isoflavanone isolated from Erythrina variegata on growth and recovery of methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2004, 24, 241–246. [Google Scholar] [CrossRef]
- Tanaka, H.; Hirata, M.; Etoh, H.; Sako, M.; Sato, M.; Murata, J.; Murata, H.; Darnaedi, D.; Fukai, T. Six New Constituents from the Roots of Erythrina variegata. Chem. Biodivers. 2004, 1, 1101–1108. [Google Scholar] [CrossRef]
- Khumalo, G.P.; Sadgrove, N.J.; Van Vuuren, S.F.; Van Wyk, B.-E. South Africa’s Best BARK Medicines Prescribed at the Johannesburg Muthi Markets for Skin, Gut, and Lung Infections: MIC’s and Brine Shrimp Lethality. Antibiotics 2021, 10, 681. [Google Scholar] [CrossRef]
- Sato, M.; Tanaka, H.; Fujiwara, S.; Hirata, M.; Yamaguchi, R.; Etoh, H.; Tokuda, C. Antibacterial property of isoflavonoids isolated from Erythrina variegata against cariogenic oral bacteria. Phytomedicine 2003, 10, 427–433. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; McGaw, L.J.; Eloff, J.N. In vitro antibacterial, antioxidant and cytotoxic activity of acetone leaf extracts of nine under-investigated Fabaceae tree species leads to potentially useful extracts in animal health and productivity. BMC Complement. Altern. Med. 2014, 14, 147. [Google Scholar] [CrossRef]
- Redko, F.; Clavin, M.L.; Weber, D.; Ranea, F.; Anke, T.; Martino, V. Antimicrobial Isoflavonoids from Erythrina crista galli Infected with Phomopsis sp. Z. Für Naturforschung C 2007, 62, 164–168. [Google Scholar] [CrossRef]
- Bunalema, L.; Kirimuhuzya, C.; Tabuti, J.R.; Waako, P.; Magadula, J.J.; Otieno, N.; Orodho, J.A.; Okemo, P. The efficacy of the crude root bark extracts of Erythrina abyssinica on rifampicin resistant Mycobacterium tuberculosis. Afr. Health Sci. 2011, 11, 587–593. [Google Scholar]
- Mabona, U.; Viljoen, A.; Shikanga, E.; Marston, A.; Van Vuuren, S. Antimicrobial activity of southern African medicinal plants with dermatological relevance: From an ethnopharmacological screening approach, to combination studies and the isolation of a bioactive compound. J. Ethnopharmacol. 2013, 148, 45–55. [Google Scholar] [CrossRef]
- Bedane, K.G.; Kusari, S.; Eckelmann, D.; Masesane, I.B.; Spiteller, M.; Majinda, R.R.T. Erylivingstone A–C with antioxidant and antibacterial activities from Erythrina livingstoniana. Fitoterapia 2015, 105, 113–118. [Google Scholar] [CrossRef]
- Kgakatsi, N.A.; Majinda, R.R.T.; Masesane, I.B.; Nwamadi, M.S.; Demissie, T.B.; Ombito, J.O.; Gobe, I. New isoflavan from Erythrina livingstoniana. Nat. Prod. Res. 2022, 38, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Tadjong Tcho, A.; Djouaka Bavoua, J.L.; Ngouonpe Wembe, A.; Gompe Bobda, E.G.; Majoumo, F.; Leuche, J.M.; Ndonfack Tiofack, E.; Mbah, J.A.; Toze, F.A.A. New flavanone and other constituents from Erythrina senegalensis A. DC. (fabaceae). Nat. Product. Res. 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chukwujekwu, J.C.; de Kock, C.A.; Smith, P.J.; Van Heerden, F.R.; Van Staden, J. Antiplasmodial activity of compounds isolated from Erythrina caffra. South Afr. J. Bot. 2016, 106, 101–103. [Google Scholar] [CrossRef]
- Nondo, R.S.O.; Moshi, M.J.; Erasto, P.; Masimba, P.J.; Machumi, F.; Kidukuli, A.W.; Heydenreich, M.; Zofou, D. Anti-plasmodial activity of Norcaesalpin D and extracts of four medicinal plants used traditionally for treatment of malaria. BMC Complement. Altern. Med. 2017, 17, 167. [Google Scholar] [CrossRef]
- Prozesky, E.A.; Meyer, J.J.M.; Louw, A.I. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected South African plants. J. Ethnopharmacol. 2001, 76, 239–245. [Google Scholar] [CrossRef]
- Ajaiyeoba, E.; Ashidi, J.; Abiodun, O.; Okpako, L.; Ogbole, O.; Akinboye, D.; Falade, C.; Bolaji, O.; Gbotosho, G.; Falade, M.; et al. Antimalarial Ethnobotany: In Vitro. Antiplasmodial Activity of Seven Plants Identified in the Nigerian Middle Belt. Pharm. Biol. 2005, 42, 588–591. [Google Scholar] [CrossRef]
- Ali, H.; König, G.M.; Khalid, S.A.; Wright, A.D.; Kaminsky, R. Evaluation of selected Sudanese medicinal plants for their in vitro activity against hemoflagellates, selected bacteria, HIV-1-RT and tyrosine kinase inhibitory, and for cytotoxicity. J. Ethnopharmacol. 2002, 83, 219–228. [Google Scholar] [CrossRef]
- Onyango, D.W.; Midiwo, J.O. In vivo Evaluation of Anti-malarial Activity of Stem and Root Extracts of Erythrina abyssinica. Eur. J. Med. Plants 2019, 27, 1–5. [Google Scholar] [CrossRef]
- Halloran, M.E.; Longini, I.M., Jr. Emerging, evolving, and established infectious diseases and interventions. Science 2014, 345, 1292–1294. [Google Scholar] [CrossRef]
- Shyr, Z.A.; Cheng, Y.S.; Lo, D.C.; Zheng, W. Drug combination therapy for emerging viral diseases. Drug Discov. Today 2021, 26, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Vere Hodge, R.A.; Field, H.J. Antiviral agents for herpes simplex virus. Adv. Pharmacol. 2013, 67, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Pujol, J.; Puertas, M.C.; Martinez-Picado, J.; Morón-López, S. Targeting Viral Transcription for HIV Cure Strategies. Microorganisms 2024, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Vidya Vijayan, K.K.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front. Immunol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Chen, P.; Zou, F.; Liu, W. Recent advancement in prevention against hepatotoxicity, molecular mechanisms, and bioavailability of gallic acid, a natural phenolic compound: Challenges and perspectives. Front. Pharmacol. 2025, 16, 1549526. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Nuwarda, R.F.; Ikram, E.H.K.; Abdul Rahim, A.S.; Gazzali, A.M.; Wahab, H.A. Neuraminidase Inhibitor of Garcinia atroviridis L. Fruits and Leaves Using Partial Purification and Molecular Characterization. Molecules 2022, 27, 949. [Google Scholar] [CrossRef]
- Ariefta, N.R.; Narita, K.; Murata, T.; Nishikawa, Y. Evaluation of the antiplasmodial efficacy of synthetic 2,5-diphenyloxazole analogs of compounds naturally derived from Oxytropis lanata. Int. J. Parasitol. Drugs Drug Resist. 2024, 25, 100540. [Google Scholar] [CrossRef]
- Hodnick, W.F.; Milosavljević, E.B.; Nelson, J.H.; Pardini, R.S. Electrochemistry of flavonoids. Relationships between redox potentials, inhibition of mitochondrial respiration, and production of oxygen radicals by flavonoids. Biochem. Pharmacol. 1988, 37, 2607–2611. [Google Scholar] [CrossRef]
- Pal, C. Redox modulating small molecules having antimalarial efficacy. Biochem. Pharmacol. 2023, 218, 115927. [Google Scholar] [CrossRef]
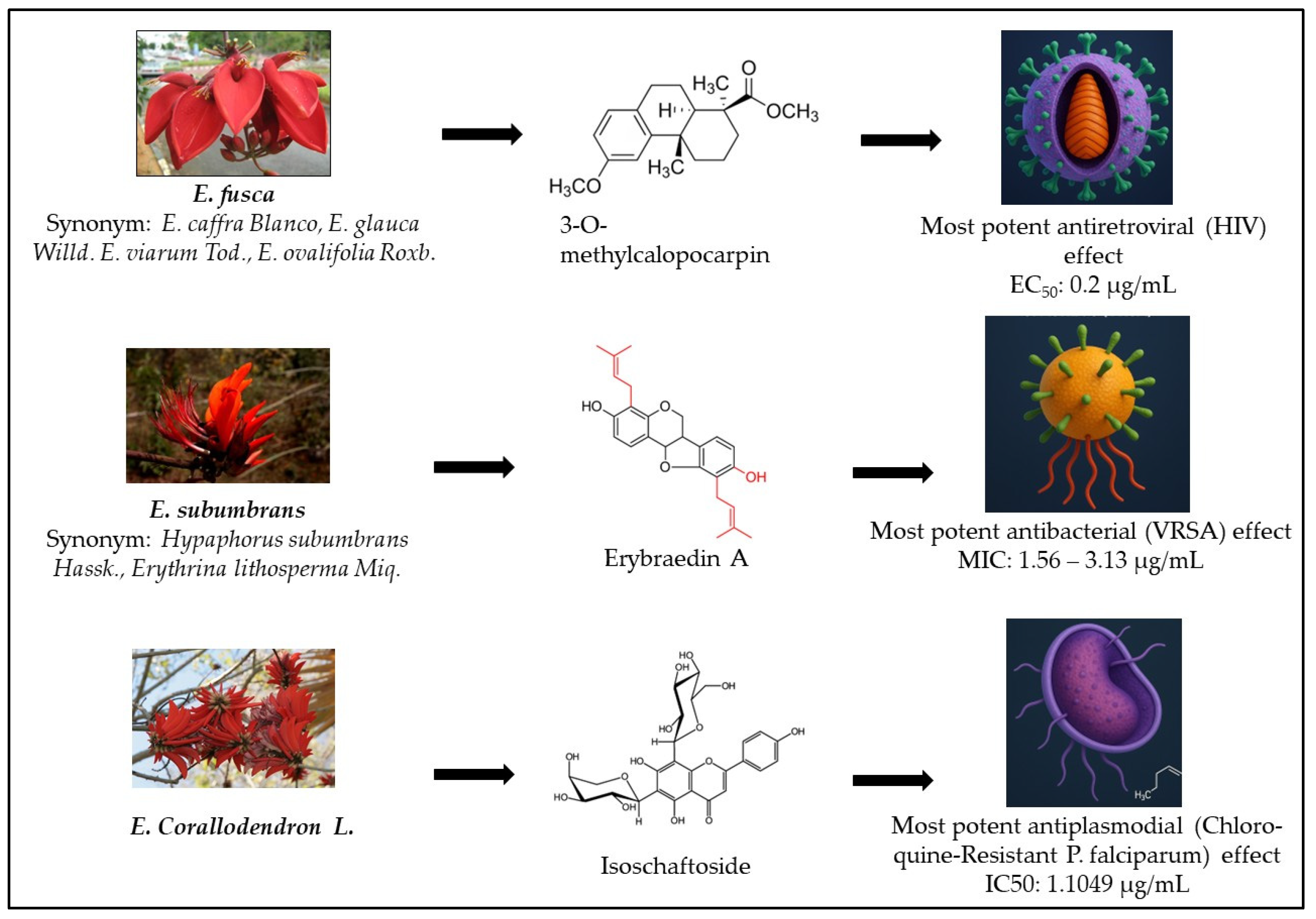
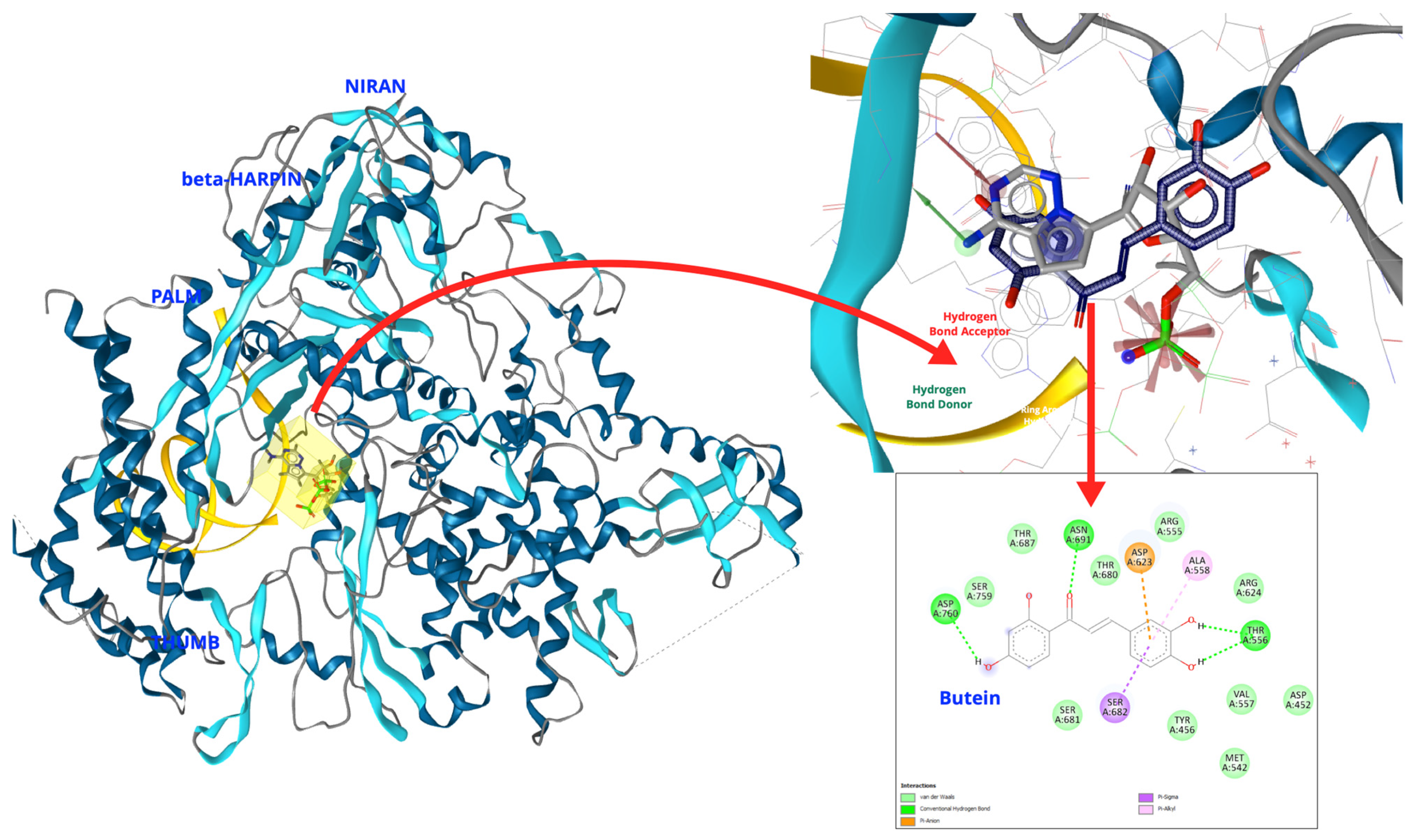
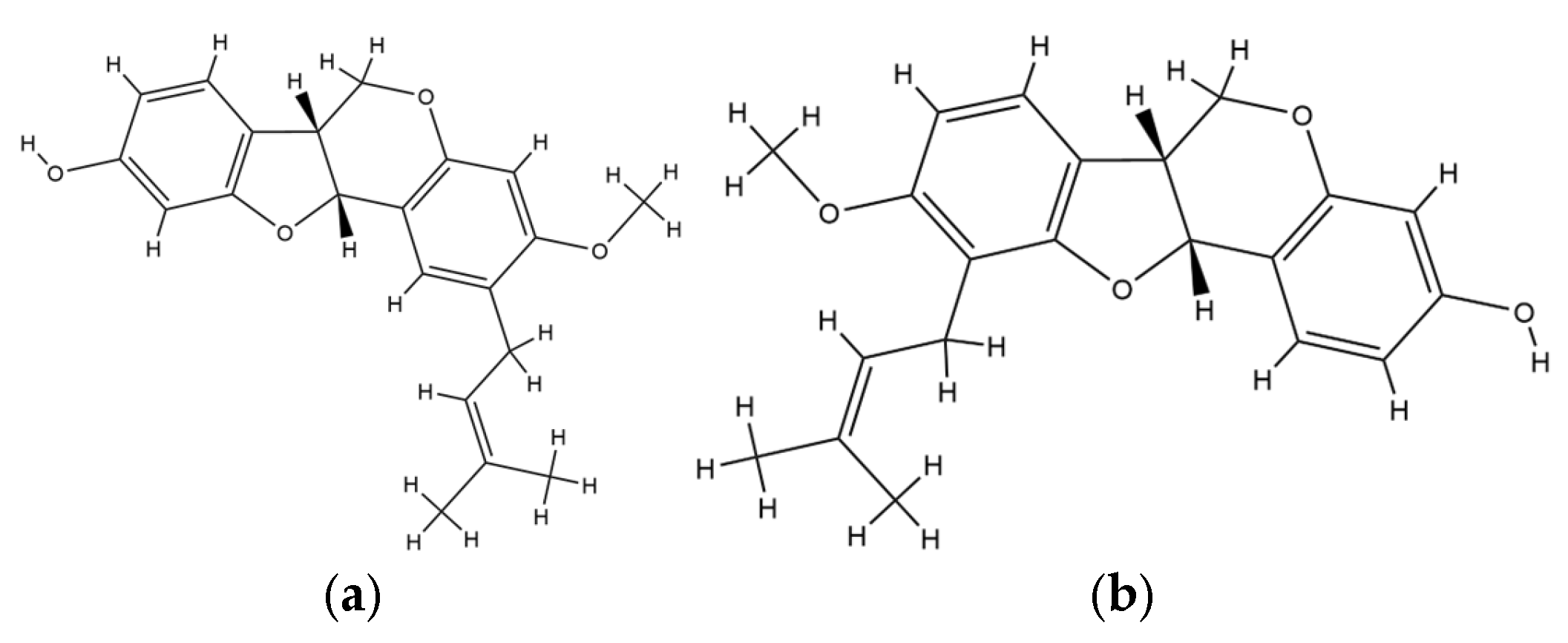
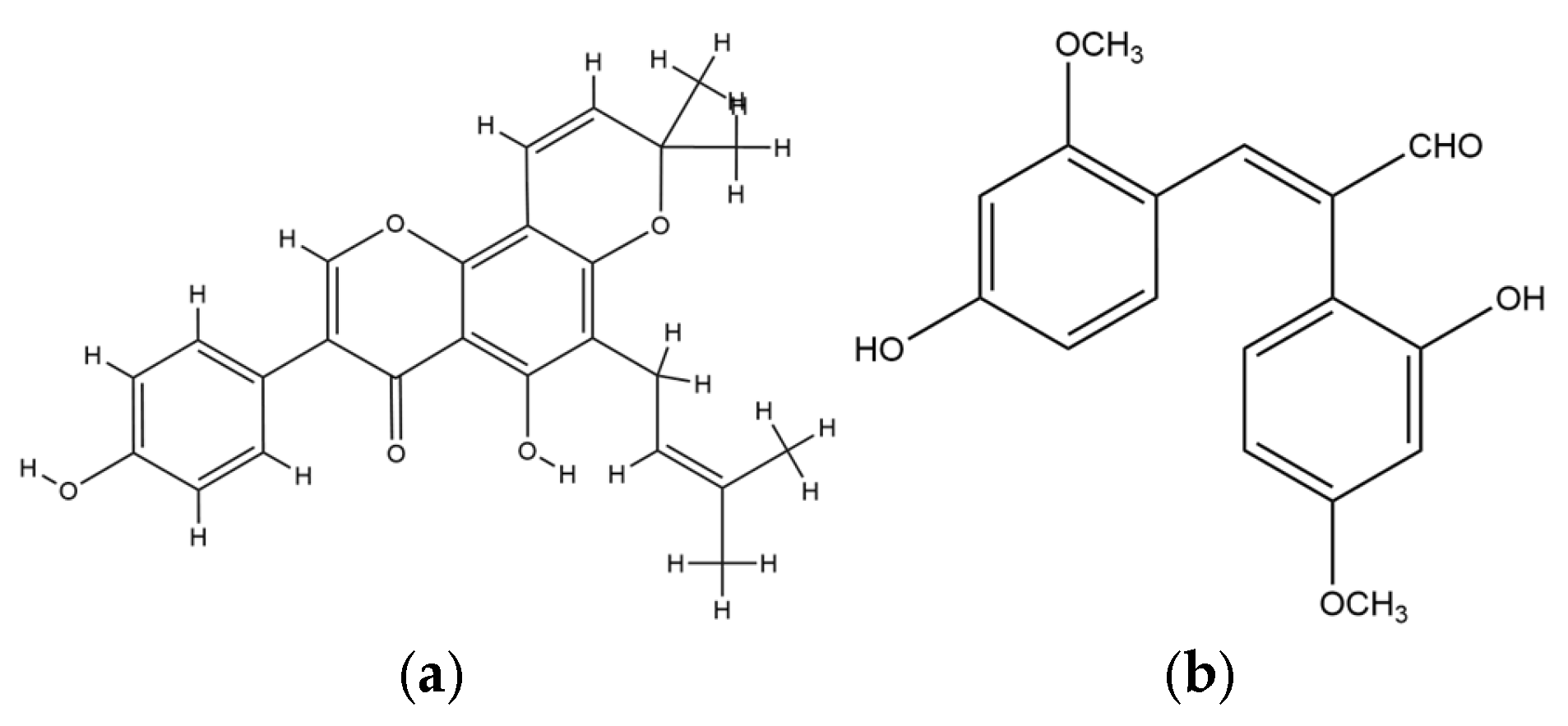
| No | Species | Compounds/Extracts | Virus | Ref. |
|---|---|---|---|---|
| 1 | E. glauca | Sandwicensin (EC50: 2 µg/mL) 3-O-methylcalopocarpin (EC50: 0.2 µg/mL) | HIV | [11] |
| 2 | E. lysistemon | 5-deoxyglyasperin F (EC50: 11.5 µg/mL) 2′-hydroxyneobavaisoflavanone (EC50: 7.6 µg/mL) | HIV | [11] |
| 3 | E. abbysinica | Crude alkaloid fraction (EC50: 53 µg/mL). The compounds identified were erythraline, erysodine, erysotrine, 8-oxoerythraline, and 11-methoxyerysodine | HIV | [12] |
| 4 | E. variegata | Apigetrin (EC50: 100.59 µg/mL) | HIV | [17] |
| 5 | E. senegalensis | Auriculatin (EC50: 1.47 µg/mL) Erysenegalensein O (EC50: 2.19 µg/mL) Erysenegalensein D (EC50: 1.10 µg/mL) Erysenegalensein N (EC50: 1.97 µg/mL) 6.8-diprenylgenistein (EC50: 0.203 µg/mL) | HIV | [10] |
| 6 | E. subumbrans | Gangetinin | SARS-CoV-2 | [14] |
| 7 | E. sigmoidea | Gangetin | SARS-CoV-2 | [13] |
| 8 | E. senagelensis | Erybraedin D | SARS-CoV-2 | [16] |
| 9 | E. variegata | Orientanol E | SARS-CoV-2 | [62] |
| 10 | E. caffra | Erycaffra F | SARS-CoV-2 | [18] |
| 11 | E. speciosa | Vitexin (EC50: 125 µg/mL) | Hepatitis Virus | [19] |
| 12 | E. senegalensis | 2. 3-dihydro-2′-hydroxyosajin (EC50: 71.8 ± 1.45 µg/mL) osajin (67.54 ± 3.56 µg/mL) 6.8-diprenylgenistein (69.41 ± 2.56 µg/mL) | Hepatitis Virus | [20] |
| 13 | E. addisonae | Erythradisson B (EC50: 8.8 µg/mL) Licoagrochalcone (EC50: 21.51 µg/mL) Abyssinone VI (EC50: 26.44 µg/mL) 5-prenylbutein (EC50: 21.93 µg/mL) | Influenza | [8] |
| 14 | E. abbysinica | Stem bark aqueous extract (IC50: 27 µg/mL) | HSV | [21] |
| 15 | E. speciosa | Vitexin (EC50: 64 µg/mL) | HSV | [19] |
| 16 | E. fusca | Stem bark decoction (EC50: 243 ± 10.9 µg/mL) | HSV | [15] |
| 17 | E. poeppigiana | Stem bark decoction (EC50: 147.6 ± 4.3 µg/mL) | HSV | [22] |
| 18 | E. variegata | Ericristagallin, Osajin, Sigmoidin A, Sigmoidin B, Sigmoidin C, Robustone, Abyssinone, Eryvarin A, Eryvarin B, Euchrenone, Lupiwighteone, Laburnetine | Dengue Virus | [61] |
| Bacteria | Erythrina | Compounds | MIC (µg/mL) | Ref |
|---|---|---|---|---|
| Staphylococcus | ||||
| S. aureus | E. caffra | 6.8-diprenylgenistein | 7.8 | [24] |
| Ethanol bark extract (specific compounds are unknown) | 39.1 | [30] | ||
| Methanol bark extract (specific compounds are unknown) | 0.313 | [41] | ||
| E. lysistemon | Erybraedin A | 2 | [35] | |
| Phaseollidin | 10 | |||
| Abyssinone V-4′-methyl-ether | 59 | |||
| Eryzerin C | 5 | |||
| Alpumisoflavone | 31 | |||
| Lysisteisoflavone | 62 | |||
| E. senegalensis | Ethanol leaf extract (specific compounds are unknown) | 25 | [35] | |
| E. stricta | Dichloromethane bark extract (specific compounds are unknown) | 7.81 | [23] | |
| E. fusca | Sandwicensin | 8 | [27] | |
| Erythrabbysin A | 64 | |||
| Erythrabbysin I | 64 | |||
| Eryvarin D | 4 | |||
| Scandeone | 8 | |||
| E. abyssinica | Methanol bark extract (specific compounds are unknown) | 23 | [28] | |
| Ethanol bark extract (specific compounds are unknown) | 62.5 | [38] | ||
| Ethyl acetate bark extract (specific compounds are unknown) | 83.3 | |||
| E. amazonia | Methanol bark extract (specific compounds are unknown) | 75 | [33] | |
| E. addisonae | Scandenone | 64 | [32] | |
| Methicillin-Resistant S. aureus (MRSA) | E. subumbrans | Ericristagallin | 0.39–1.56 | [65] |
| E. poeppigiana | Eryvarin D | 12.5 | [40] | |
| 3.9-dihydroxy-10-γ,γ- dimethylallyl- 6a,11adehydropterocarpan | 12.5 | |||
| Isolupalpigenin | 1.56–3.13 | [66] | ||
| Erythrinin B | 6.25 | |||
| Erypostyrene | 6.25 | [37] | ||
| Sandwicensin | 6.25–12.5 | |||
| Erypoegin A | 25 | |||
| Dimethylmedicarpin | 50 | |||
| Angolensin | 50 | |||
| E. sacleuxii | Erysubin F | 15.4 | [29] | |
| 7.4′-dihydroxy-8.3′-diprenylflavone | 20.5 | |||
| E. variegata | Eryvrain V | 12.5–25 | [39] | |
| Eryvarin W | 1.56–3.13 | |||
| Eryvarin X | 0.78–1.56 | |||
| Bidwillon B | 3.13–6.25 | [67] | ||
| Eryvarin Q | 3.13–6.25 | [68] | ||
| E. stricta | Dichloromethane bark extract (specific compounds are unknown) | 31.25 | [23] | |
| E. herbacea | Erybacin A | 50 | [15] | |
| Erybacin B | 12.5 | |||
| Eryvariestyrene | 12.5 | |||
| Glyasperin F | 50 | |||
| Bidwillol A | 12.5 | |||
| Phaseollinisoflavan | 50 | |||
| Erythbidin | 50 | |||
| Phaseollidin isoflavan | 12.5 | |||
| Eryvarin L | 25 | |||
| Glabrocoumarone A | 12.5 | |||
| E. fusca | Sandwicensin | 16 | [27] | |
| Erythrabbysin A | 32 | |||
| Erythrabbysin I | 64 | |||
| Eryvarin D | 4 | |||
| Vancomycin-Resistant S. aureus (VRSA) | E. subumbrans | Erycristagallin | 0.39–1.56 | [65] |
| E. zeyheri | Erybraedin A | 1.56–3.13 | [36] | |
| Eryzerin A | 12.5–25 | |||
| Eryzerin C | 6.25 | |||
| Eryzerin D | 12.5 | |||
| Eryzerin E | 12.5 | |||
| Multidrug-Resistant S. aureus (MDRSA) | E. stricta | Dichloromethane bark extract (specific compounds are unknown) | 31.25 | [23] |
| S. epidermidis | E. lysistemon | Dichloromethane bark extract (specific compounds are unknown) | 40 | [69] |
| Erybraedin A | 2 | [35] | ||
| Phaseollidin | 5 | |||
| Eryzerin C | 2 | |||
| Lysisteisoflavone | 26 | |||
| E. addisonae | Scandenone | 32 | [32] | |
| Actinomyces | ||||
| A. viscosus | E. variegata | 2-(γ,γ-dimethylallyl)-6a-hydroxyphaseollidin | 3.13 | [70] |
| Erystagallin A | 3.13 | |||
| Erycristagallin | 1.56 | |||
| Micrococcus | ||||
| M. luteus | E. caffra | Ethanol bark extract (specific compounds are unknown) | 39.1 | [30] |
| Methanol bark extract (specific compounds are unknown) | 0.156 | [41] | ||
| Enterococcus | ||||
| E. faecalis | E. caffra | Ethanol bark extract (specific compounds are unknown) | 39.1 | [30] |
| Methanol bark extract (specific compounds are unknown) | 0.156 | [41] | ||
| Acetone leaf extract (specific compounds are unknown) | 80 | [71] | ||
| E. addisonae | Scandenone | 64 | [32] | |
| Bacillus | ||||
| B. cereus | E. lysistemon | Erybraedin A | 1 | [35] |
| Phaseollidin | 10 | |||
| Abyssinone V-4′-methyl-ether | 26 | |||
| Eryzerin C | 10 | |||
| Alpumisoflavone | 31 | |||
| Lysisteisoflavone | 2 | |||
| E. caffra | Methanol bark extract (specific compounds are unknown) | 78 | [41] | |
| E. addisonae | Scandenone | 16 | [32] | |
| B. pumilus | E. caffra | Methanol bark extract (specific compounds are unknown) | 39.1 | [41] |
| B. brevis | E. cristagalli | Coumestrol | 4.4 | [72] |
| Genistein | 13.5 | |||
| Daidzein | 35 | |||
| B subtilis | E. addisonae | Scandenone | 16 | [32] |
| Mycobacterium | ||||
| M. tuberculosis | E. stricta | Erythrabbysin II | 50 | [65] |
| Erystagallin A | 12.5 | |||
| Erythrabbysin-1 | 50 | |||
| 5-hydroxysophoranone | 12.5 | |||
| Sandwicensin | 50 | |||
| E. subumbrans | Erybraedin A | 25 | ||
| Erythrabbysin II | 50 | |||
| Erystagallin A | 12.5 | |||
| Erythrabbysin-1 | 50 | |||
| Erycristagallin | 12.5 | |||
| 5-hydroxysophoranone | 12.5 | |||
| Erysubin F | 12.5 | |||
| Rifampicin-Resistant M. tuberculosis | E. abyssinica | Methanol bark extract (specific compounds are unknown) | 390 | [73] |
| M. smegmatis | E. addisonae | Scandenone | 100 | [32] |
| Propionibacterium | ||||
| P. acnes | E. lysistemon | Methanol: Dichloromethane (1:1) leaf extract (specific compounds are unknown) | 80 | [74] |
| Escherichia | ||||
| E. coli | E. sigmoidea | Neobavaisoflavone | 8–32 | [25] |
| E. livingstoniana | 5.7.3′-trihydroxy-4′-methoxy-5′-(3-methylbut2-enyl)flavanone | 5 | [75] | |
| 7.3′-dihydroxy-4′-methoxy-5′-(3- methylbut-2-enyl)flavanone | 5 | |||
| (3S,3″R)-7-hydroxy-2′-methoxy-[3″-hydroxy-2″,2″-dimethylpyrano (3′,4′)] isoflavan | 63 | [76] | ||
| E. caffra | Abyssione-V 4′-O-methyl ether | 3.9 | [24] | |
| 6.8-diprenylgenistein | 7.8 | |||
| Alpinumisoflavone | 3.9 | |||
| Methanol bark extract (specific compounds are unknown) | 0.02 | [41] | ||
| E. lysistemon | Erybraedin A | 2 | [35] | |
| Phaseollidin | 20 | |||
| Eryzerin C | 5 | |||
| Lysisteisoflavone | 6 | |||
| E. senegalensis | Ethanol leaf extract (specific compounds are unknown) | 25 | [26] | |
| E. addisonae | Scandenone | 64 | [32] | |
| Enterobacter | ||||
| E. cloacae | E. sigmoidea | Neoisoflavone | 8 | [25] |
| E. aerogenes | E. addisonae | Scandenone | 64 | [32] |
| E. cloaca | E. addisonae | Scandenone | 64 | |
| Klebsiella | ||||
| K. pneumoniae | E. sigmoidea | Neoisoflavone | 8 | [25] |
| E. caffra | Abyssione-V 4′-O-methyl ether | 3.9 | [24] | |
| 6.8-diprenylgenistein | 7.8 | |||
| Alpinumisofl avone | 3.9 | |||
| E. senegalensis | Ethanol leaf extract (specific compounds are unknown) | 6.25 | [26] | |
| Pseudomonas | ||||
| P. aeruginosa | E. sigmoidea | Neoisoflavone | 8 | [25] |
| E. lysistemon | Erybraedin A | 20 | [35] | |
| Phaseollidin | 20 | |||
| Cristacarpin | 78 | |||
| Eryzerin C | 5 | |||
| Alpumisoflavone | 20 | |||
| Lysisteisoflavone | 31 | |||
| Proteus | ||||
| P. vulgaris | E. caffra | Ethanol bark extract | 156.3 | [30] |
| Salmonella | ||||
| S. typhi | E. caffra | Methanol bark extract (specific compounds are unknown) | 0.02 | [41] |
| S. enteretidis | E. senegalensis | Calopocarpin | 62.5 | [77] |
| E. abyssinica | Methanol bark extract (specific compounds are unknown) | 29 | [28] | |
| Fungi | Erythrina Species | Compounds | MIC (µg/mL) | Ref |
|---|---|---|---|---|
| C. albicans | E. indica | Ethanol leaf extract (specific compounds are unknown) | 62.5 | [43] |
| E. senegalensis | Ethanol stem bark extract (specific compounds are unknown) | 4.00–15.63 | [42] | |
| E. stricta | Dichloromethane bark extract (specific compounds are unknown) | 125 | [23] | |
| E. poeppigiana | Erypostyrene | 50 | [37] | |
| E. sacleuxii | Erysubin F | >32.021 | [29] | |
| E. lysistemon | Aqueous extracts (specific compounds are unknown) | 25,000 | [45] | |
| C. glabrata | E. senegalensis | Ethanol stem bark extract (specific compounds are unknown) | 3.91–62.5 | [42] |
| C. krusei | E. crista-galli | Erytharbine, erysotrine, erythratidine N-oxide | 12.5–31.25 | [44] |
| C. neoformans | E. senegalensis | Hydroalcoholic leaves extract (specific compounds are unknown) | 3120 | [46] |
| A. niger | E. senegalensis | Lectin seeds extract (specific compounds are unknown | 400 | [5] |
| E. lanceolata | Dichloromethane bark extract (specific compounds are unknown) | 1250 | [31] | |
| P. camembreti | E. senegalensis | Lectin seeds extract (specific compounds are unknown | 200 | [5] |
| S. brevicaulis | E. senegalensis | Lectin seeds extract (specific compounds are unknown | 200 | [5] |
| P. oryzae | E. sacleuxii | Prostratol C and orobol | 20 | [31] |
| R. stolonifer | E. lanceolata | Dichloromethane bark extract (specific compounds are unknown) | 625 | [47] |
| Plasmodium | Erythrina Species | Compounds | IC50 (µg/mL) | Ref |
|---|---|---|---|---|
| P. falciparum | E. stricta | Erythrabbysin II | 5.5 | [65] |
| Erystagallin A | 3.8 | |||
| 5-hydroxysophoranone | 2.5 | |||
| Soyaspongenol B | 4.6 | |||
| E. subumbrans | Erybraedin A | 3.4 | ||
| Erythrabbysin II | 5.5 | |||
| Erystagallin A | 3.8 | |||
| 5-hydroxysophoranone | 2.5 | |||
| Erysubin F | 3.2 | |||
| Soyaspongenol B | 4.6 | |||
| E. latissima | Erysodine | 6.5 ± 4.7 | [50] | |
| Erysovine | 4.1 ± 0.6 | |||
| Erysotrine | 20.6 ± 8.6 | |||
| Erythraline | 7.3 ± 4.9 | |||
| E. sacleuxii | Methanol stem bark extract (specific compounds are unknown) | 1.78 ± 0.93 | [48] | |
| Methanol leaf extract (specific compounds are unknown) | 24.59 ± 10.54 | |||
| Methanol root bark extract (specific compounds are unknown) | 0.45 ± 0.09 | |||
| Acetone stem bark extract (specific compounds are unknown) | 3.8 ± 0.9 | [51] | ||
| Acetone root bark extract (specific compounds are unknown) | 2.2 ± 0.6 | |||
| E. abbysinica | Methanol stem bark extract (specific compounds are unknown) | 37.37 ± 6.46 | [49] | |
| Dichloromethane stem bark extract (specific compounds are unknown) | 5.37 ± 1.59 | |||
| aqueous stem bark extract (specific compounds are unknown) | 47.74 ± 9.15 | |||
| E. caffra | Erythrinasinate B | 24.4 ± 2.63 | [78] | |
| Lupeol | 41.7 ± 9.74 | |||
| E. ovalifolia | Erythrisenegalone | 1.69 | [52] | |
| Alpinumisoflavone | 1.98 | |||
| Phaseollidin | 1.66 | |||
| Sandwicensin | 1.83 | |||
| E. burttii | Buttinol A | 7.6 ± 0.3 | [56] | |
| Buttinol B | 19.1 ± 0.6 | |||
| Buttinol C | 9.3 ± 0.9 | |||
| Buttinol H | 13.3 ± 2.5 | |||
| Buttinol D | 4.9 ± 0.3 | |||
| 4-O-Methylsigmoidin B | 12.4 ± 1.7 | |||
| Abbysinone V | 5.7 ± 0.5 | |||
| Abbysinone V methyl ether | 10.7 ± 2.4 | |||
| Calocarpin | 19.4 ± 1.8 | |||
| E. senegalensis | Ethanol stem bark extract (specific compounds are unknown) | 1.82 | [54] | |
| E. fusca | Methanol stem bark extract (specific compounds are unknown) | 13 | [55] | |
| N-hexane stem bark extract (specific compounds are unknown) | 21 | |||
| Chloroform stem bark extract (specific compounds are unknown) | 22 | |||
| Aqueous stem bark extract (specific compounds are unknown) | 5 | |||
| E. sigmoidea | Ethanol stem bark extract (specific compounds are unknown) | 6.44 ± 0.08 | [53] | |
| Aqueous stem bark extract (specific compounds are unknown) | 29.51 ± 3.63 | |||
| E. haerdii | Ethanol leaf extract (specific compounds are unknown) | 25.6 ± 2.5 | [58] | |
| Ethanol root bark extract (specific compounds are unknown) | 11.0 ± 0.7 | |||
| Ethanol stem bark extract (specific compounds are unknown) | 8.6 ± 0.8 | |||
| E. variegata | Ethyl acetate leaf extract (specific compounds are unknown) | 16.7 | [59] | |
| N-butanol leaf extract (specific compounds are unknown) | 13.2 | |||
| 10.11-dioxoerythratidine | 9.3 | |||
| terpenoid pentacyclic glycoside | 1.8 | |||
| E. corallodendron | Isoschaftoside | 1.702 | [57] | |
| Vicenin II | 1.744 | |||
| Chloroquine-Resistant P. falciparum | E. abbysinica | Methanol stem bark extract (specific compounds are unknown) | 34.13 ± 9.79 | [49] |
| Dichloromethane stem bark extract (specific compounds are unknown) | 6.99 ± 0.76 | |||
| Aqueous stem bark extract (specific compounds are unknown) | 50.11 ± 10.23 | |||
| E. schliebenii | Ethanol root extract (specific compounds are unknown) | 1.87 ± 0.44 | [79] | |
| Aqueous stem bark extract (specific compounds are unknown) | 7.04 ± 0.72 | |||
| E. burttii | Buttinol A | 8.5 ± 0.6 | [56] | |
| Buttinol B | 21.1 ± 0.8 | |||
| Buttinol C | 9.1 ± 1.2 | |||
| Buttinol H | 20.3 ± 4.1 | |||
| Buttinol D | 6.1 ± 1.5 | |||
| 4-O-Methylsigmoidin B | 12.7 ± 2.3 | |||
| Abbysinone V | 6.6 ± 1.3 | |||
| Abbysinone V methyl ether | 11.9 ± 2.1 | |||
| Calocarpin | 17 ± 1.5 | |||
| E. lysistemon | aqueous stem bark extract (specific compounds are unknown) | 8.27 | [80] | |
| E. fusca | Methanol stem bark extract (specific compounds are unknown) | 8 | [55] | |
| N-hexane stem bark extract (specific compounds are unknown) | 5 | |||
| Chloroform stem bark extract (specific compounds are unknown) | 13 | |||
| Aqueous stem bark extract (specific compounds are unknown) | 18 | |||
| E. sacleuxii | Acetone stem bark extract (specific compounds are unknown) | 6.3 ± 1.4 | [51] | |
| Acetone root bark extract (specific compounds are unknown) | 1.34 ± 0.3 | |||
| E. haerdii | Ethanol leaf extract (specific compounds are unknown) | 36.4 ± 1.9 | [58] | |
| Ethanol root bark extract (specific compounds are unknown) | 15.2 ± 0.9 | |||
| Ethanol stem bark extract (specific compounds are unknown) | 7.6 ± 0.7 | |||
| E. variegata | Methanol leaf extract (specific compounds are unknown) | 6.8 | [59] | |
| Ethyl acetate leaf extract (specific compounds are unknown) | 26.5 | |||
| N-butanol leaf extract (specific compounds are unknown) | 5.1 | |||
| 10.11-dioxoerythratidine | 3.3 | |||
| terpenoid pentacyclic glycoside | 3.2 | |||
| E. corallodendron | Isoschaftoside | 1.1049 | [57] | |
| Vicenin II | 1.4099 | |||
| Multidrug-Resistant P. falciparum | E. senegalensis | Methanol stem bark extract (specific compounds are unknown) | 99.6 ± 1.25 | [81] |
| E. abbysinica | Methanol stem bark extract (specific compounds are unknown) | 7.81 | [82] | |
| E. subumbrans | Vogelin C | 2.8 | [34] | |
| Lespedezaflavanone B | 3.7 | |||
| Abbysinone V | 7.0 | |||
| E. sigmoidea | Ethanol stem bark extract (specific compounds are unknown) | 7.53 ± 0.22 | [53] | |
| Aqueous stem bark extract (specific compounds are unknown) | 35.23 ± 3.17 | |||
| E. fusca | Lonchocarpol A | 9.18 | [83] | |
| Phaseollidin | 9.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muchtaridi, M.; Lestyawan, S.; Fakhirah, M.A.; Rusdin, A.; Salsabila, S.; Megantara, S.; Subarnas, A.; Khairul Ikram, N.K. Therapeutic Potential of Erythrina Genus: Bioactive Phytoconstituents with Potent Antiviral and Antimicrobial Activities. Plants 2025, 14, 3053. https://doi.org/10.3390/plants14193053
Muchtaridi M, Lestyawan S, Fakhirah MA, Rusdin A, Salsabila S, Megantara S, Subarnas A, Khairul Ikram NK. Therapeutic Potential of Erythrina Genus: Bioactive Phytoconstituents with Potent Antiviral and Antimicrobial Activities. Plants. 2025; 14(19):3053. https://doi.org/10.3390/plants14193053
Chicago/Turabian StyleMuchtaridi, Muchtaridi, Samuel Lestyawan, Maitsa Alya Fakhirah, Agus Rusdin, Shela Salsabila, Sandra Megantara, Anas Subarnas, and Nur Kusaira Khairul Ikram. 2025. "Therapeutic Potential of Erythrina Genus: Bioactive Phytoconstituents with Potent Antiviral and Antimicrobial Activities" Plants 14, no. 19: 3053. https://doi.org/10.3390/plants14193053
APA StyleMuchtaridi, M., Lestyawan, S., Fakhirah, M. A., Rusdin, A., Salsabila, S., Megantara, S., Subarnas, A., & Khairul Ikram, N. K. (2025). Therapeutic Potential of Erythrina Genus: Bioactive Phytoconstituents with Potent Antiviral and Antimicrobial Activities. Plants, 14(19), 3053. https://doi.org/10.3390/plants14193053









