Morphological Acclimation of Durum Wheat Spikes in Response to Foliar Micronutrient Applications
Abstract
1. Introduction
2. Results
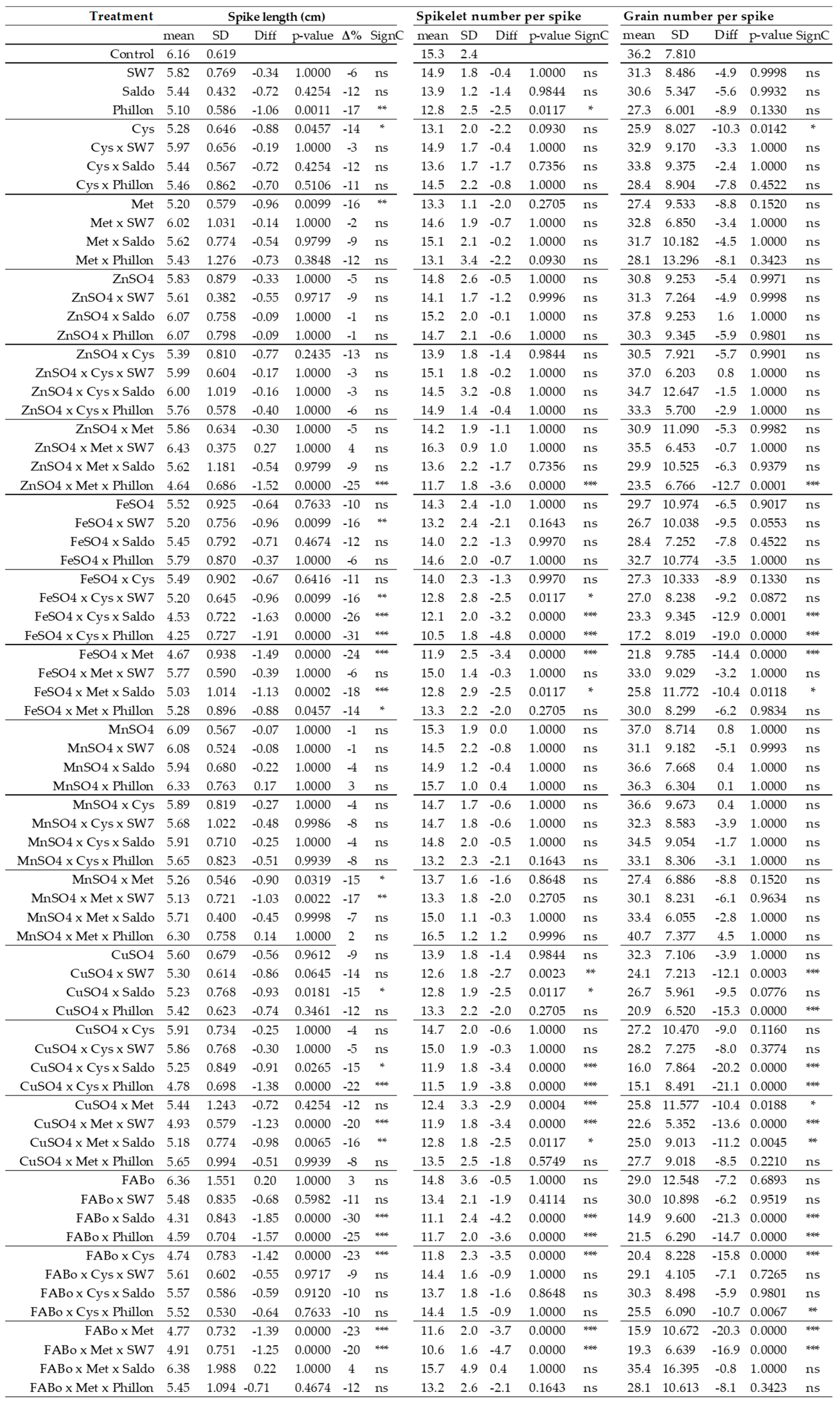
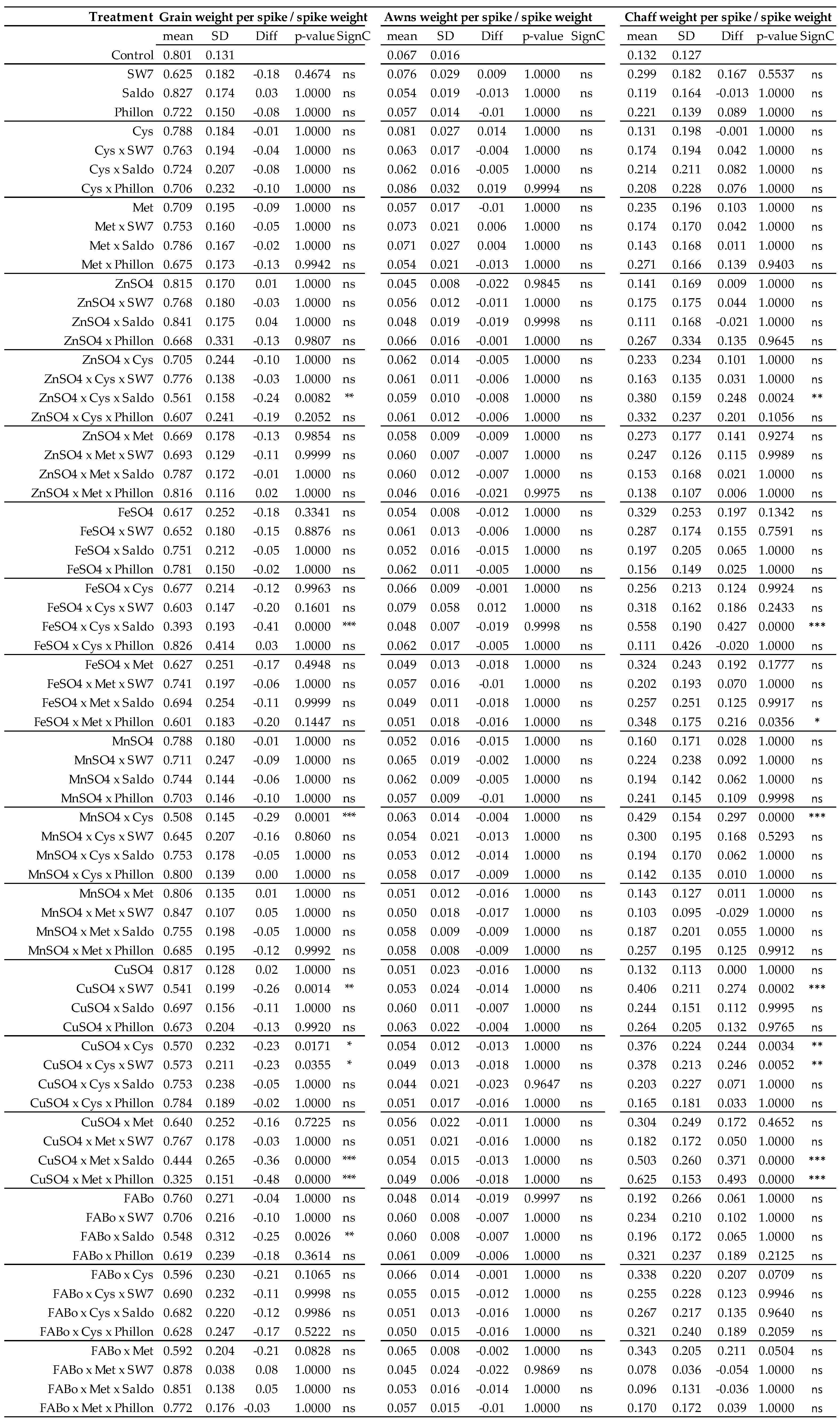
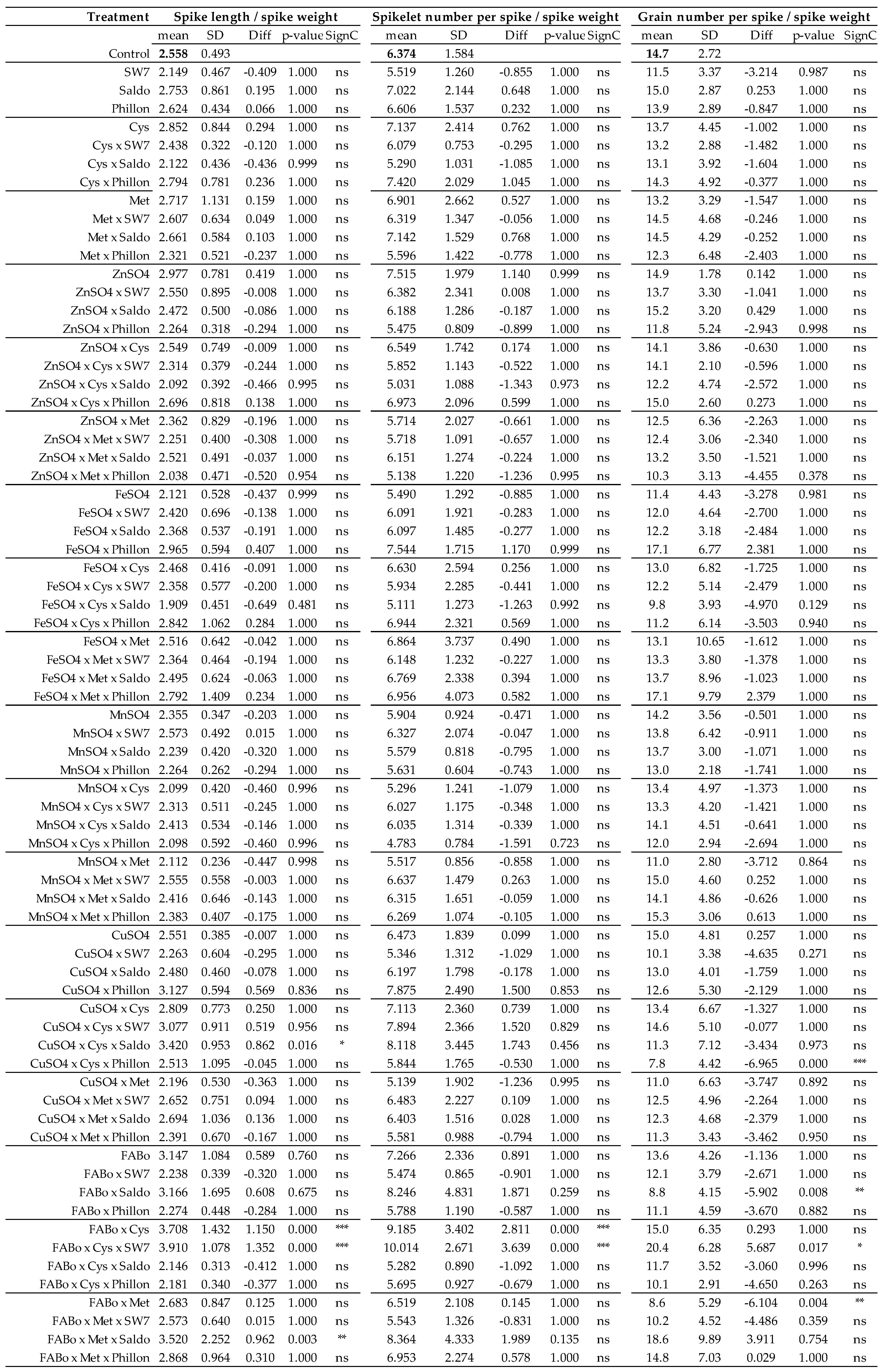
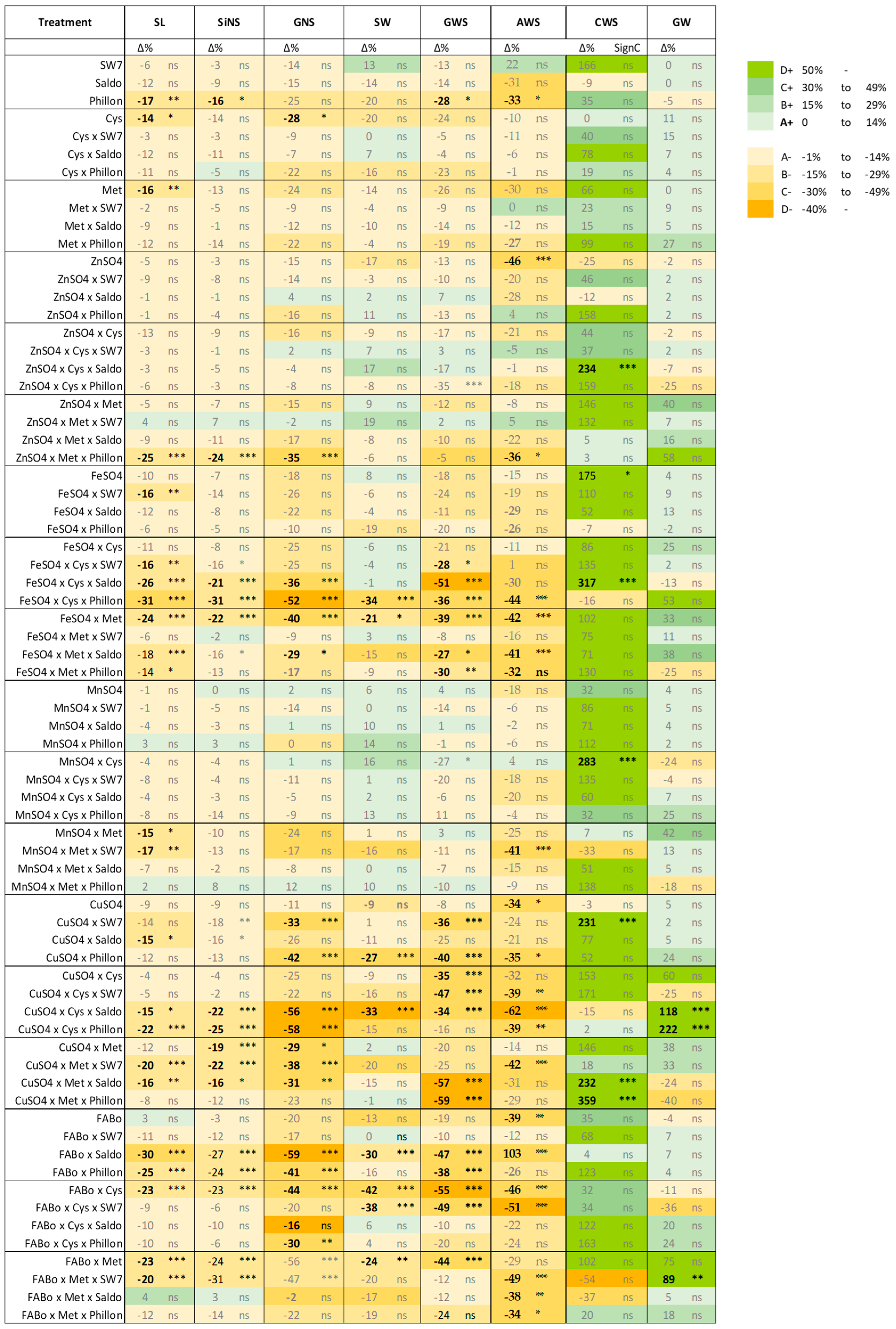
2.1. Effects on Morphological Traits
2.2. Correlations Between Morphological Traits
2.3. Comparison of the Effects on Morphological Traits
3. Discussion
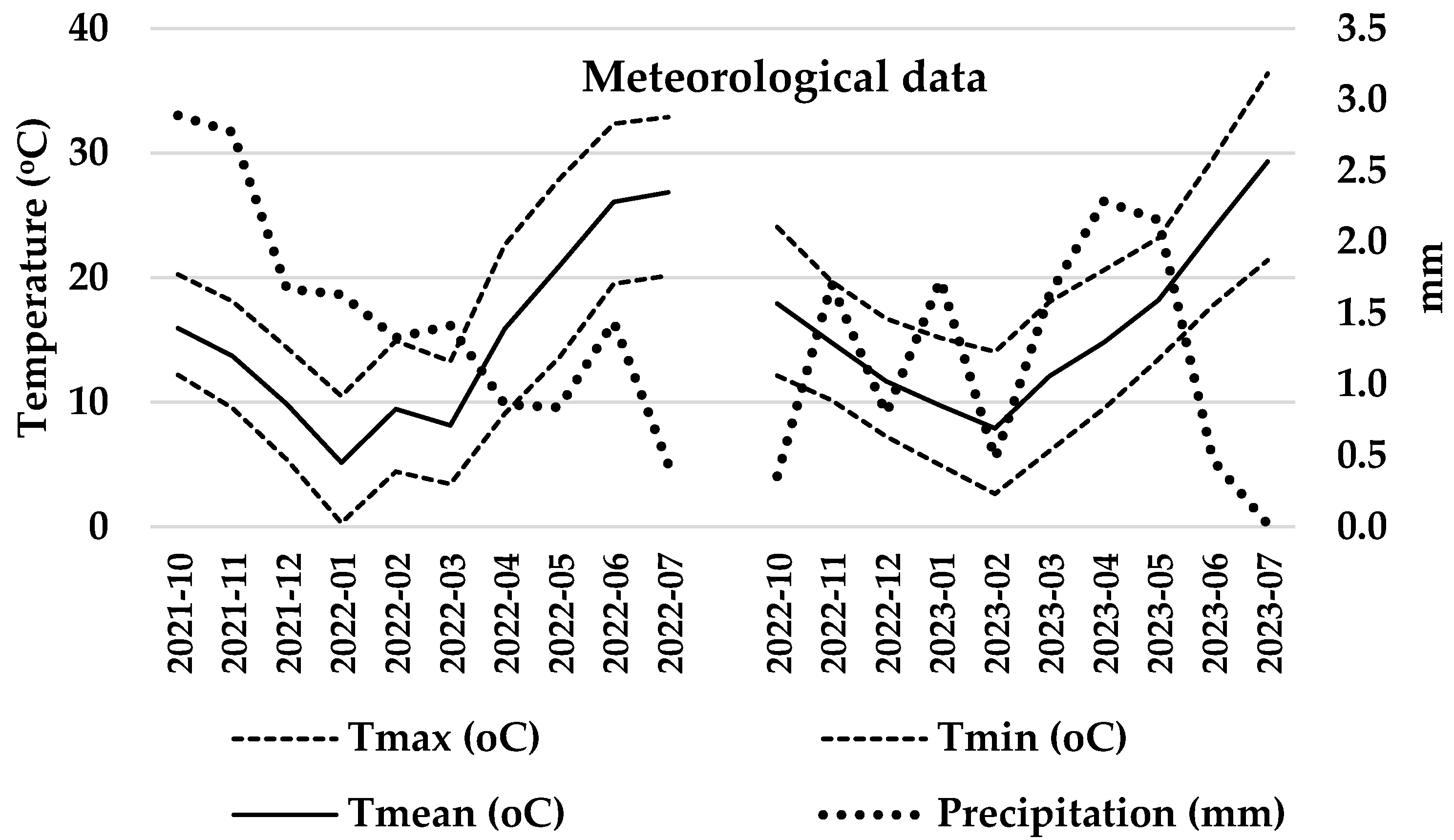
4. Materials and Methods
Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cys | Cysteine |
| Met | Methionine |
| EMi | Essential micronutrients |
| AE | Alcohol ethoxylate |
| SiE | Organosilicone-based ethoxylate |
| FABo | FytoAmino-Bo (product) |
| NA | Nicotianamine |
| SL | Spike length |
| SlNS | Spikelet number per spike |
| GNS | Grain number per spike |
| SW | Spike weight |
| GWS | Grain weight per spike |
| AWS | Awns weight per spike |
| CWS | Chaff weight per spike |
| GW | Grain weight (or weight per grain) |
| GWS/SW | Grain weight per spike/spike weight |
| AWS/SW | Awns weight per spike/spike weight |
| CWS/SW | Chaff weight per spike/spike weight |
| SlNS/SW | Spikelet number per spike/spike weight |
| GNS/SW | Grain number per spike/spike weight |
| SL/SW | Spike length/spike weight |
References
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.; Gokmen, O.; Ozturk, L.; et al. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef] [PubMed]
- Konopatskaia, I.; Vavilova, V.; Blinov, A.; Goncharov, N.P. Spike Morphology Genes in Wheat Species (Triticum L.). Proc. Latv. Acad. Sci. Sect. B 2016, 70, 45–355. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, Y.; Röder, M.S.; Reif, J.C.; Martin, W.; Ganal, M.W. Manipulation and prediction of spike morphology traits for the improvement of grain yield in wheat. Sci. Rep. 2018, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.A.; Hille RisLambers, D. Effect of environment and cultivar on source limitation to grain weight in wheat. Aust. J. Agric. Res. 1978, 29, 443–458. [Google Scholar] [CrossRef]
- Gonzαlez, F.G.; Terrile, I.I.; Falcσn, M.O. Spike fertility and duration of stem elongation as promising traits to improve potential grain number (and yield): Variation in modern Argentinean wheats. Crop Sci. 2011, 51, 1693–1702. [Google Scholar] [CrossRef]
- Zhou, H.; Riche, A.B.; Hawkesford, M.J.; Whalley, W.R.; Atkinson, B.S.; Sturrock, C.J.; Mooney, S.J. Determination of wheat spike and spikelet architecture and grain traits using X-ray Computed Tomography imaging. Plant Methods 2021, 17, 26. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Araus, J.-L.; Park, R.; Calderini, D.; Miralles, D.; Shen, T.; Zhang, J.; Parry, M.A.J. Prospects of doubling global wheat yields. Food Energy Secur. 2013, 2, 34–48. [Google Scholar] [CrossRef]
- Wolde, G.M.; Mascher, M.; Schnurbusch, T. Genetic modification of spikelet arrangement in wheat increases grain number without significantly affecting grain weight. Mol. Genet. Genom. 2019, 294, 457–468. [Google Scholar] [CrossRef]
- Royo, C.; Jose Miguel Soriano, J.M.; Alvaro, F. Wheat: A Crop in the Bottom of the Mediterranean Diet Pyramid. In Mediterranean Identities—Environment, Society, Culture; Fuerst-Bjelis, B., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 381–399. [Google Scholar] [CrossRef]
- Marzario, S.; Sica, R.; Taranto, F.; Fania, F.; Esposito, S.; De Vita, P.; Gioia, T.; Logozzo, G. Phenotypic evolution in durum wheat (Triticum durum Desf.) based on SNPs, morphological traits, UPOV descriptors and kernel-related traits. Front. Plant Sci. 2023, 14, 1206560. [Google Scholar] [CrossRef]
- Berski, W.; Ziobro, R.; Gorczyca, A.; Oleksy, A. The Effects of Sowing Density and Timing on Spike Characteristics of Durum Winter Wheat. Agriculture 2025, 15, 359. [Google Scholar] [CrossRef]
- Manhou, K.; Taghouti, M.; Moussadek, R.; Elyacoubi, H.; Bennani, S.; Zouahri, A.; Ghanimi, A.; Sanad, H.; Oueld Lhaj, M.; Hmouni, D.; et al. Performance, Agro-Morphological, and Quality Traits of Durum Wheat (Triticum turgidum L. ssp. durum Desf.) Germplasm: A Case Study in Jemâa Shaïm, Morocco. Plants 2025, 14, 1508. [Google Scholar] [CrossRef]
- Guo, Z.; Schnurbusch, T. Costs and benefits of awns. J. Exp. Bot. 2016, 67, 2533–2535. [Google Scholar] [CrossRef] [PubMed]
- Weyhrich, R.A.; Carver, B.F.; Martin, B.C. Photosynthesis and Water-Use Efficiency of Awned and Awnletted Near-Isogenic Lines of Hard Red Winter Wheat. Crop Sci. 1995, 35, 172–176. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Bonnett, D.G.; Reynolds, M.P. Awns reduce grain number to increase grain size and harvestable yield in irrigated and rainfed spring wheat. J. Exp. Bot. 2016, 67, 2573–2586. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Edwards, J. (Eds.) Procrop Wheat Growth and Development; NSW Department of Primary Industries: Sydney, Australia, 2008; ISBN 9780734718945. [Google Scholar]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Kirkwood, R.C. Use and mode of action of adjuvants for herbicides: A review of some current work. Pestic. Sci. 1993, 38, 93–102. [Google Scholar] [CrossRef]
- Hagan, N.D.; Upadhyaya, L.M.; Tabe, L.M.; Higgins, T.J.V. The redistribution of protein sulphur in transgenic rice expressing a gene for a foreign, sulfur-rich protein. Plant J. 2003, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Awazuhara, M.; Fujiwara, T.; Hayashi, H.; Watanabe-Takahashi, A.; Takahashi, H.; Saito, K. The function of SULTR2;1 sulfate transporter during seed development in Arabidopsis thaliana. Physiol. Plant. 2005, 125, 95–105. [Google Scholar] [CrossRef]
- Cakmak, I.; Gülüt, K.Y.; Marschner, H.; Graham, R.D. Effect of zinc and iron deficiency on phytosiderophore release in wheat genotypes differing in zinc efficiency. J. Plant Nutr. 1994, 17, 1–17. [Google Scholar] [CrossRef]
- Chorianopoulou, S.N.; Bouranis, D.L. The role of sulfur in agronomic biofortification with essential micronutrients. Plants 2022, 11, 1979. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Stylianidis, G.P.; Manta, V.; Karousis, E.N.; Tzanaki, A.; Dimitriadi, D.; Bouzas, E.A.; Siyiannis, V.F.; Constantinou-Kokotou, V.; Chorianopoulou, S.N.; et al. Floret Biofortification of Broccoli Using Amino Acids Coupled with Selenium under Different Surfactants: A Case Study of Cultivating Functional Foods. Plants 2023, 12, 1272. [Google Scholar] [CrossRef]
- Räsch, A.; Hunsche, M.; Mail, M.; Burkhardt, J.; Noga, G.; Pariyar, S. Agricultural adjuvants may impair leaf transpiration and photosynthetic activity. Plant Physiol. Biochem. 2018, 132, 229–237. [Google Scholar] [CrossRef]
- Burghardt, M.; Schreiber, L.; Riederer, M. Enhancement of the Diffusion of Active Ingredients in Barley Leaf Cuticular Wax by Monodisperse Alcohol Ethoxylates. J. Agric. Food Chem. 1998, 46, 1593–1602. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, Z. Molecular control of grass inflorescence development. Annu. Rev. Plant Biol. 2014, 65, 553–578. [Google Scholar] [CrossRef]
- Li, Y.P.; Fu, X.; Zhao, M.; Zhang, W.; Li, B.; An, D.; Li, J.; Zhang, A.; Liu, R.; Liu, X.G. A Genome-wide view of transcriptome dynamics during early spike development in bread wheat. Sci. Rep. 2018, 8, 15338. [Google Scholar] [CrossRef]
- Luo, X.; Yang, Y.; Lin, X.; Xiao, J. Deciphering spike architecture formation towards yield improvement in wheat. J. Genet. Genom. 2023, 50, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Ding, Y.; Long, M.; Liang, W.; Gu, X.; Liu, Y.; Wen, X. Effect of Foliar Application of Various Nitrogen Forms on Starch Accumulation and Grain Filling of Wheat (Triticum aestivum L.) Under Drought Stress. Front. Plant Sci. 2021, 12, 645379. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Ma, X.; Ma, Z.; Wang, T.; Li, Y.; Mao, J.; Chen, B. Effects of foliar fertilizer additives on grape fruit quality and endogenous hormones in leaves. BMC Plant Biol. 2025, 25, 516. [Google Scholar] [CrossRef]
- Zheng, H.; Chruszcz, M.; Lasota, P.; Lebioda, L.; Minor, W. Data mining of metal ion environments present in protein structures. J. Inorg. Biochem. 2008, 102, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Rubino, J.T.; Chenkin, M.P.; Keller, M.; Riggs-Gelasco, P.; Franz, K.J. A comparison of methionine, histidine and cysteine in copper(i)-binding peptides reveals differences relevant to copper uptake by organisms in diverse environments. Metallomics 2011, 3, 61–73. [Google Scholar] [CrossRef]
- Deepak, R.N.V.K.; Chandrakar, B.; Sankararamakrishnan, R. Comparison of metal-binding strength between methionine and cysteine residues: Implications for the design of metal-binding motifs in proteins. Biophys. Chem. 2017, 224, 32–39. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Chorianopoulou, S.N. Foliar Application of Sulfur-Containing Compounds—Pros and Cons. Plants 2023, 12, 3794. [Google Scholar] [CrossRef]
- Mondal, S.; Pramanik, K.; Panda, D.; Dutta, D.; Karmakar, S.; Bose, B. Sulfur in seeds: An overview. Plants 2022, 11, 450. [Google Scholar] [CrossRef]
- Fitzgerald, M.A.; Ugalde, T.D.; Anderson, J.W. Sulfur nutrition affects delivery and metabolism of S in developing endosperms of wheat. J. Exp. Bot. 2001, 52, 1519–1526. [Google Scholar] [CrossRef]
- Tabe, L.M.; Droux, M. Sulfur assimilation in developing lupin cotyledons could contribute significantly to the accumulation of organic sulfur reserves in seed. Plant Physiol. 2001, 126, 176–187. [Google Scholar] [CrossRef]
- Sexton, P.J.; Shibles, R.M. Activity of ATP sulfurylase in reproductive soybean. Crop Sci. 1999, 39, 131–135. [Google Scholar] [CrossRef]
- Klikocka, H.; Marks, M. Sulphur and nitrogen fertilization as a potential means of agronomic biofortification to improve the content and uptake of microelements in spring wheat grain DM. J. Chem. 2018, 2018, 9326820. [Google Scholar] [CrossRef]
- Barczak, B.; Jastrzebska, M.; Kostrzewska, M.K. Biofortification of spring barley grain with microelements through sulfur fertilization. J. Chem. 2019, 2019, 8214298. [Google Scholar] [CrossRef]
- Hancok, J.T.; Whiteman, M. Alone NO Longer: Interactions of Nitric Oxide with Reactive Oxygen Species and Hydrogen Sulfide. Adv. Bot. Res. 2016, 77, 1–14. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Nitric oxide and hydrogen sulfide modulate the NADPH-generating enzymatic system in higher plants. J. Exp. Bot. 2021, 72, 830–847. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mahmud, J.A.; Nahar, K.; Mohsin, S.M.; Parvin, K.; Fujita, M. Interaction of sulfur with phytohormones and signaling molecules in conferring abiotic stress tolerance to plants. Plant Signal. Behav. 2018, 13, e1477905. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xing, T.; Yuan, H.; Liu, Z.; Jin, Z.; Zhang, L.; Pei, Y.; Galli, A. Hydrogen Sulfide Improves Drought Tolerance in Arabidopsis thaliana by MicroRNA Expressions. PLoS ONE 2013, 8, e77047. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Shen, J.; Ge, Z.; Zhang, Z.; Shen, W.; et al. Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integr. Plant Biol. 2021, 63, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Knoche, M. Organosilicone surfactant performance in agricultural spray application: A review. Weed Res. 1994, 34, 221–239. [Google Scholar] [CrossRef]
- Yandell, B.S. Practical Data Analysis for Designed Experiments; Chapman & Hall: London, UK, 1997. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; ISBN 3-900051-07-0. [Google Scholar]

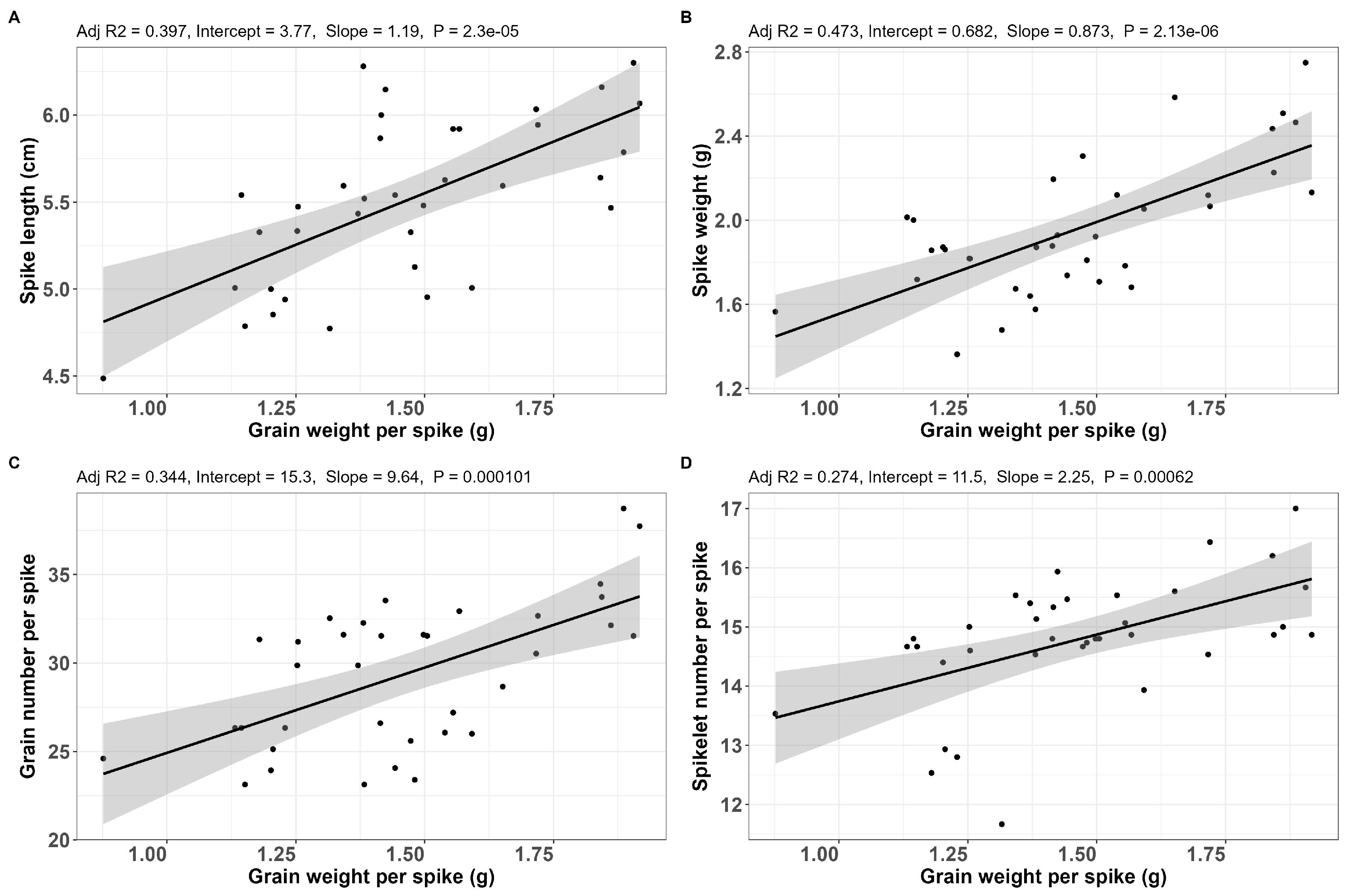

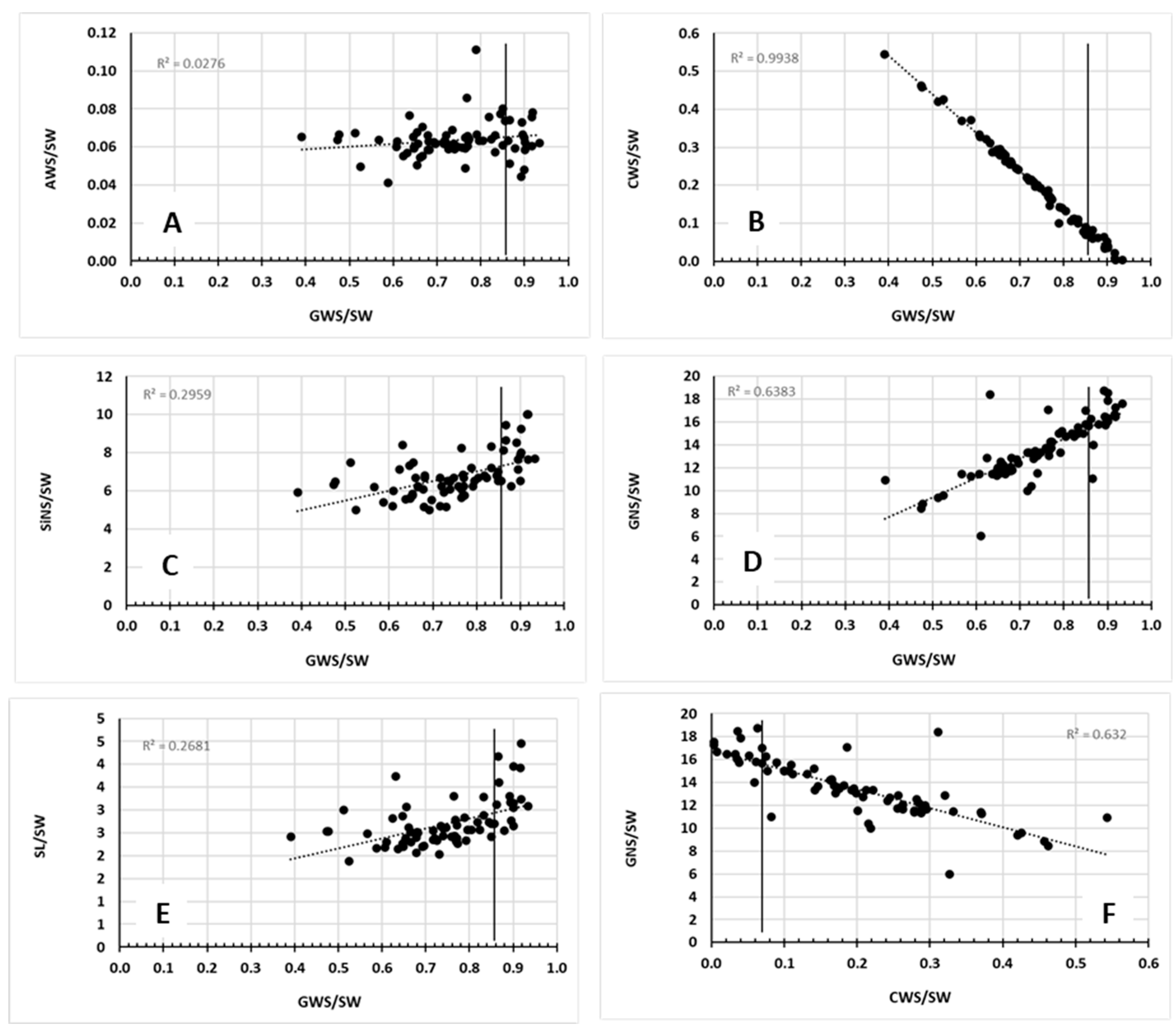
| Trait 1 | Trait 2 | Coefficient | p-Value | LB | UB |
|---|---|---|---|---|---|
| AWS | GNS | 0.22 | 5.930 × 10−2 | −0.009 | 0.433 |
| AWS | GWS | 0.19 | 1.140 × 10−1 | −0.046 | 0.402 |
| AWS | SL | 0.16 | 1.730 × 10−1 | −0.072 | 0.380 |
| AWS | SlNS | 0.22 | 6.620 × 10−2 | −0.015 | 0.428 |
| AWS | SW | 0.42 | 2.130 × 10−4 | 0.212 | 0.596 |
| GNS | GWS | 0.63 | 2.710 × 10−9 | 0.468 | 0.753 |
| GNS | SL | 0.84 | 3.370 × 10−20 | 0.754 | 0.897 |
| GNS | SlNS | 0.88 | 1.060 × 10−24 | 0.819 | 0.925 |
| GNS | SW | 0.68 | 3.910 × 10−11 | 0.536 | 0.790 |
| GWS | SL | 0.53 | 1.640 × 10−6 | 0.341 | 0.679 |
| GWS | SlNS | 0.49 | 9.940 × 10−6 | 0.297 | 0.652 |
| GWS | SW | 0.61 | 1.380 × 10−8 | 0.439 | 0.737 |
| SL | SlNS | 0.92 | 4.840 × 10−31 | 0.882 | 0.952 |
| SL | SW | 0.59 | 5.560 × 10−8 | 0.413 | 0.721 |
| SlNS | SW | 0.57 | 1.310 × 10−7 | 0.396 | 0.712 |
| Dates | Agronomic Works |
|---|---|
| Experimental 2021–2022 | |
| 22 December 2021 | Sowing |
| 7 March 2022 | Formation of the experimental plots |
| 6 May 2022 | Application of interventions |
| 8 June 2022 | Harvest day |
| Experimental 2022–2023 | |
| 4 January 2023 | Sowing |
| 24 April 2023 | Formation of the experimental plots |
| 28 June 2023 | Harvest day |
| 30 June 2023 | Harvest day |
| Experimental 2021–2022 | |||
| Reference | SW7 | ||
| Cys | Cys,SW7 | ||
| Met | Met,SW7 | ||
| ZnSO4 | ZnSO4,SW7 | ||
| ZnSO4,Cys | ZnSO4,Cys,SW7 | ||
| ZnSO4,Met | ZnSO4,Met,SW7 | ||
| FeSO4 | FeSO4,SW7 | ||
| FeSO4,Cys | FeSO4,Cys,SW7 | ||
| FeSO4,Met | FeSO4,Met,SW7 | ||
| MnSO4 | MnSO4,SW7 | ||
| MnSO4,Cys | MnSO4,Cys,SW7 | ||
| MnSO4,Met | MnSO4,Met,SW7 | ||
| CuSO4 | CuSO4,SW7 | ||
| CuSO4,Cys | CuSO4,Cys,SW7 | ||
| CuSO4,Met | CuSO4,Met,SW7 | ||
| FABo | FABo,SW7 | ||
| FABo,Cys | FABo,Cys,SW7 | ||
| FABo,Met | FABo,Met,SW7 | ||
| Experimental 2022–2023 | |||
| Reference | SW7 | Saldo | Phillon |
| Cys | Cys,SW7 | Cys,Saldo | Cys,Phillon |
| Met | Met,SW7 | Met,Saldo | Met,Phillon |
| ZnSO4 | ZnSO4,SW7 | ZnSO4,Saldo | ZnSO4,Phillon |
| ZnSO4,Cys | ZnSO4,Cys,SW7 | ZnSO4,Cys,Saldo | ZnSO4,Cys,Phillon |
| ZnSO4,Met | ZnSO4,Met,SW7 | ZnSO4,Met,Saldo | ZnSO4,Met,Phillon |
| FeSO4 | FeSO4,SW7 | FeSO4,Saldo | FeSO4,Phillon |
| FeSO4,Cys | FeSO4,Cys,SW7 | FeSO4,Cys,Saldo | FeSO4,Cys,Phillon |
| FeSO4,Met | FeSO4,Met,SW7 | FeSO4,Met,Saldo | FeSO4,Met,Phillon |
| MnSO4 | MnSO4,SW7 | MnSO4,Saldo | MnSO4,Phillon |
| MnSO4,Cys | MnSO4,Cys,SW7 | MnSO4,Cys,Saldo | MnSO4,Cys,Phillon |
| MnSO4,Met | MnSO4,Met,SW7 | MnSO4,Met,Saldo | MnSO4,Met,Phillon |
| CuSO4 | CuSO4,SW7 | CuSO4,Saldo | CuSO4,Phillon |
| CuSO4,Cys | CuSO4,Cys,SW7 | CuSO4,Cys,Saldo | CuSO4,Cys,Phillon |
| CuSO4,Met | CuSO4,Met,SW7 | CuSO4,Met,Saldo | CuSO4,Met,Phillon |
| FABo | FABo,SW7 | FABo,Saldo | FABo,Phillon |
| FABo,Cys | FABo,Cys,SW7 | FABo,Cys,Saldo | FABo,Cys,Phillon |
| FABo,Met | FABo,Met,SW7 | FABo,Met,Saldo | FABo,Met,Phillon |
| pH | 7.75 | Ca2+ | 79.90 mg L−1 | Cl− | 5.65 mg L−1 |
| Electric conductivity (20 °C) | 359 μS cm−1 | Mg2+ | 4.34 mg L−1 | SO42− | 17.00 mg L−1 |
| Hardness (as CaCO3) | 217.45 mg L−1 | Na+ | 4.61 mg L−1 | NO3− | 15.15 mg L−1 |
| K+ | 0.89 mg L−1 | NO2− | - | ||
| NH4+ | - | CO32− | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriadi, D.; Stylianidis, G.P.; Tsirogiannis, I.; Bouranis, L.D.; Chorianopoulou, S.N.; Bouranis, D.L. Morphological Acclimation of Durum Wheat Spikes in Response to Foliar Micronutrient Applications. Plants 2025, 14, 3079. https://doi.org/10.3390/plants14193079
Dimitriadi D, Stylianidis GP, Tsirogiannis I, Bouranis LD, Chorianopoulou SN, Bouranis DL. Morphological Acclimation of Durum Wheat Spikes in Response to Foliar Micronutrient Applications. Plants. 2025; 14(19):3079. https://doi.org/10.3390/plants14193079
Chicago/Turabian StyleDimitriadi, Despina, Georgios P. Stylianidis, Ioannis Tsirogiannis, Lampros D. Bouranis, Styliani N. Chorianopoulou, and Dimitris L. Bouranis. 2025. "Morphological Acclimation of Durum Wheat Spikes in Response to Foliar Micronutrient Applications" Plants 14, no. 19: 3079. https://doi.org/10.3390/plants14193079
APA StyleDimitriadi, D., Stylianidis, G. P., Tsirogiannis, I., Bouranis, L. D., Chorianopoulou, S. N., & Bouranis, D. L. (2025). Morphological Acclimation of Durum Wheat Spikes in Response to Foliar Micronutrient Applications. Plants, 14(19), 3079. https://doi.org/10.3390/plants14193079








