Morphological, Physiological, and Molecular Responses to Heat Stress in Brassicaceae
Abstract
:1. Introduction
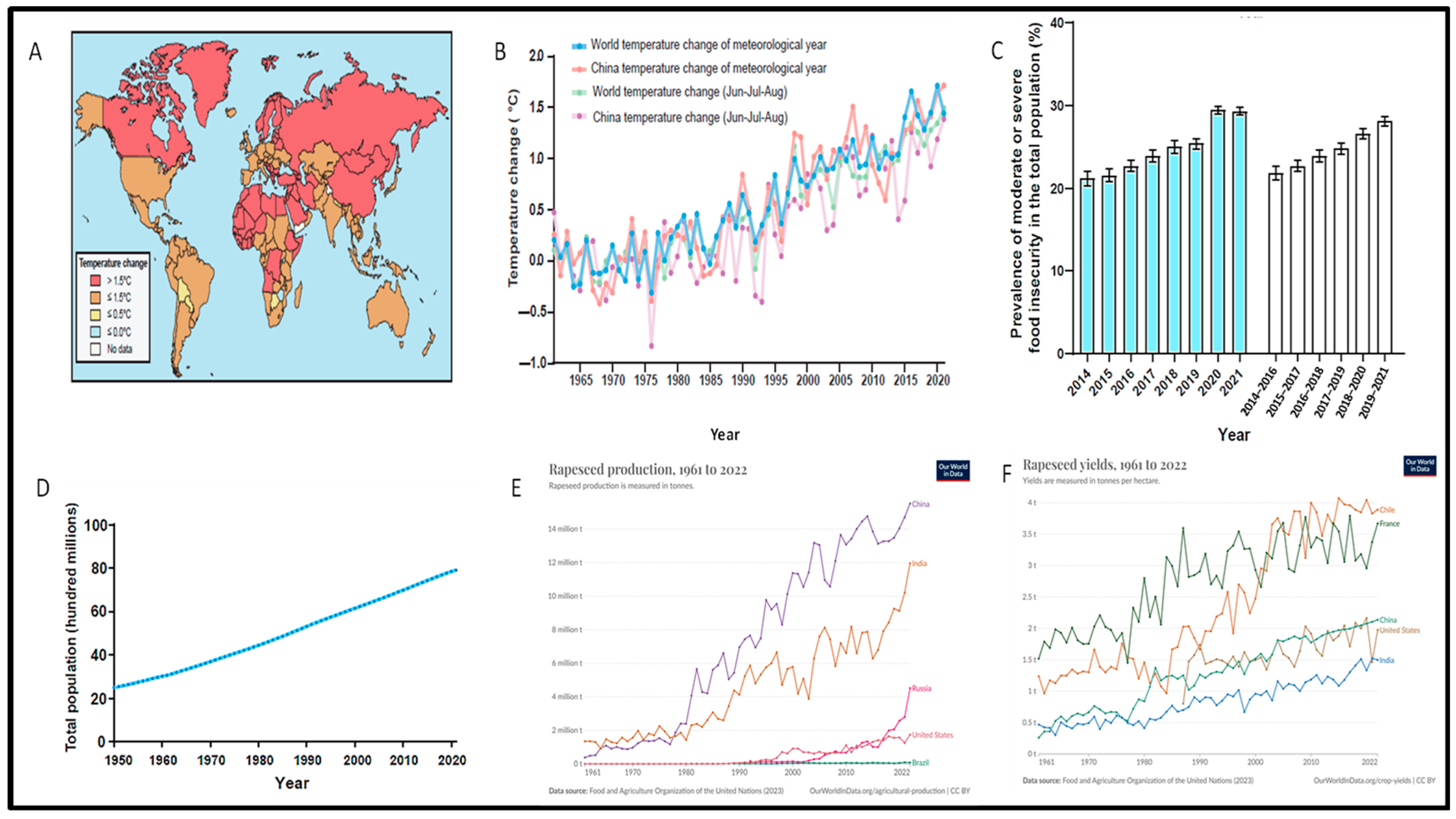
2. Global Warming Poses a Threat to Food Security
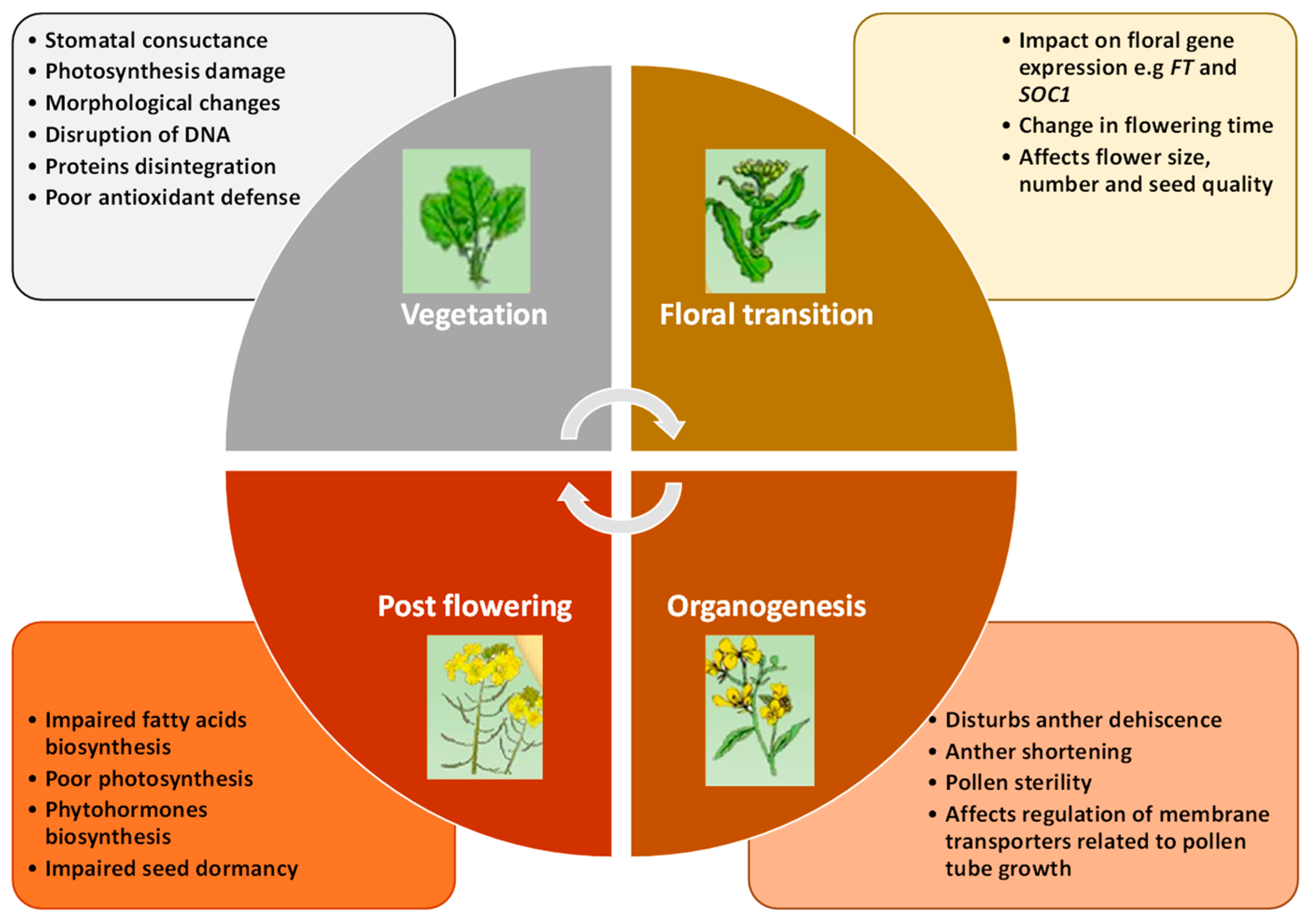
Heat Stress in Brassicaceae: Effects and Responses
3. Thermal Sensing and Responses in Thermo-Morphogenesis
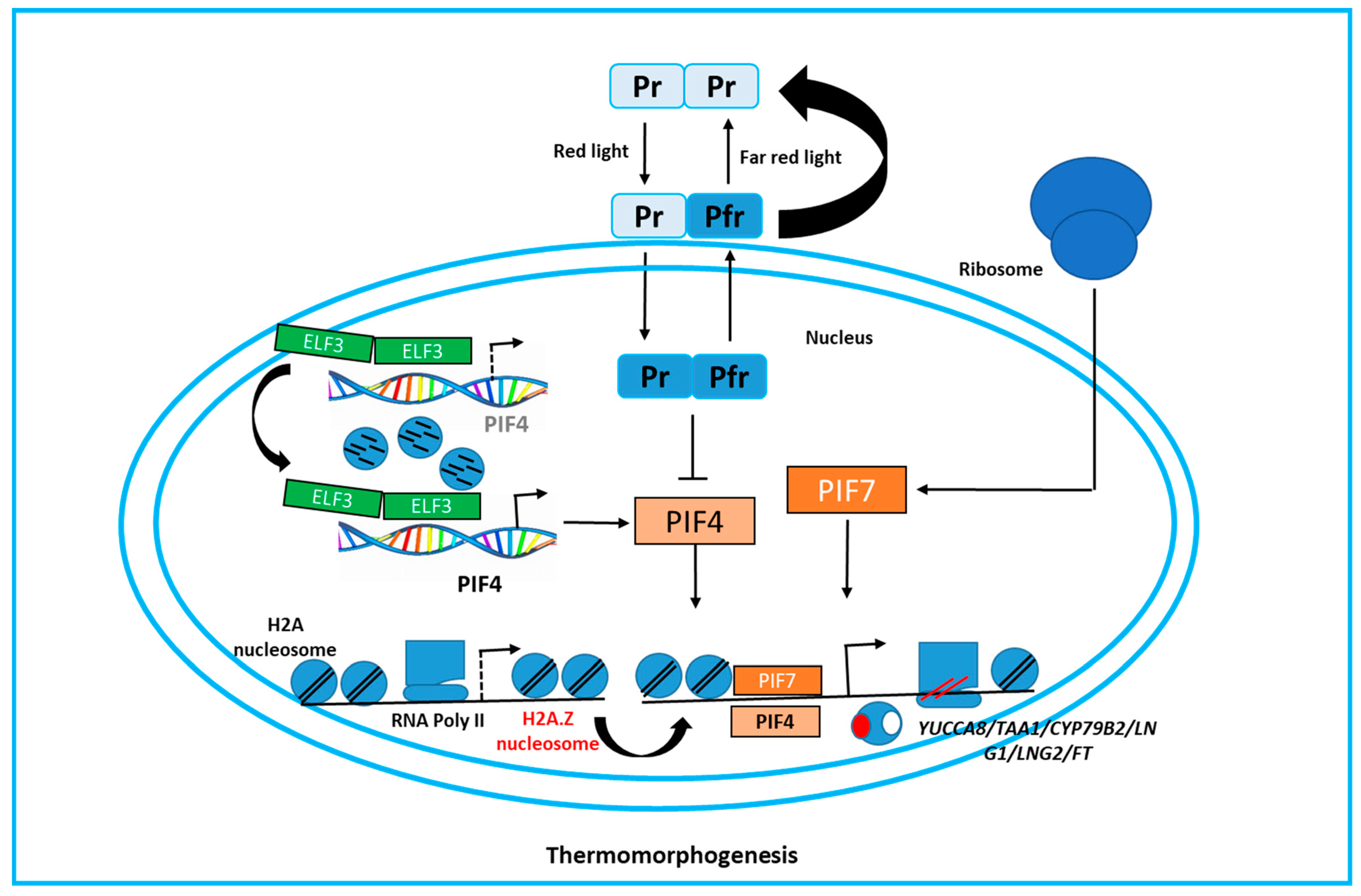
3.1. How Plants Sense Warm Temperature
3.2. The Molecular Responses of Thermo-Morphogenesis in Plants
3.3. Putative Thermo-Sensing in Plants
Heat-Induced Cell Wall Remodeling Releases Ca2+
3.4. Activation of Chemical Messengers in the Plasma Membrane
3.5. Phase Separation at Protein Levels
3.6. Subcellular Location-Specific Changes at Protein Levels
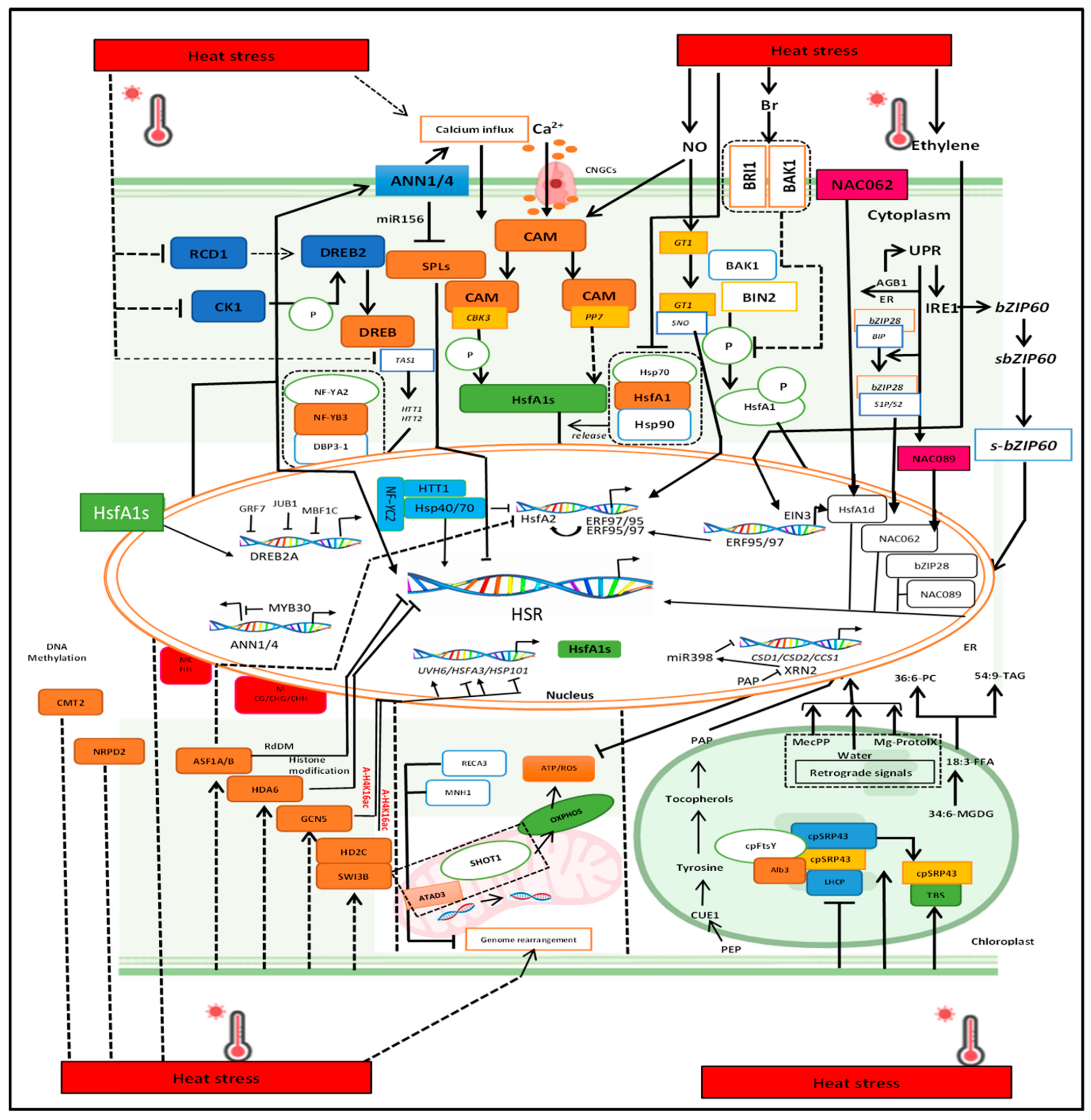
4. Thermal Tolerance Response in Plants
4.1. Transcriptional Regulation: The Central Roles of HSF and HSP Genes
4.2. Role of Signaling Molecules Including Ca2+, ROS, and Nitric Oxide
4.3. ROS-Mediated Phytohormes Signaling in the Plant Cell
4.4. Role of ncRNAs in Plant Response Under Heat Stress
4.5. Epigenetic Modification in Response to Heat Stress
4.6. Molecular Regulation of Heat Stress in Semiautonomous Organelles
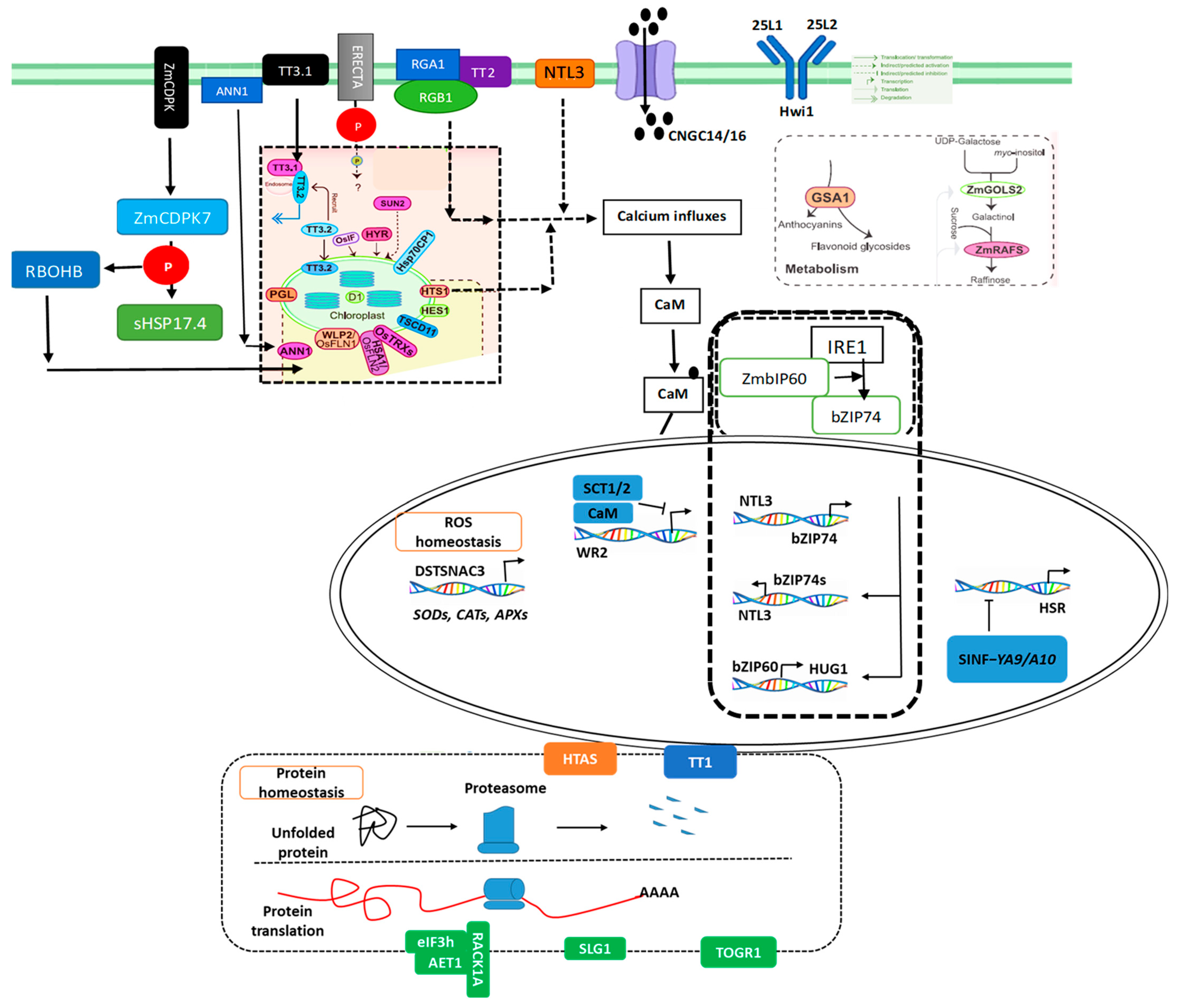
4.7. Molecular Mechanism and Genetic Control of HSRs in Crops
4.8. Ca2+ Elevation Initiates Heat Signal Transduction
4.9. Plasma Membrane-Localized Proteins Translocate into Other Cell Components to Transduce Heat Signals
4.10. HSF–HSP-Mediated Transcription Activation in the Nucleus
4.11. Regulation of Protein Degradation and Translation Maintain Protein Homeostasis Under HS
4.12. Post-Translational-Modification
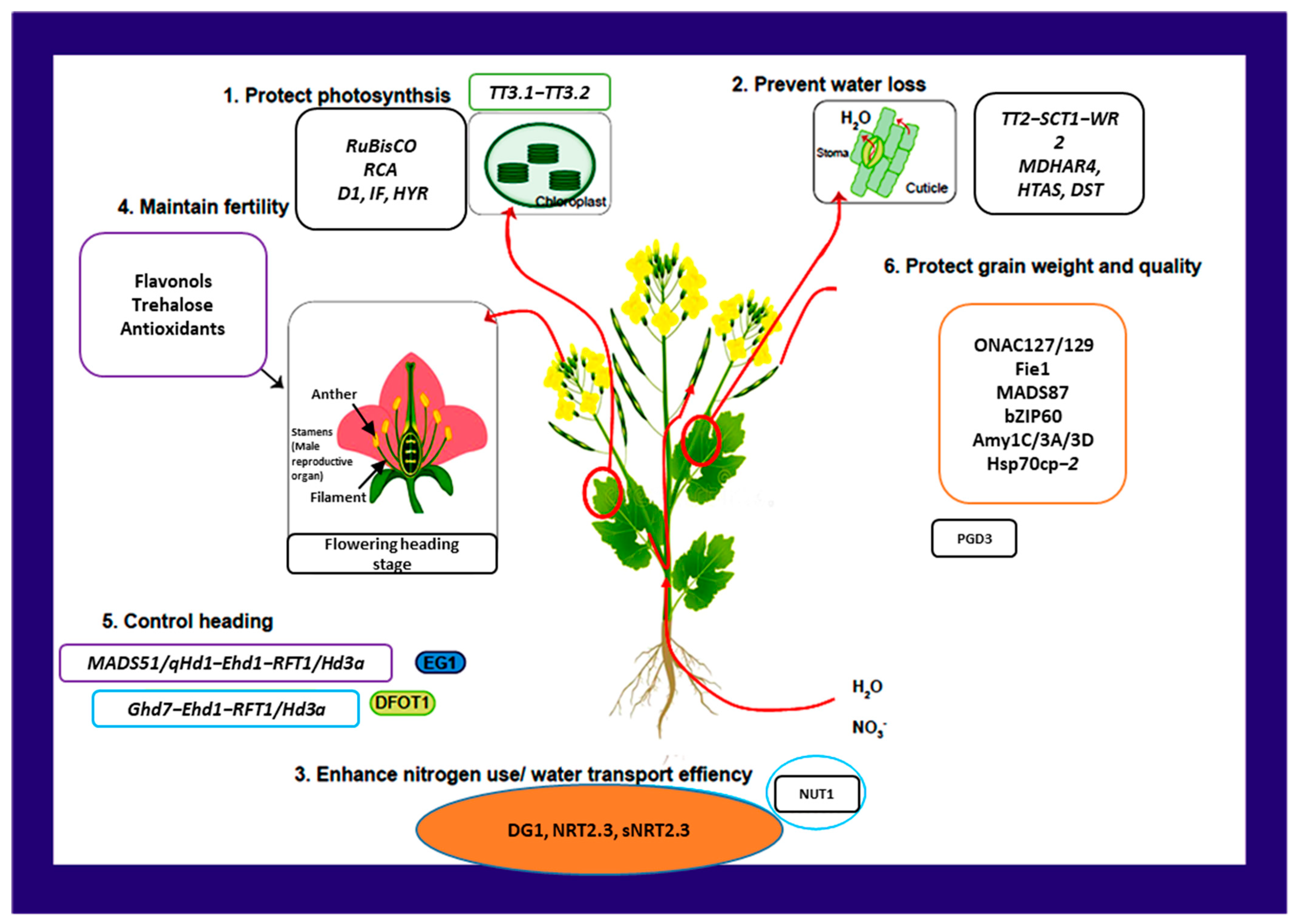
5. Maintaining Crop Productivity via Source-Sink Strategies
5.1. Source Strategies
Protecting Photosynthesis
5.2. Sink Strategies: Guaranteeing Normal Fertility and Heading Stage
6. Maintaining Grain Yield
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| ANNs | Annexins |
| ATAD3 | ATPase family AAA domain-containing protein 3 |
| ATI3 | ATG8-INTERACTING PROTEIN3 |
| CaM | Calmodulin |
| cAMP | Cyclase produces cyclic AMP |
| CBK3 | Calmodulin-binding protein kinase 3 |
| cGMP | Cyclic guanosine monophosphate |
| CK1 | (CASEIN KINASE 1) |
| CLC2 | CLATHRIN LIGHT CHAIN 2 |
| CMT2 | Chromomethylase 2 |
| CNGCs | Cyclic nucleotide-gated channels |
| CNGCs | Cyclic nucleotide-gated channels |
| CYP79B2 | Cytochrome P450 79B2 |
| DAG | Diacylglycerol |
| DFOT1 | Diurnal flower opening time 1 |
| DGK | Phospholipase C/DAG kinase |
| DREB2A | DEHYDRATION-RESPONSIVE ELEMENT BINDING 2A |
| EG1 | EXTRA GLUME 1 |
| ELF3 | EARLY FLOWERING 3 |
| ER | Endoplasmic reticulum |
| FT | Flowering locus T protein |
| GAPC | Glyceraldehyde-3-phosphate dehydrogenase |
| GRF7 | GROWTH-REGULATING FACTOR 7 |
| HDA6 | Histone deacetylase 6 |
| HIL1 | Heat-inducible lipase 1 |
| HS | Heat stress |
| HSA32 | HS-associated 32-kD protein |
| HSBP2 | HEAT SHOCK BINDING PROTEIN 2 |
| HSFs | Heat shock factors |
| HSPs | Heat shock proteins |
| HSR | Heat shock response |
| JA | Jasmonates |
| JUB1 | JUNGBRUNNEN 1 |
| LLPS | Liquid–liquid phase separation of proteins. |
| lncRNAs | Long non-coding RNAs |
| LNG1 | Longifolia 1 |
| LNG2 | Longifolia 2 |
| MBF1c | MULTIPROTEIN BRIDGING FACTOR 1C |
| mRNAs | Messenger RNAs |
| MSH1 | MutS homolog 1 |
| NBR1 | NEXT TO BRCA1 GENE 1 |
| ncRNAs | Non-coding RNAs |
| NF-YC10 | Nuclear factor Y subunit C10 |
| NRD | Negative regulatory domain |
| Pas | Phosphatidic acids |
| PE | Phosphatidylethanolamine |
| PEP | Phosphoenolpyruvate |
| PhyB | Phytochrome B |
| PIF4 | PHYTOCHROME-INTERACTING FACTOR 4 |
| PIF7 | PHYTOCHROME-INTERACTING FACTOR 7 |
| PLC/DGK | Phospholipase C/diacylglycerol [DAG] kinase generate |
| PLC | Phospholipase C |
| PLD | Phospholipase D |
| PME | Pectin methylesterases |
| PRC2 | Polycomb Repressive Complex 2 |
| PROMPTs | Promoter upstream transcripts |
| PTMs | Post-translational modifications |
| RBGD2 | RNA-binding glycine-rich D2 |
| RBOHB | Respiratory burst oxidase homolog B |
| RBOHD | Respiratory burst oxidase homolog D |
| RCA | RuBisCO activase |
| RCD1 | RADICAL-INDUCED CELL DEATH 1 |
| RdDM | RNA-directed DNA methylation |
| RNA Pol II | RNA polymerase II |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| SCT1 | Sensing Ca2+ transcription factor 1 |
| SGs | Stress granules |
| sHSPs | Small heat shock proteins |
| SlCHIP | Carboxyl terminus of the hsc70-interacting protein |
| SlMPK1 | Mitogen-activated protein kinase 1 |
| SlSPRH1 | Serine-proline-rich protein homolog |
| SPL | SQUAMOSA PROMOTER BINDING PROTEIN-LIKE |
| TAA1 | L-tryptophan--pyruvate aminotransfer-ase 1 |
| TT | Thermo-tolerance |
| UPR | Unfolded protein responses |
| UPS | Pathways ubiquitin (Ub)-26S proteasome system |
| YUCCA8 | Indole-3-pyruvate monooxygenase 8 |
References
- Muluneh, M.G. Impact of climate change on biodiversity and food security: A global perspective—A review article. Agric. Food Secur. 2021, 10, 36. [Google Scholar] [CrossRef]
- He, T.; Li, C. Harness the power of genomic selection and the potential of germplasm in crop breeding for global food security in the era with rapid climate change. Crop. J. 2020, 8, 688–700. [Google Scholar] [CrossRef]
- Jagadish, S.K.; Way, D.A.; Sharkey, T.D. Plant heat stress: Concepts directing future research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar] [CrossRef] [PubMed]
- Peer, L.A.; Dar, Z.A.; Lone, A.A.; Bhat, M.Y.; Ahamad, N. High temperature triggered plant responses from whole plant to cellular level. Plant Physiol. Rep. 2020, 25, 611–626. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.-R.; Gao, J.; Lin, H.-X.; Lin, Y. The molecular basis of heat stress responses in plants. Mol. Plant 2023, 16, 1612–1634. [Google Scholar] [CrossRef]
- Raza, A. Eco-physiological and Biochemical Responses of Rapeseed (Brassica napus L.) to Abiotic Stresses: Consequences and Mitigation Strategies. J. Plant Growth Regul. 2020, 40, 1368–1388. [Google Scholar] [CrossRef]
- E Parker, L.; McElrone, A.J.; Ostoja, S.M.; Forrestel, E.J. Extreme heat effects on perennial crops and strategies for sustaining future production. Plant Sci. 2020, 295, 110397. [Google Scholar] [CrossRef]
- Talaat, N.B. Role of reactive oxygen species signaling in plant growth and development. Chapter 10. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: New York, NY, USA, 2019; pp. 225–266. [Google Scholar] [CrossRef]
- Faizan, M.; Hayat, S.; Ahmed, S.M. Reactive Oxygen Species: Prospects in Plant Metabolism; Springer Nature: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Gajghate, R.; Chourasiya, D.; Harikrishna; Sharma, R.K. Plant morphological, physiological traits associated with adaptation against heat stress in wheat and maize. In Plant Stress Biology: Strategies and Trends; Springer: Singapore, 2020; pp. 51–81. [Google Scholar]
- Park, Y.; Nam, B.E.; Park, C. Environmentally adaptive reshaping of plant photomorphogenesis by karrikin and strigolactone signaling. J. Integr. Plant Biol. 2024, 66, 865–882. [Google Scholar] [CrossRef]
- Chung, S.; Kwon, C.; Lee, J.-H. Epigenetic control of abiotic stress signaling in plants. Genes Genom. 2021, 44, 267–278. [Google Scholar] [CrossRef]
- Pan, Y.-H.; Chen, L.; Zhu, X.-Y.; Li, J.-C.; Rashid, M.A.R.; Chen, C.; Qing, D.-J.; Zhou, W.-Y.; Yang, X.-H.; Gao, L.-J.; et al. Utilization of natural alleles for heat adaptability QTLs at the flowering stage in rice. BMC Plant Biol. 2023, 23, 256. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Mei, Y.; Xu, L.; Zhu, X.; Wang, Y.; Guo, J.; Liu, L. Genome-wide characterization of differentially expressed genes provides insights into regulatory network of heat stress response in radish (Raphanus sativus L.). Funct. Integr. Genom. 2018, 18, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Raza, A. Metabolomics: A systems biology approach for enhancing heat stress tolerance in plants. Plant Cell Rep. 2020, 41, 741–763. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Mawlong, I.; Balbeer, B.; Kumar, M.S.; Rai, P.K.; Singh, V.V. Proteomic, biochemical and peptidomics based analysis reveals heat responsive changes in the seedlings of Brassica juncea. J. Plant Biochem. Biotechnol. 2024, 33, 570–589. [Google Scholar] [CrossRef]
- Danilova, M.N.; Kudryakova, N.V.; Andreeva, A.A.; Doroshenko, A.S.; Pojidaeva, E.S.; Kusnetsov, V.V. Differential impact of heat stress on the expression of chloroplast-encoded genes. Plant Physiol. Biochem. 2018, 129, 90–100. [Google Scholar] [CrossRef]
- Møller, I.M.; Rasmusson, A.G.; Van Aken, O. Plant mitochondria–past, present and future. Plant J. 2021, 108, 912–959. [Google Scholar] [CrossRef]
- Jiang, J.; Bai, J.; Li, S.; Li, X.; Yang, L.; He, Y. HTT2 promotes plant thermotolerance in Brassica rapa. BMC Plant Biol. 2018, 18, 127. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liu, Z.; Li, X.; Wu, F.; He, Y. Heat-induced tas1 target1 mediates thermotolerance via heat stress transcription factor A1a–directed pathways in Arabidopsis. Plant Cell 2014, 26, 1764–1780. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, J.; Liu, Y.F.; Yang, L.L.; Li, W.P.; Zhang, L.M. Overexpression of Arabidopsis HsfA1a enhances diverse stress tolerance by promoting stress-induced Hsp expression. Genet. Mol. Res. 2014, 13, 1233–1243. [Google Scholar] [CrossRef]
- Kirkin, V.; Lamark, T.; Sou, Y.S.; Bjørkøy, G.; Nunn, J.L.; Bruun, J.A.; Shvets, E.; McEwan, D.G.; Clausen, T.H.; Wild, P.; et al. A Role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol. Cell 2009, 33, 505–516. [Google Scholar]
- Avin-Wittenberg, T. Autophagy and its role in plant abiotic stress management. Plant Cell Environ. 2019, 42, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, S.; Gao, C.; Zhou, J. An emerging role of non-canonical conjugation of ATG8 proteins in plant response to heat stress. Autophagy 2024, 20, 946–948. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Chakrabarty, S.; Kumar, M.; Prakash, K.; Kumari, S. Response of Early, Timely & late lenotypes of Brassica sps. grown under different temperature conditions for oil content & fatty acid composition. Int. J. Plant Soil Sci. 2023, 35, 170–179. [Google Scholar]
- Dikšaitytė, A.; Viršilė, A.; Žaltauskaitė, J.; Januškaitienė, I.; Juozapaitienė, G. Growth and photosynthetic responses in Brassica napus differ during stress and recovery periods when exposed to combined heat, drought and elevated CO2. Plant Physiol. Biochem. 2019, 142, 59–72. [Google Scholar] [CrossRef]
- Lohani, N.; Singh, M.B.; Bhalla, P.L. Short-term heat stress during flowering results in a decline in Canola seed productivity. J. Agron. Crop Sci. 2022, 208, 486–496. [Google Scholar] [CrossRef]
- Matthews, H.D.; Wynes, S. Current global efforts are insufficient to limit warming to 1.5 °C. Science 2022, 376, 1404–1409. [Google Scholar] [CrossRef]
- Kaur, G.; Brar, S.S.; Singh, C.B.; Singh, K.B. Effect of tillage and nitrogen levels on yield and water productivity of Brassica napus in north west India. Int. J. Plant Soil. Sci. 2023, 35, 32–48. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Tao, F. Impacts of climate change and climate extremes on major crops productivity in China at a global warming of 1.5 and 2.0 °C. Earth Syst. Dyn. 2018, 9, 543–562. [Google Scholar] [CrossRef]
- Raza, A.; Hafeez, M.B.; Zahra, N.; Shaukat, K.; Umbreen, S.; Tabassum, J.; Charagh, S.; Khan, R.S.A.; Hasanuzzaman, M. The plant family Brassicaceae: Introduction, biology, and importance. In The Plant Family Brassicaceae: Biology and Physiological Responses to Environmental Stresses; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–43. [Google Scholar]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef] [PubMed]
- Schiessl, S. Regulation and subfunctionalization of flowering time genes in the allotetraploid oil crop Brassica napus. Front. Plant Sci. 2020, 11, 605155. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Fujita, M. Modulation of antioxidant machinery and the methylglyoxal detoxification system in selenium-supplemented Brassica napus seedlings confers tolerance to high temperature stress. Biol. Trace Element Res. 2014, 161, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, T.; Liu, Y.; Ren, C.; Zhao, Y.; Wang, M. Isolation and characterization of gene encoding G protein subunit protein responsive to plant hormones and abiotic stresses in Brassica napus. Mol. Biol. Rep. 2010, 37, 3957–3965. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Z.; Xing, M.; Yang, Y.; Wu, X.; Liu, H.; Liang, W. Heat stress suppresses Brassica napus seed oil accumulation by inhibition of photosynthesis and BnWRI1 pathway. Plant Cell Physiol. 2019, 60, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Koscielny, C.; Hazebroek, J.; Duncan, R. Phenotypic and metabolic variation among spring Brassica napus genotypes during heat stress. Crop Pasture Sci. 2018, 69, 284–295. [Google Scholar] [CrossRef]
- Guo, R.; Wang, X.; Han, X.; Li, W.; Liu, T.; Chen, B.; Chen, X.; Wang-Pruski, G. Comparative transcriptome analyses revealed different heat stress responses in high-and low-GS Brassica alboglabra sprouts. BMC Genom. 2019, 20, 269. [Google Scholar] [CrossRef] [PubMed]
- Lohani, N.; Golicz, A.A.; Singh, M.B.; Bhalla, P.L. Genome-wide analysis of the Hsf gene family in Brassica oleracea and a comparative analysis of the Hsf gene family in B. oleracea, B. rapa and B. napus. Funct. Integr. Genom. 2019, 19, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Hao, Y.-J.; Lu, J.-X.; Lu, G.; Zhang, T. Transcriptomic analysis reveals the mechanism of thermosensitive genic male sterility (TGMS) of Brassica napus under the high temperature inducement. BMC Genom. 2019, 20, 644. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, C. Mechanisms of Cryptochrome-Mediated Photoresponses in Plants. Annu. Rev. Plant Biol. 2020, 71, 103–129. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, M.; Brini, F.; Brestic, M.; Landi, M. Interplay between low light and hormone-mediated signaling pathways in shade avoidance regulation in plants. Plant Stress 2023, 9, 100178. [Google Scholar] [CrossRef]
- Eom, S.H.; Hyun, T.K. Histone acetyltransferases (HATs) in Chinese cabbage: Insights from histone H3 acetylation and expression profiling of HATs in response to abiotic stresses. J. Am. Soc. Hortic. Sci. 2018, 143, 296–303. [Google Scholar] [CrossRef]
- Cantila, A.Y.; Chen, S.; Siddique, K.H.; Cowling, W.A. Heat shock responsive genes in Brassicaceae: Genome-wide identification, phylogeny, and evolutionary associations within and between genera. Genome 2024, 67, 464–481. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Ren, J.; Ye, X.; Jiang, X.; Liu, Z. Genome-wide identification and functional analysis of the cyclic nucleotide-gated channel gene family in Chinese cabbage. 3 Biotech 2019, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dai, Y.; Yue, L.; Chen, G.; Yuan, L.; Zhang, S.; Li, F.; Zhang, H.; Li, G.; Zhu, S. Heat stress response in Chinese cabbage (Brassica rapa L.) revealed by transcriptome and physiological analysis. PeerJ 2022, 10, e13427. [Google Scholar] [CrossRef]
- Song, X.; Hu, J.; Wu, T.; Yang, Q.; Feng, X.; Lin, H.; Feng, S.; Cui, C.; Yu, Y.; Zhou, R. Comparative analysis of long noncoding RNAs in angiosperms and characterization of long noncoding RNAs in response to heat stress in Chinese cabbage. Hortic. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ma, L.; Terzaghi, W.; Guo, Y.; Li, J. Molecular mechanisms underlying coordinated responses of plants to shade and environmental stresses. Plant J. 2024, 117, 1893–1913. [Google Scholar] [CrossRef]
- Kanojia, A.; Bhola, D.; Mudgil, Y. Light signaling as cellular integrator of multiple environmental cues in plants. Physiol. Mol. Biol. Plants 2023, 29, 1485–1503. [Google Scholar] [CrossRef] [PubMed]
- Samtani, H.; Sharma, A.; Khurana, J.P.; Khurana, P. Thermosensing in plants: Deciphering the mechanisms involved in heat sensing and their role in thermoresponse and thermotolerance. Environ. Exp. Bot. 2022, 203, 105041. [Google Scholar] [CrossRef]
- Rahaman, M.; Mamidi, S.; Rahman, M. Genome-wide association study of heat stress-tolerance traits in spring-type Brassica napus L. under controlled conditions. Crop. J. 2018, 6, 115–125. [Google Scholar] [CrossRef]
- Qiu, Y. Regulation of PIF4-mediated thermosensory growth. Plant Sci. 2020, 297, 110541. [Google Scholar] [CrossRef]
- Becskei, A.; Rahaman, S. The life and death of RNA across temperatures. Comput. Struct. Biotechnol. J. 2022, 20, 4325–4336. [Google Scholar] [CrossRef]
- Chung, B.Y.W.; Balcerowicz, M.; Di Antonio, M.; Jaeger, K.E.; Geng, F.; Franaszek, K.; Marriott, P.; Brierley, I.; Firth, A.E.; Wigge, P.A. An RNA thermoswitch regulates daytime growth in Arabidopsis. Nat. Plants 2020, 6, 522–532. [Google Scholar] [CrossRef]
- Huang, Y.; An, J.; Sircar, S.; Bergis, C.; Lopes, C.D.; He, X.; Da Costa, B.; Tan, F.-Q.; Bazin, J.; Antunez-Sanchez, J.; et al. HSFA1a modulates plant heat stress responses and alters the 3D chromatin organization of enhancer-promoter interactions. Nat. Commun. 2023, 14, 469. [Google Scholar] [CrossRef]
- Ezin, V.; Symonds, R.C. MicroRNA-mediated regulation of heat stress response. In Plant MicroRNAs and Stress Response; CRC Press: Boca Raton, FL, USA, 2024; pp. 90–119. [Google Scholar]
- Willige, B.C.; Zander, M.; Yoo, C.Y.; Phan, A.; Garza, R.M.; Wanamaker, S.A.; He, Y.; Nery, J.R.; Chen, H.; Chen, M.; et al. PHYTOCHROME-INTERACTING FACTORs trigger environmentally responsive chromatin dynamics in plants. Nat. Genet. 2021, 53, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Burko, Y.; Willige, B.C.; Seluzicki, A.; Novák, O.; Ljung, K.; Chory, J. PIF7 is a master regulator of thermomorphogenesis in shade. Nat. Commun. 2022, 13, 4942. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, C.; Zhang, S.; Shi, C.; Cheng, H.; Liu, H.; Zhong, B. Origin and adaptive evolution of UV RESISTANCE LOCUS 8-mediated signaling during plant terrestrialization. Plant Physiol. 2021, 188, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yang, W.; Pan, Q.; Zeng, Q.; Yan, C.; Bai, X.; Liu, Y.; Zhang, L.; Li, B. Effects of long-term blue light irradiation on carotenoid biosynthesis and antioxidant activities in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Food Res. Int. 2023, 174, 113661. [Google Scholar] [CrossRef]
- Yang, J.; Qu, X.; Ji, L.; Li, G.; Wang, C.; Wang, C.; Zhang, Y.; Zheng, L.; Li, W.; Zheng, X. PIF4 Promotes Expression of HSFA2 to Enhance Basal Thermotolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 6017. [Google Scholar] [CrossRef] [PubMed]
- Byeon, B.; Bilichak, A.; Kovalchuk, I. Transgenerational response to heat stress in the form of differential expression of noncoding RNA fragments in Brassica rapa plants. Plant Genome 2019, 12, 180022. [Google Scholar] [CrossRef] [PubMed]
- Jarad, M.; Antoniou-Kourounioti, R.; Hepworth, J.; Qüesta, J.I. Unique and contrasting effects of light and temperature cues on plant transcriptional programs. Transcription 2020, 11, 134–159. [Google Scholar] [CrossRef]
- Hardy, E.C.; Balcerowicz, M. Untranslated yet indispensable—UTRs act as key regulators in the environmental control of gene expression. J. Exp. Bot. 2024, 75, 4314–4331. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Luo, Q.; Yin, L.; Wu, J.; Liu, Y.; Gan, J.; Dong, A.; Shen, W.-H. OsChz1 acts as a histone chaperone in modulating chromatin organization and genome function in rice. Nat. Commun. 2020, 11, 5717. [Google Scholar] [CrossRef]
- Abelenda, J.A.; Trabanco, N.; Del Olmo, I.; Pozas, J.; Martín-Trillo, M.D.M.; Gómez-Garrido, J.; Esteve-Codina, A.; Pernas, M.; Jarillo, J.A.; Piñeiro, M. High ambient temperature impacts on flowering time in Brassica napus through both H2A. Z-dependent and independent mechanisms. Plant Cell Environ. 2023, 46, 1427–1441. [Google Scholar] [CrossRef]
- Mazzeo, M.F.; Cacace, G.; Iovieno, P.; Massarelli, I.; Grillo, S.; Siciliano, R.A. Response mechanisms induced by exposure to high temperature in anthers from thermo-tolerant and thermo-sensitive tomato plants: A proteomic perspective. PLoS ONE 2018, 13, e0201027. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A. Lipid Metabolism in Plants Under Low-Temperature Stress: A Review. In Physiological Processes in Plants Under Low Temperature Stress; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Prusty, A.; Sharma, S.; Malik, N.; Kapoor, S.; Tyagi, A.K. Functional Versatility of Multi-Protein Mediator Complex in Plant Growth and Development. Crit. Rev. Plant Sci. 2023, 42, 138–176. [Google Scholar] [CrossRef]
- Khan, A.; Khan, V.; Pandey, K.; Sopory, S.K.; Sanan-Mishra, N. Thermo-priming mediated cellular networks for abiotic stress management in plants. Front. Plant Sci. 2022, 13, 866409. [Google Scholar] [CrossRef]
- Hofmann, N.R. The plasma membrane as first responder to heat stress. Plant Cell 2009, 21, 2544. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Saito, K. Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Prog. Lipid Res. 2019, 75, 100990. [Google Scholar] [CrossRef]
- Thomas, S.E.; Balcerowicz, M.; Chung, B.Y.-W. RNA structure mediated thermoregulation: What can we learn from plants? Front. Plant Sci. 2022, 13, 938570. [Google Scholar] [CrossRef]
- Ahmad, M.; Waraich, E.A.; Skalicky, M.; Hussain, S.; Zulfiqar, U.; Anjum, M.Z.; Rahman, M.H.U.; Brestic, M.; Ratnasekera, D.; Lamilla-Tamayo, L.; et al. Adaptation strategies to improve the resistance of oilseed crops to heat stress under a changing climate: An overview. Front. Plant Sci. 2021, 12, 767150. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Iqbal, J.; Naseer, S.; Yaseen, T.; Shaukat, M.; Bibi, H.; Ahmad, Y.; Daud, H.; Abbasi, N.L.; Mahmood, T. Molecular mechanisms of plant tolerance to heat stress: Current landscape and future perspectives. Plant Cell Rep. 2021, 40, 2247–2271. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-J.; Shin, R. Calcium channels and transporters: Roles in response to biotic and abiotic stresses. Front. Plant Sci. 2022, 13, 964059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sa, G.; Zhang, Y.; Zhu, Z.; Deng, S.; Sun, J.; Li, N.; Li, J.; Yao, J.; Zhao, N. Paxillus involutus-Facilitated Cd2+ Influx through Plasma Membrane Ca2+-Permeable Channels Is Stimulated by H2O2 and H+-ATPase in Ectomycorrhizal Populus × canescens under Cadmium Stress. Front. Plant Sci. 2017, 7, 1975. [Google Scholar] [CrossRef]
- Saha, R.; Mitra, R.K. Thermo-Resistive Phase Behavior of Trivalent Ion-Induced Microscopic Protein-Rich Phases: Correlating with Ion-Specific Protein Hydration. Langmuir 2023, 39, 4601–4610. [Google Scholar] [CrossRef]
- Levental, I.; Lyman, E. Regulation of membrane protein structure and function by their lipid nano-environment. Nat. Rev. Mol. Cell Biol. 2022, 24, 107–122. [Google Scholar] [CrossRef]
- Li, B.; Gao, K.; Ren, H.; Tang, W. Molecular mechanisms governing plant responses to high temperatures. J. Integr. Plant Biol. 2018, 60, 757–779. [Google Scholar] [CrossRef] [PubMed]
- Hongal, D.A.; Raju, D.; Kumar, S.; Talukdar, A.; Das, A.; Kumari, K.; Dash, P.K.; Chinnusamy, V.; Das Munshi, A.; Behera, T.K.; et al. Elucidating the role of key physio-biochemical traits and molecular network conferring heat stress tolerance in cucumber. Front. Plant Sci. 2023, 14, 1128928. [Google Scholar] [CrossRef] [PubMed]
- Zakany, F.; Kovacs, T.; Panyi, G.; Varga, Z. Direct and indirect cholesterol effects on membrane proteins with special focus on potassium channels. Biochim. et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2020, 1865, 158706. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, P.; Singh, S. A review report: Low temperature stress for crop production. Int. J. Pure Appl. Biosci. 2018, 6, 575–598. [Google Scholar] [CrossRef]
- Kan, Y.; Lin, H.-X. Molecular regulation and genetic control of rice thermal response. Crop. J. 2021, 9, 497–505. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Liu, Z.; Dai, L.; Zhang, M.; Wang, L.; Zhao, J.; Liu, M. Genome-wide identification of CNGC genes in Chinese jujube (Ziziphus jujuba Mill.) and ZjCNGC2 mediated signalling cascades in response to cold stress. BMC Genom. 2020, 21, 191. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Rashid, B.; Ali, Q.; Husnain, T. Mechanisms of heat sensing and responses in plants. It is not all about calcium ions. Biol. Plant. 2018, 62, 409–420. [Google Scholar] [CrossRef]
- Wu, J.; Nadeem, M.; Galagedara, L.; Thomas, R.; Cheema, M. Recent insights into cell responses to cold stress in plants: Signaling, defence, and potential functions of phosphatidic acid. Environ. Exp. Bot. 2022, 203, 105068. [Google Scholar] [CrossRef]
- D’ambrosio, J.M.; Gonorazky, G.; Sueldo, D.J.; Moraga, J.; Di Palma, A.A.; Lamattina, L.; Collado, I.G.; Laxalt, A.M. The sesquiterpene botrydial from Botrytis cinerea induces phosphatidic acid production in tomato cell suspensions. Planta 2018, 247, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, X.; Li, W. Exogenously-supplied trehalose inhibits the growth of wheat seedlings under high temperature by affecting plant hormone levels and cell cycle processes. Plant Signal. Behav. 2021, 16, 1907043. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cao, X.; Deng, X. Epigenetic and transcription factors synergistically promote the high temperature response in plants. Trends Biochem. Sci. 2023, 48, 788–800. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.; Han, C.; Wang, S.; Bai, M.; Song, C. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, H.; Paulo, M.C. Genetic Analysis on Brassica oleracea Breeding Traits. Doctoral Thesis, Wageningen University, Wageningen, The Netherlands, 2018. [Google Scholar]
- Friedrich, T.; Oberkofler, V.; Trindade, I.; Altmann, S.; Brzezinka, K.; Lämke, J.; Gorka, M.; Kappel, C.; Sokolowska, E.; Skirycz, A. Heteromeric HSFA2/HSFA3 complexes drive transcriptional memory after heat stress in Arabidopsis. Nat. Commun. 2021, 12, 3426. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jiang, J.; Sun, S.; Wang, X. Brassinosteroids promote thermotolerance through releasing BIN2-mediated phosphorylation and suppression of HsfA1 transcription factors in Arabidopsis. Plant Commun. 2022, 3, 100419. [Google Scholar] [CrossRef] [PubMed]
- Kozeko, L.Y. The Role of HSP90 Chaperones in Stability and Plasticity of ontogenesis of plants under normal and stressful conditions (Arabidopsis thaliana). Cytol. Genet. 2019, 53, 143–161. [Google Scholar] [CrossRef]
- Han, D.; Yu, Z.; Lai, J.; Yang, C. Post-translational modification: A strategic response to high temperature in plants. aBIOTECH 2022, 3, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Boehning, M.; Hummel, B.; Aprile-Garcia, F.; Pandit, A.S.; Eisenhardt, N.; Khavaran, A.; Niskanen, E.; Vos, S.M.; Palvimo, J.J.; et al. Stress-induced nuclear condensation of NELF drives transcriptional downregulation. Mol. Cell 2021, 81, 1013–1026.e11. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef]
- Choudry, M.W.; Riaz, R.; Nawaz, P.; Ashraf, M.; Ijaz, B.; Bakhsh, A. CRISPR-Cas9 mediated understanding of plants’ abiotic stress-responsive genes to combat changing climatic patterns. Funct. Integr. Genom. 2024, 24, 132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhu, Z. Transcription factors in the regulation of plant heat responses. Crit. Rev. Plant Sci. 2023, 42, 385–398. [Google Scholar] [CrossRef]
- Jha, U.C.; Nayyar, H.; Roychowdhury, R.; Prasad, P.V.; Parida, S.K.; Siddique, K.H. Non-coding RNAs (ncRNAs) in plants: Master regulators for adapting to extreme temperature conditions. Plant Physiol. Biochem. 2023, 205, 108164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant responses to heat stress: Physiology, transcription, noncoding RNAs, and epigenetics. Int. J. Mol. Sci. 2020, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Jha, U.C.; Nayyar, H.; Jha, R.; Khurshid, M.; Zhou, M.; Mantri, N.; Siddique, K.H. Long non-coding RNAs: Emerging players regulating plant abiotic stress response and adaptation. BMC Plant Biol. 2020, 20, 466. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Priming crops for the future: Rewiring stress memory. Trends Plant Sci. 2021, 27, 699–716. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, X.; Li, J.; Dang, S.; Ma, H.; Zhang, Y. Comparative transcriptomic and metabolomic analyses provide insights into the responses to high temperature stress in Alfalfa (Medicago sativa L.). BMC Plant Biol. 2024, 24, 776. [Google Scholar] [CrossRef]
- Ghosh, S.; Adhikari, S.; Adhikari, A.; Hossain, Z. Contribution of plant miRNAome studies towards understanding heavy metal stress responses: Current status and future perspectives. Environ. Exp. Bot. 2021, 194, 104705. [Google Scholar] [CrossRef]
- Ruan, M.; Zhao, H.; Wen, Y.; Chen, H.; He, F.; Hou, X.; Song, X.; Jiang, H.; Ruan, Y.-L.; Wu, L. The complex transcriptional regulation of heat stress response in maize. Stress Biol. 2024, 4, 24. [Google Scholar] [CrossRef]
- Verma, N.; Giri, S.K.; Singh, G.; Gill, R.; Kumar, A. Epigenetic regulation of heat and cold stress responses in crop plants. Plant Gene 2022, 29, 100351. [Google Scholar] [CrossRef]
- Entrambasaguas, L.; Ruocco, M.; Verhoeven, K.J.F.; Procaccini, G.; Marín-Guirao, L. Gene body DNA methylation in seagrasses: Inter- and intraspecific differences and interaction with transcriptome plasticity under heat stress. Sci. Rep. 2021, 11, 14343. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Du, X.; Hu, H.; Du, J. Molecular mechanisms of the RNA polymerases in plant RNA-directed DNA methylation. Trends Biochem. Sci. 2023, 49, 247–256. [Google Scholar] [CrossRef]
- Pecinka, A.; Chevalier, C.; Colas, I.; Kalantidis, K.; Varotto, S.; Krugman, T.; Michailidis, C.; Vallés, M.-P.; Muñoz, A.; Pradillo, M. Chromatin dynamics during interphase and cell division: Similarities and differences between model and crop plants. J. Exp. Bot. 2020, 71, 5205–5222. [Google Scholar] [CrossRef]
- Ruberti, C.; Lai, Y.; Brandizzi, F. Recovery from temporary endoplasmic reticulum stress in plants relies on the tissue-specific and largely independent roles of bZIP 28 and bZIP 60, as well as an antagonizing function of BAX-Inhibitor 1 upon the pro-adaptive signaling mediated by bZIP 28. Plant J. 2018, 93, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, A.; Hu, C.; Li, Y. Current advances and future prospects of ER stress signaling and its chemical mitigation in plants. Plant Growth Regul. 2024, 104, 89–93. [Google Scholar] [CrossRef]
- Yang, Z.-T.; Fan, S.-X.; Wang, J.-J.; An, Y.; Guo, Z.-Q.; Li, K.; Liu, J.-X. The plasma membrane-associated transcription factor NAC091 regulates unfolded protein response in Arabidopsis thaliana. Plant Sci. 2023, 334, 111777. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Choudhury, S.R. Heterotrimeric G-proteins mediated hormonal responses in plants. Cell. Signal. 2020, 76, 109799. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, M.-J.; Wang, J.-J.; Lu, H.-P.; Liu, J.-X. bZIP17 regulates heat stress tolerance at reproductive stage in Arabidopsis. aBIOTECH 2021, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Locato, V.; Cimini, S.; De Gara, L. ROS and redox balance as multifaceted players of cross-tolerance: Epigenetic and retrograde control of gene expression. J. Exp. Bot. 2018, 69, 3373–3391. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, J.-F.; Kan, Y.; Shan, J.-X.; Ye, W.-W.; Dong, N.-Q.; Guo, T.; Xiang, Y.-H.; Yang, Y.-B.; Li, Y.-C.; et al. A genetic module at one locus in rice protects chloroplasts to enhance thermotolerance. Science 2022, 376, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Vertegaal, A.C.O. Signalling mechanisms and cellular functions of SUMO. Nat. Rev. Mol. Cell Biol. 2022, 23, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Ziehe, D.; Dünschede, B.; Schünemann, D. Molecular mechanism of SRP-dependent light-harvesting protein transport to the thylakoid membrane in plants. Photosynth. Res. 2018, 138, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Siegel, A.; Shan, S.-O.; Grimm, B.; Wang, P. Chloroplast SRP43 autonomously protects chlorophyll biosynthesis proteins against heat shock. Nat. Plants 2021, 7, 1420–1432. [Google Scholar] [CrossRef]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 2020, 44, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, Q.; Wu, Y.; Huang, F.; Ismayil, A.; Zhang, D.; Li, H.; Gu, H.; Ludman, M.; Fátyol, K.; et al. A calmodulin-binding transcription factor links calcium signaling to antiviral RNAi defense in plants. Cell Host Microbe 2021, 29, 1393–1406.e7. [Google Scholar] [CrossRef] [PubMed]
- Cejudo, F.J.; González, M.-C.; Pérez-Ruiz, J.M. Redox regulation of chloroplast metabolism. Plant Physiol. 2020, 186, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Beaugelin, I.; Chevalier, A.; D’Alessandro, S.; Ksas, B.; Havaux, M. Endoplasmic reticulum-mediated unfolded protein response is an integral part of singlet oxygen signalling in plants. Plant J. 2020, 102, 1266–1280. [Google Scholar] [CrossRef]
- Lee, K.P.; Kim, C. Photosynthetic ROS and retrograde signaling pathways. New Phytol. 2024, 244, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, N.; Li, R.; Ma, X.; Zhang, L. Comparative transcriptome and flavonoids components analysis reveal the structural genes responsible for the yellow seed coat color of Brassica rapa L. PeerJ 2021, 9, e10770. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liao, L.; Xie, S.; Yao, M.; Xie, P.; Liu, W.; Kang, Y.; Huang, L.; Wang, M.; Qian, L.; et al. Comprehensive analyses of the annexin (ANN) gene family in Brassica rapa, Brassica oleracea and Brassica napus reveals their roles in stress response. Sci. Rep. 2020, 10, 4295. [Google Scholar] [CrossRef] [PubMed]
- Hussain, J.; Bibi, G.; Shafiq, S. Calcium Dependent Protein Kinases in Plants. In Protein Kinases and Stress Signaling in Plants; Pandey, G.K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Guo, Y.; Sun, Y.; Wang, Z.; Wang, Y.; Guan, L.; Wang, L.; Zhou, Q. Transcriptome and metabolome integrated analysis revealed the effects and potential mechanism of hydrogen peroxide on antioxidant system in postharvest broccoli. Postharvest Biol. Technol. 2023, 206, 112547. [Google Scholar] [CrossRef]
- Qin, M.; Li, H.; Zhao, N.; Zhang, Y.; Zhang, B.; Liang, F.; Zuo, K.; Guo, N.; Tao, S.; Liu, X.; et al. Integrated genomics, QTL mapping, and co-expression analyses identifying candidates of low-temperature tolerance in Brassica napus L. Ind. Crop. Prod. 2022, 187, 115437. [Google Scholar] [CrossRef]
- Qin, M.; Li, H.; Guo, Z.; Zhu, Y.; Wang, R.; Zhang, M.; Zhang, Q.; Xu, Y.; Song, J.; Huang, Z.; et al. Phenotypic damage and transcriptomic responses of flower buds in rapeseed (Brassica napus L.) under low-temperature stress. Ind. Crop. Prod. 2023, 198, 116669. [Google Scholar] [CrossRef]
- Ray, S.D. Ca2+, The Miracle Molecule in Plant Hormone Signaling During Abiotic Stress. In Mechanism of Plant Hormone Signaling Under Stress; Pandey, G.K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Rao, S.; Gupta, A.; Bansal, C.; Sorin, C.; Crespi, M.; Mathur, S. A conserved HSF: miR169: NF-YA loop involved in tomato and Arabidopsis heat stress tolerance. Plant J. 2022, 112, 7–26. [Google Scholar] [CrossRef] [PubMed]
- Bansal, C.; Balyan, S.; Mathur, S. Inferring the regulatory network of the miRNA-mediated response to individual and combined heat and drought stress in tomato. J. Plant Biochem. Biotechnol. 2021, 30, 862–877. [Google Scholar] [CrossRef]
- Mondal, S.; Karmakar, S.; Panda, D.; Pramanik, K.; Bose, B.; Singhal, R.K. Crucial plant processes under heat stress and tolerance through heat shock proteins. Plant Stress 2023, 10, 100227. [Google Scholar] [CrossRef]
- Rao, S.; Das, J.R.; Mathur, S. Exploring the master regulator heat stress transcription factor HSFA1a-mediated transcriptional cascade of HSFs in the heat stress response of tomato. J. Plant Biochem. Biotechnol. 2021, 30, 878–888. [Google Scholar] [CrossRef]
- Song, N.; Wang, J.; Qin, Q.; Su, A.; Cheng, Y.; Si, W.; Cheng, B.; Fan, J.; Jiang, H. ZmHSFA2B self-regulatory loop is critical for heat tolerance in maize. Plant Biotechnol. J. 2024, 23, 284–301. [Google Scholar] [CrossRef]
- Pandey, V.; Singh, S. Plant Adaptation and Tolerance to Heat Stress: Advance Approaches and Future Aspects. Comb. Chem. High Throughput Screen. 2024, 27, 1701–1715. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batool, I.; Ayyaz, A.; Qin, T.; Wu, X.; Chen, W.; Hannan, F.; Zafar, Z.U.; Naeem, M.S.; Farooq, M.A.; Zhou, W. Morphological, Physiological, and Molecular Responses to Heat Stress in Brassicaceae. Plants 2025, 14, 152. https://doi.org/10.3390/plants14020152
Batool I, Ayyaz A, Qin T, Wu X, Chen W, Hannan F, Zafar ZU, Naeem MS, Farooq MA, Zhou W. Morphological, Physiological, and Molecular Responses to Heat Stress in Brassicaceae. Plants. 2025; 14(2):152. https://doi.org/10.3390/plants14020152
Chicago/Turabian StyleBatool, Iram, Ahsan Ayyaz, Tongjun Qin, Xiaofen Wu, Weiqi Chen, Fakhir Hannan, Zafar Ullah Zafar, Muhammad Shahbaz Naeem, Muhammad Ahsan Farooq, and Weijun Zhou. 2025. "Morphological, Physiological, and Molecular Responses to Heat Stress in Brassicaceae" Plants 14, no. 2: 152. https://doi.org/10.3390/plants14020152
APA StyleBatool, I., Ayyaz, A., Qin, T., Wu, X., Chen, W., Hannan, F., Zafar, Z. U., Naeem, M. S., Farooq, M. A., & Zhou, W. (2025). Morphological, Physiological, and Molecular Responses to Heat Stress in Brassicaceae. Plants, 14(2), 152. https://doi.org/10.3390/plants14020152





