Structure of Plant Populations in Constructed Wetlands and Their Ability for Water Purification
Abstract
:1. Introduction
2. Results
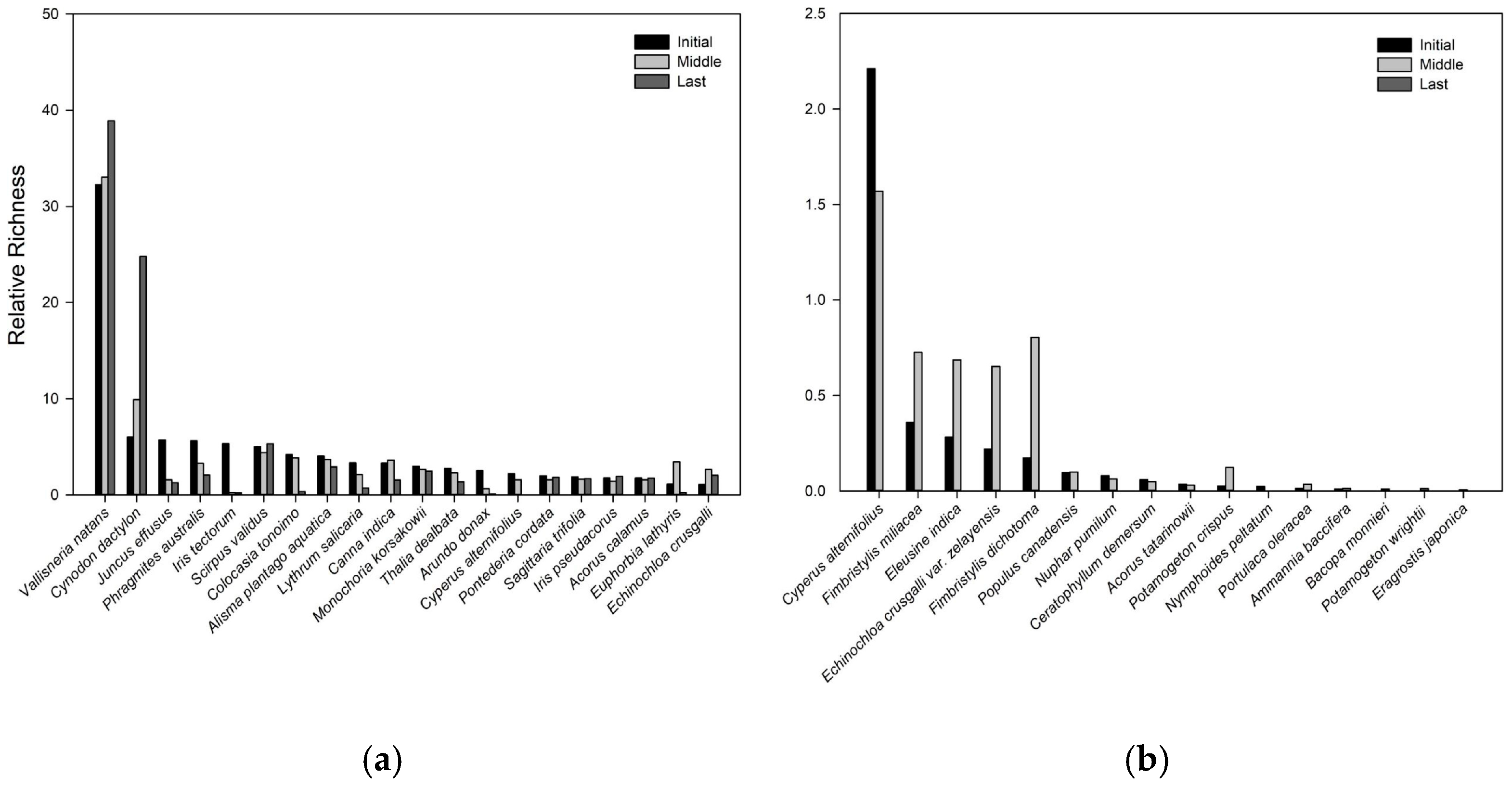
2.1. Plant Species Composition in CW
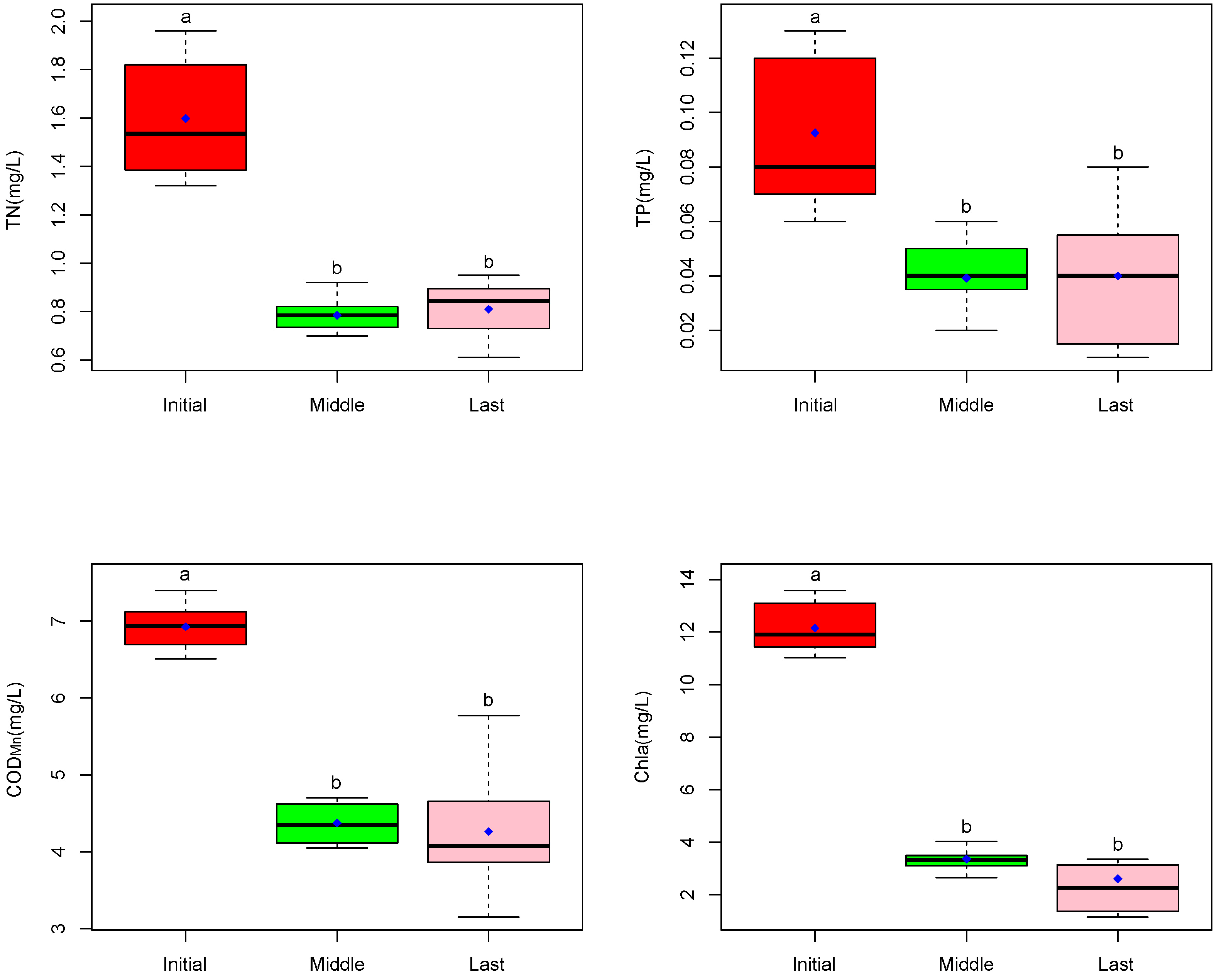
2.2. Species Diversity and Purification Effects of CW
2.3. The Relationship Between Environmental Data and Plant Species in CW
3. Discussion
4. Materials and Methods
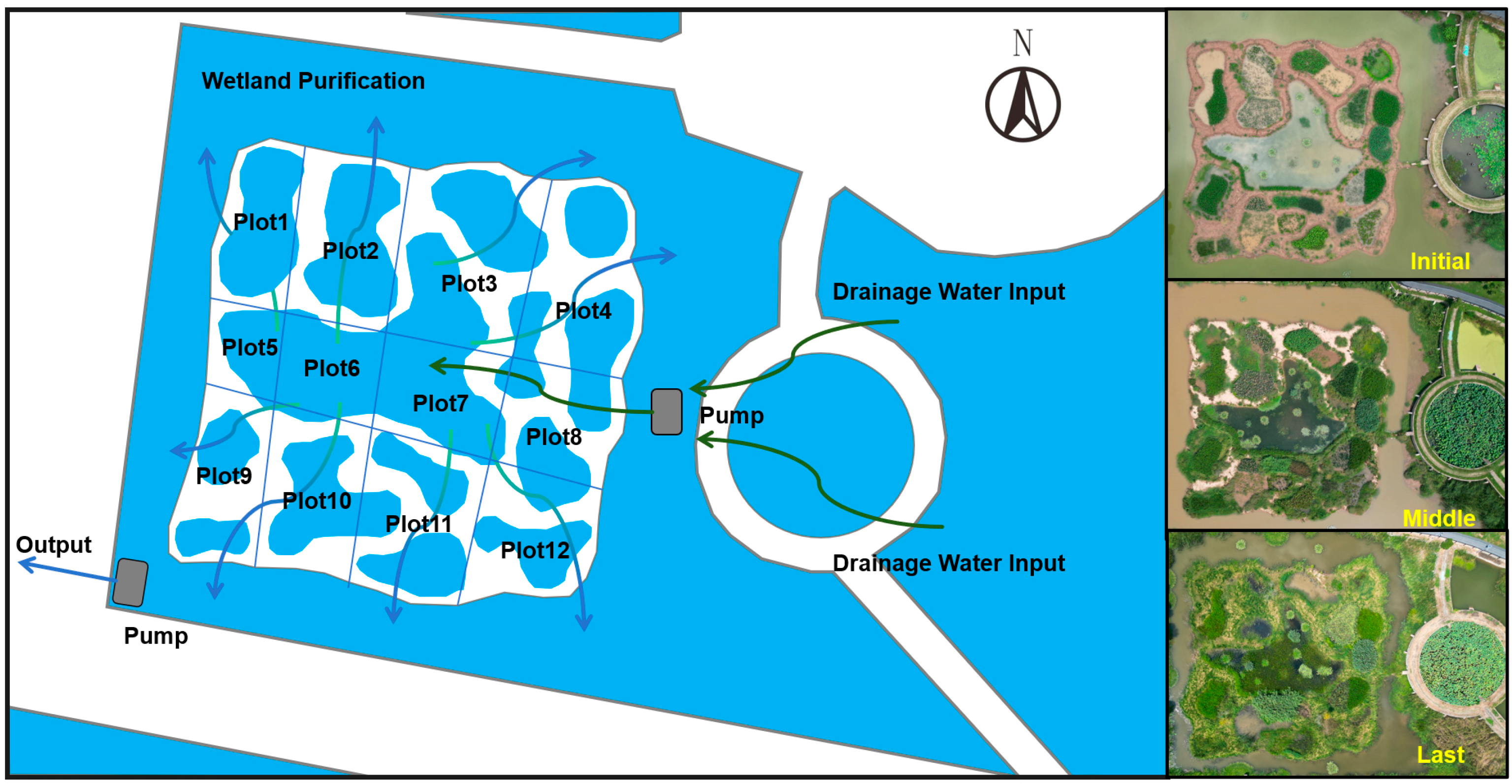
4.1. Study Site and Experimental Design
4.2. Data Collection
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Licata, M.; Gennaro, M.C.; Tuttolomondo, T.; Leto, C.; La Bella, S. Research Focusing on Plant Performance in Constructed Wetlands and Agronomic Application of Treated Wastewater-A Set of Experimental Studies in Sicily (Italy). PLoS ONE 2019, 14, e0219445. [Google Scholar] [CrossRef]
- Parde, D.; Patwa, A.; Shukla, A.; Vijay, R.; Killedar, D.J.; Kumar, R. A Review of Constructed Wetland on Type, Treatment and Technology of Wastewater. Environ. Technol. Innov. 2021, 21, 101261. [Google Scholar] [CrossRef]
- Vymazal, J. The Historical Development of Constructed Wetlands for Wastewater Treatment. Land 2022, 11, 174. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Ngo, H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A Review on the Sustainability of Constructed Wetlands for Wastewater Treatment: Design and Operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef]
- Wu, H.; Wang, R.; Yan, P.; Wu, S.; Chen, Z.; Zhao, Y.; Cheng, C.; Hu, Z.; Zhuang, L.; Guo, Z.; et al. Constructed Wetlands for Pollution Control. Nat. Rev. Earth Environ. 2023, 4, 218–234. [Google Scholar] [CrossRef]
- Choudhury, M.I.; Segersten, J.; Hellman, M.; Mckie, B.G.; Hallin, S.; Ecke, F. Importance of Plant Species for Nitrogen Removal Using Constructed Floating Wetlands in a Cold Climate. Ecol. Eng. 2019, 138, 126–132. [Google Scholar] [CrossRef]
- Jeppesen, E.; Peder Jensen, J.; Søndergaard, M.; Lauridsen, T.; Junge Pedersen, L.; Jensen, L. Top-down Control in Freshwater Lakes: The Role of Nutrient State, Submerged Macrophytes and Water Depth. In Shallow Lakes ’95; Kufel, L., Prejs, A., Rybak, J.I., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 151–164. [Google Scholar]
- Patyal, V.; Jaspal, D.; Khare, K. Materials in Constructed Wetlands for Wastewater Remediation: A Review. Water Environ. Res. 2021, 93, 2853–2872. [Google Scholar] [CrossRef] [PubMed]
- Nuamah, L.A.; Li, Y.; Pu, Y.; Nwankwegu, A.S.; Haikuo, Z.; Norgbey, E.; Banahene, P.; Bofah-Buoh, R. Constructed Wetlands, Status, Progress, and Challenges. The Need for Critical Operational Reassessment for a Cleaner Productive Ecosystem. J. Clean. Prod. 2020, 269, 122340. [Google Scholar] [CrossRef]
- García-Ávila, F.; Patiño-Chávez, J.; Zhinín-Chimbo, F.; Donoso-Moscoso, S.; Flores del Pino, L.; Avilés-Añazco, A. Performance of Phragmites Australis and Cyperus Papyrus in the Treatment of Municipal Wastewater by Vertical Flow Subsurface Constructed Wetlands. Int. Soil Water Conserv. Res. 2019, 7, 286–296. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Ho, S.; Wang, L.; Yang, J. Effect of Plant Species Compositions on Performance of Lab-Scale Constructed Wetland through Investigating Photosynthesis and Microbial Communities. Bioresour. Technol. 2017, 229, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Białowiec, A.; Davies, L.; Albuquerque, A.; Randerson, P.F. Nitrogen Removal from Landfill Leachate in Constructed Wetlands with Reed and Willow: Redox Potential in the Root Zone. J. Environ. Manag. 2012, 97, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Zaplata, M.; Winter, S.; Fischer, A.; Kollmann, J.; Ulrich, W. Species-Driven Phases and Increasing Structure in Early-Successional Plant Communities. Am. Nat. 2013, 181, E17–E27. [Google Scholar] [CrossRef]
- Zajac, R.N.; Whitlatch, R.B.; Thrush, S.F. Recolonization and Succession in Soft-Sediment Infaunal Communities: The Spatial Scale of Controlling Factors. Hydrobiologia 1998, 375, 227–240. [Google Scholar] [CrossRef]
- Mori, A.S.; Furukawa, T.; Sasaki, T. Response Diversity Determines the Resilience of Ecosystems to Environmental Change. Biol. Rev. 2013, 88, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Naiman, R.J.; Decamps, H. The Ecology of Interfaces: Riparian Zones. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef]
- Redoglio, A.; Sperfeld, E. What drives growth responses of nitrogen and phosphorus (co-) limited primary producer communities? Front. Ecol. Evol. 2024, 12, 1368445. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, H.-J.; Wang, H.-Z.; Li, Y.; Liang, X.-M.; Xu, C.; Jeppesen, E. Does the Responses of Vallisneria Natans (Lour.) Hara to High Nitrogen Loading Differ between the Summer High-Growth Season and the Low-Growth Season? Sci. Total Environ. 2017, 601, 1513–1521. [Google Scholar] [CrossRef]
- Lin, Q.; Fan, M.; Peng, X.; Ma, J.; Zhang, Y.; Yu, F.; Wu, Z.; Liu, B. Response of Vallisneria Natans to Aluminum Phytotoxicity and Their Synergistic Effect on Nitrogen, Phosphorus Change in Sediments. J. Hazard. Mater. 2020, 400, 123167. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Zheng, X.; Huang, P.; Li, P.; You, J. Long-Term Improvement of Sediment in Situ Restoration and REDOX Characteristics by Vallisneria Natans Coupling with Carbon Fiber. Ecotoxicol. Environ. Saf. 2023, 266, 115547. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Sun, J.; Chen, Y.; Cao, Y. High Density Promotes the Germination of the Recalcitrant Seeds of Submerged Macrophyte Vallisneria Natans. Aquat. Bot. 2024, 195, 103810. [Google Scholar] [CrossRef]
- Zhi, Y.; Cao, Y.; Sun, J.; Li, W.; Jeppesen, E. Indirect Effects of Extreme Precipitation on the Growth of Vallisneria Denseserrulata Makino. Environ. Exp. Bot. 2018, 153, 229–235. [Google Scholar] [CrossRef]
- Yan, P.; Peng, Y.; Fan, Y.; Zhang, M.; Chen, J.; Gu, X.; Sun, S.; He, S. Effects of Ferrous Addition to Vallisneria Natans: An Attempt to Apply Ferrous to Submerged Macrophyte Restoration. Environ. Res. 2023, 237, 117022. [Google Scholar] [CrossRef]
- Zuo, J.-C.; Liu, L.-D.; Kong, D.-R.; Zhang, S.-M.; Hu, D.-C.; Xie, W.-H. Effects of Sediment Burial on Seed Germination and Seedling Establishment of Vallisneria Natans. J. Freshw. Ecol. 2016, 31, 289–297. [Google Scholar] [CrossRef]
- Tan, S.-D.; Zhang, S.-J.; Zhang, K.-R.; Dang, H.-S.; Li, M.; Zhang, Q.-F. Effect of Long-Time and Deep Submergence on Recovery Growth and Photosynthesis of Three Grass Species in Three Gorges Reservoir Area. Plant Sci. J. 2009, 27, 391–396. [Google Scholar]
- Yu, S.; Lai, S.; Wang, S.; Pan, J.; Fu, C.; Wang, S. On Effect of Copper Stress on Physiological Characteristics in Suitable Plant of Cynodon Dactylon and Its Cu Uptake in the Three Gorges Reservoir Area. J. Southwest China Norm. Univ. Nat. Sci. Ed. 2013, 38, 64–69. [Google Scholar]
- Li, Q. Influence of Sand Burial and Drought on Growth Recovery of Cynodon Dactylon in a Water-Level-Fluctuating Zone of the Three Gorges Reservoir. Acta Ecol. Sin. 2016, 36, 200–208. [Google Scholar]
- Li, Q.; Ding, W.; Wang, S.; Zhu, Q.; Yang, J.; Ke, S.; Qin, L.; Yang, L.; Zheng, J.; Meng, Y. Influence of Multi-Year High Water Level Running on Growth Recovery of Cynodon Dactylon Population in Water-Level-Fluctuating Zone of the Three Gorges Reservoir. Acta Ecol. Sin. 2020, 40, 985–992. [Google Scholar]
- Zhang, P.; Wang, P.; Jin, S. Study on the Morphology and Mechanical Properties of Cynodon Dactylon in the Riparian Zone Slopes of a Large Reservoir. Appl. Sci. 2024, 14, 2888. [Google Scholar] [CrossRef]
- Zaplata, M.K.; Nhabanga, A.; Stalmans, M.; Volpers, T.; Burkart, M.; Sperfeld, E. Grasses cope with high-contrast ecosystem conditions in thelarge outflow of the Banhine wetlands, Mozambique. Afr. J. Ecol. 2021, 59, 190–203. [Google Scholar] [CrossRef]
- Yamauchi, T.; Sumi, K.; Morishita, H.; Nomura, Y. Root Anatomical Plasticity Contributes to the Different Adaptive Responses of Two Phragmites Species to Water-Deficit and Low-Oxygen Conditions. Funct. Plant Biol. 2024, 51, FP23231. [Google Scholar] [CrossRef]
- Walsh, G.E.; Weber, D.E.; Nguyen, M.T.; Esry, L.K. Responses of Wetland Plants to Effluents in Water and Sediment. Environ. Exp. Bot. 1991, 31, 351–358. [Google Scholar] [CrossRef]
- Clarke, E.; Baldwin, A.H. Responses of Wetland Plants to Ammonia and Water Level. Ecol. Eng. 2002, 18, 257–264. [Google Scholar] [CrossRef]
- Tang, H.; Dai, Y.; Fan, Y.; Song, X.; Wang, F.; Liang, W. Effect of Vallisneria Spiralis on Water Quality and Sediment Nitrogen at Different Growth Stages in Eutrophic Shallow Lake Mesocosms. Pol. J. Environ. Stud. 2021, 30, 2341–2351. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; Ortega-Pineda, G.; Zitácuaro-Contreras, I.; Vidal-Álvarez, M.; Martínez-Aguilar, K.E.; Álvarez-Hernández, L.M.; Zamora-Castro, S. Removal of Nitrogen, Phosphates, and Chemical Oxygen Demand from Community Wastewater by Using Treatment Wetlands Planted with Ornamental Plants in Different Mineral Filter Media. Nitrogen 2024, 5, 903–914. [Google Scholar] [CrossRef]
- Saeed, T.; Sun, G. A Comparative Study on the Removal of Nutrients and Organic Matter in Wetland Reactors Employing Organic Media. Chem. Eng. J. 2011, 171, 439–447. [Google Scholar] [CrossRef]
- Wu, H.; Li, W.; Zhang, J.; Li, C.; Zhang, J.; Xie, H. Application of Using Surface Constructed Wetland for Removal of Chemical Oxygen Demand and Ammonium in Polluted River Water. Desalin. Water Treat. 2012, 44, 142–150. [Google Scholar] [CrossRef]
- Ruan, W.; Cai, H.; Xu, X.; Man, Y.; Wang, R.; Tai, Y.; Chen, Z.; Vymazal, J.; Chen, J.; Yang, Y.; et al. Efficiency and Plant Indication of Nitrogen and Phosphorus Removal in Constructed Wetlands: A Field-Scale Study in a Frost-Free Area. Sci. Total Environ. 2021, 799, 149301. [Google Scholar] [CrossRef]
- Scheffer, M. Ecology of Shallow Lakes; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Van Dyke, M.N.; Levine, J.M.; Kraft, N.J. Small Rainfall Changes Drive Substantial Changes in Plant Coexistence. Nature 2022, 611, 507–511. [Google Scholar] [CrossRef]
- Huang, J.; Kankanamge, N.R.; Chow, C.; Welsh, D.T.; Li, T.; Teasdale, P.R. Removing Ammonium from Water and Wastewater Using Cost-Effective Adsorbents: A Review. J. Environ. Sci. 2018, 63, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, F.G.; Legendre, P.; Borcard, D. Forward Selection of Explanatory Variables. Ecology 2008, 89, 2623–2632. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Xian, L.; Liu, F. Structure of Plant Populations in Constructed Wetlands and Their Ability for Water Purification. Plants 2025, 14, 162. https://doi.org/10.3390/plants14020162
Yu J, Xian L, Liu F. Structure of Plant Populations in Constructed Wetlands and Their Ability for Water Purification. Plants. 2025; 14(2):162. https://doi.org/10.3390/plants14020162
Chicago/Turabian StyleYu, Junshuang, Ling Xian, and Fan Liu. 2025. "Structure of Plant Populations in Constructed Wetlands and Their Ability for Water Purification" Plants 14, no. 2: 162. https://doi.org/10.3390/plants14020162
APA StyleYu, J., Xian, L., & Liu, F. (2025). Structure of Plant Populations in Constructed Wetlands and Their Ability for Water Purification. Plants, 14(2), 162. https://doi.org/10.3390/plants14020162





