Responses of the Leaf Characteristics of Nymphoides peltata to a Water Depth Gradient in the Qionghai Lake, Western Sichuan Plateau, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Experimental Methods and Design
2.2.1. Measurement of N. peltata Leaf Morphological Characteristics
2.2.2. Measurement of Water Salinity and pH
2.3. Statistical Analysis
3. Results
3.1. Physical and Chemical Characteristics of Water
3.2. Stand Characteristics of Nymphoides peltata
3.3. Main Characteristics (Leaf and Leaf Petiole) of Nymphoides peltata Along a Water Depth Gradient
3.4. Results of the RDA of the Leaf Traits of and Environmental Factors Affecting Nymphoides peltata
3.5. Correlation Analysis of the Leaf Characteristics of Nymphoides peltata
4. Discussion
4.1. Response of N. peltata Leaf Traits to Differences in Water Depth in Shallow Water Areas
4.2. Response of the Leaf Traits of the Floating Heart-Growing N. peltata to Differences in Water Depth in Deep-Water Areas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Liu, H.M.; Kou, X.; Xu, Z.C.; Yu, X.W.; Cap, X.A.; Wen, L.; Ma, L.Q.; Wang, L.X. Functional traits of dominant plants and their adaptations in lakeshore wetlands of the Inner Mongolia Plateau. Acta Ecol. Sin. 2022, 42, 7773–7789. [Google Scholar] [CrossRef]
- Suding, K.N.; Lavorel, S.; Chapin, F.S.; Cornelissen, J.H.C.; Díaz, S.; Garnier, E.; Goldberg, D.; Navas, M.-L. Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Change Biol. 2008, 14, 1125–1140. [Google Scholar] [CrossRef]
- Limberger, R.; Daugaard, U.; Gupta, A.; Krug, R.M.; Lemmen, K.D.; van Moorsel, S.J.; Suleiman, M.; Zuppinger-Dingley, D.; Petchey, O.L. Functional diversity can facilitate the collapse of an undesirable ecosystem state. Ecol. Lett. 2023, 26, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Felix, J.A.; Stevenson, P.C.; Koricheva, J. Plant neighbourhood diversity effects on leaf traits: A meta-analysis. Funct. Ecol. 2023, 37, 3150–3163. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Rolland, G.; Dauzat, M.; Bédiée, A.; Baldazzi, V.; Bertin, N.; Guédon, Y.; Granier, C. Are compound leaves more complex than simple ones? A multi-scale analysis. Ann. Bot. 2018, 122, 1173–1185. [Google Scholar] [CrossRef]
- Anderegg, L.D.L.; Loy, X.; Markham, I.P.; Elmer, C.M.; Hovenden, M.J.; HilleRisLambers, J.; Mayfield, M.M. Aridity drives coordinated trait shifts but not decreased trait variance across the geographic range of eight Australian trees. New Phytol. 2021, 229, 1375–1387. [Google Scholar] [CrossRef]
- He, Y.Q.; Shi, X.J.; Chen, G.J.; Lai, M.Y.; Zeng, J.Y.; Wei, K.; Deng, C.Y. Response and adaptation of leaf functional traits of Eurya emarginata to environmental factors. Acta Ecol. Sin. 2022, 46, 2418–2429. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, X.; Li, D.; Xu, G.; Guo, Y. Spatial heterogeneity of vegetation extent and the response to water level fluctuations and micro-topography in Poyang Lake, China. Ecol. Indicat. 2021, 124, 107420. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Gao, W.J.; Yuan, S.B.; Jiang, X.; Zhao, Y.; Cui, Y.; Wang, H. Effects of sub-monthly and sub-daily water level variations on water level fluctuation requirements of Phragmites australis and Phalaris arundinacea. Water Biol. Secur. 2024, 4, 100317. [Google Scholar] [CrossRef]
- Zhu, G.R.; Li, W.; Zhang, M.; Ni, L.Y.; Wang, S.R. Adaptation of submerged macrophytes to both water depth and flood intensity as revealed by their mechanical resistance. Hydrobiologia 2012, 696, 77–93. [Google Scholar] [CrossRef]
- Wei, H.; He, F.; Xu, D.; Zhou, Q.; Xiao, E.; Zhang, L.; Wu, Z. A comparison of the growth and photosynthetic response of Vallisneria natans (Lour.) Hara a long-term water depth gradient under flowing static water. J. Freshw. Ecol. 2018, 33, 223–237. [Google Scholar] [CrossRef]
- Luo, W.P.; Xie, Y.H.; Song, F.B. Survival strategies of wetland plants in flooding environments. Chin. J. Ecol. 2007, 26, 1478–1485. [Google Scholar] [CrossRef]
- Hussner, A.; Meyer, C.; Busch, J. The influence of water level and nutrient availability on growth and root system development of Myriophyllum aquaticum. Weed Res. 2009, 49, 73–80. [Google Scholar] [CrossRef]
- Wang, L.H.; Yang, L.; Liu, L.; He, L.; Jiang, W.X.; Shen, H.L.; Zhu, T.S.; Pan, B.Z. Species diversity and functional diversity of submerged vegetation community in response to water depth gradient in Nansi Lake, China. Acta Ecol. Sin. 2020, 40, 6233–6242. [Google Scholar] [CrossRef]
- Wang, L.; Hu, X.Q.; Zhang, Z.L. Physiological and ecological adaptation strategies of Vallisneria natans to different water depths and sediments. Chin. J. Ecol. 2021, 40, 2421–2430. [Google Scholar] [CrossRef]
- Yao, J.; Li, Y.; Wei, L.P.; Jiang, S.S.; Yang, Y.S.; Hou, J.H. Changes of allometric relationships among leaf traits in different ontogenetic stages of Acer mono from different types of forests in Donglingshan of Beijing. Acta Ecol. Sin. 2013, 33, 3907–3915. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Richards, J.H.; Troxler, T.G.; Lee, D.W.; Zimmerman, M.S. Experimental determination of effects of water depth on Nymphaea odorata growth, morphology and biomass allocation. Aquat. Bot. 2011, 95, 9–16. [Google Scholar] [CrossRef]
- Mohammadi, B.; Ahmadi, F.; Mehdizadeh, S.; Guan, Y.; Pham, Q.B.; Linh, N.T.T.; Tri, D.Q. Developing Novel Robust Models to Improve the Accuracy of Daily Streamflow Modeling. Water Resour. Manag. 2020, 34, 3387–3409. [Google Scholar] [CrossRef]
- Mohammadi, B.; Guan, Y.; Aghelpour, P.; Emamgholizadeh, S.; Pillco Zol’a, R.; Zhang, D. Simulation of Titicaca lake water level fluctuations using hybrid machine learning technique integrated with grey wolf optimizer algorithm. Water 2020, 12, 3015. [Google Scholar] [CrossRef]
- Zhang, P.X.; Zhao, C.Z.; Huang, C.L.; Li, G.Y.; Wu, X.S.; Wang, S.H.; Liu, D.Y. Changes in Soil Organic Carbon Content Under Different Inundation Gradients in Peat Bogs on the China’s Qinghai-Tibet Plateau. Wetlands 2023, 43, 104. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, C.Z.; Wang, J.W.; Wen, J.; Li, Z.Q.; Ma, J.Y. Morphological and photosynthetic physiological characteristics of Saussurea salsa in response to flooding in salt marshes of Xiao Sugan Lake, Gansu, China. Chin. J. Plant Ecol. 2019, 43, 685–696. [Google Scholar] [CrossRef]
- Yu, L.F.; Yu, D. Differential responses of the floating-leaved aquatic plant Nymphoides peltata to gradual versus rapid increases in water levels. Aquat. Bot. 2011, 94, 71–76. [Google Scholar] [CrossRef]
- Catia, G.; Scremin-Dias, E. Compared leaf anatomy of Nymphaea (Nymphaeaceae) species from Brazilian flood plain. Braz. J. Biol. 2013, 73, 809–817. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, D.; Ji, X.S.; Zhang, S.J.; Huang, Q.; Wang, W.L. Effects of water depth gradient on physiological characteristics of Vallisneria natans. J. Freshw. Ecol. 2019, 34, 405–417. [Google Scholar] [CrossRef]
- Xin, K.J.; Cao, Y.; Xie, Q.Z.; Liang, R.H.; Huang, H.X.; Chen, Y.T.; Qi, J.J. Effects of water level changes on the morphological and physiology of the submerged macrophyte Vallisneria natans. J. Freshw. Ecol. 2022, 37, 405–424. [Google Scholar] [CrossRef]
- Scremin-Dias, E.; Silveira, B.B.; Fabiano, V.S.; Catian, G. Vegetative organs morphological plasticity of Ludwigia grandiflora in flooded and flood-free habitats. Plant Syst. Evol. 2023, 309, 14. [Google Scholar] [CrossRef]
- Chou, Q.C.; Zhang, W.; Chen, J.F.; Ren, W.J.; Yuan, C.B.; Wen, Z.H.; Zhang, X.L.; Cao, T.; Ni, L.Y.; Jeppesen, E. Phenotypic responses of a submerged macrophyte (Vallisneria natans) to low light combined with water depth. Aquat. Bot. 2022, 176, 103462. [Google Scholar] [CrossRef]
- Wang, J.J.; Xie, T.; Que, T.Y.; Zhang, S.W.; Qian, X.Q.; Lyu, S.P. Dynamics in phosphorus and bacterial community structure as influenced by Vallisneria natans in sediment of Qinhulake. Chin. J. Ecol. 2022, 41, 1787–1795. [Google Scholar] [CrossRef]
- Zhang, M.T.; Liu, J.X.; Su, J.H.; Chai, B.F. Diversity Patterns and Influencing Factors of Epibiotic in Vallisneria natans and Planktonic Bacteria Communities. Environ. Sci. 2023, 44, 252–261. [Google Scholar] [CrossRef]
- Yin, J.; Fan, P.; Zhong, G.D.; Wu, Z.H. Responses of Vallisneria natans (Lour.) Hara to the combined effects of Mn and Ph. Ecotoxicology 2019, 28, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H. Effects of Competition on the Keystone Species, Redundant Species and Resource Allocation of Floating -Leaved Aquatic Plant Communities. Ph.D. Thesis, Wuhan University, Wuhan, China, 2005. [Google Scholar]
- Wang, Y.; Wang, Q.F.; Guo, Y.H.; Barrett, S.C.H. Reproductive consequences of interactions between clonal growth and sexual reproduction in Nymphoides peltate: A distylous aquatic plant. New Phytol. 2005, 165, 329–336. [Google Scholar] [CrossRef]
- Bao, X.M.; Chen, K.N.; Fan, C.X. Effects on nutrient level of a eutrophic lake with reconstructing of floating-leaved aquatic vegetation. Ecol. Environ. 2005, 14, 807–811. [Google Scholar]
- Nguyen, N.H.T.; Bickel, T.O.; Perrett, C.; Adkins, S. Alien invasive macrophyte put into the shade: The native floating-leaved macrophyte Nymphoides indica reduces Cabomba caroliniana growth performance through competition for light. Freshw. Biol. 2021, 66, 1123–1135. [Google Scholar] [CrossRef]
- Du, F.F.; Liu, X.J.; Chen, S.Z.; Li, N.W.; Yao, D.R. Experimental Study on Tolerance and Photosynthetic Characteristics of Nymphoides peltata under Salt Stress. Wetl. Sci. 2022, 20, 565–572. [Google Scholar] [CrossRef]
- Li, H.; Dong, W.J.; Wu, Z.Y. Analysis on the construction and management of Qionghai National Wetland Park in Sichuan. Water Resour. Dev. Manag. 2021, 7, 54–57. [Google Scholar]
- Netto, A.T.; Campostrini, E.; Oliveira, J.G.; Bressan-Smith, R.E. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 reading in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Li, Q.; Jun, W.; Zhao, C.Z.; Zhao, L.C.; Dan, K. The relationship between the main leaf traits and photosynthetic physiological characteristics of Phragmites australis under different habitats of a salt marsh at Qinwangchuan, China. AoB PLANTS 2022, 14, 1–11. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2005. [Google Scholar]
- Li, Q.; Zhao, C.Z.; Kang, M.P.; Li, X.Y. The relationship of the main root-shoot morphological characteristics and biomass allocation of Saussurea salsa under different habitat conditions in Sugan lake wetland on the northern margin of the Qinghai-Tibet Plateau. Ecol. Indic. 2021, 128, 107836. [Google Scholar] [CrossRef]
- Li, S.J.; Wang, H.; Gou, W.; White, J.F.; Kingsley, K.L.; Wu, G.Q.; Su, P.X. Leaf functional traits of dominant desert plants in the Hexi Corridor, Northwestern China: Trade-off relationships and adversity strategies. Glob. Ecol. Conserv. 2021, 28, e01666. [Google Scholar] [CrossRef]
- He, N.P.; Li, Y.; Liu, C.C.; Xu, L.; Li, M.X.; Zhang, J.H.; He, J.S.; Tang, Z.Y.; Han, X.G.; Ye, Q.; et al. Plant trait networks: Improved resolution of the dimensionality of adaptation. Trends Ecol. Evol. 2020, 35, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Larson, J.E.; Vose, G. Leaf traits and performance vary with plant age and water availability in Artemisia californica. Ann. Bot. 2021, 127, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Li, Y.L.; Zhang, T.H.; Zhao, X.Y.; Huang, Y.X.; Song, L.L. Research advances of plant leaf traits at different ecology scales. J. Desert Res. 2012, 32, 33–41. [Google Scholar]
- Wilson, P.J.; Thompson, K.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Song, L.L.; Fan, J.W.; Wu, S.H.; Zhong, H.P.; Wang, N. Response characteristics of leaf traits of common species along an altitudinal gradient in Hongchiba Grassland, Chongqing. Acta Ecol. Sin. 2012, 32, 2759–2767. [Google Scholar] [CrossRef]
- Lusk, C.H.; Grierson, E.R.P.; Laughlin, D.C. Large leaves in warm, moist environments confer an advantage in seedling light interception efficiency. New Phytol. 2019, 223, 1319–1327. [Google Scholar] [CrossRef]
- De Wit, R.D. Lake and Reservoir Sedimentation: Processes, Measurements, and Management; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Campitelli, B.E.; Stinchcombe, J.R. Natural selection maintains a single-locus leaf shape cline in ivyleaf morning glory, Ipomoea hederacea. Mol. Ecol. 2013, 22, 552–564. [Google Scholar] [CrossRef]
- Adebowale, A.; Naidoo, Y.; Lamb, J.; Nicholas, A. Comparative foliar epidermal micromorphology of Southern African Strychnos L. (Loganiaceae): Taxonomic, ecological and cytological considerations. Plant Syst. Evol. 2014, 300, 127–138. [Google Scholar] [CrossRef]
- McDonald, P.G.; Fonseca, C.R.; McCoverton, J.; Westoby, M. Leaf Size Divergence Along Rainfall Soil-Nutr. Gradients: Is Method Size Reduct. Common Among Clades? Funct. Ecol. 2003, 17, 50–57. [Google Scholar] [CrossRef]
- Afzal, A.; Duiker, S.W.; Watson, J.E. Leaf thickness to predict plant water status. Biosyst. Eng. 2017, 156, 148–156. [Google Scholar] [CrossRef]
- Funk, J.L.; Vitousek, P.M. Resource use efficiency and plant invasion in low resource systems. Nature 2007, 446, 1079–1081. [Google Scholar] [CrossRef] [PubMed]

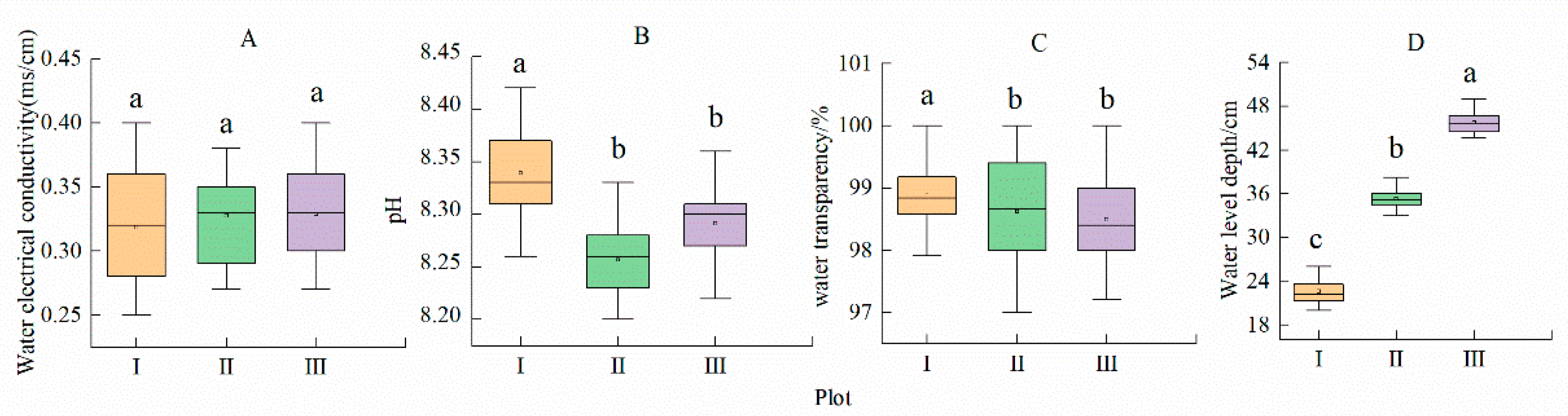

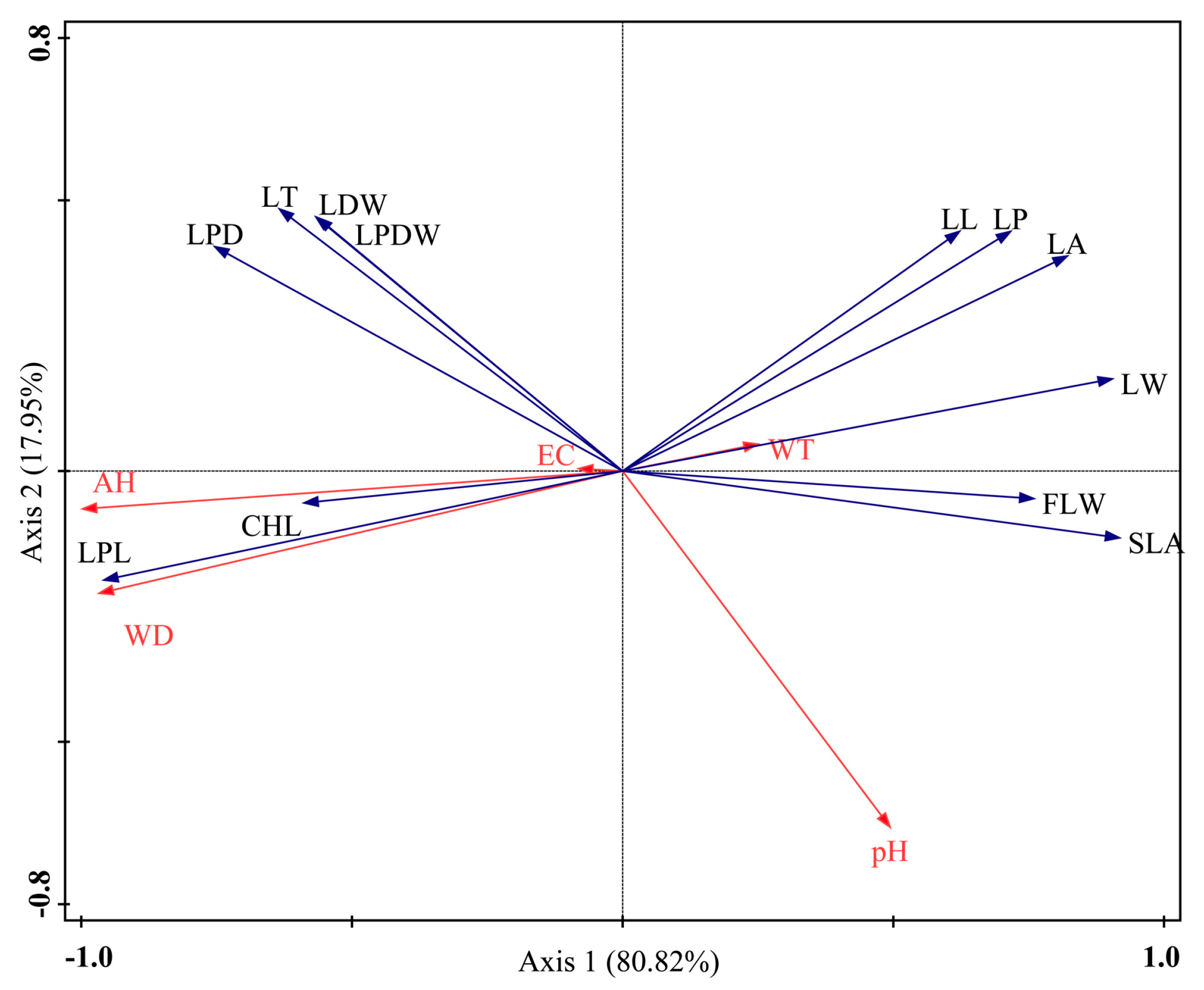

| Plot | I | II | III | Plasticity Index |
|---|---|---|---|---|
| Leaf thickness (mm) | 0.14 ± 0.01 c | 0.43 ± 0.01 a | 0.27 ± 0.01 b | 0.67 |
| Leaf area (cm2) | 14.51 ± 0.04 a | 13.61 ± 0.14 b | 9.66 ± 0.08 c | 0.33 |
| Leaf perimeter (mm) | 15.15 ± 0.01 a | 15.06 ± 0.05 a | 13.40 ± 0.08 b | 0.12 |
| Leaf length (mm) | 4.47 ± 0.01 a | 4.45 ± 0.02 a | 3.87 ± 0.06 b | 0.13 |
| Leaf width (mm) | 4.19 ± 0.02 a | 3.70 ± 0.05 b | 3.25 ± 0.01 c | 0.22 |
| Leaf petiole length (cm) | 23.14 ± 0.40 c | 35.37 ± 0.29 b | 46.04± 0.41 a | 0.50 |
| Fresh leaf weight (g) | 0.94 ± 0.07 a | 0.52 ± 0.02 b | 0.31 ± 0.02 c | 0.67 |
| Leaf dry weight (g) | 0.05 ± 0.001 c | 0.09 ± 0.002 a | 0.07 ± 0.001 b | 0.40 |
| Specific leaf area (cm2/g) | 284.05 ± 5.42 a | 151.88 ± 2.86 b | 142.19 ± 3.35 c | 0.50 |
| Leaf petiole diameter(mm) | 1.02 ± 0.01 c | 1.58 ± 0.01 a | 1.38 ± 0.01 b | 0.35 |
| Leaf petiole dry weight (g) | 0.03 ± 0.001 c | 0.07 ± 0.001 a | 0.05 ± 0.001 b | 0.57 |
| Chlorophyll (SPAD) | 31.71 ± 0.32 b | 34.11± 0.48 a | 34.90 ± 0.10 a | 0.09 |
| Statistic | Axis 1 | Axis 2 | Total Variance | |
|---|---|---|---|---|
| EC | −0.08 | 0.003 | 1 | |
| pH | 0.48 | −0.46 | ||
| WT | 0.25 | −0.03 | ||
| WD | −0.95 | −0.16 | ||
| AH | −0.98 | −0.05 | ||
| Eigenvalues | 0.56 | 0.12 | ||
| function traits–environment correlations | 0.98 | 0.70 | ||
| Explained variation (cumulative) | of function traits data | 55.65 | 68.02 | |
| of function traits–environment relation | 80.82 | 98.77 | ||
| All eigenvalues | 1 | |||
| Canonical eigenvalues | 0.69 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Chen, L.; Qiu, Y.; Li, X.; Nan, Z.; Yao, S.; Chen, Z.; Zhang, Y.; Zhao, C. Responses of the Leaf Characteristics of Nymphoides peltata to a Water Depth Gradient in the Qionghai Lake, Western Sichuan Plateau, China. Plants 2025, 14, 919. https://doi.org/10.3390/plants14060919
Li Q, Chen L, Qiu Y, Li X, Nan Z, Yao S, Chen Z, Zhang Y, Zhao C. Responses of the Leaf Characteristics of Nymphoides peltata to a Water Depth Gradient in the Qionghai Lake, Western Sichuan Plateau, China. Plants. 2025; 14(6):919. https://doi.org/10.3390/plants14060919
Chicago/Turabian StyleLi, Qun, Lan Chen, Yumei Qiu, Xiaoyan Li, Zhe Nan, Shulin Yao, Zhenghong Chen, Yuhan Zhang, and Chengzhang Zhao. 2025. "Responses of the Leaf Characteristics of Nymphoides peltata to a Water Depth Gradient in the Qionghai Lake, Western Sichuan Plateau, China" Plants 14, no. 6: 919. https://doi.org/10.3390/plants14060919
APA StyleLi, Q., Chen, L., Qiu, Y., Li, X., Nan, Z., Yao, S., Chen, Z., Zhang, Y., & Zhao, C. (2025). Responses of the Leaf Characteristics of Nymphoides peltata to a Water Depth Gradient in the Qionghai Lake, Western Sichuan Plateau, China. Plants, 14(6), 919. https://doi.org/10.3390/plants14060919






