Impact of Polystyrene Microplastics on Soil Properties, Microbial Diversity and Solanum lycopersicum L. Growth in Meadow Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil and MPs
2.2. Pot Experiment
2.3. Soil Properties and Enzyme Activity Analysis
2.4. Plant Growth and Enzyme Activity Analysis
2.5. Rhizosphere Microbial Community
2.6. Date Analysis
3. Result and Discussion
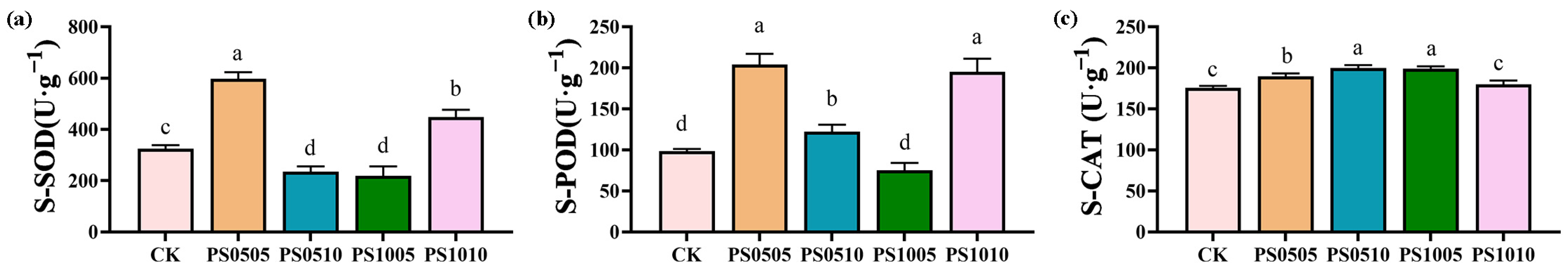
3.1. Plant Growth and Antioxidant Enzyme Activity
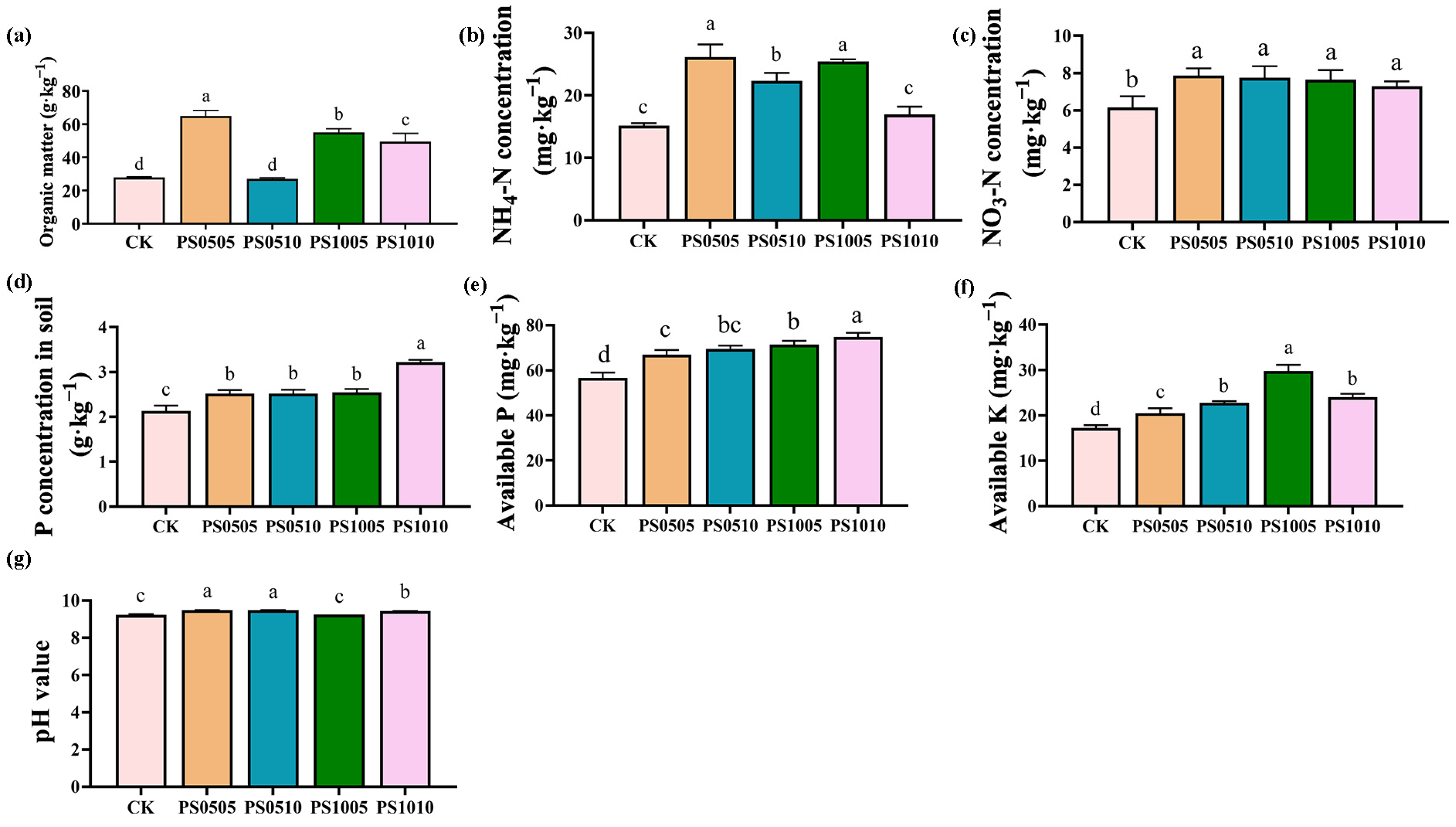
3.2. Soil Physicochemical/Biochemical Properties
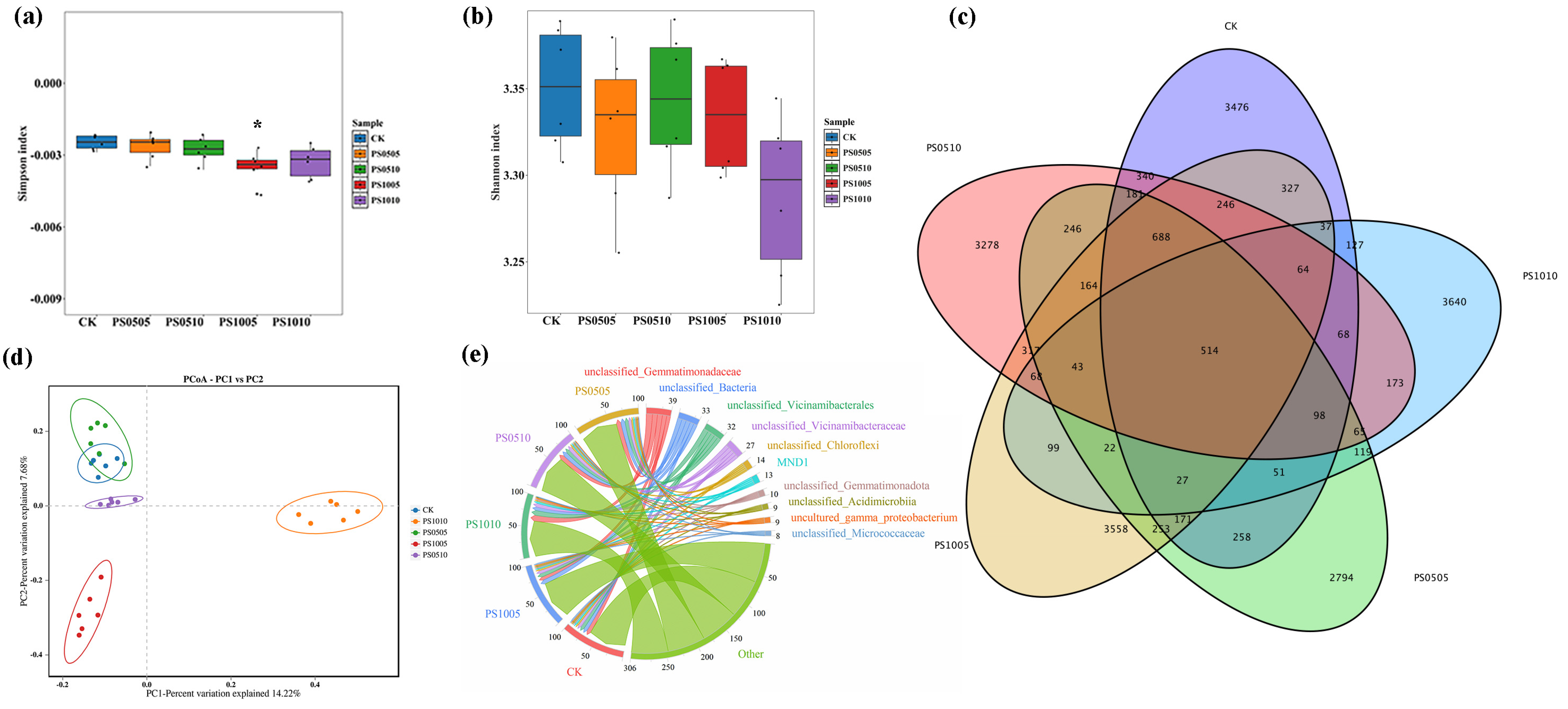
3.3. Diversity and Composition of Rhizosphere Soil Microbial Communities
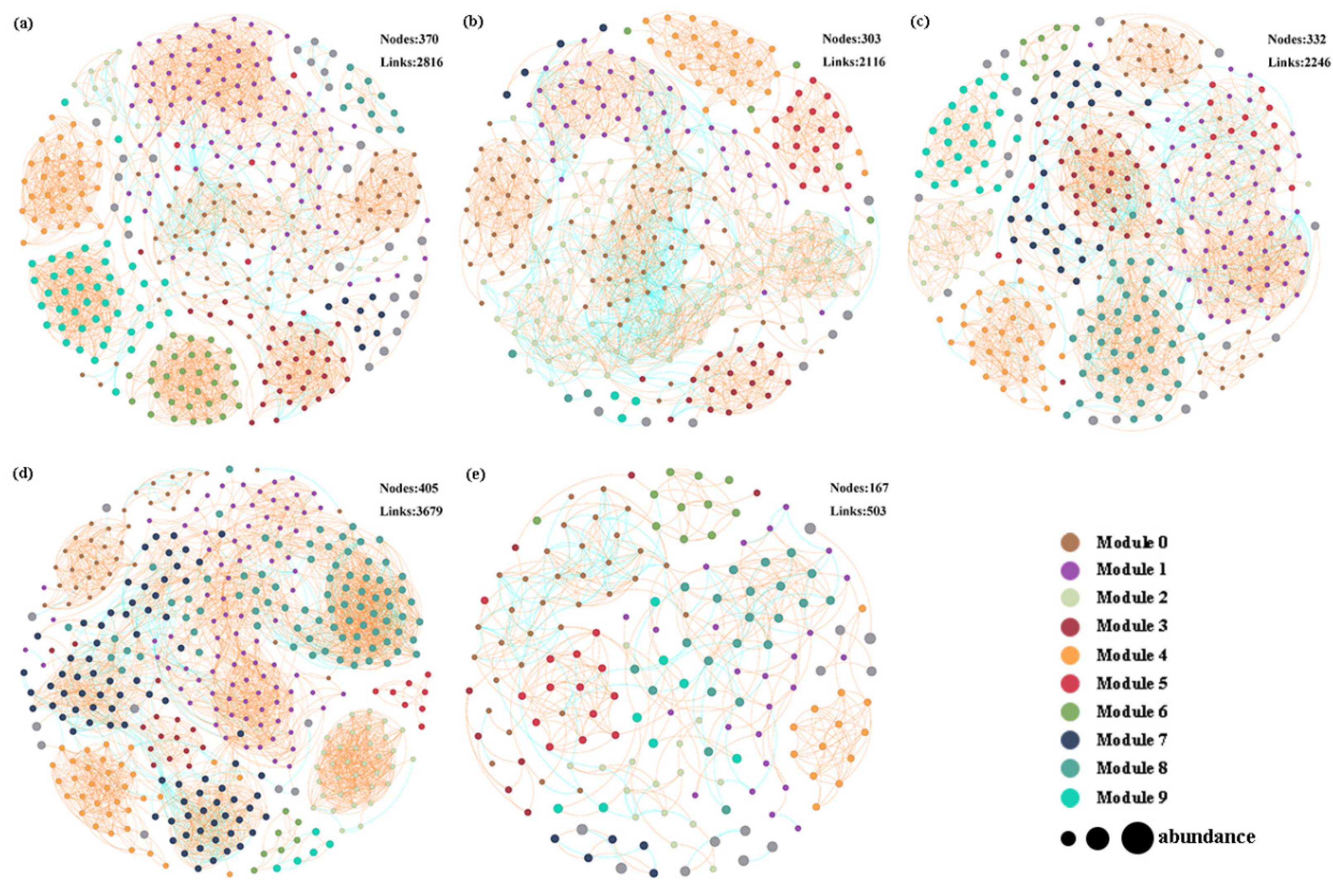
3.4. Co-Occurrence Networks Analysis
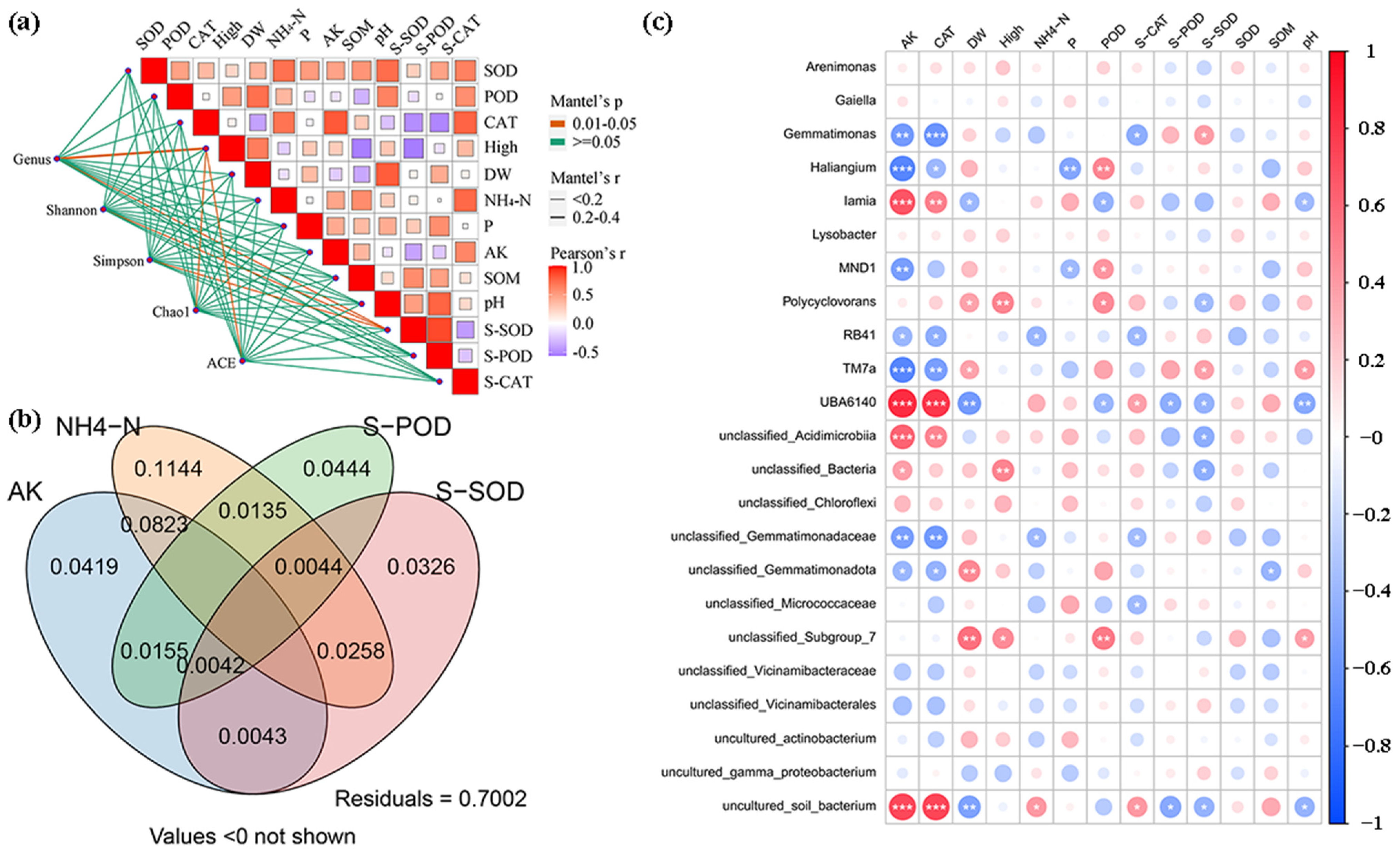
3.5. Correlation Analysis
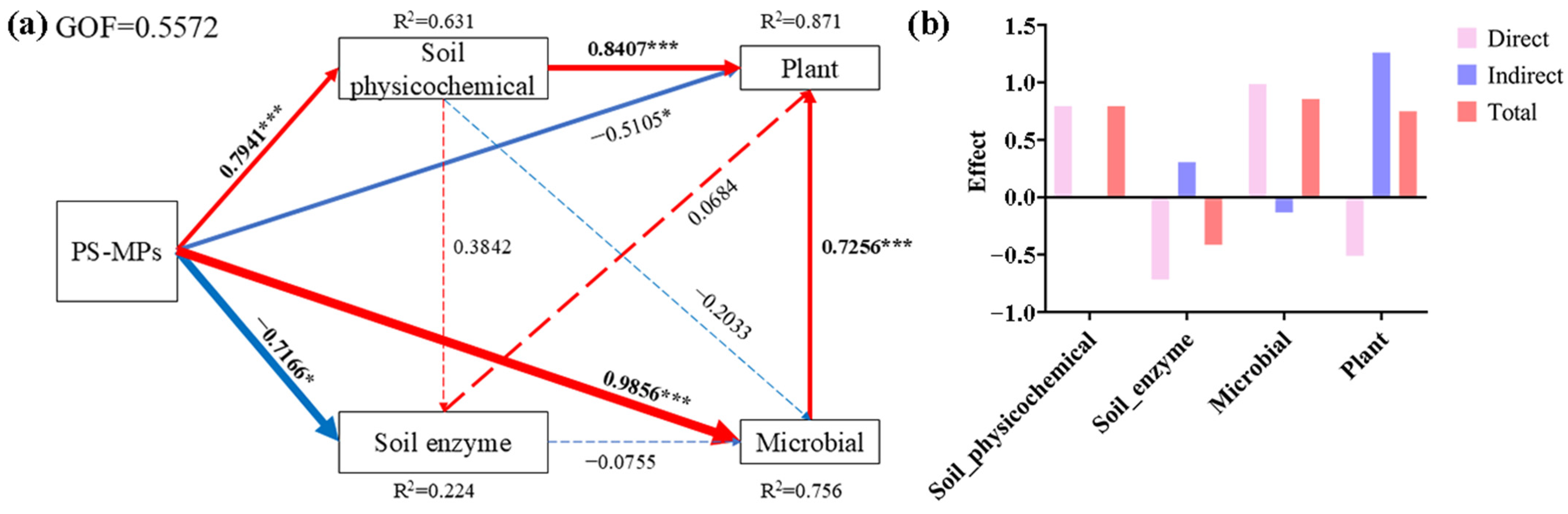
3.6. Effects of PS-MPs on the Soil–Microbial–Plant System
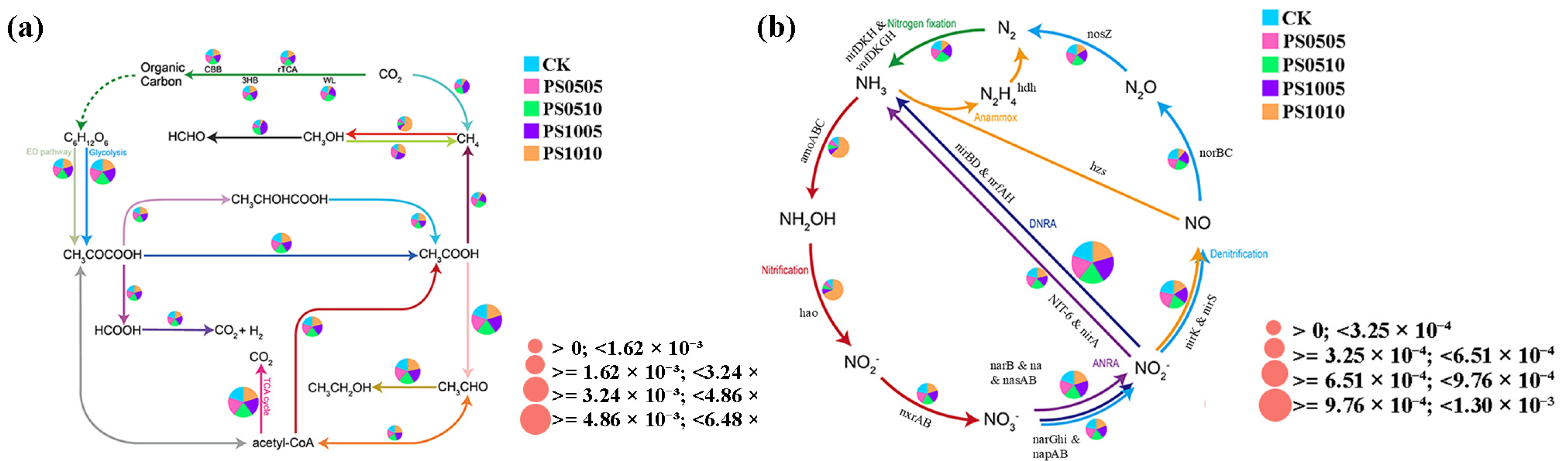
3.7. Examination of Predictive Functional Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Li, Y.; Liang, X.; Lu, S.; Ren, J.; Zhang, Y.; Han, Z.; Gao, B.; Sun, K. Effects of microplastics on soil carbon pool and terrestrial plant performance. Carbon Res. 2024, 3, 37. [Google Scholar] [CrossRef]
- Eskander, S.B.; Saleh, H.M.; Tawfik, M.E.; Bayoumi, T.A. Towards potential applications of cement-polymer composites based on recycled polystyrene foam wastes on construction fields: Impact of exposure to water ecologies. Case Stud. Constr. Mater. 2021, 15, e00664. [Google Scholar] [CrossRef]
- Zhao, S.; Rillig, M.C.; Bing, H.; Cui, Q.; Qiu, T.; Cui, Y.; Penuelas, J.; Liu, B.; Bian, S.; Monikh, F.A.; et al. Microplastic pollution promotes soil respiration: A global-scale meta-analysis. Glob. Change Biol. 2024, 30, e17415. [Google Scholar] [CrossRef]
- Liu, E.K.; He, W.Q.; Yan, C.R. ‘White revolution’ to ‘white pollution’—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 091001. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Hu, C.; Ge, T.; Wang, L.; Xing, J.; He, X.; Zhao, Y. Comparative Evaluation of Analytical Techniques for Quantifying and Characterizing Polyethylene Microplastics in Farmland Soil Samples. Agriculture 2024, 14, 554. [Google Scholar] [CrossRef]
- Tian, H.; Du, Y.; Luo, X.; Dong, J.; Chen, S.; Hu, X.; Zhang, M.; Liu, Z.; Abolfathi, S. Understanding visible light and microbe-driven degradation mechanisms of polyurethane plastics: Pathways, property changes, and product analysis. Water Res. 2024, 259, 121856. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Miranda, R.R.; Eddingsaas, N.C.; Chu, J.; Freezman, I.M.; Tyler, A.C.; Hudson, A.O. Polystyrene Degradation by Exiguobacterium sp. RIT 594: Preliminary Evidence for a Pathway Containing an Atypical Oxygenase. Microorganisms 2022, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yi, L.; Mandeep, K.; Zhisheng, Y.; Taizheng, C.; Ming, X. Phytotoxic Effects of Polyethylene Microplastics on the Growth of Food Crops Soybean (Glycine max) and Mung Bean (Vigna radiata). Int. J. Environ. Res. Public Health 2021, 18, 10629. [Google Scholar] [CrossRef]
- Zhai, Y.; Bai, J.; Chang, P.; Liu, Z.; Wang, Y.; Liu, G.; Cui, B.; Peijnenburg, W.; Vijver, M.G. Microplastics in terrestrial ecosystem: Exploring the menace to the soil-plant-microbe interactions. Trends Anal. Chem. 2024, 174, 117667. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Q.; Adams, C.A.; Sun, Y.; Zhang, S. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater. 2021, 424, 127531. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Bueno, R.P.; Romero-González, R.; González-Fernández, M.J.; Guil-Guerrero, J.L. Phytochemical composition and in vitro anti-tumour activities of selected tomato varieties. J. Sci. Food Agric. 2017, 97, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef]
- Gravel, V.; Blok, W.; Hallmann, E.; Carmona-Torres, C.; Wang, H.; Peppel, A.; Golec, A.F.C.; Dorais, M.; Meeteren, U.; Heuvelink, E.; et al. Differences in N uptake and fruit quality between organically and conventionally grown greenhouse tomatoes. Agron. Sustain. Dev. 2010, 30, 797–806. [Google Scholar] [CrossRef]
- Fucheng, G.; Haijun, L.; Xiaoguo, M.; Hu, G.; Ying, Z.; Ruimiao, L.; Kai, C.; Lin, Y. Effects of Organic Fertilizer Application on Tomato Yield and Quality: A Meta-Analysis. Appl. Sci. 2023, 13, 2184. [Google Scholar] [CrossRef]
- Noemi, F.M.M.; Miguel, E.D.; Jorge, D.A.; Adolfo, G.A.G.; Fernando, A.Z.J.; Javier, M.C.F.; Angel, P.Q.R.; Olivas, O.G.I. Preharvest nitrogen application affects quality and antioxidant status of two tomato cultivars. Bragantia 2020, 79, 134–144. [Google Scholar] [CrossRef]
- Kosterna, E. The effect of covering and mulching on the soil temperature, growth and yield of tomato. Folia Hortic. 2015, 26, 91–101. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Peijnenburg, W.J.G.M.; Li, R.; Yang, J.; Zhou, Q. Confocal measurement of microplastics uptake by plants. MethodsX 2019, 7, 100750. [Google Scholar] [CrossRef]
- Mészáros, E.; Bodor, A.; Kovács, E.; Papp, S.; Kovács, K.; Perei, K.; Feigl, G. Impacts of Plastics on Plant Development: Recent Advances and Future Research Directions. Plants 2023, 12, 3282. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Dey, T.K.; Jamal, M. Microplastic/nanoplastic toxicity in plants: An imminent concern. Environ. Monit. Assess. 2022, 195, 27. [Google Scholar] [CrossRef]
- Iswahyudi, I.; Widodo, W.; Warkoyo, W.; Sutanto, A.; Garfansa, M.P.; Mujiyanti, W.A.; Sholeh, M.S. Investigating the Impact of Microplastics Type of Polyethylene, Polypropylene, and Polystyrene on Seed Germination and Early Growth of Rice Plants. Environ. Qual. Manag. 2024, 34, e22287. [Google Scholar] [CrossRef]
- Qiuge, Z.; Mengsai, Z.; Fansong, M.; Yongli, X.; Wei, D.; Yaning, L. Effect of Polystyrene Microplastics on Rice Seed Germination and Antioxidant Enzyme Activity. Toxics 2021, 9, 179. [Google Scholar] [CrossRef]
- Zhou, C.-Q.; Lu, C.-H.; Mai, L.; Bao, L.-J.; Liu, L.-Y.; Zeng, E.Y. Response of rice (Oryza sativa L.) roots to nanoplastic treatment at seedling stage. J. Hazard. Mater. 2021, 401, 123412. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Z.; Zhu, D.; Lindhardt, J.H.; Lin, S.-M.; Ke, X.; Cui, L. Long-Term Fertilization History Alters Effects of Microplastics on Soil Properties, Microbial Communities, and Functions in Diverse Farmland Ecosystem. Environ. Sci. Technol. 2021, 55, 4658–4668. [Google Scholar] [CrossRef]
- Meiling, Y.; Shaohong, Z.; Lilan, Z.; Shiyuan, D. The effects of three different microplastics on enzyme activities and microbial communities in soil. Water Environ. Res. 2020, 93, 24–32. [Google Scholar] [CrossRef]
- Moharana, T.; Patnaik, A.; Mishra, C.S.K.; Behera, B.P.; Samal, R.R. High-density polyethylene microplastics in agricultural soil: Impact on microbes, enzymes, and carbon-nitrogen ratio. J. Environ. Qual. 2024, 53, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Kublik, S.; Gschwendtner, S.; Magritsch, T.; Radl, V.; Rillig, M.C.; Schloter, M. Microplastics in soil induce a new microbial habitat, with consequences for bulk soil microbiomes. Front. Environ. Sci. 2022, 10, 989267. [Google Scholar] [CrossRef]
- Mebius, L.J. A Rapid Method for the Determination of Organic Carbon in Soil. Anal. Chim. Acta 1960, 22, 120–124. [Google Scholar] [CrossRef]
- Guo, W.; Andersen, M.N.; Qi, X.-B.; Li, P.; Li, Z.-Y.; Fan, X.-Y.; Zhou, Y. Effects of reclaimed water irrigation and nitrogen fertilization on the chemical properties and microbial community of soil. J. Integr. Agric. 2017, 16, 679–690. [Google Scholar] [CrossRef]
- Tenenhaus, M.; Vinzi, V.E.; Chatelin, Y.M.; Lauro, C. PLS path modeling. Comput. Stat. Data Anal. 2005, 48, 159–205. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, M.; Shahbaz, M.; Hu, Z.e.; Zhu, Z.; Lu, S.; Yu, Y.; Yao, H.; Chen, J.; Ge, T. Microplastics in soil can increase nutrient uptake by wheat. J. Hazard. Mater. 2022, 438, 129547. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass. Front. Plant Sci. 2021, 12, 714541. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, J.; Zhang, Z.; Ren, Z.; Li, X.; Zhang, R.; Ma, X. Uptake and effect of carboxyl-modified polystyrene microplastics on cotton plants. J. Hazard. Mater. 2024, 466, 133581. [Google Scholar] [CrossRef]
- Fan, P.; Tan, W.; Yu, H. Effects of different concentrations and types of microplastics on bacteria and fungi in alkaline soil. Ecotoxicol. Environ. Saf. 2022, 229, 113045. [Google Scholar] [CrossRef]
- Silva, A.A.R.; Capitulino, J.D.; Lima, G.S.; Azevedo, C.A.V.; Arruda, T.F.L.; Souza, A.R.; Gheyi, H.R.; Soares, L.A.A. Hydrogen peroxide in attenuation of salt stress effects on physiological indicators and growth of soursop. Braz. J. Biol. 2024, 84, e261211. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qi, W.; Cao, X.; Hu, J.; Li, Y.; Peng, J.; Hu, C.; Qu, J. Microplastic residues in wetland ecosystems: Do they truly threaten the plant-microbe-soil system? Environ. Int. 2021, 156, 106708. [Google Scholar] [CrossRef]
- Zhu, F.; Yan, Y.; Doyle, E.; Zhu, C.; Jin, X.; Chen, Z.; Wang, C.; He, H.; Zhou, D.; Gu, C. Microplastics altered soil microbiome and nitrogen cycling: The role of phthalate plasticizer. J. Hazard. Mater. 2022, 427, 127944. [Google Scholar] [CrossRef]
- Shi, J.; Wang, J.; Lv, J.; Wang, Z.; Peng, Y.; Wang, X. Microplastic presence significantly alters soil nitrogen transformation and decreases nitrogen bioavailability under contrasting temperatures. J. Environ. Manag. 2022, 317, 115473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pei, L.; Zhao, Y.; Shan, J.; Zheng, X.; Xu, G.; Sun, Y.; Wang, F. Effects of microplastics and nitrogen deposition on soil multifunctionality, particularly C and N cycling. J. Hazard. Mater. 2023, 451, 131152. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, B.; Zhang, W.; Liang, F.; Wu, J.; Song, Z.; Zhu, Y. Polyethylene Microplastic Particles Alter the Nature, Bacterial Community and Metabolite Profile of Reed Rhizosphere Soils. Water 2023, 15, 1505. [Google Scholar] [CrossRef]
- Wu, S.; Lu, H.; Yi, Z.; Chen, G.; Sun, H. Microplastic Has No Effect on Rice Yield and Gaseous N Emission from an Infertile Soil with High Inorganic N Inputs. Plants 2024, 13, 1279. [Google Scholar] [CrossRef] [PubMed]
- Lahimer, M.C.; Ayed, N.; Horriche, J.; Belgaied, S. Characterization of plastic packaging additives: Food contact, stability and toxicity. Arab. J. Chem. 2017, 10, S1938–S1954. [Google Scholar] [CrossRef]
- Rong, L.; Zhao, L.; Zhao, L.; Cheng, Z.; Yao, Y.; Yuan, C.; Wang, L.; Sun, H. LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci. Total Environ. 2021, 773, 145640. [Google Scholar] [CrossRef] [PubMed]
- DeBruyn, J.M.; Radosevich, M.; Wommack, K.E.; Polson, S.W.; Hauser, L.J.; Fawaz, M.N.; Korlach, J.; Tsai, Y.-C. Genome Sequence and Methylome of Soil Bacterium Gemmatirosa kalamazoonensis KBS708T, a Member of the Rarely Cultivated Gemmatimonadetes Phylum. Genome Announc. 2014, 2, e00226. [Google Scholar] [CrossRef]
- Liu, C.; Zhuang, J.; Wang, J.; Fan, G.; Feng, M.; Zhang, S. Soil bacterial communities of three types of plants from ecological restoration areas and plant-growth promotional benefits of Microbacterium invictum (strain X-18). Front. Microbiol. 2022, 13, 926037. [Google Scholar] [CrossRef]
- Huber, K.J.; Overmann, J. Vicinamibacteraceae fam. nov., the first described family within the subdivision 6 Acidobacteria. Int. J. Syst. Evol. Microbiol. 2018, 68, 2331–2334. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Liu, J.; Wang, J.; Han, W.; Shen, Z.; Muraina, T.O.; Chen, J.; Sun, D. Comparison of soil microbial community between reseeding grassland and natural grassland in Songnen Meadow. Sci. Rep. 2020, 10, 16884. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem and environmental sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef]
- Krasilnikov, N. On the Role of Soil Bacteria in Plant Nutrition. J. Gen. Appl. Microbiol. 1961, 7, 128–144. [Google Scholar] [CrossRef][Green Version]
- Claudia, K.; Julien, V.; Andrea, P.-H.; Tanja, S.; Svetlana, A.; Sylvie, J.; Robert, S.; Susan, A.; Dieter, S.; Rapheal, M.; et al. The Arabidopsis thaliana MND1 homologue plays a key role in meiotic homologous pairing, synapsis and recombination. J. Cell Sci. 2006, 119, 2486–2496. [Google Scholar] [CrossRef]
- Yifeng, L.; Yan, Z.; Yongfeng, D.; Wei, J.; Yanping, Z.; Jinju, G.; Lili, D.; Hongqiang, R. Response to Comment on “Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver”. Environ. Sci. Technol. 2016, 50, 12523–12524. [Google Scholar] [CrossRef]
- Schöpfer, L.; Schnepf, U.; Marhan, S.; Brümmer, F.; Kandeler, E.; Pagel, H. Hydrolyzable microplastics in soil—Low biodegradation but formation of a specific microbial habitat? Biol. Fertil. Soils 2022, 58, 471–486. [Google Scholar] [CrossRef]
- Narayana, N.K.; Kingery, W.L.; Shankle, M.W.; Shanmugam, S.G. Differential Response of Soil Microbial Diversity and Community Composition Influenced by Cover Crops and Fertilizer Treatments in a Dryland Soybean Production System. Agronomy 2022, 12, 618. [Google Scholar] [CrossRef]
- Ren, X.; Tang, J.; Liu, X.; Liu, Q. Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environ. Pollut. 2020, 256, 113347. [Google Scholar] [CrossRef]
- Anouk, V.T.P.; Klein, M.; Caldas, V.; Galvez, L.O.; Broersma, C.; Hoebe, N.; Sanders, I.R.; Shimizu, T.; Kiers, E.T. Decreasing relatedness among mycorrhizal fungi in a shared plant network increases fungal network size but not plant benefit. Ecol. Lett. 2021, 25, 509–520. [Google Scholar] [CrossRef]
- Comte, J.; Lovejoy, C.; Crevecoeur, S.; Vincent, W.F. Co-occurrence patterns in aquatic bacterial communities across changing permafrost landscapes. Biogeosci. Discuss. 2015, 12, 10233–10269. [Google Scholar] [CrossRef]
- Rajcoomar, S.; Amoah, I.D.; Abunama, T.; Mohlomi, N.; Bux, F.; Kumari, S. Biofilm formation on microplastics in wastewater: Insights into factors, diversity and inactivation strategies. J. Environ. Sci. Technol. 2023, 21, 4429–4444. [Google Scholar] [CrossRef]
- Ezzedine, J.A.; Janicot, A.; Rasconi, S.; Domaizon, I.; Jacquet, S. Short-Term Dynamics of Bdellovibrio and Like Organisms in Lake Geneva in Response to a Simulated Climatic Extreme Event. Microb. Ecol. 2021, 84, 717–729. [Google Scholar] [CrossRef]
- Durán, P.; Tortella, G.; Sadowsky, M.J.; Viscardi, S.; Barra, P.J.; Mora, M.d.l.L. Engineering Multigenerational Host-Modulated Microbiota against Soilborne Pathogens in Response to Global Climate Change. Biology 2021, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lou, Y.; Yan, X.; Pan, H.; Wang, H.; Yang, Q.; Sun, Y.; Zhuge, Y. The Microbiome and Antibiotic Resistome in Soil under Biodegradable Composite Carbon Source Amendment. J. Xenobiotics 2023, 13, 424–438. [Google Scholar] [CrossRef]
- Xu, Z.; Li, R.; Liu, T.; Zhang, G.; Wu, S.; Xu, K.; Zhang, Y.; Wang, Q.; Kang, J.; Zhang, Z.; et al. Effect of inoculation with newly isolated thermotolerant ammonia-oxidizing bacteria on nitrogen conversion and microbial community during cattle manure composting. J. Environ. Manag. 2022, 317, 115474. [Google Scholar] [CrossRef] [PubMed]
- Guihua, L.; Weishui, Y.; Fanhua, M.; Jianfeng, Z.; Changai, L. Pathways and Drivers of Gross N Transformation in Different Soil Types under Long-Term Chemical Fertilizer Treatments. Agriculture 2023, 13, 300. [Google Scholar] [CrossRef]
- MCrawford, N.M.; Forde, B.G. Molecular and developmental biology of inorganic nitrogen nutrition. Arab. Book 2002, 1, e0011. [Google Scholar] [CrossRef]
- Koichi, F. Regulation of Adenine Nucleotide Metabolism by Adenylate Kinase Isozymes: Physiological Roles and Diseases. Int. J. Mol. Sci. 2023, 24, 5561. [Google Scholar] [CrossRef]
- Liron, F.; Kartik, C.; Dror, A.; Edris, T.; Amanda, K.; Hadas, M. Accelerating Microbial Activity of Soil Aquifer Treatment by Hydrogen Peroxide. Energies 2022, 15, 3852. [Google Scholar] [CrossRef]
- Tally, F.P.; Goldin, B.R.; Jacobus, N.V.; Gorbach, S.L. Superoxide dismutase in anaerobic bacteria of clinical significance. Infect. Immun. 1977, 16, 20–25. [Google Scholar] [CrossRef]
- Najmuldeen, H.; Alghamdi, R.; Alghofaili, F.; Yesilkaya, H. Functional assessment of microbial superoxide dismutase isozymes suggests a differential role for each isozyme. Free Radic. Biol. Med. 2019, 134, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Li, Y.; Wang, C.; Qiao, J.; Zhu, K.; Sun, Y.; Hu, Q. Effect of Sod Production on Physical, Chemical, and Biological Properties of Soils in North and South China. Agriculture 2024, 14, 1786. [Google Scholar] [CrossRef]
- Awet, T.T.; Kohl, Y.; Meier, F.; Straskraba, S.; Grün, A.L.; Ruf, T.; Jost, C.; Drexel, R.; Tunc, E.; Emmerling, C. Effects of polystyrene nanoparticles on the microbiota and functional diversity of enzymes in soil. Environ. Sci. Eur. 2018, 30, 11. [Google Scholar] [CrossRef]
- Jabin, P.P.N.; Ismail, S. Solubilization of Insoluble Potassium by Different Microbial Isolates in vitro Condition. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3600–3607. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Mostafa, A.A.; Zedan, A.; Elbltagy, H.M.; Dawoud, S.F.M.; Elbanna, B.A.; El-Shazly, S.A.; El-Sadawy, A.A.; Sharaf-Eldin, A.M.; Darweesh, M.; et al. Potential Effect of Biochar on Soil Properties, Microbial Activity and Vicia faba Properties Affected by Microplastics Contamination. Agronomy 2023, 13, 149. [Google Scholar] [CrossRef]
- Maslovska, O.; Komplikevych, S.; Hnatush, S. Oxidative stress and protection against it in bacteria. Stud. Biol. 2023, 17, 153–172. [Google Scholar] [CrossRef]
- Jiang, L.; Song, M.; Yang, L.; Zhang, D.; Sun, Y.; Shen, Z.; Luo, C.; Zhang, G. Exploring the Influence of Environmental Factors on Bacterial Communities within the Rhizosphere of the Cu-tolerant plant, Elsholtzia splendens. Sci. Rep. 2016, 6, 36302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Lozano, Y.M.; Rillig, M.C. Microplastics Increase Soil pH and Decrease Microbial Activities as a Function of Microplastic Shape, Polymer Type, and Exposure Time. Front. Environ. Sci. 2021, 9, 1. [Google Scholar] [CrossRef]
- Xu, H.; Chen, C.; Pang, Z.; Zhang, G.; Zhang, W.; Kan, H. Effects of microplastics concentration on plant root traits and biomass: Experiment and meta-analysis. Ecotoxicol. Environ. Saf. 2024, 285, 117038. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liang, J.; Yang, Y.; Jiang, H.; Tian, X. Effect of polylactic acid microplastics on soil properties, soil microbials and plant growth. Chemosphere 2023, 329, 138504. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, L.; Guo, M.; Lin, X.; Wang, R.; Guo, S. Effects of microplastic properties and dissolved organic matter on phosphorus availability in soil and aqueous mediums. Environ. Pollut. 2024, 340, 122784. [Google Scholar] [CrossRef] [PubMed]
- Dieser, M.; E Broemsen, E.L.J.; A Cameron, K.; King, G.M.; Achberger, A.; Choquette, K.; Hagedorn, B.; Sletten, R.; Junge, K.; Christner, B.C. Molecular and biogeochemical evidence for methane cycling beneath the western margin of the Greenland Ice Sheet. ISME J. 2014, 8, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Ferry, J.G. Methane from acetate. J. Bacteriol. 1992, 174, 5489–5495. [Google Scholar] [CrossRef]
- Olivia, C.; Rolando, C.; Lorna, G.; Aminael, S.-R. Assessing the Effect of Pretreatments on the Structure and Functionality of Microbial Communities for the Bioconversion of Microalgae to Biogas. Front. Microbiol. 2018, 9, 1388. [Google Scholar] [CrossRef]
- Visser, M.; Pieterse, M.M.; Pinkse, M.W.H.; Nijsse, B.; Verhaert, P.D.E.M.; de Vos, W.M.; Schaap, P.J.; Stams, A.J.M. Unravelling the one-carbon metabolism of the acetogen Sporomusa strain An4 by genome and proteome analysis. Environ. Microbiol. 2016, 18, 2843–2855. [Google Scholar] [CrossRef] [PubMed]
- Soufi, H.H.; Tran, D.; Louca, S. Microbiology of Big Soda Lake, a multi-extreme meromictic volcanic crater lake in the Nevada desert. Environ. Microbiol. 2024, 26, e16578. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Gao, J.; Wang, F.; Yang, W.; Han, L.; Lin, D.; Min, B.; Zhi, Y.; Grieger, K.; et al. Effect of microplastics on ecosystem functioning: Microbial nitrogen removal mediated by benthic invertebrates. Sci. Total Environ. 2021, 754, 142133. [Google Scholar] [CrossRef]
- Deng, Y.; Liang, C.; Zhu, X.; Zhu, X.; Chen, L.; Pan, H.; Xun, F.; Tao, Y.; Xing, P. Methylomonadaceae was the active and dominant methanotroph in Tibet lake sediments. ISME Commun. 2024, 4, ycae032. [Google Scholar] [CrossRef]
- Seeley, M.E.; Song, B.; Passie, R.; Hale, R.C. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat. Commun. 2020, 11, 2372. [Google Scholar] [CrossRef] [PubMed]
- Ahmerkamp, S.; Marchant, H.K.; Peng, C.; Probandt, D.; Littmann, S.; Kuypers, M.M.M.; Holtappels, M. The effect of sediment grain properties and porewater flow on microbial abundance and respiration in permeable sediments. Sci. Rep. 2020, 10, 3573. [Google Scholar] [CrossRef]
- Zhou, Z.; Hua, J.; Xue, J. Polyethylene microplastic and soil nitrogen dynamics: Unraveling the links between functional genes, microbial communities, and transformation processes. J. Hazard. Mater. 2023, 458, 131857. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Suo, Y.; Wang, J.; Chen, B.; Wang, K.; Yang, X.; Zhu, Y.; Zhang, J.; Lu, M.; Liu, Y. Impact of Polystyrene Microplastics on Soil Properties, Microbial Diversity and Solanum lycopersicum L. Growth in Meadow Soils. Plants 2025, 14, 256. https://doi.org/10.3390/plants14020256
Liu S, Suo Y, Wang J, Chen B, Wang K, Yang X, Zhu Y, Zhang J, Lu M, Liu Y. Impact of Polystyrene Microplastics on Soil Properties, Microbial Diversity and Solanum lycopersicum L. Growth in Meadow Soils. Plants. 2025; 14(2):256. https://doi.org/10.3390/plants14020256
Chicago/Turabian StyleLiu, Shuming, Yan Suo, Jinghuizi Wang, Binglin Chen, Kaili Wang, Xiaoyu Yang, Yaokun Zhu, Jiaxing Zhang, Mengchu Lu, and Yunqing Liu. 2025. "Impact of Polystyrene Microplastics on Soil Properties, Microbial Diversity and Solanum lycopersicum L. Growth in Meadow Soils" Plants 14, no. 2: 256. https://doi.org/10.3390/plants14020256
APA StyleLiu, S., Suo, Y., Wang, J., Chen, B., Wang, K., Yang, X., Zhu, Y., Zhang, J., Lu, M., & Liu, Y. (2025). Impact of Polystyrene Microplastics on Soil Properties, Microbial Diversity and Solanum lycopersicum L. Growth in Meadow Soils. Plants, 14(2), 256. https://doi.org/10.3390/plants14020256





