Functional Studies on the LiAG1 Gene of Lilium ‘Ice Pink Queen’ in Flower Development
Abstract
:1. Introduction
2. Results
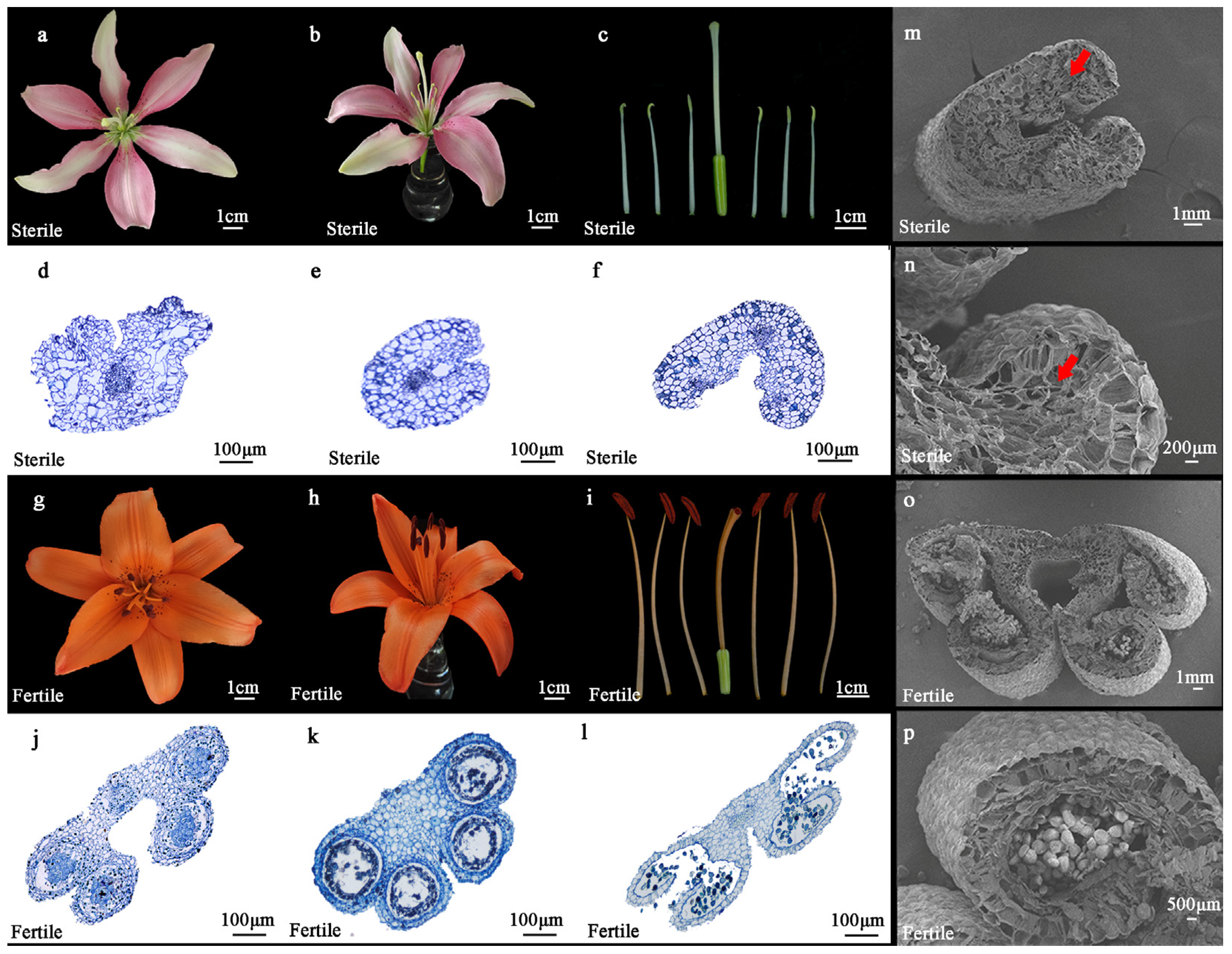
2.1. Morphological Characteristics of Lilium Asiatica Hybrida ‘Ice Pink Queen’ and ‘Elite’
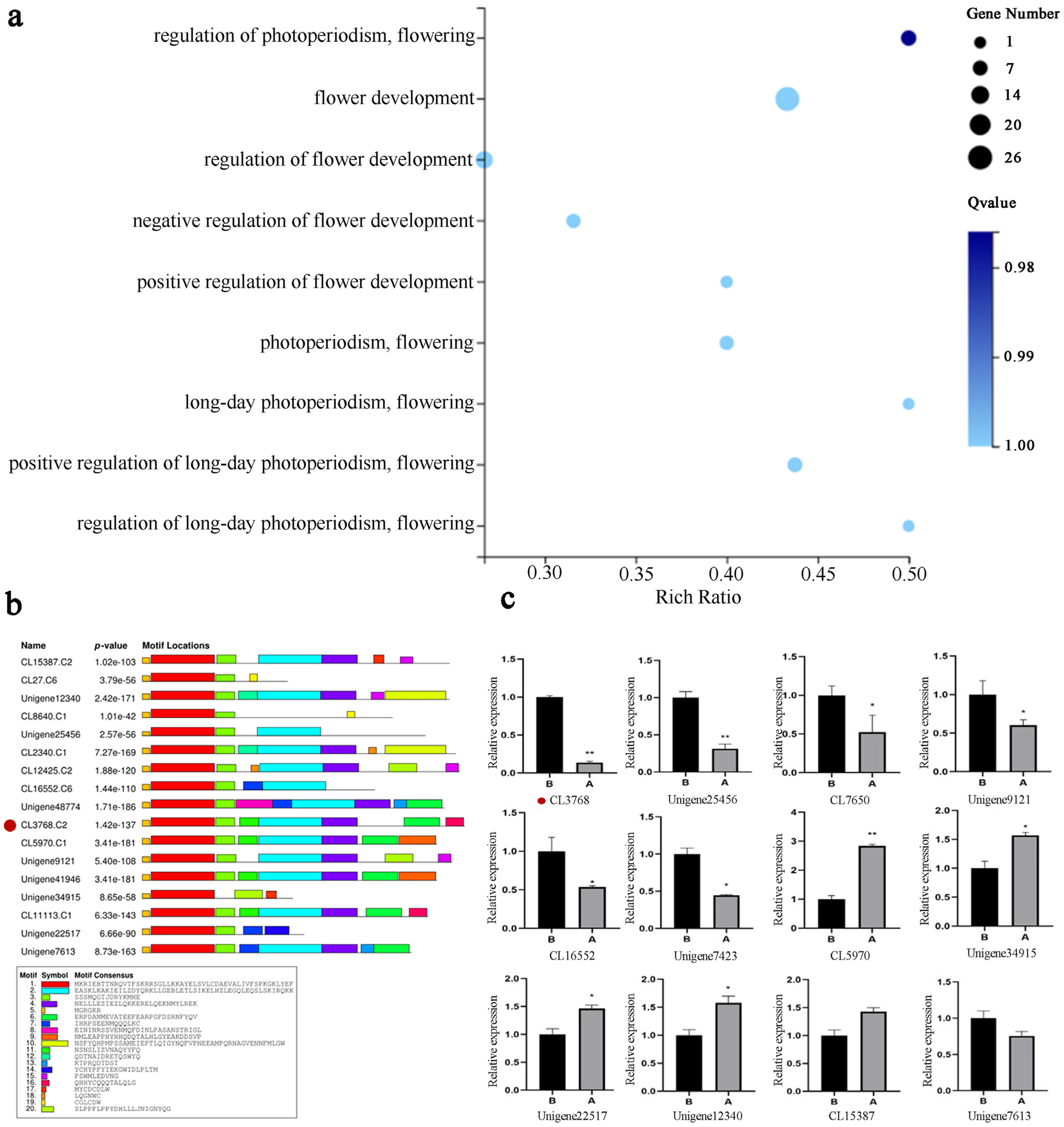
2.2. Transcriptome Data Analysis of Flower Buds in the ‘Ice Pink Queen’ and ’Elite’
2.3. LiAG1 Is a MADS-Box Member in Lily and Is a Nuclear Localisation Protein
2.4. LiAG1 Gene Is Mainly Expressed in Anthers
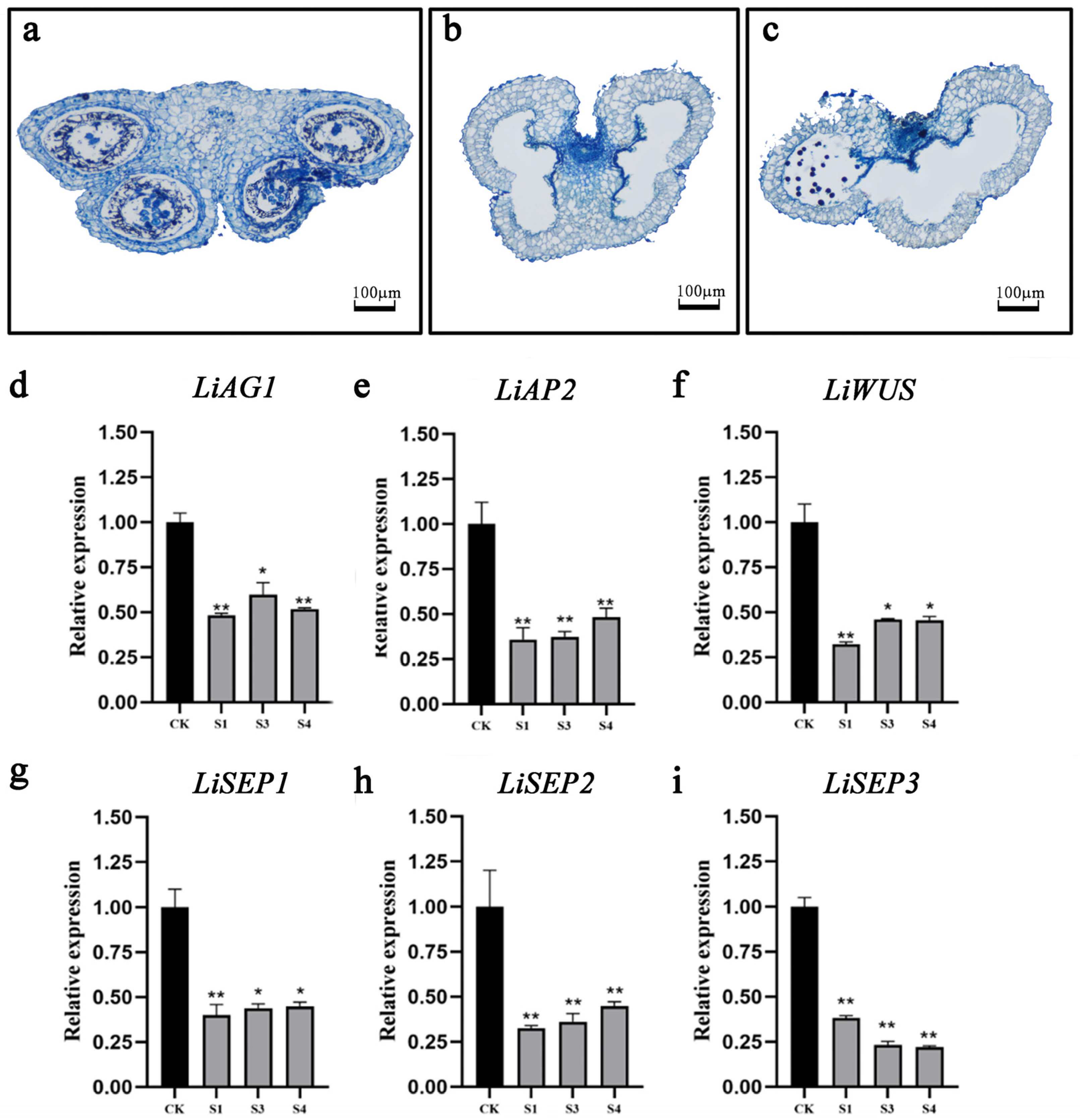
2.5. Silenced LiAG1 Affects Anther Development
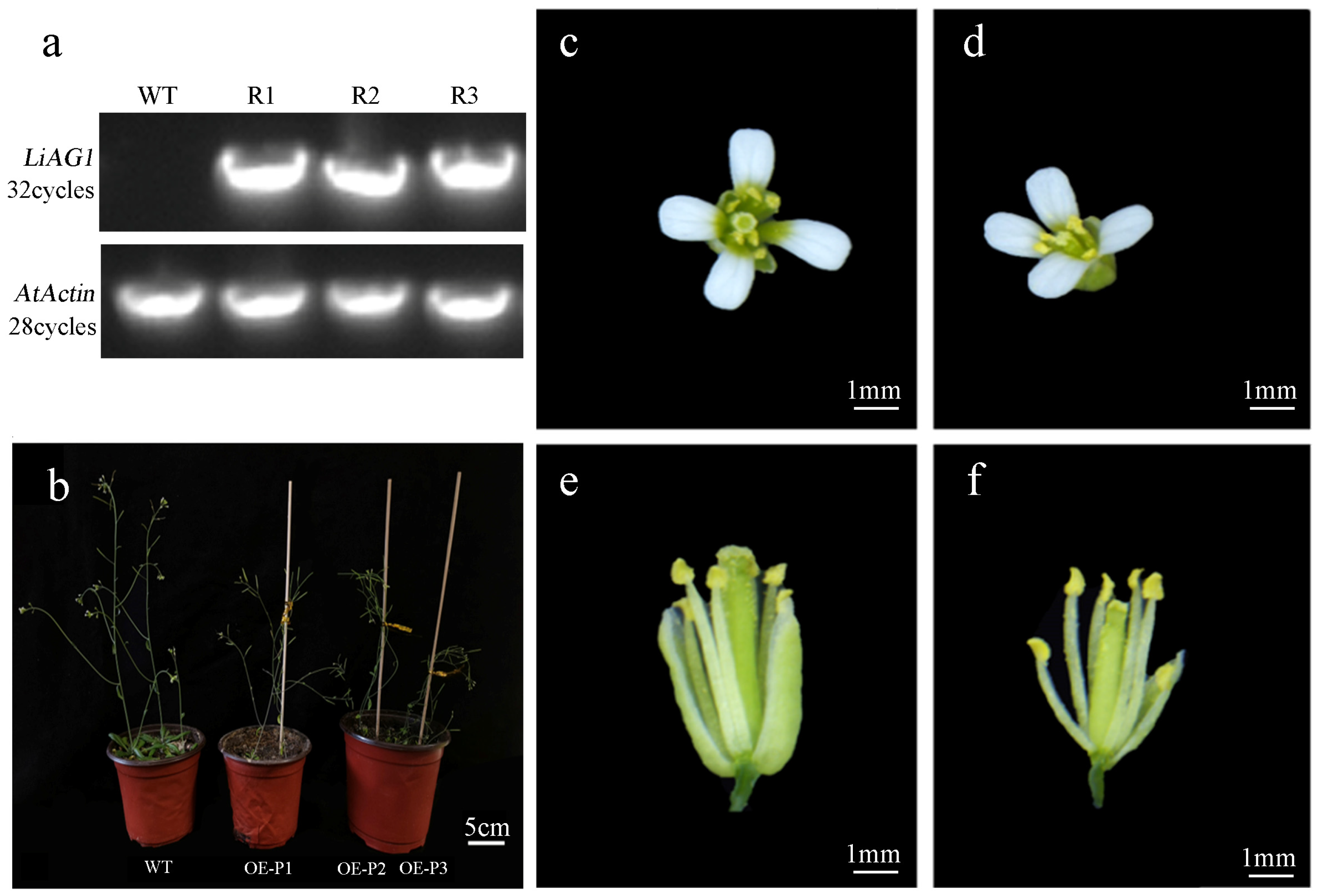
2.6. Overexpression of LiAG1 Can Promote Early Flowering and Stigma Shortening of Arabidopsis
2.7. Expression of Key Flowering-Related Genes of LiAG1 Transgenic Arabidopsis thaliana
2.8. Overexpression of LiAG1 Can Promote Early Flowering and Stigma Shortening in Tobacco
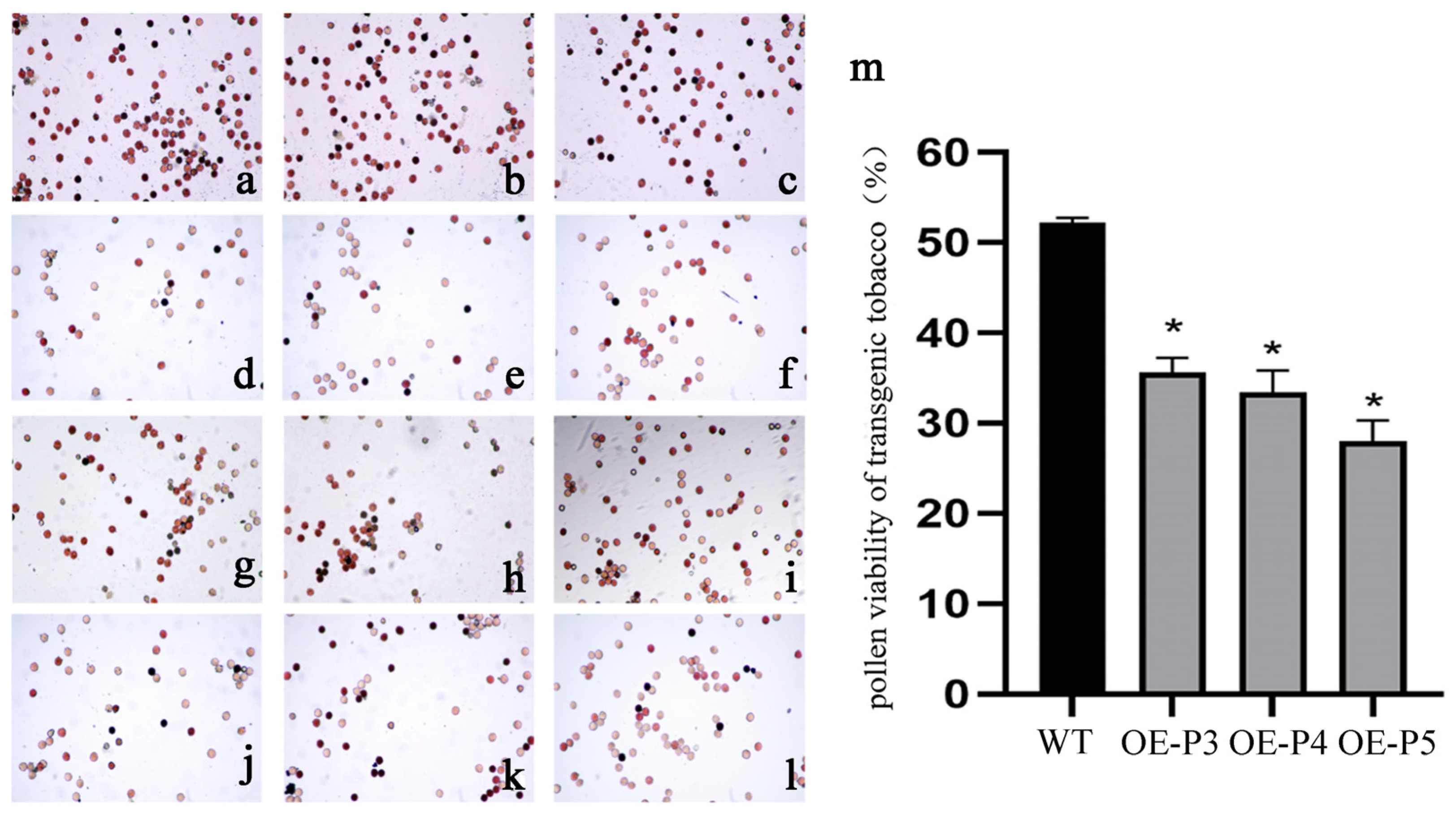
2.9. Overexpression of LiAG1 Decreased the Viability of Tobacco Pollen
2.10. Expression of Key Flowering-Related Genes in LiAG1 Transgenic Tobacco
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Morphological and Histological Analysis
4.3. Transcriptome Differential Expression Gene Screening
4.4. RNA Extraction and Gene Cloning
4.5. Sequence Comparison and Phylogenetic Analysis
4.6. Subcellular Localisation of LiAG1
4.7. Organ-Specific Expression of LiAG1
4.8. Virus-Induced LiAG1 Gene Silencing and Identification
4.9. Generation of Transgenic Arabidopsis Thaliana Strains and Identification of Positive Plants
4.10. Production and Phenotypic Identification of Transgenic Tobacco Lines ‘95’
4.11. Observation of Pollen Viability
4.12. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maio, K.A.; Moubayidin, L. ‘Organ’ising Floral Organ Development. Plants 2024, 13, 1595. [Google Scholar] [CrossRef] [PubMed]
- Bazakos, C.; Michailidis, M.; Tourvas, N.; Alexiou, K.G.; Mellidou, I.; Polychroniadou, C.; Boutsika, A.; Xanthopoulou, A.; Moysiadis, T.; Skodra, C.; et al. Genetic mosaic of the Mediterranean fig: Comprehensive genomic insights from a gene bank collection. Physiol. Plant. 2024, 176, e14482. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Ma, X.; Zhang, J.; Ming, R. Genome-wide analysis of MADS-box genes and their expression patterns in unisexual flower development in dioecious spinach. Sci. Rep. 2024, 14, 18635. [Google Scholar] [CrossRef]
- Song, G.Q.; Chen, Q. Overexpression of the MADS-box gene K-domain increases the yield potential of blueberry. Plant Sci. Int. J. Exp. Plant Biol. 2018, 276, 22–31. [Google Scholar] [CrossRef]
- Theißen, G.; Gramzow, L. Chapter 8—Structure and Evolution of Plant MADS Domain Transcription Factors. In Plant Transcription Factors; Gonzalez, D.H., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 127–138. [Google Scholar]
- Becker, A.; Theissen, G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenetics Evol. 2003, 29, 464–489. [Google Scholar] [CrossRef]
- Theißen, G.; Becker, A.; Di Rosa, A.; Kanno, A.; Kim, J.T.; Münster, T.; Winter, K.-U.; Saedler, H. A short history of MADS-box genes in plants. Plant Mol. Biol. 2004, 42, 115–149. [Google Scholar] [CrossRef]
- Callens, C.; Tucker, M.R.; Zhang, D.; Wilson, Z.A. Dissecting the role of MADS-box genes in monocot floral development and diversity. J. Exp. Bot. 2018, 69, 2435–2459. [Google Scholar] [CrossRef]
- Chen, M.-K.; Hsu, W.-H.; Lee, P.-F.; Thiruvengadam, M.; Chen, H.-I.; Yang, C.-H. The MADS box gene, FOREVER YOUNG FLOWER, acts as a repressor controlling floral organ senescence and abscission in Arabidopsis. Plant J. 2011, 68, 168–185. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.; Yong, X.; Ahmad, S.; Yang, W.; Cheng, T.; Wang, J.; Zhang, Q. SEP-class genes in Prunus mume and their likely role in floral organ development. BMC Plant Biol. 2017, 17, 10. [Google Scholar] [CrossRef]
- Ito, T.; Wellmer, F.; Yu, H.; Das, P.; Ito, N.; Alves-Ferreira, M.; Riechmann, J.L.; Meyerowitz, E.M. The homeotic protein AGAMOUS controls microsporogenesis by regulation of SPOROCYTELESS. Nature 2004, 430, 356–360. [Google Scholar] [CrossRef]
- Krizek, B.A.; Prost, V.; Macias, A. AINTEGUMENTA Promotes Petal Identity and Acts as a Negative Regulator of AGAMOUS. Plant Cell 2000, 12, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ye, H.; Li, X.; Lv, X.; Lou, J.; Chen, Y.; Yu, S.; Zhang, L. Genome-Wide Analysis of the MADS-Box Gene Family in Hibiscus syriacus and Their Role in Floral Organ Development. Int. J. Mol. Sci. 2023, 25, 406. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wang, D.; Li, Y.; Niu, M.; Liu, C.; Liu, L.; Wang, J.; Li, J. Genome-wide identification and expression pattern analysis of MIKC-Type MADS-box genes in Chionanthus retusus, an androdioecy plant. BMC Genom. 2024, 25, 662. [Google Scholar] [CrossRef] [PubMed]
- ÓMaoiléidigh, D.S.; Wuest, S.E.; Rae, L.; Raganelli, A.; Ryan, P.T.; Kwasniewska, K.; Das, P.; Lohan, A.J.; Loftus, B.; Graciet, E.; et al. Control of reproductive floral organ identity specification in Arabidopsis by the C function regulator AGAMOUS. Plant Cell 2013, 25, 2482–2503. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Oizumi, K.; Kubota, S.; Bagheri, A.; Shafaroudi, S.M.; Nakano, M.; Kanno, A. Double flower formation in Tricyrtis macranthopsis is related to low expression of AGAMOUS ortholog gene. Sci. Hortic. 2015, 193, 337–345. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.; Liu, D.; Li, F.; Lu, H. Exon skipping of AGAMOUS homolog PrseAG in developing double flowers of Prunus lannesiana (Rosaceae). Plant Cell Rep. 2013, 32, 227–237. [Google Scholar] [CrossRef]
- Dastmalchi, S.; Moshtaghi, N.; Sharifi, A. Role of AGAMOUS Gene in Increasing Tepals of Amaryllis. J. Agric. Sci. Technol. 2023, 25, 975–988. [Google Scholar]
- Yanofsky, M.F.; Ma, H.; Bowman, J.L.; Drews, G.N.; Feldmann, K.A.; Meyerowitz, E.M. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 1990, 346, 35–39. [Google Scholar] [CrossRef]
- Kennedy, A.; Geuten, K. The Role of FLOWERING LOCUS C Relatives in Cereals. Front. Plant Sci. 2020, 11, 617340. [Google Scholar] [CrossRef]
- Wei, J.; Liu, D.; Liu, G.; Tang, J.; Chen, Y. Molecular Cloning, Characterization, and Expression of MiSOC1: A Homolog of the Flowering Gene SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 from Mango (Mangifera indica L). Front. Plant Sci. 2016, 7, 1758. [Google Scholar] [CrossRef]
- Zhao, S.; Luo, Y.; Zhang, Z.; Xu, M.; Wang, W.; Zhao, Y.; Zhang, L.; Fan, Y.; Wang, L. ZmSOC1, a MADS-box transcription factor from Zea mays, promotes flowering in Arabidopsis. Int. J. Mol. Sci. 2014, 15, 19987–20003. [Google Scholar] [CrossRef] [PubMed]
- Feyissa, B.A.; Amyot, L.; Nasrollahi, V.; Papadopoulos, Y.; Kohalmi, S.E.; Hannoufa, A. Involvement of the miR156/SPL module in flooding response in Medicago sativa. Sci. Rep. 2021, 11, 3243. [Google Scholar] [CrossRef] [PubMed]
- Wigge, P.A.; Kim, M.C.; Jaeger, K.E.; Busch, W.; Schmid, M.; Lohmann, J.U.; Weigel, D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 2005, 309, 1056–1059. [Google Scholar] [CrossRef]
- Pajon, M.; Febres, V.J.; Moore, G.A. Expression patterns of flowering genes in leaves of ‘Pineapple’ sweet orange [Citrus sinensis (L.) Osbeck] and pummelo (Citrus grandis Osbeck). BMC Plant Biol. 2017, 17, 146. [Google Scholar] [CrossRef]
- Kavai-ool, U.N.; Kupriyanova, E.V.; Ezhova, T.A. Different Influence of Mutant Alleles of the APETALA1 Gene on the Development of Reproductive Organs in abruptus Mutant Flowers of the of Arabidopsis thaliana (L.) Heynh. Russ. J. Dev. Biol. 2011, 42, 267–271. [Google Scholar] [CrossRef]
- Chu, T.; Xie, H.; Xu, Y.; Ma, R. Regulation pattern of the FRUITFULL (FUL) gene of Arabidopsis thaliana. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2010, 26, 1546–1554. [Google Scholar]
- Kazama, T.; Itabashi, E.; Fujii, S.; Nakamura, T.; Toriyama, K. Mitochondrial ORF79 levels determine pollen abortion in cytoplasmic male sterile rice. Plant J. Cell Mol. Biol. 2016, 85, 707–716. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.-H.; Wang, S.; Zhang, Y.; Huang, T.; Cai, Y.-D. Prediction and analysis of essential genes using the enrichments of gene ontology and KEGG pathways. PLoS ONE 2017, 12, e0184129. [Google Scholar] [CrossRef]
- Kramer, E.M.; Jaramillo, M.A.; Di Stilio, V.S. Patterns of Gene Duplication and Functional Evolution During the Diversification of the AGAMOUS Subfamily of MADS Box Genes in Angiosperms. Genetics 2004, 166, 1011–1023. [Google Scholar] [CrossRef]
- Dinh, T.T.; Girke, T.; Liu, X.; Yant, L.; Schmid, M.; Chen, X. The floral homeotic protein APETALA2 recognizes and acts through an AT-rich sequence element. Development 2012, 139, 1978–1986. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, R.; Xu, Y.; Wang, M.; Zhang, J.; Bai, M.; Han, C.; Xiang, F.; Wang, Z.-Y.; Mysore, K.S.; et al. AGLF provides C-function in floral organ identity through transcriptional regulation of AGAMOUS in Medicago truncatula. Proc. Natl. Acad. Sci. USA 2019, 116, 5176–5181. [Google Scholar] [CrossRef] [PubMed]
- Galimba, K.D.; Tolkin, T.R.; Sullivan, A.M.; Melzer, R.; Theißen, G.; Di Stilio, V.S. Loss of deeply conserved C-class floral homeotic gene function and C- and E-class protein interaction in a double-flowered ranunculid mutant. Proc. Natl. Acad. Sci. USA 2012, 109, E2267–E2275. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lou, X.; Li, J.; Pu, M.; Mirbahar, A.A.; Liu, D.; Sun, J.; Zhan, K.; He, L.; Zhang, A. Cloning and Functional Analysis of MADS-box Genes, TaAG-A and TaAG-B, from a Wheat K-type Cytoplasmic Male Sterile Line. Front. Plant Sci. 2017, 8, 1081. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.-K.; Liu, M.-Q.; Wu, G.-J.; Wang, J.-Y.; Zhong, Y.; Li, H.-Y.; Tang, H.-L.; Zeng, W.; Ma, L.-X.; Zhang, Y.; et al. Ectopic expression of an AGAMOUS homologue gene in Jatropha curcas causes early flowering and heterostylous phenotypes. Gene 2021, 766, 145141. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Zhang, J.; Pang, C.; Yu, H.; Guo, D.; Jiang, H.; Ding, M.; Chen, Z.; Tao, Q.; Gu, H.; et al. The molecular mechanism of SPOROCYTELESS/NOZZLE in controlling Arabidopsis ovule development. Cell Res. 2015, 25, 121–134. [Google Scholar] [CrossRef]
- Pina-Martins, F.; Caperta, A.D.; Conceição, S.I.R.; Nunes, V.L.; Marques, I.; Paulo, O.S. A first look at sea-lavenders genomics–can genome wide SNP information tip the scales of controversy in the Limonium vulgare species complex? BMC Plant Biol. 2023, 23, 34. [Google Scholar] [CrossRef]
- Nardeli, S.M.; Arge, L.W.P.; Artico, S.; de Moura, S.M.; Tschoeke, D.A.; Guedes, F.A.D.; Grossi-de-Sa, M.F.; Martinelli, A.P.; Alves-Ferreira, M. Global gene expression profile and functional analysis reveal the conservation of reproduction-associated gene networks in Gossypium hirsutum. Plant Reprod. 2024, 37, 215–227. [Google Scholar] [CrossRef]
- Shi, L.; Li, C.J.; Lv, G.F.; Li, X.; Feng, W.T.; Bi, Y.J.; Wang, W.H.; Wang, Y.Q.; Zhu, L.; Tang, W.Q.; et al. The adaptor protein ECAP, the corepressor LEUNIG, and the transcription factor BEH3 interact and regulate microsporocyte generation in Arabidopsis. Plant Cell 2024, 36, 2531–2549. [Google Scholar] [CrossRef]
- Chen, W.Q.; Wang, P.; Liu, C.; Han, Y.T.; Zhao, F. Male Germ Cell Specification in Plants. Int. J. Mol. Sci. 2024, 25, 6643. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, L.; Wang, W.; Qi, W.; Cao, Z.; Li, H.; Bao, M.; He, Y. Identification, characterization and functional analysis of AGAMOUS subfamily genes associated with floral organs and seed development in Marigold (Tagetes erecta). BMC Plant Biol. 2020, 20, 439. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, L.; Li, Z.; Lei, M.; Xu, L. Cloning and Overexpression Vector Construction of AfAG from Aechmea fasciata. Mol. Plant Breed. 2016, 14, 389–395. [Google Scholar]
- Liu, Y.; Zhang, D.; Ping, J.; Li, S.; Chen, Z.; Ma, J. Innovation of a Regulatory Mechanism Modulating Semi-determinate Stem Growth through Artificial Selection in Soybean. PLoS Genet. 2016, 12, e1005818. [Google Scholar] [CrossRef] [PubMed]
- Serwatowska, J.; Roque, E.; Gómez-Mena, C.; Constantin, G.D.; Wen, J.; Mysore, K.S.; Lund, O.S.; Johansen, E.; Beltrán, J.P.; Cañas, L.A. Two euAGAMOUS genes control C-function in Medicago truncatula. PLoS ONE 2014, 9, e103770. [Google Scholar] [CrossRef] [PubMed]
- Criswell, S.; Gaylord, B.; Pitzer, C.R. Histological methods for plant tissues. J. Histotechnol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Alhomaidi, E. Scanning electron microscopic exploration of intricate pollen morphology and antimicrobial potentials of gourd family. Microsc. Res. Tech. 2024, 87, 999–1008. [Google Scholar] [CrossRef]
- Ding, X.S.; Schneider, W.L.; Chaluvadi, S.R.; Mian, M.A.; Nelson, R.S. Characterization of a Brome mosaic virus strain and its use as a vector for gene silencing in monocotyledonous hosts. Mol. Plant-Microbe Interact. MPMI 2006, 19, 1229–1239. [Google Scholar] [CrossRef]
- Hu, F.; Ren, C.; Bao, R.; Luo, F. Comparison of Genomic DNA Extraction Methods From Hyacinth. J. Shenyang Agric. Univ. 2011, 42, 570–573. [Google Scholar]
- Zhang, X.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, D.; Dai, J.; Yang, T.; Zhang, J.; Che, D.; Fan, J. Cloning and Functional Characterization of 2-C-methyl-D-erythritol-4-phosphate cytidylyltransferase (LiMCT) Gene in Oriental Lily (Lilium ‘Sorbonne’). Mol. Biotechnol. 2024, 66, 56–67. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, H.; Yu, J.; Chen, J.; Luo, M.; Lei, B. Regulation of Leaf Bud by REVOLUTA in Tobacco Based on CRISPR/Cas9 System. Biotechnol. Bull. 2023, 39, 197–208. [Google Scholar]
- Yao, Z.; Liu, Y.; Dai, P.; Xiang, D.; Zhang, X.; Zhao, T.; Wang, Z. Wild Tobacco Pollen Viability, Stigma Receptivity and Reproductive Characteristics. Acta Bot. Boreali-Occident. Sin. 2015, 35, 614–621. [Google Scholar]





| Gene ID | Nr |
|---|---|
| CL3308.Contig4_All | PREDICTED: GATA transcription factor 19-like |
| CL14787.Contig1_All | exportin-T isoform X1 |
| CL14787.Contig2_All | FRIGIDA-like protein 4a |
| CL2611.Contig1_All | FRIGIDA-like protein 4a |
| CL30.Contig4_All | nodulin homeobox-like isoform X2 |
| CL3768.Contig2_All | agamous-like protein |
| CL3527.Contig1_All | E3 ubiquitin-protein ligase BRE1-like 2 |
| CL3713.Contig2_All | hypothetical protein COCNU_14G003670 |
| CL5787.Contig2_All | probable histidine kinase 3 |
| CL6866.Contig1_All | FRIGIDA-like protein 4b |
| CL6885.Contig3_All | flowering-promoting factor 1-like protein 1 |
| CL8146.Contig2_All | pheophorbide a oxygenase, chloroplastic |
| CL860.Contig2_All | allene oxide cylase |
| CL8635.Contig3_All | hypothetical protein C4D60_Mb05t18560 |
| CL9067.Contig5_All | FTL1 |
| Unigene26098_All | E3 ubiquitin-protein ligase BRE1-like 2 |
| Unigene30599_All | E3 ubiquitin-protein ligase BRE1-like 2 |
| Unigene37308_All | E3 ubiquitin-protein ligase BRE1-like 2 |
| Unigene42154_All | G-protein coupled receptor 1 |
| Unigene6100_All | phosphatidyl ethanolamine-binding protein |
| Unigene8761_All | uncharacterised protein LOC103705616 isoform X1 |
| Unigene9076_All | FRIGIDA-like protein 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, L.; Dai, J.; Fu, R.; Wu, N.; Yu, J.; Dong, J.; Yang, T.; Fan, J. Functional Studies on the LiAG1 Gene of Lilium ‘Ice Pink Queen’ in Flower Development. Plants 2025, 14, 323. https://doi.org/10.3390/plants14030323
Xue L, Dai J, Fu R, Wu N, Yu J, Dong J, Yang T, Fan J. Functional Studies on the LiAG1 Gene of Lilium ‘Ice Pink Queen’ in Flower Development. Plants. 2025; 14(3):323. https://doi.org/10.3390/plants14030323
Chicago/Turabian StyleXue, Lili, Jingqi Dai, Ruyu Fu, Nana Wu, Jiaxuan Yu, Jie Dong, Tao Yang, and Jinping Fan. 2025. "Functional Studies on the LiAG1 Gene of Lilium ‘Ice Pink Queen’ in Flower Development" Plants 14, no. 3: 323. https://doi.org/10.3390/plants14030323
APA StyleXue, L., Dai, J., Fu, R., Wu, N., Yu, J., Dong, J., Yang, T., & Fan, J. (2025). Functional Studies on the LiAG1 Gene of Lilium ‘Ice Pink Queen’ in Flower Development. Plants, 14(3), 323. https://doi.org/10.3390/plants14030323






