Current Biological Insights of Castanea sativa Mill. to Improve Crop Sustainability to Climate Change
Abstract
1. Introduction
2. Knowing C. sativa Plasticity Anticipating Climate Changes
2.1. Botanical Level
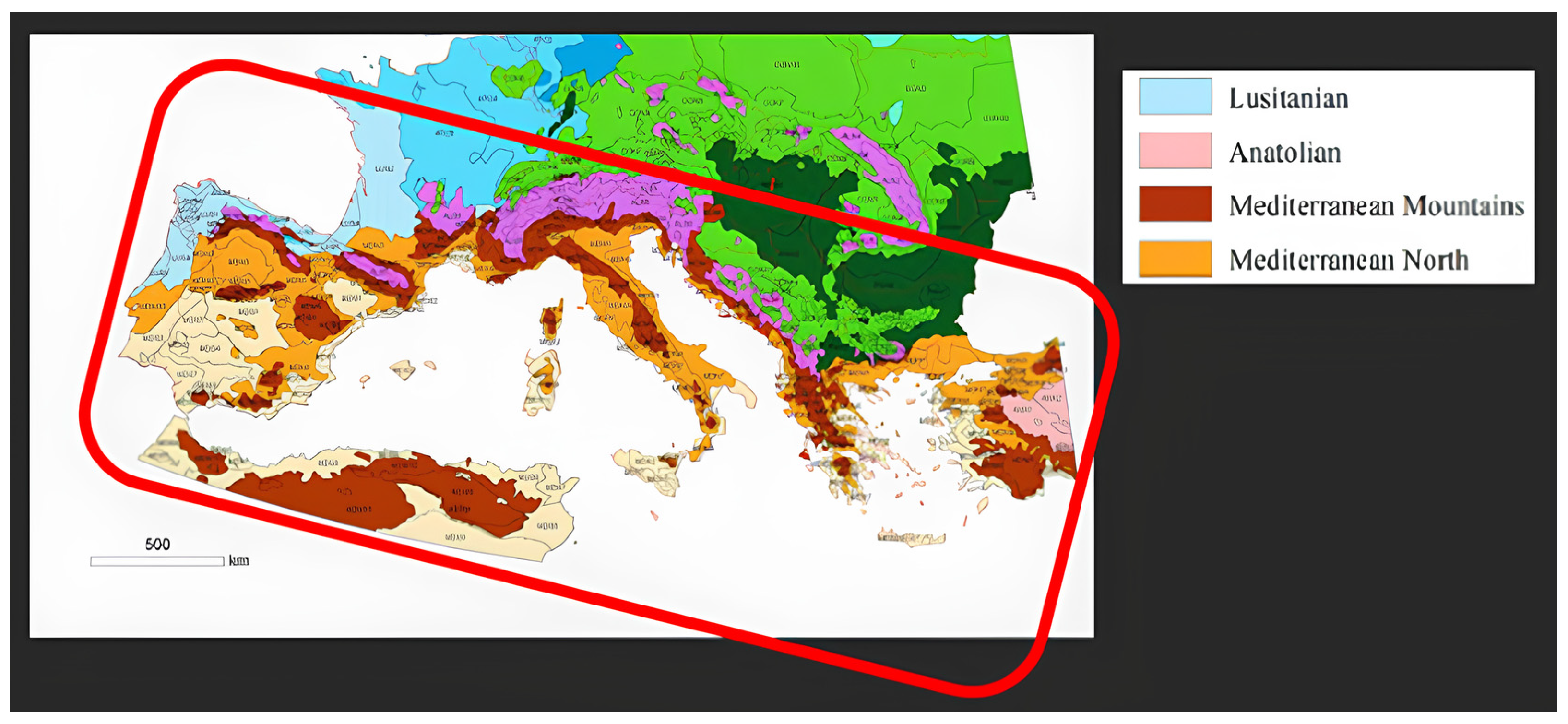
2.2. Ecological Level
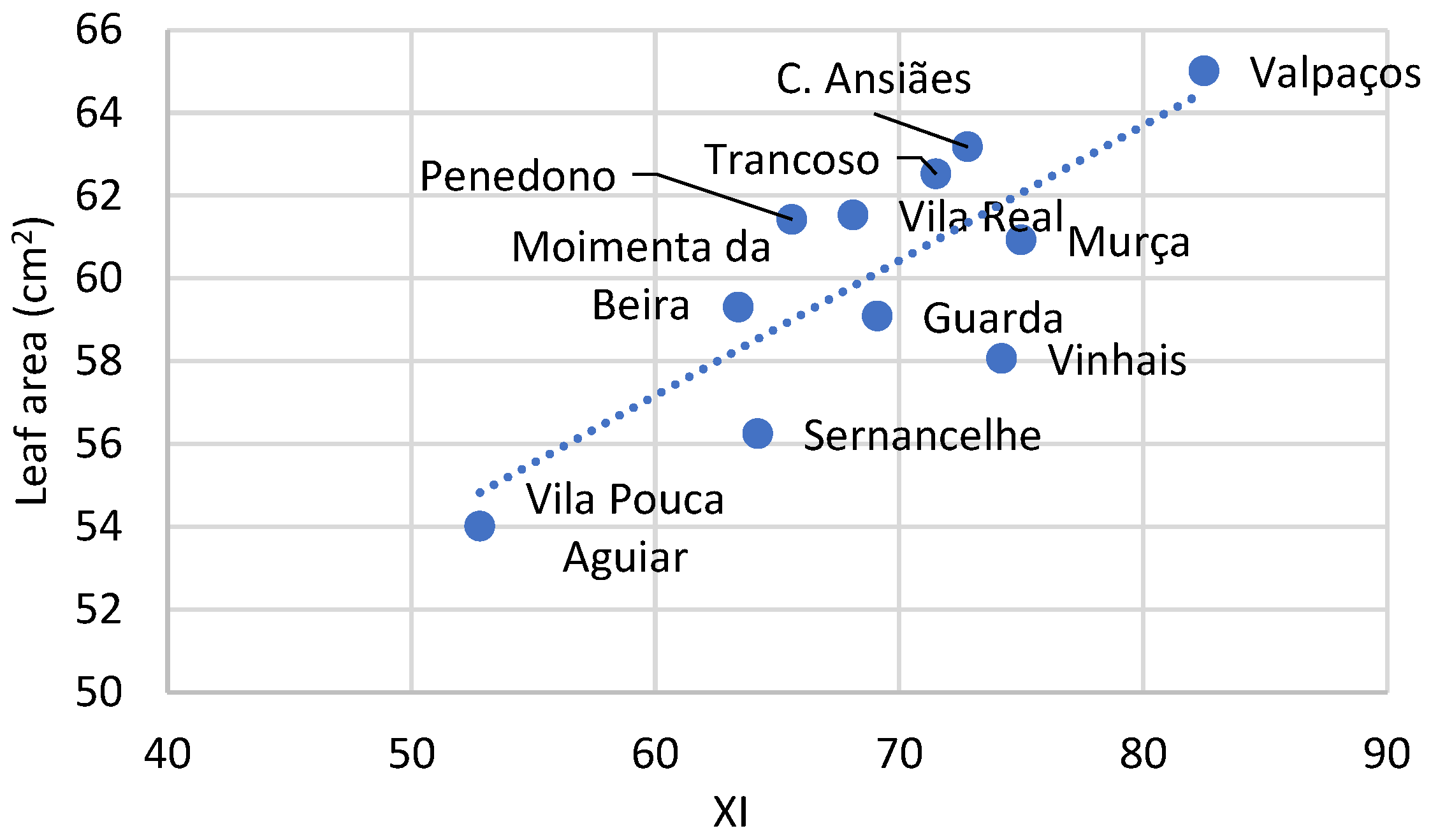
2.3. Morphological and Phenological Level
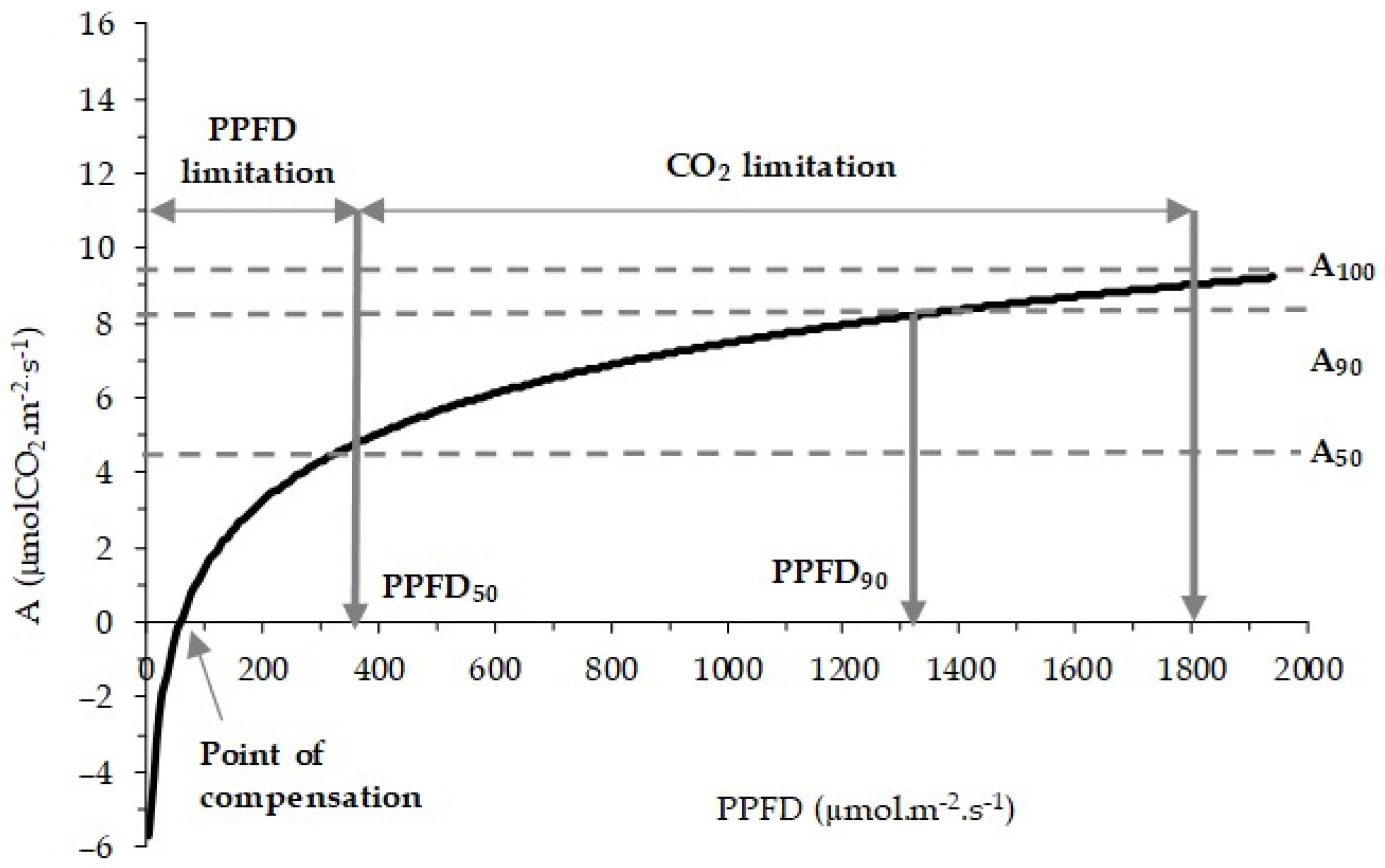
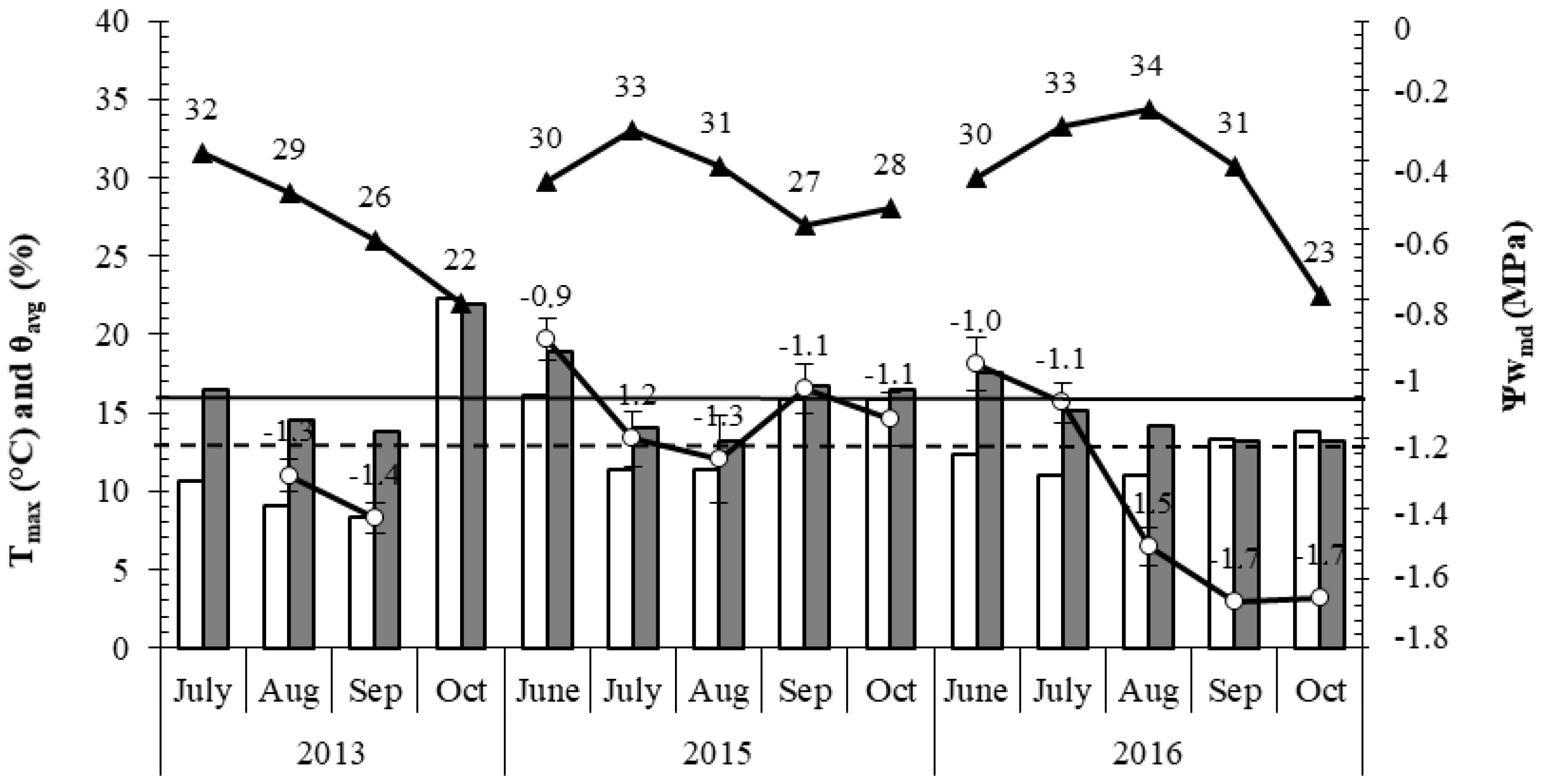
2.4. Ecophysiological Level
2.5. Biochemical Level
3. Chestnut Adaptative Strategies to Climate Changes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larue, C.; Barreneche, T.; Petit, R.J. Efficient Monitoring of Phenology in Chestnuts. Sci. Hortic. 2021, 281, 109958. [Google Scholar] [CrossRef]
- Conedera, M.; Krebs, P.; Tinner, W.; Pradella, M.; Torriani, D. The Cultivation of Castanea sativa (Mill.) in Europe, from Its Origin to Its Diffusion on a Continental Scale. Veg. Hist. Archaeobot 2004, 13, 161–179. [Google Scholar] [CrossRef]
- McEvoy, T. Positive Impact Forestry: A Sustainable Approach to Managing Woodlands; Island Press: Washington, DC, USA, 2012. [Google Scholar]
- Food and Agriculture Organization Crops. Available online: https://www.fao.org/faostat/en/#home (accessed on 4 June 2022).
- INE—Instituto Nacional de Estatística Chestnut Production. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_pesquisa&frm_accao=PESQUISAR&frm_show_page_num=1&frm_modo_pesquisa=PESQUISA_SIMPLES&frm_modo_texto=MODO_TEXTO_ALL&frm_texto=castanha&frm_imgPesquisar= (accessed on 3 June 2022).
- Perlin, A.P.; Gomes, C.M.; Motke, F.D.; Kruglianskas, I.; Zaluski, F.C. Climate Change Mitigation, Adaptation Practices, and Business Performance in Brazilian Industrial Companies. Sustainability 2022, 14, 11506. [Google Scholar] [CrossRef]
- Míguez-Soto, B.; Fernández-Cruz, J.; Fernández-López, J. Mediterranean and Northern Iberian Gene Pools of Wild Castanea sativa Mill. Are Two Differentiated Ecotypes Originated under Natural Divergent Selection. PLoS ONE 2019, 14, e0211315. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, G.; Nuzzo, V.; Xiloyannis, C.; Dichio, B. Climate Change Mitigation and Adaptation in Agriculture: The Case of the Olive. J. Water Clim. Change 2018, 9, 633–642. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Butt, M.; Irfan, M.; Rizwan, M.S.; Ali, H.; Cheema, M.A. Interactive Effect of Biochar and Silicon on Improving Morpho-Physiological and Biochemical Attributes of Maize by Reducing Drought Hazards. J. Soil. Sci. Plant Nutr. 2020, 20, 1819–1826. [Google Scholar] [CrossRef]
- Tricker, P.J.; ElHabti, A.; J Schmidt, J. The Physiological and Genetic Basis of Combined Drought and Heat Tolerance in Wheat. J. Exp. 2018, 69, 3195–3210. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant Adaptations to the Combination of Drought and High Temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Parmar, N.; Singh, K.H.; Sharma, D.; Singh, L.; Kumar, P.; Nanjundan, J.; Khan, Y.J.; Chauhan, D.K.; Thakur, A.K. Genetic Engineering Strategies for Biotic and Abiotic Stress Tolerance and Quality Enhancement in Horticultural Crops: A Comprehensive Review. 3 Biotech 2017, 7, 239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yan, Y.; Wang, C.; Tang, M.; Wu, G. Adaptation and Mitigation for Combating Climate Change–from Single to Joint. Ecosyst. Health Sustain. 2018, 4, 85–94. [Google Scholar] [CrossRef]
- Iglesias, A.; Quiroga, S.; Moneo, M.; Garrote, L. From Climate Change Impacts to the Development of Adaptation Strategies: Challenges for Agriculture in Europe. Clim. Change 2012, 112, 143–168. [Google Scholar] [CrossRef]
- Linnenluecke, M.K.; Zhou, C.; Smith, T.; Thompson, N.; Nucifora, N. The Impact of Climate Change on the Australian Sugarcane Industry. J. Clean. Prod. 2020, 246, 118974. [Google Scholar] [CrossRef]
- Allard, R.F. Climate Change Adaptation: Infrastructure and Extreme Weather. In Industry, Innovation and Infrastructure; Springer: Berlin/Heidelberg, Germany, 2021; pp. 105–116. [Google Scholar] [CrossRef]
- Hallegatte, S. Strategies to Adapt to an Uncertain Climate Change. Glob. Environ. Change 2009, 19, 240–247. [Google Scholar] [CrossRef]
- Mellano, M.G. COLUTAD®: Un Nuovo Portinnesto per C. sativa. Castanea 2020, 15, 14–15. [Google Scholar]
- Lang, P.; Dane, F.; Kubisiak, T.L.; Huang, H. Molecular Evidence for an Asian Origin and a Unique Westward Migration of Species in the Genus Castanea via Europe to North America. Mol. Phylogenet Evol. 2007, 43, 49–59. [Google Scholar] [CrossRef]
- Kubitzki, K. Fagaceae. In Flowering Plants Dicotyledons; Springer: Berlin/Heidelberg, Germany, 1993; pp. 301–309. [Google Scholar] [CrossRef]
- Staton, M.; Addo-Quaye, C.; Cannon, N.; Yu, J.; Zhebentyayeva, T.; Huff, M.; Islam-Faridi, N.; Fan, S.; Georgi, L.L.; Nelson, C.D.; et al. A Reference Genome Assembly and Adaptive Trait Analysis of Castanea mollissima ‘Vanuxem,’ a Source of Resistance to Chestnut Blight in Restoration Breeding. Tree Genet. Genomes 2020, 16, 1–23. [Google Scholar] [CrossRef]
- Lados, B.B.; Benke, A.; Borovics, A.; Köbölkuti, Z.A.; Molnár, C.É.; Nagy, L.; Tóth, E.G.; Cseke, K. What We Know about Turkey Oak (Quercus cerris L.)—From Evolutionary History to Species Ecology. Forestry 2024, 97, 497–511. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Ballester, A.; Corredoira, E.; Vieitez, A.M.; Agnanostakis, S.; Costa, R.; Bounous, G.; Botta, R.; Beccaro, G.L.; Kubisiak, T.L.; et al. Chestnut. In Fruit Breeding; Springer: New York, NY, USA, 2012; pp. 729–769. ISBN 9781441907622. [Google Scholar]
- Beccaro, G.; Bounous, G.; Biaggi, M.D.; Donno, D.; Marinoni, D.T.; Zou, F.; Mellano, M.G. Botany, Anatomy, and Nut Composition. In The Chestnut Handbook; Routledge: London, UK, 2019; pp. 5–34. [Google Scholar] [CrossRef]
- Rutter, P.A.; Miller, G.; Payne, J.A. Chestnuts (Castanea). In Genetic Resources of Temperate Fruit and Nut Crops; International Society for Horticultural Science: Leuven, Belgium, 1991; Volume 290, pp. 761–790. [Google Scholar]
- Fernandes, P.; Colavolpe, M.B.; Serrazina, S.; Costa, R.L. European and American Chestnuts: An Overview of the Main Threats and Control Efforts. Front. Plant Sci. 2022, 13, 951844. [Google Scholar] [CrossRef] [PubMed]
- Aravanopoulos, F.A. Breeding of Fast Growing Forest Tree Species for Biomass Production in Greece. Biomass Bioenergy 2010, 34, 1531–1537. [Google Scholar] [CrossRef]
- Mattioni, C.; Cherubini, M.; Micheli, E.; Villani, F.; Bucci, G. Role of Domestication in Shaping Castanea sativa Genetic Variation in Europe. Tree Genet. Genomes 2008, 4, 563–574. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Costa, R.M.L.; Ramos-Cabrer, A.M.; Ribeiro, C.A.M.; da Silva, M.F.S.; Manzano, G.; Barreneche, T. Variation in Grafted European Chestnut and Hybrids by Microsatellites Reveals Two Main Origins in the Iberian Peninsula. Tree Genet. Genomes 2010, 6, 701–715. [Google Scholar] [CrossRef]
- Mattioni, C.; Martin, M.A.; Chiocchini, F.; Cherubini, M.; Gaudet, M.; Pollegioni, P.; Velichkov, I.; Jarman, R.; Chambers, F.M.; Paule, L.; et al. Landscape Genetics Structure of European Sweet Chestnut (Castanea sativa Mill): Indications for Conservation Priorities. Tree Genet. Genomes 2017, 13, 1–14. [Google Scholar] [CrossRef]
- Stukenbrock, E.H.; McDonald, B.A. The Origins of Plant Pathogens in Agro-Ecosystems. Annu. Rev. Phytopathol. 2008, 46, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Lauteri, M.; Pliura, A.; Monteverdi, M.C.; Brugnoli, E.; Villani, F.; Eriksson, G. Genetic Variation in Carbon Isotope Discrimination in Six European Populations of Castanea sativa Mill. Originating from Contrasting Localities. J. Evol. Biol. 2004, 17, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Tzonev, R.; Lyubenova, M.; Hinkov, G.; Karakiev, T. Ecological and Syntaxonomic Characteristics of the Floristic Complex in the Sweet Chestnut (Castanea sativa Mill.) Forests of Belasitsa Mountain; Academia.Edu: London, UK, 2011. [Google Scholar]
- Mattioni, C.; Martin, M.A.; Pollegioni, P.; Cherubini, M.; Villani, F. Microsatellite Markers Reveal a Strong Geographical Structure in European Populations of Castanea sativa (Fagaceae): Evidence for Multiple Glacial Refugia. Am. J. Bot. 2013, 100, 951–961. [Google Scholar] [CrossRef]
- Metzger, M.; Leemans, R.; Earth, D.S.-I.J. A Multidisciplinary Multi-Scale Framework for Assessing Vulnerabilities to Global Change. Int. J. Appl. Earth Obs. Geoinf. 2005, 7, 253–267. [Google Scholar] [CrossRef]
- Freitas, T.R.; Santos, J.A.; Silva, A.P.; Fraga, H. Influence of Climate Change on Chestnut Trees: A Review. Plants 2021, 10, 1463. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Fraga, H.; Belo-Pereira, M.; Santos, J.A. Assessment of Growing Thermal Conditions of Main Fruit Species in Portugal Based on Hourly Records from a Weather Station Network. Appl. Sci. 2019, 9, 3782. [Google Scholar] [CrossRef]
- Rodrigues, A.; Gonçalves, A.; Costa, R.; Gomes, A. GIS-Based Assessment of the Chestnut Expansion Potential: A Case-Study on the Marvão Productive Area, Portugal. Agriculture 2021, 11, 1260. [Google Scholar] [CrossRef]
- Pereira, M.G.; Caramelo, L.; Gouveia, C.; Gomes-Laranjo, J.; Magalhães, M. Assessment of Weather-Related Risk on Chestnut Productivity. Nat. Hazards Earth Syst. Sci. 2011, 11, 2729–2739. [Google Scholar] [CrossRef]
- Nguyen, J.L.; Schwartz, J.; Dockery, D.W. The Relationship between Indoor and Outdoor Temperature, Apparent Temperature, Relative Humidity, and Absolute Humidity. Indoor Air 2014, 24, 103–112. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Morel, O.; Perlerou, C.; Robin, C.; Diamandis, S.; Vannini, A. Occurrence and Distribution of Phytophthora Species in European Chestnut Stands, and Their Association with Ink Disease and Crown Decline. Eur. J. Plant Pathol. 2005, 111, 169–180. [Google Scholar] [CrossRef]
- Instituto Português do Mar e da Atmosfera. Available online: https://www.ipma.pt/pt/index.html (accessed on 28 December 2024).
- Mota, M.; Marques, T.; Pinto, T.; Raimundo, F.; Borges, A.; Caço, J.; Gomes-Laranjo, J. Relating Plant and Soil Water Content to Encourage Smart Watering in Chestnut Trees. Agric. Water Manag. 2018, 203, 30–36. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.; Afonso, S.; Gonçalves, B. Compared Leaf Anatomy and Water Relations of Commercial and Traditional Prunus dulcis (Mill.) Cultivars under Rain-Fed Conditions. Sci. Hortic. 2018, 229, 226–232. [Google Scholar] [CrossRef]
- Mota, M.; Pinto, T.; Vilela, A.; Marques, T.; Borges, A.; Caco, J.; Ferreira-Cardoso, J.; Raimundo, F.; Gomes-Laranjo, J. Irrigation positively affects the chestnut’s quality: The chemical composition, fruit size and sensory attributes. Sci. Hortic. 2018, 238, 177–186. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Staggenborg, S.A.; Ristic, Z. Impacts of Drought and/or Heat Stress on Physiological, Developmental, Growth, and Yield Processes of Crop Plants. In Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 301–355. [Google Scholar] [CrossRef]
- Gomes-Laranjo, J.; Pinto, T.; Anjos, R.; Crespi, A.L.; Peixoto, F.; Pimentel-Pereira, M.; Coutinho, J.P.; Ferreira-Cardoso, J.; Gomes-da-Costa, L.; Araújo-Alves, J.; et al. O Castanheiro Do Ponto de Vista Botânico e Ecofisiológico. In Castanheiros; Gomes-Laranjo, J., Ferreira-Cardoso, J., Portela, E., Abreu, C.G., Eds.; Universidade Trás-os-Montes e Alto Douro: Vila Real, Portugal, 2007; pp. 43–162. [Google Scholar]
- Karthika, K.S.; Rashmi, I.; Parvathi, M.S. Biological Functions, Uptake and Transport of Essential Nutrients in Relation to Plant Growth. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–49. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef]
- Lv, X.; Li, Y.; Chen, R.; Rui, M.; Y Wang, Y. Stomatal Responses of Two Drought-Tolerant Barley Varieties with Different ROS Regulation Strategies under Drought Conditions. Antioxidants 2023, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Matthew, C.; Yang, P.; Huang, Y.; Nie, B.; Nan, Z. Leaf Morphology, Functional Trait and Altitude Response in Perennial Vetch (Vicia unijuga A. Braun), Alfalfa (Medicago sativa L.) and Sainfoin (Onobrychis viciifolia Scop.). Planta 2023, 257, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D.; Bresta, P. Protective and Defensive Roles of Non-Glandular Trichomes against Multiple Stresses: Structure–Function Coordination. J. Res. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Jara-Chiquito, J.L.; Pujade-Villar, J.; Ferreira, B.G.; Álvarez, R. Histological Changes Induced by the Cynipid Wasp Dryocosmus kuriphilus (Hymenoptera: Cynipidae) in Leaves of the Chestnut Castanea sativa (Fagaceae): Mechanisms of Galling Impact on Host Vigor. Arthropod Plant Interact. 2021, 15, 223–233. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, J.; Kang, H.; Hui, N.; Yin, S.; Chen, Z.; Du, B.; Liu, C. Spatial Variations in Leaf Trichomes and Their Coordination with Stomata in Quercus variabilis across Eastern Asia. Authorea 2023, Preprints. [Google Scholar] [CrossRef]
- Ciordia, M.; Feito, I.; Pereira-Lorenzo, S.; Fernández, A.; Majada, J. Adaptive Diversity in Castanea sativa Mill. Half-Sib Progenies in Response to Drought Stress. Environ. Exp. Bot. 2012, 78, 56–63. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, H.; Abbott, A. Dormancy behaviors and underlying regulatory mechanisms: From perspective of pathways to epigenetic regulation. In Advances in Plant Dormancy; Anderson, J.V., Ed.; Springer: Fargo, ND, USA, 2015; pp. 35–47. ISBN 978-3-319-14450-4. [Google Scholar]
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean Olive Orchards under Climate Change: A Review of Future Impacts and Adaptation Strategies. Agronomy 2020, 11, 56. [Google Scholar] [CrossRef]
- Santos, J.A.; Costa, R.; Fraga, H. Climate Change Impacts on Thermal Growing Conditions of Portuguese Grapevine Varieties. In Proceedings of the E3S Web of Conferences; Springer: Dordrecht, The Netherlands, 2018; Volume 50, pp. 273–286. [Google Scholar]
- Santamaría, M.E.; Rodríguez, R.; Cañal, M.J.; Toorop, P.E. Transcriptome Analysis of Chestnut (Castanea sativa) Tree Buds Suggests a Putative Role for Epigenetic Control of Bud Dormancy. Ann. Bot. 2011, 108, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Furones-Pérez, P.; Fernández-López, J. Morphological and Phenological Description of 38 Sweet Chestnut Cultivars (Castanea sativa Miller) in a Contemporary Collection. SJAR 2009, 7, 829–843. [Google Scholar] [CrossRef]
- Pérez-Girón, J.C.; Álvarez-Álvarez, P.; Díaz-Varela, E.R.; Mendes-Lopes, D.M. Influence of Climate Variations on Primary Production Indicators and on the Resilience of Forest Ecosystems in a Future Scenario of Climate Change: Application to Sweet Chestnut Agroforestry Systems in the Iberian Peninsula. Ecol. Indic. 2020, 113, 106199. [Google Scholar] [CrossRef]
- Freitas, T. Study of Climate Change Impacts and the Adaptation Measures in Agricultural, Chestnut and Almond Species, in Portuguese Territory. Ph.D. Thesis, University of Trás-os-Montes e Alto Douro, Vila Real, Portugal, 2024. [Google Scholar]
- Pinto, T.; Pereira, M.G.; Gonçalves, M.; Silva, P.; Gomes-Laranjo, J. Calendarização Dos Estados Fenológicos Em Cultivares de Castanheiro. In ClimCast—Os Novos Desafios Para o Souto no Contexto das Alterações Climáticas; Gomes-Laranjo, J., Pires, A., Pinto, J.A., Marques, D., Martins, A., Carneiro, R., Eds.; RefCast—Associação Portuguesa da Castanha: Vila Real, Portugal, 2022; Volume 1, pp. 179–190. ISBN 978-989-53782-3-4. [Google Scholar]
- Almeida, P.; Dinis, L.T.; Coutinho, J.; Pinto, T.; Anjos, R.; Ferreira-Cardoso, J.; Pimentel-Pereira, M.; Peixoto, F.; Gomes-Laranjo, J. Effect of Temperature and Radiation on Photosynthesis Productivity in Chestnut Populations (Castanea sativa Mill. Cv. Judia). Acta Agron. Hung. 2007, 55, 193–203. [Google Scholar] [CrossRef]
- Beccaro, G.L.; Donno, D.; Lione, G.G.; De Biaggi, M.; Gamba, G.; Rapalino, S.; Riondato, I.; Gonthier, P.; Mellano, M.G. Castanea spp. Agrobiodiversity Conservation: Genotype Influence on Chemical and Sensorial Traits of Cultivars Grown on the Same Clonal Rootstock. Foods 2020, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Bacelar, E.A.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Ferreira, H.F.; Correia, C.M. Changes in Growth, Gas Exchange, Xylem Hydraulic Properties and Water Use Efficiency of Three Olive Cultivars under Contrasting Water Availability Regimes. Environ. Exp. Bot. 2007, 60, 183–192. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Sustain. Agric. 2009, 29, 153–188. [Google Scholar] [CrossRef]
- Papendick, R.I.; Campbell, G.S. Theory and Measurement of Water Potential. In Water Potential Relations in Soil Microbiology; Wiley: New York, NY, USA, 2015; pp. 1–22. [Google Scholar] [CrossRef]
- Gomes-Laranjo, J.; Coutinho, J.P.; Galhano, V.; Ferreira-Cardoso, J.V. Differences in Photosynthetic Apparatus of Leaves from Different Sides of the Chestnut Canopy. Photosynthetica 2008, 46, 63–72. [Google Scholar] [CrossRef]
- Marques, T.; Ferreira-Pinto, A.; Pinto, T.; Gomes-Laranjo, J. Predicting the Behavior of Chestnut Cultivars Grafted on ColUTAD under Different Edaphoclimatic Conditions. Acta Hortic. 2023, 1400, 49–58. [Google Scholar] [CrossRef]
- Gombos, Z.; Murata, N. Genetic Engineering of the Unsaturation of Membrane Glycerolipid: Effects on the Ability of the Photosynthetic Machinery to Tolerate Temperature Stress. In Lipids in Photosynthesis: Structure, Function and Genetics; Springer: Berlin/Heidelberg, Germany, 1998; pp. 249–262. [Google Scholar] [CrossRef]
- Murata, N.; Siegenthaler, P.A. Lipids in Photosynthesis: An Overview. In Lipids in Photosynthesis: Structure, Function and Genetics; Kluwer Academic Publishers: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 1998; Volume 6, pp. 1–20. ISBN 0-306-48087-5. [Google Scholar]
- Webb, M.S.; Green, B.R. Biochemical and Biophysical Properties of Thylakoid Acyl Lipids. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 1991, 1060, 133–158. [Google Scholar] [CrossRef]
- Borges, O.P.; Carvalho, J.S.; Correia, P.R.; Silva, A.P. Lipid and Fatty Acid Profiles of Castanea sativa Mill. Chestnuts of 17 Native Portuguese Cultivars. J. Food Compos. Anal. 2007, 20, 80–89. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, I.J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 37. [Google Scholar] [CrossRef]
- Carneiro-Carvalho, A.; Pinto, T.; Gomes-Laranjo, J.; Anjos, R. The Potential of SiK® Fertilization in the Resilience of Chestnut Plants to Drought—A Biochemical Study. Front. Plant Sci. 2023, 14, 1120226. [Google Scholar] [CrossRef]
- Boardman, N.K. Comparative Photosynthesis of Sun and Shade. Annu. Rev. Plant Biol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Martínez, S.; Fuentes, C.; Carballo, J. Antioxidant Activity, Total Phenolic Content and Total Flavonoid Content in Sweet Chestnut (Castanea sativa Mill.) Cultivars Grown in Northwest Spain under Different Environmental Conditions. Foods 2022, 11, 3519. [Google Scholar] [CrossRef]
- Zoratti, L.; Karppinen, K.; Escobar, A.L.; Häggman, H.; Jaakola, L. Light-Controlled Flavonoid Biosynthesis in Fruits. Front. Plant Sci. 2014, 5, 534. [Google Scholar] [CrossRef]
- Poljak, I.; Vahčić, N.; Liber, Z.; Šatović, Z.; Idžojtić, M. Morphological and Chemical Variation of Wild Sweet Chestnut (Castanea sativa Mill.) Populations. Forests 2022, 13, 55. [Google Scholar] [CrossRef]
- Garrett, H. Plants of the Metroplex: Newly Revised Edition; University of Texas Press: Austin, TX, USA, 1998. [Google Scholar]
- Jacobsen, S.E. New Climate-Proof Cropping Systems in Dry Areas of the Mediterranean Region. J. Agron. Crop Sci. 2014, 200, 399–401. [Google Scholar] [CrossRef]
- Kirda, C. Deficit Irrigation Practices. Available online: https://www.fao.org/4/y3655e/y3655e03.htm (accessed on 22 December 2024).
- Gomes-Laranjo, J. Seminário: Impacto Das Alterações Climáticas Antropogénicas Na Fisiologia e Produtividade Do Castanheiro (Castanea sativa Mill.); Universidade de Trás-os-Montes e Alto Douro: Vila Real, Portugal, 2023. [Google Scholar]
- Perulli, G.; Boini, A.; Morandi, B.; Grappadelli, L.; Manfrini, L. Irrigation improves tree physiological performances and nut quality in sweet chestnut. Italus Hortus. 2022, 29, 156. [Google Scholar] [CrossRef]
- Mota, M.; Pinto, T.; Marques, T.; Borges, A.; Caço, J.; Raimundo, F.; Gomes-Laranjo, J. Study on Yield Values of Two Irrigation Systems in Adult Chestnut Trees and Comparison with Non-Irrigated Chestnut Orchard. Rev. De Ciências Agrárias 2018, 41, 236–248. [Google Scholar] [CrossRef]
- Repullo-Ruibérriz de Torres, M.A.; Ordóñez-Fernández, R.; Giráldez, J.V.; Márquez-García, J.; Laguna, A.; Carbonell-Bojollo, R. Efficiency of Four Different Seeded Plants and Native Vegetation as Cover Crops in the Control of Soil and Carbon Losses by Water Erosion in Olive Orchards. Land. Degrad. Dev. 2018, 29, 2278–2290. [Google Scholar] [CrossRef]
- Michalopoulos, G.; Kasapi, K.A.; Koubouris, G.; Psarras, G. Adaptation of Mediterranean Olive Groves to Climate Change Through Sustainable Cultivation Practices. Climate 2020, 8, 54. [Google Scholar] [CrossRef]
- Correia, C.M.; Brito, C.; Sampaio, A.; Dias, A.A. Leguminous Cover Crops Improve the Profitability and the Sustainability of Rainfed Olive (Olea europaea L.) Orchards: From Soil Biology to Physiology of Yield. Procedia Environ. Sci. 2015, 29, 282–283. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Gattinger, A.; Gimeno, B.S. Managing Soil Carbon for Climate Change Mitigation and Adaptation in Mediterranean Cropping Systems: A Meta-Analysis. Agric. Ecosyst. Environ. 2013, 168, 25–36. [Google Scholar] [CrossRef]
- Toprak, S. Balanced Basic Fertilization in Sweet Chestnut (Castanea sativa Mill.): Vertical Distribution of Plant Nutrients in Different Soil Textures. Alinteri J. Agric. Sci. 2021, 36, 7–13. [Google Scholar] [CrossRef]
- Broadbent, P.; Baker, K.F. Behaviour of Phytophthora cinnamomi in Soils Suppressive and Conducive to Root Rot. Aust. J. Agric. Res. 1974, 25, 121–137. [Google Scholar] [CrossRef]
- Kraayenhof, R.; Sterk, G.J.; Sang, H.W.W.F.; Krab, K. Monovalent Cations Differentially Affect Membrane Surface Properties and Membrane Curvature, as Revealed by Fluorescent Probes and Dynamic Light Scattering. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1996, 1282, 293–302. [Google Scholar] [CrossRef]
- Nesbitt, H.J.; Malajczuk, N.; Glenn, A.R. Effect of Organic Matter on the Survival of Phytophthora cinnamomi Rands in Soil. Soil. Biol. Biochem. 1979, 11, 133–136. [Google Scholar] [CrossRef]
- Coffey, M.D.; Guillemet, F. Avocado Rootstocks; California Avocado Society: Ventura, CA, USA, 1987; Volume 71, pp. 173–179. [Google Scholar]
- Lee, B.S.; Zentmyer, G.A. Influence of Calcium Nitrate and Ammonium Sulfate on Phytophthora Root Rot of Persea indica. Phytopathology 2012, 72, 1558–1564. [Google Scholar] [CrossRef]
- Malajczuk, N.; Boughton, T.J.; Campbell, N.A.; Litchfield, R.T. Interaction between the Fungicide Metalaxyl and Soil Microorganisms on Survival of Phytophthora cinnamomi. Ann. Appl. Biol. 1983, 103, 57–61. [Google Scholar] [CrossRef]
- Alvarez, J.; Datnoff, L.E. The Economic Potential of Silicon for Integrated Management and Sustainable Rice Production. Crop Prot. 2001, 20, 43–48. [Google Scholar] [CrossRef]
- Brecht, M.O.; Datnoff, L.E.; Kucharek, T.A.; Nagata, R.T. Influence of Silicon and Chlorothalonil on the Suppression of Gray Leaf Spot and Increase Plant Growth in St. Augustinegrass. Plant Dis. 2007, 88, 338–344. [Google Scholar] [CrossRef]
- Xu, R.; Huang, J.; Guo, H.; Wang, C.; Zhan, H. Functions of Silicon and Phytolith in Higher Plants. Plant Signal. Behav. 2023, 18, 2198848. [Google Scholar] [CrossRef] [PubMed]
- Bokor, B.; Santos, C.S.; Kostoláni, D.; Machado, J.; da Silva, M.N.; Carvalho, S.M.P.; Vaculík, M.; Vasconcelos, M.W. Mitigation of Climate Change and Environmental Hazards in Plants: Potential Role of the Beneficial Metalloid Silicon. J. Hazard. Mater. 2021, 416, 12619. [Google Scholar] [CrossRef]
- Carneiro-Carvalho, A.; Anjos, R.; Pinto, T.; Gomes-Laranjo, J. Stress Oxidative Evaluation on SiK®-Supplemented Castanea sativa Mill. Plants Growing under High Temperature. J. Soil. Sci. Plant Nutr. 2021, 21, 415–425. [Google Scholar] [CrossRef]
- Li, R.; Sun, Y.; Wang, H.; Wang, H. Advances in Understanding Silicon Transporters and the Benefits to Silicon-Associated Disease Resistance in Plants. Appl. Sci. 2022, 12, 3282. [Google Scholar] [CrossRef]
- Castellana, S.; Martin, M.Á.; Solla, A.; Alcaide, F.; Villani, F.; Cherubini, M.; Neale, D.; Mattioni, C. Signatures of Local Adaptation to Climate in Natural Populations of Sweet Chestnut (Castanea sativa Mill.) from Southern Europe. Ann. For. Sci. 2021, 78, 1–21. [Google Scholar] [CrossRef]
- Diegues, S.F.F. Instalação de Soutos Demonstração Com o Porta-Enxerto ColUTAD Em Situação Climática Contrastante No NE Transmontano; Dissertação, Instituto Politécnico de Bragança: Bragança, Portugal, 2019. [Google Scholar]
- Song, G.; Chen, Q.; Callow, P.; Mandujano, M.; Han, X.; Cuenca, B.; Bonito, G.; Medina-Mora, C.; Fulbright, D.W.; Guyer, D.E. Efficient Micropropagation of Chestnut Hybrids (Castanea spp.) Using Modified Woody Plant Medium and Zeatin Riboside. Hortic. Plant J. 2021, 7, 174–180. [Google Scholar] [CrossRef]
- Fernandes, P.; Machado, H.; do Céu Silva, M.; Costa, R.L. A Histopathological Study Reveals New Insights into Responses of Chestnut (Castanea spp.) to Root Infection by Phytophthora cinnamomi. Phytopathology 2021, 111, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.; Batista, J.; Pereira, A.; Droga, R.; Espírito Santo, J.; Pires, A.; Carmo, M.; Seco, F.; Freitas, M.; Ramos, C.; et al. ClimCast—Os Novos Desafios Para o Souto No Contexto Das Alterações Climáticas, 1st ed.; Gomes-Laranjo, J., Pires, A., Pinto, J.A., Marques, D., Martins, A., Carneiro, R., Eds.; RefCast—Associação Portuguesa da Castanha: Vila Real, Portugal, 2022; Volume 1, ISBN 9789895378234. [Google Scholar]
- Calheiros, T.; Pereira, M.G.; Pinto, J.G.; Caramelo, L.; Gomes-Laranjo, J.; Dacamara, C.C. Assessing Potential Changes of Chestnut Productivity in Europe under Future Climate Conditions. In EGU General Assembly Conference Abstracts; EGU General Assembly: Vienna, Austria, 2012; pp. 13–14. [Google Scholar]
- Lobell, D.B.; Field, C.B.; Cahill, K.N.; Bonfils, C. Impacts of Future Climate Change on California Perennial Crop Yields: Model Projections with Climate and Crop Uncertainties. Agric. For. Meteorol. 2006, 141, 208–218. [Google Scholar] [CrossRef]
- Tubiello, F.N.; Ewert, F. Simulating the Effects of Elevated CO2 on Crops: Approaches and Applications for Climate Change. Eur. J. Agron. 2002, 18, 57–74. [Google Scholar] [CrossRef]
- Engel, E.C.; Weltzin, J.F.; Norby, R.J.; Classen, A.T. Responses of an Old-Field Plant Community to Interacting Factors of Elevated [CO2], Warming, and Soil Moisture. J. Plant Ecol. 2009, 2, 1–11. [Google Scholar] [CrossRef]
- Scheben, A.; Edwards, D. Bottlenecks for Genome-Edited Crops on the Road from Lab to Farm. Genome Biol. 2018, 19. [Google Scholar] [CrossRef]
- Dalgleish, H.J.; Nelson, C.D.; Scrivani, J.A.; Jacobs, D.F. Consequences of Shifts in Abundance and Distribution of American Chestnut for Restoration of a Foundation Forest Tree. Forests 2016, 7, 4. [Google Scholar] [CrossRef]




| Cultivar | Chlorophyll (μg·cm−2) | Carotenoids (μg·cm−2) | Total (μg·cm−2) | Cla/Clb | Cl/Car |
|---|---|---|---|---|---|
| Judia | 113.3 | 29.3 | 142.6 | 3.60 | 3.87 |
| Longal | 103.0 | 26.3 | 129.3 | 4.01 | 3.92 |
| Martaínha | 85.7 | 19.5 | 105.2 | 4.35 | 4.39 |
| Treatments | Macronutrients (g kg−1) | Micronutrients (mg kg−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | B | Fe | Zn | Mn | Cu | ||
| Average | NI | 19.5 | 2.5 | 11.5 | 7.2 | 2.1 | 0.7 | 29.0 | 25.0 | 19.8 | 718.0 | 10.8 |
| TI | 21.5 | 2.6 | 12.8 | 7.2 | 2.2 | 0.7 | 29.3 | 21.0 | 26.0 | 640.8 | 9.80 | |
| Variation (%) | NI | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| TI | 109.9 | 106.1 | 110.9 | 100.0 | 106.0 | 100.0 | 100.9 | 84.00 | 131.6 | 89.20 | 90.70 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, T.; Ferreira-Pinto, A.; Fevereiro, P.; Pinto, T.; Gomes-Laranjo, J. Current Biological Insights of Castanea sativa Mill. to Improve Crop Sustainability to Climate Change. Plants 2025, 14, 335. https://doi.org/10.3390/plants14030335
Marques T, Ferreira-Pinto A, Fevereiro P, Pinto T, Gomes-Laranjo J. Current Biological Insights of Castanea sativa Mill. to Improve Crop Sustainability to Climate Change. Plants. 2025; 14(3):335. https://doi.org/10.3390/plants14030335
Chicago/Turabian StyleMarques, Tiago, Andrea Ferreira-Pinto, Pedro Fevereiro, Teresa Pinto, and José Gomes-Laranjo. 2025. "Current Biological Insights of Castanea sativa Mill. to Improve Crop Sustainability to Climate Change" Plants 14, no. 3: 335. https://doi.org/10.3390/plants14030335
APA StyleMarques, T., Ferreira-Pinto, A., Fevereiro, P., Pinto, T., & Gomes-Laranjo, J. (2025). Current Biological Insights of Castanea sativa Mill. to Improve Crop Sustainability to Climate Change. Plants, 14(3), 335. https://doi.org/10.3390/plants14030335







