A Comprehensive Review on Graptopetalum paraguayense’s Phytochemical Profiles, Pharmacological Activities, and Development as a Functional Food
Abstract
1. Introduction
2. Methods
3. Botanical Description of G. paraguayense
4. Toxicity Studies and Safety Profile of G. paraguayense
5. Phytochemicals of G. paraguayense
| Sr. No. | Compound Name | Amount (µg/g) | Type of Extract/Fraction | Part of Plant | Pharmacological Activity | References |
|---|---|---|---|---|---|---|
| 1 | Genistin | 0.27 ± 0.08 # | Ethyl acetate fraction of extract | Leaf | PNS | [14] |
| 2 | Daidzin | 1.16 ± 0.09 # | Ethyl acetate fraction of extract | Leaf | PNS | [14] |
| 3 | Quercetin | 7.8 ± 0.1 | Aqueous extract | Immature plant leaf | PNS | [12] |
| 4.3 ± 0.1 | Aqueous extract | Intermediately mature plant leaf | ||||
| 2.8 ± 0.1 | Aqueous extract | Mature plant leaf | ||||
| 4 | Gallic acid | 11.0 ± 1.5 | Aqueous extract | Immature plant leaf | Antioxidant activity | [12] |
| 8.4 ± 1.1 | Aqueous extract | Intermediately mature plant leaf | ||||
| 6.8 ± 0.8 | Aqueous extract | Mature plant leaf | ||||
| 5 | Oxalic acid | 27.2 ± 1.8 | Aqueous extract | Immature plant leaf | PNS | [12] |
| 22.3 ± 2.1 | Aqueous extract | Intermediately mature plant leaf | ||||
| 20.4 ± 1.8 | Aqueous extract | Mature plant leaf | ||||
| 6 | Hydroxybutanedioic acid | 17.6 ± 0.6 | Aqueous extract | Immature plant leaf | PNS | [12] |
| 15.5 ± 0.7 | Aqueous extract | Intermediately mature plant leaf | ||||
| 14.3 ± 0.5 | Aqueous extract | Mature plant leaf | ||||
| 7 | Rutin | 0.8 ± 0.1 | Aqueous extract | Immature plant leaf | PNS | [12] |
| 0.5 ± 0.1 | Aqueous extract | Intermediately mature plant leaf | ||||
| 0.4 ± 0.1 | Aqueous extract | Mature plant leaf | ||||
| 8 | Quercetin 3-O-[6″-(3-hydroxyl-3- methylglutaroyl)]-β-d-glucopyranoside | 4.8 | Methanolic extract | Leaf | Antioxidant activity | [11] |
| 9 | Kampferol 3-O-[6″-(3-hydroxyl-3-methylglutaroyl)]-β-d-glucopyranoside | 5.7 | Methanolic extract | Leaf | Antioxidant activity | [11] |
| 10 | Quercetin 3-O-[6″-(3-hydroxyl-3-methylglutaroyl)- 2″-acetyl]-β-d-glucopyranoside | 4.3 | Methanolic extract | Leaf | Antioxidant activity | [11] |
| 11 | Kampferol 3- O-[6″-(3-hydroxyl-3-methylglutaroyl)-2″-acetyl]-β-dglucopyranoside | 2.5 | Methanolic extract | Leaf | Antioxidant activity | [11] |
| 12 | Isoquercetin | NP | Methanolic extract | Leaf | Antioxidant activity | [11] |
| 13 | Kaempferol 3-O-β-d-glucopyranoside | NP | Methanolic extract | Leaf | Antioxidant activity | [11] |
| 14 | Kaempferol | NP | Methanolic extract | Leaf | Antioxidant activity | [11] |
| 15 | Isoquercitrin-6-(3-hydroxy-3-methylglutarate) | 90.52 ± 2.69 # | Aqueous extract | Leaf | Antioxidant activity | [16] |
| 16 | Astragalin-6-(3-hydroxy-3-methylglutarate) | 23.23 ± 0.5 # | Aqueous extract | Leaf | Antioxidant activity | [16] |
| 17 | Isoquercitrin -2-acetyl-6-(3-hydroxy-3-methylglutarate) | 77.35 ± 0.55 # | Aqueous extract | Leaf | Antioxidant activity | [16] |
| 18 | Astragalin-2-acetyl-6(3-hydroxy-3-methylglutarate) | 40.62 ± 0.29 # | Aqueous extract | Leaf | Antioxidant activity | [16] |
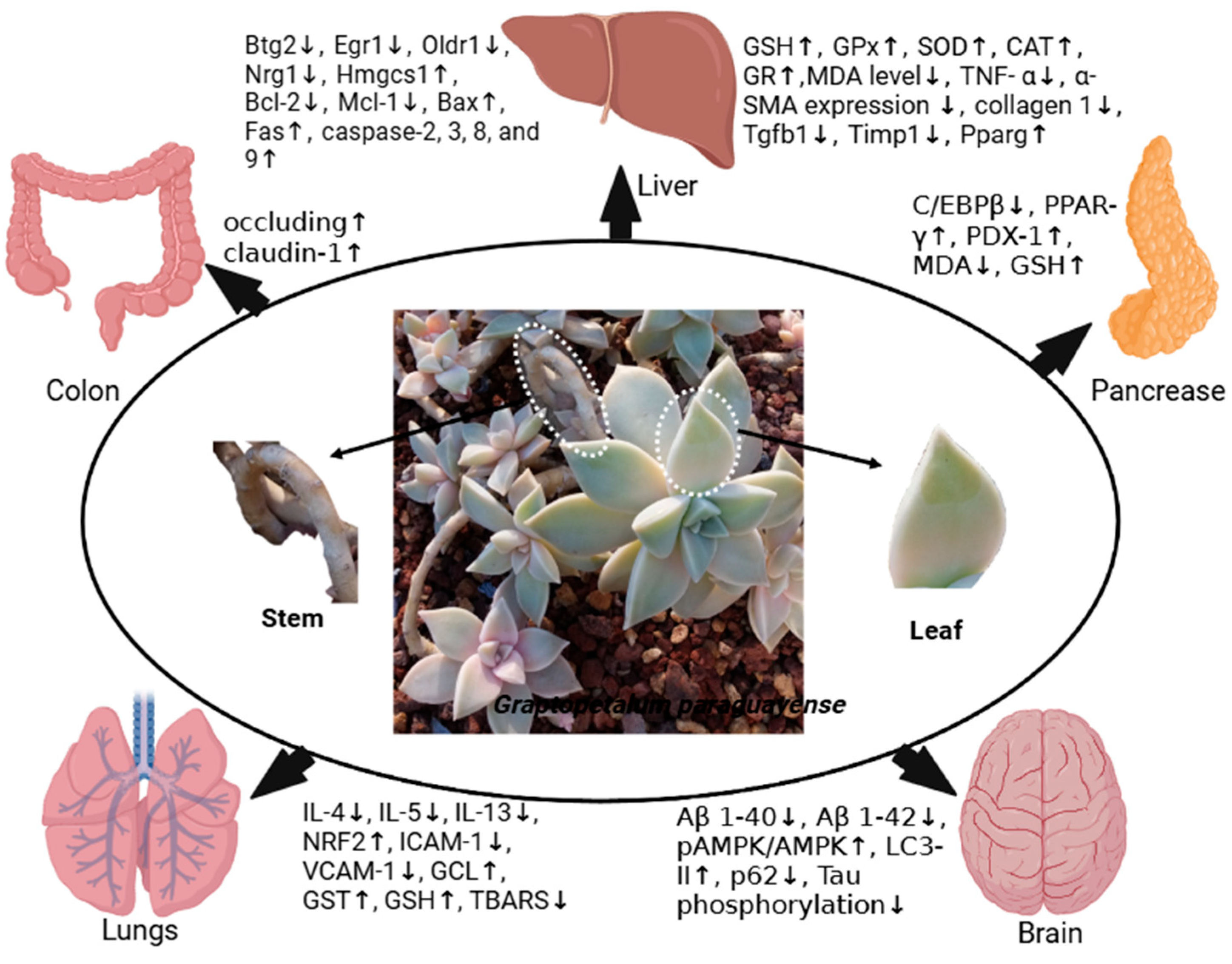
6. Pharmacological Activities of G. paraguayense
6.1. Anticancer Effects
6.1.1. In Vitro Anticancer Effects
6.1.2. In Vivo Anticancer Effects
6.2. Anti-Alzheimer Disease Effects of G. paraguayense
6.2.1. In Vitro Anti-AD Effects of G. paraguayense
6.2.2. In Vivo Anti-Alzheimer Disease Effects of G. paraguayense
6.3. Anti-Aging Effects of G. paraguayense
6.4. Antihypertensive Activities of G. paraguayense
6.4.1. In Vitro Antihypertensive Activities of G. paraguayense
6.4.2. In Vivo Antihypertensive Effects of G. paraguayense
6.4.3. Antihypertensive Effects of G. paraguayense in Clinical Studies
6.5. Antioxidant Activities of G. paraguayense
6.5.1. In Vitro Antioxidant Activities of G. paraguayense
6.5.2. In Vivo Antioxidant Activities of G. paraguayense
6.5.3. Antioxidant Activities of G. paraguayense in Clinical Studies
6.6. Hepatoprotective Activity
6.7. Anti-Diabetic Activity
6.8. Tyrosinase Inhibitory Activity
6.9. Antiviral Activity
6.10. Antibacterial Activity
6.11. Anti-Inflammatory Activity
| Sr. No. | Activity | Plant Part/Extract Type | Method | Result | Ref. |
|---|---|---|---|---|---|
| 1. | Anticancer activity | Stem extract aqueous solution | Anti-proliferative effect on HepG2 cells | HepG2 cells ↓ | [19] |
| Cell cycle and apoptosis study by flow cytometry | G0/G1 ↑ and apoptotic cells ↑ | ||||
| Leaf extract in 30% DMSO | Anti-proliferative effect on Huh7 and Mahlavu cells | The IC50 values were 500 and 250 μg/mL for Huh7 and Mahlavu cells, respectively | [20] | ||
| Fraction of leaf extract in 30% DMSO (30DE3) | Anti-proliferative effect on Huh7, PLC5, and Mahlavu cells | The IC50 values were 50, 37.5, and 75 μg/mL, for Huh7, Mahlavu, and PLC5 cells, respectively | |||
| WB | |||||
| ROS was measured through hydroethidine fluorescence for detecting the intracellular level of superoxide production | The production of superoxide↓ and intracellular peroxide ↓ | ||||
| Leaf extract in 50% ethanol (62.5–500 μg/mL) | Viability of A375.S2 cells (melanoma cells) and caspase-3 activity by flow cytometry and stained with PI | Viability of the cells ↓ (IC50 250 μg/mL), apoptotic cells ↑ | [21] | ||
| Leaf extract in 50% ethanol (250 μg/mL) | DAPI staining, DNA electrophoresis, and flow cytometry for studying DNA damage, apoptosis, and caspase 3 activity | Chromatin condensation ↑, caspase-3 activity ↑ | |||
| Protein expression through WB | Cyclin A ↓, Cyclin B ↓, CDC2 ↓, CDC25c ↓, CHK1 ↑, CHK2 ↑, Weel ↑, p21 ↑, p53 ↑, SOD, catalase, and AIF ↑, Bax ↑, caspase-9 ↑, Bcl2 ↓ | ||||
| Effect on calcium signaling | Intracellular levels of Ca2+ ↑, GRP78 ↑, GADD153 ↑, and caspase-7 ↑ | ||||
| Mitochondria-dependent apoptotic signals in A375 | MMP (ΔΨm) in A375.S2 cells with an early time period of (0.5–2 h) exposure. In addition, the levels of Bax, caspase-9, and AIF in cells were stimulated after GE50 treatment, but the level of Bcl-2 was attenuated in cells | ||||
| 500 μg/mL | GADD153, Endo G, cytochrome c, and AIF nuclear translocation in A375.S2 cells through Confocal microscopy | Nuclear translocation of GADD153↑, Endo G ↑, and AIF ↑; Release of cytochrome c↑ from mitochondria to cytosol. | |||
| 250 μg/mL | Activity of MDA, SOD, and catalase studied through spectrophotometry-based methods. | GPx ↑, SOD ↑, and CAT ↑ | |||
| 250 μg/mL | ROS production and redox status and 2,7-dichlorodihydrofluorescein diacetate (10 μM), Indo-1-AM (2.5 μg/mL), and 3,3′-dihexyloxacarbocyanine iodide (500 nM) dye were used to determine ROS, Ca2+, and MMP | ROS ↓, MMP ↓, and Ca2+ ↑ | |||
| Extract of leaves in water (LEW) and its precipitate (LEWP) | Caco-2 cells studied through MTT assay | Caco-2 cells ↓ IC50: values were >1 and 0.12 mg/mL for LEW and LEWP, respectively | [6] | ||
| 2 | Anti-Alzheimer disease activity | 10, 30, and 50 μg/mL of 30DE3 | ELISA for studying Aβ 1-40 and Aβ 1-42 in SH-SY5Y-APP695 cells | Aβ 1-40 and Aβ 1-42 secretion ↓ | [9] |
| 5 μg/mL of 30DE3 | Microarray L1000 expression profiling and gene set enrichment analysis (GSEA) of HT29 cells | Significant genes were enriched in AD, HD, and AMPK signaling pathways | |||
| 5 μg/mL of 30DE3 | Studying the phosphorylation of AMPK in glial U87 cells through WB | pAMPK/AMPK ↑ | |||
| 5, 25, and 50 μg/mL of 30DE3 | Glial (U87) and neuronal (SH-SY5Y-APP695) cells and microtubule-associated protein 1A/1B-light chain 3 (LC3) through WB for studying the autophagy | LC3-II ↑ | |||
| WB on SH-SY5Y-APP695 cells | p62 ↓ | ||||
| 5, 10, 20, and 50 μg/mL of 30DE3 | Neurons differentiated from human-induced pluripotent stem cells from AD patients | Aβ 1-40 and Aβ 1-42 secretion ↓ | [24] | ||
| Tau phosphorylation in AD-iNs, measured using Western blotting | Tau phosphorylation ↓ at Ser214 | ||||
| 3 | Antihypertensive activity | Extract of leaves in water and 50% and 95% ethanol | FAPGG substrate-based assay for ACE inhibition | IC50 values were 46.8 ± 2.5, 19.6 ± 2.5, and 13.7 ± 2.0c, for water, 50% ethanol, and 95% ethanol, respectively | [7] |
| 4 | Antioxidant activity | Extract of leaves in water and 50% and 95% ethanol | Superoxide-radical-scavenging activity | IC50 values were 0.27 ± 0.01, 1.07 ± 0.24, and 0.19 ± 0.01 for water, 50 ethanol, and 95% ethanol extracts, respectively | [5] |
| DPPH-radical-scavenging activity | IC50 values were 1.65 ± 0.08, 0.29 ± 0.05, and 1.44 ± 0.16 for water, 50 ethanol, and 95% ethanol extracts, respectively | ||||
| Lipid peroxidation inhibition | IC50 values were 1.29 ± 0.20, 1.49 ± 0.17, and 1.34 ± 0.20 for water, 50 ethanol, and 95% ethanol extracts, respectively | ||||
| Extract of stem in water and 50% and 95% ethanol | Superoxide-radical-scavenging activity | IC50 values were 0.28 ± 0.01, 2.15 ± 0.19, and 3.82 ± 0.25 for water, 50 ethanol, and 95% ethanol extracts, respectively | [19] | ||
| DPPH-radical-scavenging activity | IC50 values were 0.35 ± 0.02, 0.32 ± 0.01, and 0.51 ± 0.02 for water, 50 ethanol, and 95% ethanol extracts, respectively | ||||
| ABTS+ | IC50 values were 0.87 ± 0.01, 0.76 ± 0.00, and 1.71 ± 0.06 for water, 50 ethanol, and 95% ethanol extracts, respectively | ||||
| Lipid peroxidation inhibition | IC50 values were 0.68 ± 0.02, 1.12 ± 0.03, and 0.67 ± 0.01 for water, 50 ethanol, and 95% ethanol extracts, respectively | ||||
| Leaf extract (from immature, intermediately mature, and mature plants) | GPx, GR, CAT, and SOD enzyme activities on normal liver cells (FL83B cell line) | Immature leaf extract: GPx, GR, CAT, and SOD enzyme activities were 4.1 ± 0.3, 4.7 ± 0.1, 2.2 ± 0.2, and 1.4 ± 0.1, respectively; Intermediately mature leaf extract: GPx, GR, CAT, and SOD enzyme activities were 3.9 ± 0.3, 4.0 ± 0.2, 2.0 ± 0.2, and 1.3 ± 0.1, respectively; Mature leaf extract: GPx, GR, CAT, and SOD enzyme activities were 2.7 ± 0.4, 4.2 ± 0.3, 2.1 ± 0.3, and 1.0 ± 0.1, respectively. | [12] | ||
| Leaf juice from immature plants fermented through La, Lpl, and Lpr | GPx, GR, CAT, and SOD enzyme activities were 4.5 ± 0.1, 5.1 ± 0.2, 2.3 ± 0.2, and 1.9 ± 0.1, respectively, for La fermented leaf juice; GPx, GR, CAT, and SOD enzyme activities were 5.0 ± 0.2, 5.3 ± 0.1, 2.9 ± 0.2, and 2.1 ± 0.1, respectively, for Lpl fermented leaf juice; GPx, GR, CAT, and SOD enzyme activities were 4.1 ± 0.2, 4.9 ± 0.3, 2.3 ± 0.1, and 1.7 ± 0.1, respectively, for Lpr fermented leaf juice | ||||
| Aqueous extract of leaves partitioned in n-hexane, ethyl acetate, n-butanol, and water fractions and compounds isolated from ethyl acetate fraction (C1–C6) | DPPH- and ABTS-scavenging assays | Among the fractions, ethyl acetate fraction showed the highest DPPH-scavenging activity, and for compounds C1–C5, the DPPH-scavenging activities were 86.88 ± 0.68, 40.18 ± 1.57, 11.57 ± 3.04, 43.19 ± 1.37, and 9.65 ± 1.79%, respectively | [16] | ||
| Ethyl acetate fraction showed the highest ABTS-scavenging activity | |||||
| Antiglycation activity fluorescence intensities were measured using a spectrofluorometer | Among the fractions, ethyl acetate fraction showed the highest glycation inhibition activity, and for compounds C1–C5, the glycation inhibition activities were 68.11 ± 4.24, 44.82 ± 6.35, 14.98 ± 3.46, 32.97 ± 4.91, and 19.48 ± 6.13, respectively | ||||
| Compounds (C6–C12) isolated from methanolic leave extract | DPPH | IC50 values for compounds C6–C12 were 7.88 ± 0.57, 144.93 ± 3.56, 8.40 ± 0.06, 108.47 ± 6.94, 11.06 ± 0.34, 351.89 ± 17.53, and 19.18 ± 0.91 μM, respectively | [11] | ||
| ABTS | IC50 values for compounds C6–C12 were 5.69 ± 0.31, 96.81 ± 2.40, 9.22 ± 0.67, 152.21 ± 12.16, 14.50 ± 1.24, 277.44 ± 10.01, and 15.28 ± 0.06 μM, respectively | ||||
| Lipid peroxidation inhibition | IC50 values for compounds C6–C12 were 10.76 ± 0.10, 9.10 ± 0.17, 46.54 ± 1.01, 17.45 ± 0.18, 1.67 ± 0.01, 330.64 ± 12.63, and 2.96 ± 0.06 μM, respectively | ||||
| Extract of leaves in water (LEW) and its precipitate (LEWP) | DPPH | SC50 values were 4.14 and 1.45 mg/mL for LEW and LEWP, respectively | [6] | ||
| ABTS | SC50 values were 0.30 and 0.62 mg/mL for LEW and LEWP, respectively | ||||
| Superoxide anion scavenging | SC50 values were 0.18 and 0.37 mg/mL for LEW and LEWP, respectively | ||||
| 5. | Anti-inflammatory activity | Extract of leaves in water (LEW) and its precipitate (LEWP), 0.125–0.50 mg/mL | LPS-induced BV-2 cells and ELISA for studying their expression | TNF-α ↓ and IL-6 ↓ (LEW treatment) TNF-α ↑ and IL-6 ↑ (LEWP treatment) | [6] |
| 6. | Tyrosinase inhibitory activity | Extract in water and 50% and 95% ethanol | Mushroom tyrosinase on Dopa oxidation inhibition | IC50 values were 0.80 ± 0.02, 1.14 ± 0.05, and 2.83 ± 0.05 mg/mL for 95% ethanol, 50% ethanol, and water extracts, respectively | [49] |
| 7. | Antibacterial activity | Methanolic extract of leaves | The BMD assay for SA, EF, EC, PA, SP, and MRSA | MIC values were 2.5, 5, >5, >5, 2.5, and 5 mg/mL for SA, EF, EC, PA, SP, and MRSA, respectively | [50] |
| Biofilm formation assay for MRSA | MBIC50 was 1.6 mg/mL | ||||
| 8. | Antiviral activity | Methanolic extract of leaves | Cytopathic effect reduction assay | Protection values were 97.5, 65.5, 25.5, and 13% for HSV-1 strain Victoria, HSV-1 strain DD, HSV-2 strain Bja, and HSV-2 strain PU, respectively | [50] |
| 30DE3 | RT-qPCR-based expression analysis on Hep3B/T2 | PGC-1α ↓, G6Pase ↓, and PEPCK ↓ | [54] | ||
| WB | PGC-1α ↓, HNF-4α ↓, FOXO1 ↓ | ||||
| Luciferase promoter activity assay | HBV core promoter activity ↓ | ||||
| ELISA | HBV surface antigen (HBsAg) secretion and wild-type HBV DNA | ||||
| 1.3ES2 RT-qPCR | PGC-1α ↓, G6Pase ↓, PEPCK ↓, HBV mRNA ↓, wild-type HBV DNA ↓ | ||||
| 1.3ES2 WB | HBV core protein levels ↓ |
| Sr. No. | Activity | Material and Dose | Model | Method | Results | Reference |
|---|---|---|---|---|---|---|
| 1 | Anticancer activity | 0.6 and 1.8 g/rat of lyophilized G. paraguayense powder and 0.036 g/rat of 30DE3 powder per day for 3 weeks | Male Wistar albino rats; disease induced through the carcinogen DEN | Collagen content in liver, measured by levels of hepatic hydroxyproline | Collagen content ↓ (in all treatments) | [20] |
| Tumor burden histopathology | Number of tumors ↓ (in all treatments) | |||||
| 2 | Anti-AD activity | 30DE3 powder, 300 mg/kg/day | APPswe/PS1dE9 (APP/PS1) double-transgenic mice | Thioflavin-S (ThS) fluorescent staining of brain sections | Deposition of Aβ ↓ (in the cerebral hemisphere) | [9] |
| ELISA analysis of cerebral cortex tissues | Soluble and in soluble Aβ1-40 levels ↓ | |||||
| WB analysis | pAMPK ↑ and AMPK ↑ | |||||
| 0, 20, and 40 μg/mL of 30DE3. | Transgenic Caenorhabditis elegans carrying GFP::LGG-1 | GFP fluorescence-based method | GFP::LGG-1 ↑ | |||
| Nuclear translocation of HLH-30/TFEB ↑ | ||||||
| 3 | Anti-aging activity | 20 μg/mL of 30DE3 | Transgenic Caenorhabditis elegans carrying 35 polyglutamine repeats (Q35) | Thrashing assay | Mobility ↑ | [9] |
| Wild-type Caenorhabditis elegans | Lifespan analysis | Lifespan ↑ | ||||
| daf-16 null mutant Caenorhabditis elegans | Lifespan analysis | Lifespan ↑ | ||||
| 4. | Antihypertensive activity | Extract of leaves in 50% ethanol, 2.5 g/kg BW daily for four consecutive weeks | Spontaneously hypertensive rats (SHRs) and age-matched normotensive WKY rats | SBP, DBP, and MBP were observed | SBP ↓, DBP ↓ and MBP ↓, normalized with treatment | [27] |
| FAPGG substrate-based assay for ACE inhibition | ACE activity ↓ (normalized in plasma, kidneys, and lungs) | |||||
| 2,2′-azinobis-(3-ethyl benzothiazoline-6-sulfonic acid (ABTS) | TAS ↑ (in plasma) | |||||
| TBARS method: α-Tocopherol and GSH in tissue homogenates were measured by an HPLC-based method | MDA ↓ (in heart, liver, and brain) | |||||
| α-Tocopherol and GSH in tissue analyzed with HPLC | GSH level ↑ (in heart and brain) | |||||
| α-tocopherol ↑ (in heart, liver, and brain) | ||||||
| Antioxidant enzyme activities in organs | Catalase ↑ and GPx ↑ activities (in heart, liver, and brain) | |||||
| 4 g of the water extract of G. paraguayense | Participants with metabolic syndrome (n = 54) | Blood pressure and fasting blood glucose levels | SBP ↓ and, fasting blood glucose ↓ | [29] | ||
| Lipid profile | LDL-C ↓, TC ↓ (p = 0.08), TG ↓, HDL-C ↑ | |||||
| Activities of SOD and CAT | Activities of SOD ↑ and CAT ↑ | |||||
| 5 | Antioxidant activity | Extract of leaves in 50% ethanol | Male Wistar rats; oxidative stress induced by t-BHP | TBA-reactive substance (TBARS) method | MDA level ↓ (in heart tissues) | [33] |
| SOD activity was determined spectrophotometrically | SOD activity ↑ (in liver tissue) | |||||
| Extract of leaves in 50% ethanol, 0.25 g/100 g BW for six weeks | CCl4-induced oxidative stress in Sprague–Dawley (SD) rats | HPLC-based method was used to measure levels of vitamin C, vitamin E, and GSH | Vitamin C ↑, vitamin E ↑, and GSH ↑ | [15] | ||
| Serum TAS by kit-based method | TAS ↑ | |||||
| MDA and GSH levels and GPx, SOD, CAT, and GST activities in liver | GPx ↑, SOD ↑, and CAT ↑, and GST ↑ | |||||
| 100 g of G. paraguayense was provided for eight weeks | 18 subjects suffering from hypercholesterolemia | Plasma levels of MDA, ascorbic acid and α-tocopherol | Plasma MDA ↓, ascorbic acid ↑, α-Tocopherol ↑ | [28] | ||
| Activities of GSH, GPx, SOD, and CAT in erythrocytes | GSH ↑, GPx ↑, and CAT ↑ | |||||
| Water extract of leaves, 4 g/day for 12 weeks | MS subjects (26 treatment, 28 placebo) | Red blood cell (RBC) SOD and CAT activity | SOD ↑ and CAT ↑ | [8] | ||
| Particle-enhanced immunonephelometry with an image analyzer and ELISA | CRP ↓, IL-6 ↓, TNF-α ↓ | |||||
| 6 | Hepatoprotective activity | Water extract of G. paraguayense, 50 to 300 mg/kg BW | SD rats; CCl4-induced hepatotoxicity | Serum biochemical assays; BUN, CRE, ALT, and AST of rat serum were determined | TC ↓, TG ↓, ALT ↓, and AST ↓ | [10] |
| GSH levels in liver | GSH level ↑ | |||||
| Antioxidant enzyme activity | GPx ↑, SOD ↑, CAT ↑, and GR ↑ | |||||
| MDA levels in liver | MDA levels ↓ | |||||
| Immunoassay for TNF-α expression | TNF- α ↓ | |||||
| Histopathology | Damage to liver tissues ↓ | |||||
| 80% ethanolic extract of leaves (1.4 g/kg) for 6 weeks study | Dimethylnitrosamine (DMN)-induced liver fibrosis in SD rats | Histopathology | BW ↑, LW ↑, necrosis ↓ and inflammatory effects ↓ | [37] | ||
| Various doses of G. paraguayense for 5 days (from days 5 to 10) | Immunocytochemical (IHC) staining analysis of the activation of cultured rat HSCs. | IHC staining of α-SMA expression and stress fiber formation in liver | α-SMA expression ↓ and stress fiber formation ↓ | |||
| Culture of HSCs | Culture of HSCs | α-SMA expression ↓ and collagen 1 ↓ | ||||
| RT-qPCR and microarray for expressions of known and novel genes of liver damage | Tgfb1 ↓, Timp1 ↓, Pparg ↑, Btg2 ↓, Egr1 ↓, Oldr1 ↓, Nrg1 ↓, and Hmgcs1 ↑ | |||||
| 80% ethanolic extract of leaves, 10–100 μg | HSCs isolated from the liver of SD rats | Survival of HSCs | Survival of HSCs ↓ | |||
| Microarray analysis | 64% of 254 liver damage-related genes were restored | |||||
| Methanolic extract of leaves, 400 mg/kg per day for six weeks | DMN-induced liver fibrosis in SD rats | Weight of body and organs and survival rate were calculated | BW ↑, LW ↑, SW ↓ and survival rate ↑; Necrosis ↑ and inflammatory effects ↑ | [39] | ||
| Methanolic extract of leaves, 400 mg/mL | Cultured HSCs of SD rats | Caspase activity assay | Activity of caspase-2, 3, 8, and 9 ↑ | |||
| WB | Bcl-2 ↓, Mcl-1 ↓, Bax ↑, and Fas ↑ | |||||
| 250, 500, 750, and 1000 μg/mL of 30DE | HSC-T6 | MTT assay | IC50: 366 μg/mL | [40] | ||
| 5, 25, 50, 75, and 100 μg/mL of 30DE3 | HSC-T6 and LX-2 cells | MTT assay | IC50: 20.8 and 22.5 μg/mL for HSC-T6 and LX-2 cells, respectively (30DE3) | |||
| 5, 10, and 15 μg/mL of 30DE3 | HSC-T6 cells | Wound healing assay and Transwell invasion assays for studying migration/invasion | Migration/invasion of HSC-T6 cells ↓ | |||
| Water extract of G. paraguayense | Male Sprague–Dawley rats | Serum biochemical assays | ALT ↓ and ALP ↓ | [41] | ||
| Immunoassay of serum samples | IL-6 ↓ and TNF-α ↓ | |||||
| WB | TGF-1 ↓ | |||||
| RP-HPLC coupled with UV detection in serum and liver | MGL ↓ | |||||
| Male 5-week-old C57BL/6J mice | Length of large intestine | Length of large intestine ↑ | ||||
| WB | Occluding ↑, claudin-1 ↑ | |||||
| Intestinal microflora by PCR analysis | Bacteroidetes/Firmicutes ↑ | |||||
| 7 | Anti-diabetic activity | Ethanolic extract of leaves, 300 mg/kg BW, administered by intraperitoneal injection for 12-weeks | C57BL/6 mice | Oral glucose tolerance test (OGTT) | Glucose levels ↓ | [46] |
| Glucose uptake of hepatic cells | Glucose uptake of hepatic cells ↑ | |||||
| Immunohistochemistry (IHC) staining | Number/area of islet cells and insulin levels ↑ | |||||
| WB | C/EBPβ ↓, PPAR-γ ↑, and PDX-1 ↑ | |||||
| Assay for lipid peroxidation products | MDA ↓, | |||||
| Assay for glutathione (GSH) | GSH ↑ | |||||
| 8 | Anti-airway-inflammation activity | Ethanol extract of leaves, 50 and 200 mg/kg | Ovalbumin-sensitized BALB/C Mice | Histopathology of lungs | Infiltration of inflammatory cells into lung tissues ↓ | [57] |
| TLC and DLC of bronchoalveolar lavage fluid (BALF) | Total cells ↓, eosinophils ↓, lymphocytes ↓, and neutrophils ↓ | |||||
| BALF | IL-4 ↓, IL-5 ↓, and IL-13 ↓ | |||||
| Flow cytometry | T cells (CD4 and CD8) ↓ | |||||
| PAS staining of lung sections | Mucus production ↓ | |||||
| Immunohistochemistry of lung tissues | NRF2 ↑ | |||||
| RT-qPCR | ICAM-1 ↓, VCAM-1 ↓, GCL ↑, and GST ↑ | |||||
| Thiobarbituric acid-reactive substance (TBARS) assay | GSH ↑ and generation of TBARS↓ | |||||
| Serum levels of IgE | IgE ↓ | |||||
| WB | GCL ↑ and GST ↑ GATA3 ↓ (in CD4 T cells and blood monocytes) |
7. Discussion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kalicharan, B.; Naidoo, Y.; van Staden, J. Ethnopharmacology and biological activities of the Aizoaceae. J. Ethnopharmacol. 2023, 303, 115988. [Google Scholar] [CrossRef] [PubMed]
- Razia, S.; Park, H.; Shin, E.; Shim, K.-S.; Cho, E.; Kang, M.C.; Kim, S.Y. Synergistic effect of Aloe vera flower and Aloe gel on cutaneous wound healing targeting MFAP4 and its associated signaling pathway: In-vitro study. J. Ethnopharmacol. 2022, 290, 115096. [Google Scholar] [CrossRef] [PubMed]
- Maan, A.A.; Nazir, A.; Khan, M.K.I.; Ahmad, T.; Zia, R.; Murid, M.; Abrar, M. The therapeutic properties and applications of Aloe vera: A review. J. Herb. Med. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Hsu, S.-L. Pharmaceutical Use of Graptopetalum and Related Plants. U.S. Patent 7,364,758, 29 April 2008. [Google Scholar]
- Chung, Y.-C.; Chen, S.-J.; Hsu, C.-K.; Chang, C.-T.; Chou, S.-T. Studies on the antioxidative activity of Graptopetalum paraguayense E. Walther. Food Chem. 2005, 91, 419–424. [Google Scholar] [CrossRef]
- Ai, L.; Chung, Y.-C.; Jeng, K.-C.G.; Lai, P.F.-H.; Yeh, S.-C.; Lee, K.C.; Lin, S.-Y.; Xia, Y.; Wang, G.; Cui, S.W. Antioxidant hydrocolloids from herb Graptopetalum paraguayense leaves show anti-colon cancer cells and anti-neuroinflammatory potentials. Food Hydrocoll. 2017, 73, 51–59. [Google Scholar] [CrossRef]
- Chen, S.-J.; Chang, C.-T.; Chung, Y.-C.; Chou, S.-T. Studies on the inhibitory effect of Graptopetalum paraguayense E. Walther extracts on the angiotensin converting enzyme. Food Chem. 2007, 100, 1032–1036. [Google Scholar] [CrossRef]
- Chen, S.J.; Yen, C.H.; Liu, J.T.; Tseng, Y.F.; Lin, P.T. Anti-inflammatory effect of water extracts of Graptopetalum paraguayense supplementation in subjects with metabolic syndrome: A preliminary study. J. Sci. Food Agric. 2016, 96, 1772–1776. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-X.; Le, P.T.N.; Tzeng, T.-T.; Tran, T.-H.; Nguyen, A.T.; Cheng, I.H.-J.; Huang, C.-Y.F.; Shiao, Y.-J.; Ching, T.-T. Graptopetalum paraguayense extract ameliorates proteotoxicity in aging and age-related diseases in model systems. Nutrients 2021, 13, 4317. [Google Scholar] [CrossRef] [PubMed]
- Duh, P.-D.; Lin, S.-L.; Wu, S.-C. Hepatoprotection of Graptopetalum paraguayense E. Walther on CCl4-induced liver damage and inflammation. J. Ethnopharmacol. 2011, 134, 379–385. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Peng, H.-Y.; Hsu, S.-L.; Jong, T.-T.; Chou, S.-T. Chemical characterization and antioxidative activity of four 3-hydroxyl-3-methylglutaroyl (HMG)-substituted flavonoid glycosides from Graptopetalum paraguayense E. Walther. Bot. Stud. 2015, 56, 8. [Google Scholar] [CrossRef][Green Version]
- Wu, S.-C.; Su, Y.-S.; Cheng, H.-Y. Antioxidant properties of Lactobacillus-fermented and non-fermented Graptopetalum paraguayense E. Walther at different stages of maturity. Food Chem. 2011, 129, 804–809. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Chou, S.-T.; Jhan, J.-K.; Liao, J.-W.; Chen, S.-J. In vitro and in vivo safety of aqueous extracts of Graptopetalum paraguayense E. Walther. J. Ethnopharmacol. 2012, 140, 91–97. [Google Scholar] [CrossRef]
- Kao, T.-K.; Ou, Y.-C.; Raung, S.-L.; Chen, W.-Y.; Yen, Y.-J.; Lai, C.-Y.; Chou, S.-T.; Chen, C.-J. Graptopetalum paraguayense E. Walther leaf extracts protect against brain injury in ischemic rats. Am. J. Chin. Med. 2010, 38, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.-W.; Chen, S.-J.; Peng, H.-C.; Liao, J.-W.; Chou, S.-T. Antioxidant activity of Graptopetalum paraguayense E. Walther leaf extract counteracts oxidative stress induced by ethanol and carbon tetrachloride co-induced hepatotoxicity in rats. Antioxidants 2019, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Shen, S.R.; Li, Y.H.; Lo, C.Y.; Lee, B.H.; Wu, S.C. Anti-Glycation of Active Compounds Purified from G raptopetalum Paraguayense. J. Food Biochem. 2016, 40, 161–169. [Google Scholar] [CrossRef]
- Alshehri, A.; Ahmad, A.; Tiwari, R.K.; Ahmad, I.; Alkhathami, A.G.; Alshahrani, M.Y.; Asiri, M.A.; Almeleebia, T.M.; Saeed, M.; Yadav, D.K.; et al. In vitro evaluation of antioxidant, anticancer, and anti-inflammatory activities of ethanolic leaf extract of Adenium obesum. Front. Pharmacol. 2022, 13, 847534. [Google Scholar] [CrossRef]
- Suthar, S.K.; Monga, J.; Sharma, M.; Lee, S.-Y. Synthesis, biological evaluation, and in silico studies of lantadene-derived pentacyclic triterpenoids as anticancer agents targeting IKK-β. J. Biomol. Struct. Dyn. 2024. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Chung, J.-G.; Chung, Y.-C.; Chou, S.-T. In vitro antioxidant and antiproliferative activity of the stem extracts from Graptopetalum paraguayense. Am. J. Chin. Med. 2008, 36, 369–383. [Google Scholar] [CrossRef]
- Hsu, W.H.; Chang, C.C.; Huang, K.W.; Chen, Y.C.; Hsu, S.L.; Wu, L.C.; Tsou, A.P.; Lai, J.M.; Huang, C.Y. Evaluation of the medicinal herb Graptopetalum paraguayense as a treatment for liver cancer. PLoS ONE 2015, 10, e0121298. [Google Scholar] [CrossRef]
- Peng, H.-Y.; Lin, Y.-F.; Chen, Y.-H.; Chou, S.-T. The cytotoxic effects of Graptopetalum paraguayense (N.E.Br.) E. Walther extract on human melanoma cells. J. Herb. Med. 2023, 40, 100675. [Google Scholar] [CrossRef]
- Yoon, D.H.; Hong, S.-M.; Ko, E.J.; Jeon, R.O.; Kim, S.Y. Anti-neuroinflammatory Effects of Active Compound SPA1413 via Suppression of the MAPK and JAK/STAT Signaling Pathways. Biol. Pharm. Bull. 2023, 46, 1517–1526. [Google Scholar] [CrossRef]
- Alam, J.; Jaiswal, V.; Sharma, L. Screening of Antibiotics against β-amyloid as anti-amyloidogenic agents: A drug repurposing approach. Curr. Comput.-Aided Drug Des. 2021, 17, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-C.; Fann, M.-J.; Tran, T.T.; Chen, S.-C.; Devina, T.; Cheng, I.H.-J.; Lien, C.-C.; Kao, L.-S.; Wang, S.-J.; Fuh, J.-L.; et al. Assessing the therapeutic potential of Graptopetalum paraguayense on Alzheimer’s disease using patient iPSC-derived neurons. Sci. Rep. 2019, 9, 19301. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Hussain, T.; Khan, M.A.; Jaiswal, V. Exploring AT2R and its polymorphism in different diseases: An approach to develop AT2R as a drug target beyond hypertension. Curr. Drug Targets 2022, 23, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Jaiswal, V.; Khan, M.A. In silico approach for exploring the role of AT1R polymorphism on its function, structure and drug interactions. Curr. Comput.-Aided Drug Des. 2021, 17, 927–935. [Google Scholar] [CrossRef]
- Chung, Y.C.; Chen, S.J.; Peng, H.Y.; Chou, S.T. Antihypertensive and antioxidant effects of the Graptopetalum paraguayense E. Walther extract in spontaneously hypertensive rats. J. Sci. Food Agric. 2009, 89, 2678–2686. [Google Scholar] [CrossRef]
- Yu-Ling, L.; Hsin-Yi, P.; Hui-Min, H.; Ching-Hsiu, L.; Su-Tze, C. Effects of Graptopetalum paraguayense consumption on serum lipid profiles and antioxidative status in hypercholesteremic subjects. J. Sci. Food Agric. 2011, 91, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-H.; Chen, S.-J.; Liu, J.-T.; Tseng, Y.-F.; Lin, P.-T. Effects of water extracts of Graptopetalum paraguayense on blood pressure, fasting glucose, and lipid profiles of subjects with metabolic syndrome. BioMed Res. Int. 2013, 2013, 809234. [Google Scholar] [CrossRef]
- Alok, S.; Jain, S.K.; Verma, A.; Kumar, M.; Mahor, A.; Sabharwal, M. Herbal antioxidant in clinical practice: A review. Asian Pac. J. Trop. Biomed. 2014, 4, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Park, M.; Lee, H.-J. Comparative transcriptome analysis of the expression of antioxidant and immunity genes in the spleen of a Cyanidin 3-O-Glucoside-treated alzheimer’s mouse model. Antioxidants 2021, 10, 1435. [Google Scholar] [CrossRef]
- Khanam, A.; Ahmad, S.; Husain, A.; Rehman, S.; Farooqui, A.; Yusuf, M.A. Glycation and antioxidants: Hand in the glove of antiglycation and natural antioxidants. Curr. Protein Pept. Sci. 2020, 21, 899–915. [Google Scholar] [CrossRef]
- Chou, S.T.; Chung, Y.C.; Teng, K.Y.; Yeh, J.Y. Effects of Graptopetalumparaguayense extract on tert-butylhydroperoxide-induced oxidative stress. J. Sci. Food Agric. 2008, 88, 429–434. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Ro, H.; Jung, J.Y.; Chang, J.H.; Chung, W.; Kim, A.J. The Fatty Liver Index’s Association with Incident Chronic Kidney Disease in Korean Middle-Aged Adults: A Community-Based Cohort Study. J. Clin. Med. 2024, 13, 1616. [Google Scholar] [CrossRef]
- Kim, J.; Shin, M.-S.; Jang, A.Y.; Kim, S.; Heo, S.; Cha, E.; An, M. Advance Directives and Factors Associated with the Completion in Patients with Heart Failure. Int. J. Environ. Res. Public Health 2021, 18, 1780. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-J.; Yang, C.-H.; Huang, S.-F.; Yuo, Y.-L.; Hsieh, H.-C.; Tseng, T.-L.; Chen, C.-H.; Hsu, S.-L.; Huang, C.-Y.F. Evaluation of the Chinese medicinal herb, Graptopetalum paraguayense, as a therapeutic treatment for liver damage in rat models. Evid.-Based Complement. Altern. Med. 2012, 2012, 256561. [Google Scholar] [CrossRef]
- Shan, L.; Wang, F.; Zhai, D.; Meng, X.; Liu, J.; Lv, X. Matrix metalloproteinases induce extracellular matrix degradation through various pathways to alleviate hepatic fibrosis. Biomed. Pharmacother. 2023, 161, 114472. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-J.; Chang, C.-C.; Yang, C.-H.; Hsieh, S.-J.; Wu, Y.-C.; Lai, J.-M.; Tseng, T.-L.; Huang, C.-Y.F.; Hsu, S.-L. Graptopetalum paraguayense ameliorates chemical-induced rat hepatic fibrosis in vivo and inactivates stellate cells and Kupffer cells in vitro. PLoS ONE 2013, 8, e53988. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsu, W.-H.; Liao, S.-C.; Chyan, Y.-J.; Huang, K.-W.; Hsu, S.-L.; Chen, Y.-C.; Siu, M.-L.; Chang, C.-C.; Chung, Y.-S.; Huang, C.-Y.F. Graptopetalum paraguayense inhibits liver fibrosis by blocking TGF-β signaling in vivo and in vitro. Int. J. Mol. Sci. 2019, 20, 2592. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Shen, S.-R.; Lee, P.-S.; Huang, X.-S.; Chang, W.-C.; Wu, S.-C. Protective effects of Graptopetalum paraguayense E. Walther against methylglyoxal-induced liver damage and microflora imbalances caused by high-fructose induction. Fermentation 2023, 9, 366. [Google Scholar] [CrossRef]
- Oh, S.; Rho, N.-K.; Byun, K.-A.; Yang, J.Y.; Sun, H.J.; Jang, M.; Kang, D.; Son, K.H.; Byun, K. Combined Treatment of Monopolar and Bipolar Radiofrequency Increases Skin Elasticity by Decreasing the Accumulation of Advanced Glycated End Products in Aged Animal Skin. Int. J. Mol. Sci. 2022, 23, 2993. [Google Scholar] [CrossRef]
- Bayarsaikhan, G.; Bayarsaikhan, D.; Oh, P.C.; Kang, W.C.; Lee, B. CUPRAC-Reactive Advanced Glycation End Products as Prognostic Markers of Human Acute Myocardial Infarction. Antioxidants 2021, 10, 434. [Google Scholar] [CrossRef]
- Lee, D.; Park, J.Y.; Lee, S.; Kang, K.S. In vitro studies to assess the α-glucosidase inhibitory activity and insulin secretion effect of isorhamnetin 3-o-glucoside and quercetin 3-o-glucoside isolated from Salicornia herbacea. Processes 2021, 9, 483. [Google Scholar] [CrossRef]
- Lim, H.M.; Park, S.-H. Regulation of reactive oxygen species by phytochemicals for the management of cancer and diabetes. Crit. Rev. Food Sci. Nutr. 2023, 63, 5911–5936. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Lee, C.-C.; Cheng, Y.-H.; Chang, W.-C.; Hsu, W.-H.; Wu, S.-C. Graptopetalum paraguayense and resveratrol ameliorates carboxymethyllysine (CML)-induced pancreas dysfunction and hyperglycemia. Food Chem. Toxicol. 2013, 62, 492–498. [Google Scholar] [CrossRef]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Lee, Y.H.; Yoo, A.Y.; Amna, S.; Park, J.K. Evaluation of the effect of molecular weight change of konjac glucomannan on antioxidant and tyrosinase activities. Macromol. Res. 2024, 32, 401–413. [Google Scholar] [CrossRef]
- Huang, K.-F.; Chen, Y.-W.; Chang, C.-T.; Chou, S.-T. Studies on the inhibitory effect of Graptopetalum paraguayense E. Walther extracts on mushroom tyrosinase. Food Chem. 2005, 89, 583–587. [Google Scholar] [CrossRef]
- Zaharieva, M.M.; Genova-Kalou, P.; Dincheva, I.; Badjakov, I.; Krumova, S.; Enchev, V.; Najdenski, H.; Markova, N. Anti-Herpes Simplex virus and antibacterial activities of Graptopetalum paraguayense E. Walther leaf extract: A pilot study. Biotechnol. Biotechnol. Equip. 2019, 33, 1251–1259. [Google Scholar] [CrossRef]
- Easterbrook, P.J.; Luhmann, N.; Bajis, S.; Min, M.S.; Newman, M.; Lesi, O.; Doherty, M.C. WHO 2024 hepatitis B guidelines: An opportunity to transform care. Lancet Gastroenterol. Hepatol. 2024, 9, 493–495. [Google Scholar] [CrossRef]
- Lee, J.-K.; Choi, J.-W.; Park, I.; Kim, N.-E.; Kwon, H.C.; Kwon, J.; Song, Y.-J. Roseoside Is a Bioactive Compound in Kirengeshoma koreana Nakai Extract with Potent In Vitro Antiviral Activity Against Hepatitis C Virus. Molecules 2024, 29, 5130. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.-Y.; Kwon, H.C.; Jang, D.S.; Song, Y.-J. Antiviral Activities of Ethyl Pheophorbides a and b Isolated from Aster pseudoglehnii against Influenza Viruses. Molecules 2023, 28, 41. [Google Scholar] [CrossRef] [PubMed]
- Jhuang, H.-J.; Hsu, W.-H.; Lin, K.-T.; Hsu, S.-L.; Wang, F.-S.; Chou, C.-K.; Lee, K.-H.; Tsou, A.-P.; Lai, J.-M.; Yeh, S.-F.; et al. Gluconeogenesis, lipogenesis, and HBV replication are commonly regulated by PGC-1α-dependent pathway. Oncotarget 2015, 6, 7788–7803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, S.J.; Yoon, S.; Kim, K.-K.; Kim, D.; Lee, H.E.; Kim, K.G.; Shin, S.K.; Park, I.B.; Kim, S.M.; Lee, D.H. A Composite Blood Biomarker Including AKR1B10 and Cytokeratin 18 for Progressive Types of Nonalcoholic Fatty Liver Disease. Diabetes Metab. J. 2024, 48, 740–751. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of oxidative stress in metabolic syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Cheng, Y.-H.; Wu, S.-C. Graptopetalum paraguayense Ameliorates Airway Inflammation and Allergy in Ovalbumin-(OVA-) Sensitized BALB/C Mice by Inhibiting Th2 Signal. Evid.-Based Complement. Altern. Med. 2013, 2013, 237096. [Google Scholar] [CrossRef]
- Islam, M.Z.; Park, B.-J.; Lee, Y.-T. Bioactive Phytochemicals and Antioxidant Capacity of Wheatgrass Treated with Salicylic Acid under Organic Soil Cultivation. Chem. Biodivers. 2021, 18, e2000861. [Google Scholar] [CrossRef]
- Choi, J.; An, J.; Lee, H.-D.; Kim, W.J.; Lee, S.; Lee, S. Comprehensive Analysis of Phenolic Compounds, Carotenoids, and Antioxidant Activities in Lactuca sativa var. longifolia Cultivated in a Smart Farm System. Processes 2023, 11, 2993. [Google Scholar] [CrossRef]
- Saito, K. Phytochemical genomics—A new trend. Curr. Opin. Plant Biol. 2013, 16, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Reyes-González, C.E.; Torres-Morán, J.P.; Ramírez-Hernández, B.C.; Portillo, L.; Pimienta-Barrios, E.; Torres-Morán, M.I. Morphological changes of mexican native succulent plants in a vertical greenery system compared with pot conditions. HortTechnology 2018, 28, 304–309. [Google Scholar] [CrossRef]
- Tan, C.K.; Chong, H.C.; Tan, E.H.P.; Tan, N.S. Getting ‘Smad’ about obesity and diabetes. Nutr. Diabetes 2012, 2, e29. [Google Scholar] [CrossRef]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef]
- Song, G.Y.; Kim, S.M.; Back, S.; Yang, S.B.; Yang, Y.M. Atractylodes Lancea and Its Constituent, Atractylodin, Ameliorates Metabolic Dysfunction-Associated Steatotic Liver Disease via AMPK Activation. Biomol. Ther. 2024, 32, 778–792. [Google Scholar] [CrossRef]
- Goyal, A.; Agrawal, A.; Verma, A.; Dubey, N. The PI3K-AKT pathway: A plausible therapeutic target in Parkinson’s disease. Exp. Mol. Pathol. 2023, 129, 104846. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Doerge, D.; Padilla-Banks, E.; Woodling, K.A.; Kissling, G.E.; Newbold, R. Oral Exposure to Genistin, the Glycosylated Form of Genistein, during Neonatal Life Adversely Affects the Female Reproductive System. Environ. Health Perspect. 2009, 117, 1883–1889. [Google Scholar] [CrossRef]
- Niho, N.; Shibutani, M.; Tamura, T.; Toyoda, K.; Uneyama, C.; Takahashi, N.; Hirose, M. Subchronic toxicity study of gallic acid by oral administration in F344 rats. Food Chem. Toxicol. 2001, 39, 1063–1070. [Google Scholar] [CrossRef]
- Bsc, S.N. Oxalate content of foods and its effect on humans. Asia Pac. J. Clin. Nutr. 1999, 8, 64–74. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaiswal, V.; Lee, H.-J. A Comprehensive Review on Graptopetalum paraguayense’s Phytochemical Profiles, Pharmacological Activities, and Development as a Functional Food. Plants 2025, 14, 349. https://doi.org/10.3390/plants14030349
Jaiswal V, Lee H-J. A Comprehensive Review on Graptopetalum paraguayense’s Phytochemical Profiles, Pharmacological Activities, and Development as a Functional Food. Plants. 2025; 14(3):349. https://doi.org/10.3390/plants14030349
Chicago/Turabian StyleJaiswal, Varun, and Hae-Jeung Lee. 2025. "A Comprehensive Review on Graptopetalum paraguayense’s Phytochemical Profiles, Pharmacological Activities, and Development as a Functional Food" Plants 14, no. 3: 349. https://doi.org/10.3390/plants14030349
APA StyleJaiswal, V., & Lee, H.-J. (2025). A Comprehensive Review on Graptopetalum paraguayense’s Phytochemical Profiles, Pharmacological Activities, and Development as a Functional Food. Plants, 14(3), 349. https://doi.org/10.3390/plants14030349







