MicroRNAs in Plant Genetic Regulation of Drought Tolerance and Their Function in Enhancing Stress Adaptation
Abstract
1. Introduction
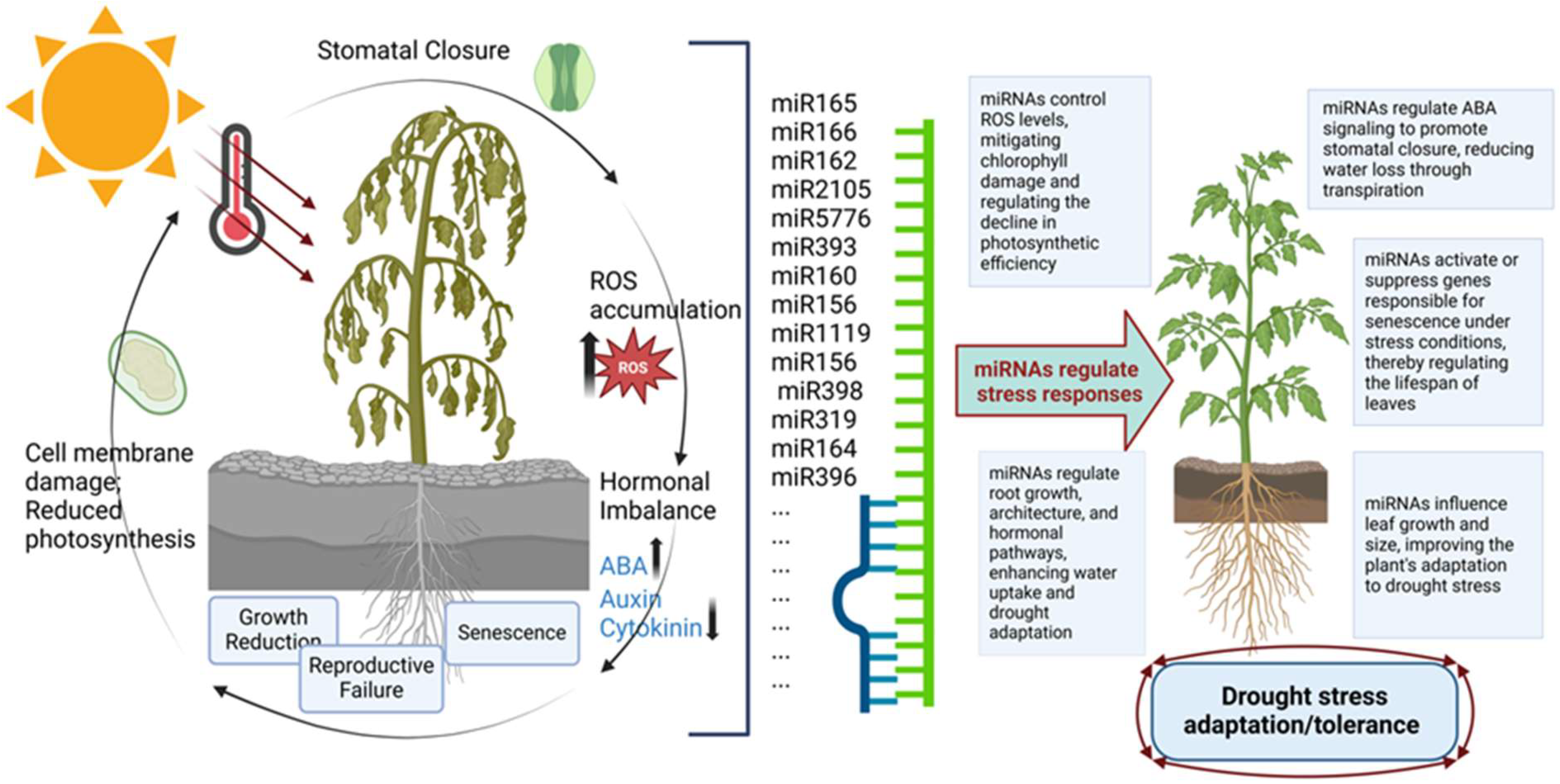
2. Mechanism of miRNA in the Genetic Regulation of Drought Stress Tolerance in Plants
2.1. MicroRNA Biogenesis and Processing Pathway in Plants
2.2. Signaling Pathways Associated with Stress Tolerance Regulated by miRNAs
2.2.1. MicroRNAs in Hormonal Signaling Pathways
2.2.2. MicroRNAs in Antioxidant-Based Pathways
2.2.3. MicroRNAs in Calcium Signaling and Natural Antisense Transcript-Based Pathways
| Pathway | Key miRNAs | Target Genes | Stress Role | Refs. |
|---|---|---|---|---|
| Hormonal Pathways (ABA) | miR159, miR165/166, miR162, miR2105, miR5776-x, miR393 | ABI4, BG1, OsbZIP86, NCED3, PaNCED2, RACK1A | Regulates ABA signaling, stomatal closure, root architecture, and dormancy | [48,49,50,51,52,53,54,55,56,57] |
| Hormonal Pathways (Auxin) | miR160, miR156ab, miR393 | ARF10, ARF16, ARF17, TIR1/AFB2, MdYUCCAs, MdPINs, MdLBD18, RACK1A | Controls auxin signaling, root/shoot growth, and stress tolerance | [63,64,65,66,67,68,69] |
| Antioxidant Pathways | miR1119, csn-miR156f-2-5p, miR398 | SOD, CAT, CsSPL14, CSD1, CSD2, COX5b | Maintains ROS homeostasis, photosynthetic efficiency, and antioxidant activity | [77,78,79] |
| Calcium Signaling Pathways | miR319, miR164, miR396 | TCP factors, NAC factors, GRFs | Regulates calcium responses, water uptake, and stomatal behavior | [77,82,83,84] |
| NAT-Based Pathways | miR398 (NAT398b, NAT398c) | ROS regulatory genes | Optimizes miRNA responses, ROS regulation, and cellular balance | [87] |
3. In Silico Tools for miRNA Target Prediction in Drought Tolerance Mechanisms
3.1. Overview of miRNA Target Prediction
3.2. Applications of In Silico Tools for miRNA Research in Drought Tolerance
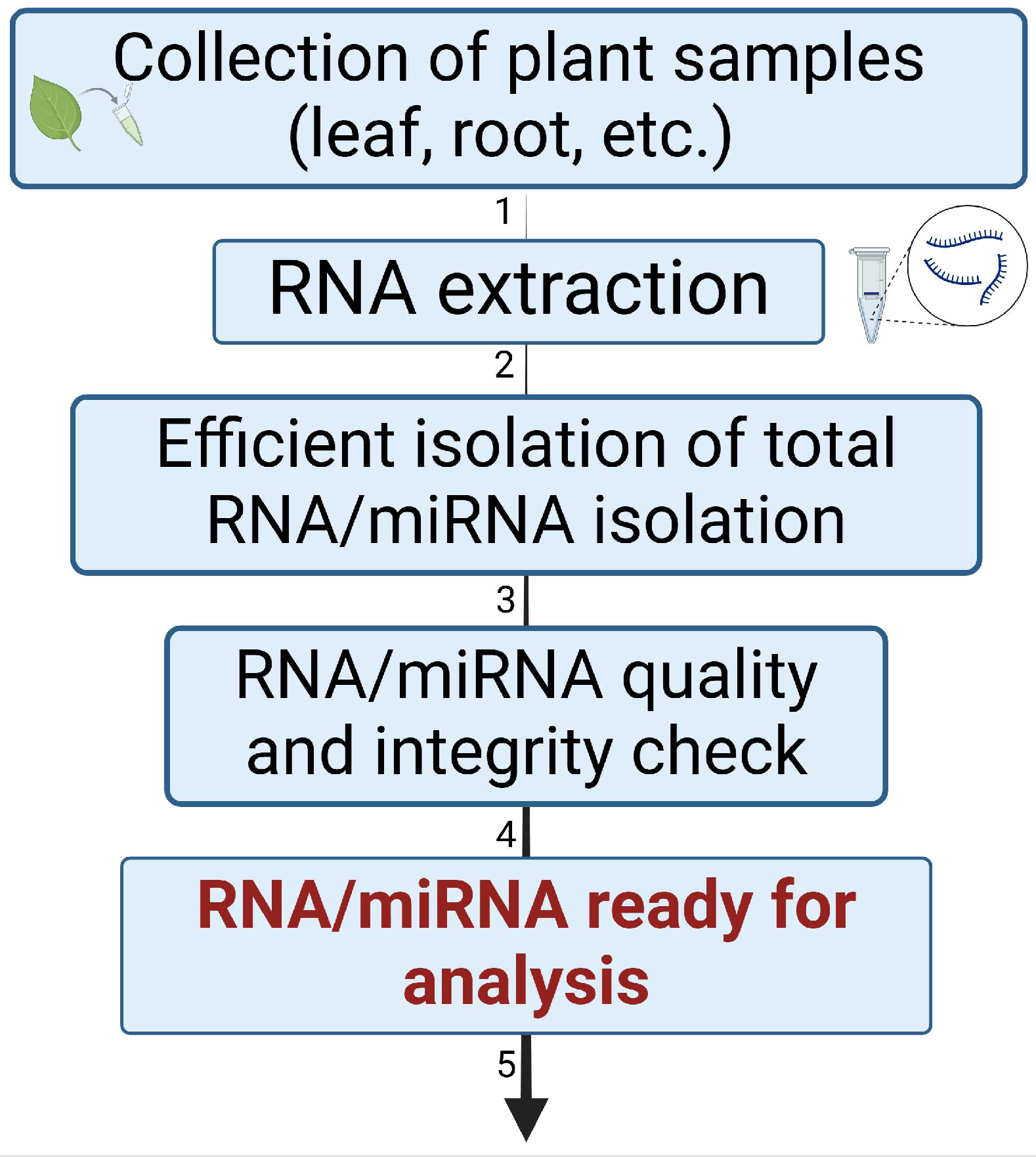
4. Experimental Approaches for miRNA Isolation and Analysis in Drought Stress Research
4.1. Isolation of Total RNA/miRNA
4.2. Microarray Analysis for miRNA Profiling
4.3. High-Throughput Sequencing for miRNA Discovery
4.4. RACE-PCR for miRNA Target Validation
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lieber, M.; Chin-Hong, P.; Kelly, K.; Dandu, M.; Weiser, S.D. A systematic review and meta-analysis assessing the impact of droughts, flooding, and climate variability on malnutrition. Glob. Public Health 2022, 17, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Anstett, D.N.; Branch, H.A.; Angert, A.L. Regional differences in rapid evolution during severe drought. Evol. Lett. 2021, 5, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ Physio-Biochemical and Phyto-Hormonal Responses to Alleviate the Adverse Effects of Drought Stress: A Comprehensive Review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef]
- Monroe, J.G.; Powell, T.; Price, N.; Mullen, J.L.; Howard, A.; Evans, K.; Lovell, J.T.; McKay, J.K. Drought adaptation in Arabidopsis thaliana by extensive genetic loss-of-function. Elife 2018, 7, e41038. [Google Scholar] [CrossRef]
- Marques, I.; Hu, H. Molecular Insight of Plants Response to Drought Stress: Perspectives and New Insights towards Food Security. Int. J. Mol. Sci. 2024, 25, 4988. [Google Scholar] [CrossRef]
- Singh, V.; Gupta, K.; Singh, S.; Jain, M.; Garg, R. Unravelling the molecular mechanism underlying drought stress response in chickpea via integrated multi-omics analysis. Front. Plant Sci. 2023, 14, 1156606. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, X.; Wang, C.; Wang, Y. miRNAs: Primary modulators of plant drought tolerance. J. Plant Physiol. 2024, 301, 154313. [Google Scholar] [CrossRef]
- Luo, G.; Li, L.; Yang, X.; Yu, Y.; Gao, L.; Mo, B.; Chen, X.; Liu, L. MicroRNA1432 regulates rice drought stress tolerance by targeting the CALMODULIN-LIKE2 gene. Plant Physiol. 2024, 195, 1954–1968. [Google Scholar] [CrossRef]
- Belkozhayev, A.; Niyazova, R.; Kamal, M.A.; Ivashchenko, A.; Sharipov, K.; Wilson, C.M. Differential microRNA expression in the SH-SY5Y human cell model as potential biomarkers for Huntington’s disease. Front. Cell Neurosci. 2024, 18, 1399742. [Google Scholar] [CrossRef]
- Belkozhayev, A.M.; Al-Yozbaki, M.; George, A.; Ye Niyazova, R.; Sharipov, K.O.; Byrne, L.J.; Wilson, C.M. Extracellular Vesicles, Stem Cells and the Role of miRNAs in Neurodegeneration. Curr. Neuropharmacol. 2022, 20, 1450–1478. [Google Scholar] [CrossRef] [PubMed]
- Aalto, A.P.; Pasquinelli, A.E. Small non-coding RNAs mount a silent revolution in gene expression. Curr. Opin. Cell Biol. 2012, 24, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Belkozhayev, A.; Niyazova, R.; Wilson, C.; Jainakbayev, N.; Pyrkova, A.; Ashirbekov, Y.; Akimniyazova, A.; Sharipov, K.; Ivashchenko, A. Bioinformatics Analysis of the Interaction of miRNAs and piRNAs with Human mRNA Genes Having di- and Trinucleotide Repeats. Genes 2022, 13, 800. [Google Scholar] [CrossRef]
- Rakhmetullina, A.; Pyrkova, A.; Aisina, D.; Ivashchenko, A. In silico prediction of human genes as potential targets for rice miRNAs. Comput. Biol. Chem. 2020, 87, 107305. [Google Scholar] [CrossRef]
- Válóczi, A.; Várallyay, É.; Kauppinen, S.; Burgyán, J.; Havelda, Z. Spatio-Temporal Accumulation of microRNAs Is Highly Coordinated in Developing Plant Tissues. Plant J. 2006, 47, 140–151. [Google Scholar] [CrossRef]
- Parizotto, E.A.; Dunoyer, P.; Rahm, N.; Himber, C.; Voinnet, O. In Vivo Investigation of the Transcription, Processing, Endonucleolytic Activity, and Functional Relevance of the Spatial Distribution of a Plant miRNA. Genes Dev. 2015, 29, 465. [Google Scholar] [CrossRef]
- Villani, V.; Di Marco, G.; Iacovelli, F.; Bernardi, G.; Gismondi, A.; Canini, A. Profile and Potential Bioactivity of the miRNome and Metabolome Expressed in Malva sylvestris L. Leaf and Flower. BMC Plant Biol. 2023, 23, 439. [Google Scholar] [CrossRef]
- Gismondi, A.; Di Marco, G.; Camoni, L.; Pezzotti, M.; Ruffini Castiglione, M.; Canini, A. MicroRNA Expression Profiles in Moringa oleifera Lam. Seedlings at Different Growth Conditions. J. Plant Growth Regul. 2023, 42, 2115–2123. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Zhang, X.; Chen, D.; Cheng, Y.; Shen, F. Plant MicroRNAs in Cross-Kingdom Regulation of Gene Expression. Int. J. Mol. Sci. 2018, 19, 2007. [Google Scholar] [CrossRef]
- Gómez-Martín, C.; Zhou, H.; Medina, J.M.; Aparicio-Puerta, E.; Shi, B.; Hackenberg, M. Genome-Wide Analysis of microRNA Expression Profile in Roots and Leaves of Three Wheat Cultivars under Water and Drought Conditions. Biomolecules 2023, 13, 440. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Shen, T.; Luo, Q.; Xu, M.; Yang, Z. The miRNA-mRNA Regulatory Modules of Pinus massoniana Lamb. in Response to Drought Stress. Int. J. Mol. Sci. 2023, 24, 14655. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, Y.; Tao, X.; Fahim, A.M.; Zhang, X.; Han, C.; Yang, G.; Wang, W.; Pu, Y.; Liu, L.; et al. Integrated miRNA and mRNA Transcriptome Analysis Reveals Regulatory Mechanisms in the Response of Winter Brassica rapa to Drought Stress. Int. J. Mol. Sci. 2024, 25, 10098. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Song, Y.; Xue, X.; Xue, R.; Jiang, H.; Zhou, Y.; Qi, X.; Wang, Y. Differential Transcription Profiling Reveals the MicroRNAs Involved in Alleviating Damage to Photosynthesis under Drought Stress during the Grain Filling Stage in Wheat. Int. J. Mol. Sci. 2024, 25, 5518. [Google Scholar] [CrossRef]
- Lopos, L.C.; Panthi, U.; Kovalchuk, I.; Bilichak, A. Modulation of Plant MicroRNA Expression: Its Potential Usability in Wheat (Triticum aestivum L.) Improvement. Curr. Genom. 2023, 24, 197–206. [Google Scholar] [CrossRef]
- Geng, A.; Lian, W.; Wang, Y.; Liu, M.; Zhang, Y.; Wang, X.; Chen, G. Molecular Mechanisms and Regulatory Pathways Underlying Drought Stress Response in Rice. Int. J. Mol. Sci. 2024, 25, 1185. [Google Scholar] [CrossRef]
- Sarsekeyeva, F.K.; Sadvakasova, A.K.; Sandybayeva, S.K.; Kossalbayev, B.D.; Huang, Z.; Zayadan, B.K.; Akmukhanova, N.R.; Leong, Y.K.; Chang, J.-S.; Allakhverdiev, S.I. Microalgae- and Cyanobacteria-Derived Phytostimulants for Mitigation of Salt Stress and Improved Agriculture. Algal Res. 2024, 82, 103686. [Google Scholar] [CrossRef]
- Jeena, G.S.; Singh, N.; Shikha; Shukla, R.K. An insight into microRNA biogenesis and its regulatory role in plant secondary metabolism. Plant Cell Rep. 2022, 41, 1651–1671. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, Y.; Chen, S.; Shi, M.; Jiang, X.; He, Z.; Gao, S. Mechanisms of MicroRNA Biogenesis and Stability Control in Plants. Front. Plant Sci. 2022, 13, 844149. [Google Scholar] [CrossRef]
- Liu, C.; Axtell, M.J.; Fedoroff, N.V. The helicase and RNaseIIIa domains of Arabidopsis Dicer-Like1 modulate catalytic parameters during microRNA biogenesis. Plant Physiol. 2012, 159, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, L. Analyzing the microRNA Transcriptome in Plants Using Deep Sequencing Data. Biology 2012, 1, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, S.; Yu, B. microRNA biogenesis, degradation and activity in plants. Cell Mol. Life Sci. 2015, 72, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Bajczyk, M.; Jarmolowski, A.; Jozwiak, M.; Pacak, A.; Pietrykowska, H.; Sierocka, I.; Swida-Barteczka, A.; Szewc, L.; Szweykowska-Kulinska, Z. Recent Insights into Plant miRNA Biogenesis: Multiple Layers of miRNA Level Regulation. Plants 2023, 12, 342. [Google Scholar] [CrossRef] [PubMed]
- Guleria, P.; Mahajan, M.; Bhardwaj, J.; Yadav, S.K. Plant small RNAs: Biogenesis, mode of action and their roles in abiotic stresses. Genom. Proteom. Bioinform. 2011, 9, 183–199. [Google Scholar] [CrossRef]
- de Mello, A.S.; Ferguson, B.S.; Shebs-Maurine, E.L.; Giotto, F.M. MicroRNA Biogenesis, Gene Regulation Mechanisms, and Availability in Foods. Noncoding RNA 2024, 10, 52. [Google Scholar] [CrossRef]
- Samad, A.F.A.; Sajad, M.; Nazaruddin, N.; Fauzi, I.A.; Murad, A.M.A.; Zainal, Z.; Ismail, I. MicroRNA and Transcription Factor: Key Players in Plant Regulatory Network. Front. Plant Sci. 2017, 8, 565. [Google Scholar] [CrossRef]
- Li, M.; Yu, B. Recent advances in the regulation of plant miRNA biogenesis. RNA Biol. 2021, 18, 2087–2096. [Google Scholar] [CrossRef]
- Ghifari, A.S.; Saha, S.; Murcha, M.W. The biogenesis and regulation of the plant oxidative phosphorylation system. Plant Physiol. 2023, 192, 728–747. [Google Scholar] [CrossRef]
- Ni, C.; Buszczak, M. The homeostatic regulation of ribosome biogenesis. Semin. Cell Dev. Biol. 2023, 136, 13–26. [Google Scholar] [CrossRef]
- Pegler, J.L.; Oultram, J.M.J.; Grof, C.P.L.; Eamens, A.L. Profiling the Abiotic Stress Responsive microRNA Landscape of Arabidopsis thaliana. Plants 2019, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Shriram, V.; Kumar, V.; Devarumath, R.M.; Khare, T.S.; Wani, S.H. MicroRNAs as potential targets for abiotic stress tolerance in plants. Front. Plant Sci. 2016, 7, 817. [Google Scholar] [CrossRef] [PubMed]
- Islam, W.; Waheed, A.; Naveed, H.; Zeng, F. MicroRNAs mediated plant responses to salt stress. Cells 2022, 11, 2806. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, J.; Zou, J.J.; Cai, X.; Zeng, X.; Xing, W. A systematic review on the role of miRNAs in plant response to stresses under the changing climatic conditions. Plant Stress 2024, 14, 100674. [Google Scholar] [CrossRef]
- Tuteja, N. Abscisic acid and abiotic stress signaling. Plant Signal Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Abhilasha, A.; Roy Choudhury, S. Molecular and Physiological Perspectives of Abscisic Acid Mediated Drought Adjustment Strategies. Plants 2021, 10, 2769. [Google Scholar] [CrossRef]
- Fu, T.; Wang, C.; Yang, Y.; Yang, X.; Wang, J.; Zhang, L.; Wang, Z.; Wang, Y. Function identification of miR159a, a positive regulator during poplar resistance to drought stress. Hortic. Res. 2023, 10, uhad221. [Google Scholar] [CrossRef]
- Tiwari, M. Blessing in disguise: A loss of miR159 makes plant drought tolerant and ABA sensitive. Physiol. Plant. 2022, 174, e13763. [Google Scholar] [CrossRef]
- Contreras-Cubas, C.; Rabanal, F.A.; Arenas-Huertero, C.; Ortiz, M.A.; Covarrubias, A.A.; Reyes, J.L. The Phaseolus vulgaris miR159a precursor encodes a second differentially expressed microRNA. Plant Mol. Biol. 2012, 80, 103–115. [Google Scholar] [CrossRef]
- Eldem, V.; Çelikkol Akçay, U.; Ozhuner, E.; Bakır, Y.; Uranbey, S.; Unver, T. Genome-wide identification of miRNAs responsive to drought in peach (Prunus persica) by high-throughput deep sequencing. PLoS ONE 2012, 7, e50298. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhao, C.; Zhou, J.; Yang, Y.; Wang, P.; Zhu, X.; Tang, G.; Bressan, R.A.; Zhu, J.K. The miR165/166 Mediated Regulatory Module Plays Critical Roles in ABA Homeostasis and Response in Arabidopsis thaliana. PLoS Genet. 2016, 12, e1006416. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Gao, Z.; Wang, F.; Xu, T.; Qi, M.; Liu, Y.; Li, T. MicroRNA162 regulates stomatal conductance in response to low night temperature stress via abscisic acid signaling pathway in tomato. Front. Plant Sci. 2023, 14, 1045112. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, M.; Yang, S.; Gao, C.; Su, Y.; Zeng, X.; Jiao, Z.; Xu, W.; Zhang, M.; Xia, K. miR2105 and OsSAPK10 co-regulate OsbZIP86 to mediate drought-induced ABA biosynthesis via OsNCED3 in rice. Plant Physiol. 2022, 189, 889–905. [Google Scholar] [CrossRef]
- Premachandran, Y. Triggered in distress: A miRNA-controlled switch for drought-induced ABA biosynthesis in rice. Plant Physiol. 2022, 189, 447–449. [Google Scholar] [CrossRef]
- Xu, W.; Chen, C.; Bao, W.; Chen, Y.; Chen, J.; Zhao, H.; Zhu, G.; Wuyun, T.N.; Wang, L. Integrated transcriptome and miRNA analysis provides insight into the floral buds dormancy in Prunus armeniaca. Plant Growth Regul. 2024, 104, 869–883. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, J.; Zhang, N.; Wu, J.; Si, H. Roles of microRNAs in abiotic stress response and characteristics regulation of plants. Front. Plant Sci. 2022, 13, 919243. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, H.; Li, N.; Batley, J.; Wang, Y. The miR393-Target Module Regulates Plant Development and Responses to Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2022, 23, 9477. [Google Scholar] [CrossRef]
- Si-Ammour, A.; Windels, D.; Arn-Bouldoires, E.; Kutter, C.; Ailhas, J.; Meins, F.; Vazquez, F. miR393 and Secondary siRNAs Regulate Expression of the TIR1/AFB2 Auxin Receptor Clade and Auxin-Related Development of Arabidopsis Leaves. Plant Physiol. 2011, 157, 683–691. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, M.; Xu, X.; Li, H.; Deng, W. Roles of Auxin in the Growth, Development, and Stress Tolerance of Horticultural Plants. Cells 2022, 11, 2761. [Google Scholar] [CrossRef]
- Chen, H.; Li, Z.; Xiong, L. A plant microRNA regulates the adaptation of roots to drought stress. FEBS Lett. 2012, 586, 1742–1747. [Google Scholar] [CrossRef] [PubMed]
- Marzi, D.; Brunetti, P.; Saini, S.S.; Yadav, G.; Puglia, G.D.; Dello Ioio, R. Role of transcriptional regulation in auxin-mediated response to abiotic stresses. Front. Genet. 2024, 15, 1394091. [Google Scholar] [CrossRef] [PubMed]
- Hao, K.; Wang, Y.; Zhu, Z.; Wu, Y.; Chen, R.; Zhang, L. miR160: An Indispensable Regulator in Plants. Front. Plant Sci. 2022, 13, 833322. [Google Scholar] [CrossRef]
- Pessino, S.; Cucinotta, M.; Colono, C.; Costantini, E.; Perrone, D.; Di Marzo, M.; Callizaya Terceros, G.; Petrella, R.; Mizzotti, C.; Azzaro, C.; et al. Auxin response factor 10 insensitive to miR160 regulation induces apospory-like phenotypes in Arabidopsis. iScience 2024, 27, 111115. [Google Scholar] [CrossRef]
- Safi, A. A microRNA with a mega impact on plant growth: miR156ab spray keeps drought away. Plant Physiol. 2023, 192, 1666–1668. [Google Scholar] [CrossRef]
- Guo, C.; Xu, Y.; Shi, M.; Lai, Y.; Wu, X.; Wang, H.; Zhu, Z.; Poethig, R.S.; Wu, G. Repression of miR156 by miR159 Regulates the Timing of the Juvenile-to-Adult Transition in Arabidopsis. Plant Cell 2017, 29, 1293–1304. [Google Scholar] [CrossRef]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.W.; Weigel, D.; Poethig, R.S. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef]
- Jerome Jeyakumar, J.M.; Ali, A.; Wang, W.-M.; Thiruvengadam, M. Characterizing the Role of the miR156-SPL Network in Plant Development and Stress Response. Plants 2020, 9, 1206. [Google Scholar] [CrossRef]
- Liu, H.H.; Tian, X.; Li, Y.J.; Wu, C.A.; Zheng, C.C. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 2008, 14, 836–843. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Carbonell, T.; Gomes, A.V. MicroRNAs in the regulation of cellular redox status and its implications in myocardial ischemia-reperfusion injury. Redox Biol. 2020, 36, 101607. [Google Scholar] [CrossRef]
- Banerjee, J.; Khanna, S.; Bhattacharya, A. MicroRNA Regulation of Oxidative Stress. Oxid. Med. Cell Longev. 2017, 2017, 2872156. [Google Scholar] [CrossRef]
- Kaya, C.; Uğurlar, F.; Adamakis, I.S. Epigenetic Modifications of Hormonal Signaling Pathways in Plant Drought Response and Tolerance for Sustainable Food Security. Int. J. Mol. Sci. 2024, 25, 8229. [Google Scholar] [CrossRef]
- Bakhshi, B.; Fard, E.M. The Arrangement of MicroRNAs in the Regulation of Drought Stress Response in Plants: A Systematic Review. Plant Mol. Biol. Rep. 2023, 41, 369–387. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, C.; Tian, C.; Yang, N.; Zhang, C.; Zheng, A.; Chen, Y.; Lai, Z.; Guo, Y. Identification and Validation of the miR156 Family Involved in Drought Responses and Tolerance in Tea Plants (Camellia sinensis (L.) O. Kuntze. Plants 2024, 13, 201. [Google Scholar] [CrossRef]
- Ding, Y.; Tao, Y.; Zhu, C. Emerging roles of microRNAs in the mediation of drought stress response in plants. J. Exp. Bot. 2013, 64, 3077–3086. [Google Scholar] [CrossRef]
- Li, L.; Yu, D.; Zhao, F.; Pang, C.; Song, M.; Wei, H.; Fan, S.; Yu, S. Genome-wide analysis of the calcium-dependent protein kinase gene family in Gossypium raimondii. J. Integr. Agric. 2015, 14, 29–41. [Google Scholar] [CrossRef]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into Drought Stress Signaling in Plants and the Molecular Genetic Basis of Cotton Drought Tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hu, L. MicroRNA: A Dynamic Player from Signalling to Abiotic Tolerance in Plants. Int. J. Mol. Sci. 2023, 24, 11364. [Google Scholar] [CrossRef] [PubMed]
- Aliniaeifard, S.; Shomali, A.; Seifikalhor, M.; Lastochkina, O. Calcium Signaling in Plants Under Drought. In Salt and Drought Stress Tolerance in Plants: Signaling and Communication in Plants; Hasanuzzaman, M., Tanveer, M., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Peng, X.; Feng, C.; Wang, Y.T.; Zhang, X.; Wang, Y.Y.; Sun, Y.T.; Xiao, Y.Q.; Zhai, Z.F.; Zhou, X.; Du, B.Y.; et al. miR164g-MsNAC022 acts as a novel module mediating drought response by transcriptional regulation of reactive oxygen species scavenging systems in apple. Hortic. Res. 2022, 9, uhac192. [Google Scholar] [CrossRef]
- Santos, F.; Capela, A.M.; Mateus, F.; Nóbrega-Pereira, S.; Bernardes de Jesus, B. Non-coding antisense transcripts: Fine regulation of gene expression in cancer. Comput. Struct. Biotechnol. J. 2022, 20, 5652–5660. [Google Scholar] [CrossRef]
- Wight, M.; Werner, A. The functions of natural antisense transcripts. Essays Biochem. 2013, 54, 91–101. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Yang, J.; He, Y. Natural antisense transcripts of MIR398 genes suppress microR398 processing and attenuate plant thermotolerance. Nat. Commun. 2020, 11, 5351. [Google Scholar] [CrossRef]
- Jiao, P.; Ma, R.; Wang, C.; Chen, N.; Liu, S.; Qu, J.; Guan, S.; Ma, Y. Integration of mRNA and microRNA analysis reveals the molecular mechanisms underlying drought stress tolerance in maize (Zea mays L.). Front. Plant Sci. 2022, 13, 932667. [Google Scholar] [CrossRef]
- Luo, C.; Bashir, N.H.; Li, Z.; Liu, C.; Shi, Y.; Chu, H. Plant microRNAs regulate the defense response against pathogens. Front. Microbiol. 2024, 15, 1434798. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. The miRNA-target interactions: An underestimated intricacy. Nucleic Acids Res. 2024, 52, 1544–1557. [Google Scholar] [CrossRef]
- Siddika, T.; Heinemann, I.U. Bringing MicroRNAs to Light: Methods for MicroRNA Quantification and Visualization in Live Cells. Front. Bioeng. Biotechnol. 2021, 8, 619583. [Google Scholar] [CrossRef]
- Afonso-Grunz, F.; Müller, S. Principles of miRNA-mRNA interactions: Beyond sequence complementarity. Cell Mol. Life Sci. 2015, 72, 3127–3141. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, Y.; Wang, K.; Wen, Z.; Li, M.; Zhang, L.; Guang, X. In silico method for systematic analysis of feature importance in microRNA-mRNA interactions. BMC Bioinform. 2009, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Iyer, N.J.; Jia, X.; Sunkar, R.; Tang, G.; Mahalingam, R. microRNAs responsive to ozone-induced oxidative stress in Arabidopsis thaliana. Plant Signal Behav. 2012, 7, 484–491. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Chen, W.; Fu, Z.; Zhao, S.; E, Y.; Zhang, H.; Zhang, B.; Sun, M.; Han, P.; et al. Integration of mRNA and miRNA analysis reveals the molecular mechanisms of sugar beet (Beta vulgaris L.) response to salt stress. Sci. Rep. 2023, 13, 22074. [Google Scholar] [CrossRef]
- Das, R.; Mondal, S.K. Plant miRNAs: Biogenesis and its functional validation to combat drought stress with special focus on maize. Plant Gene 2021, 27, 100294. [Google Scholar] [CrossRef]
- Klapproth, C.; Zötzsche, S.; Kühnl, F.; Fallmann, J.; Stadler, P.F.; Findeiß, S. Tailored machine learning models for functional RNA detection in genome-wide screens. NAR Genom. Bioinform. 2023, 5, lqad072. [Google Scholar] [CrossRef]
- Mahood, E.H.; Kruse, L.H.; Moghe, G.D. Machine learning: A powerful tool for gene function prediction in plants. Appl. Plant Sci. 2020, 8, e11376. [Google Scholar] [CrossRef]
- Bolger, M.E.; Arsova, B.; Usadel, B. Plant genome and transcriptome annotations: From misconceptions to simple solutions. Brief Bioinform. 2018, 19, 437–449. [Google Scholar] [CrossRef]
- Son, A.; Park, J.; Kim, W.; Lee, W.; Yoon, Y.; Ji, J.; Kim, H. Integrating Computational Design and Experimental Approaches for Next-Generation Biologics. Biomolecules 2024, 14, 1073. [Google Scholar] [CrossRef]
- Arif, K.M.T.; Okolicsanyi, R.K.; Haupt, L.M.; Griffiths, L.R. A combinatorial in silico approach for microRNA-target identification: Order out of chaos. Biochimie 2021, 187, 121–130. [Google Scholar] [CrossRef]
- Murmu, S.; Sinha, D.; Chaurasia, H.; Sharma, S.; Das, R.; Jha, G.K.; Archak, S. A review of artificial intelligence-assisted omics techniques in plant defense: Current trends and future directions. Front. Plant Sci. 2024, 15, 1292054. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhao, L.; Zhao, S.; Niu, X.; Li, L.; Gao, H.; Liu, J.; Wang, L.; Zhang, T.; Cheng, R.; et al. Utilizing machine learning and bioinformatics analysis to identify drought-responsive genes affecting yield in foxtail millet. Int. J. Biol. Macromol. 2024, 277, 134288. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Han, J.H. MicroRNA: Biological and computational perspective. Genom. Proteom. Bioinform. 2005, 3, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Dweep, H.; Sticht, C.; Gretz, N. In-Silico Algorithms for the Screening of Possible microRNA Binding Sites and Their Interactions. Curr. Genom. 2013, 14, 127–136. [Google Scholar] [CrossRef]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef]
- Chaudhary, S.; Grover, A.; Sharma, P.C. MicroRNAs: Potential Targets for Developing Stress-Tolerant Crops. Life 2021, 11, 289. [Google Scholar] [CrossRef]
- Tang, Q.; Lv, H.; Li, Q.; Zhang, X.; Li, L.; Xu, J.; Wu, F.; Wang, Q.; Feng, X.; Lu, Y. Characteristics of microRNAs and Target Genes in Maize Root under Drought Stress. Int. J. Mol. Sci. 2022, 23, 4968. [Google Scholar] [CrossRef]
- Dai, H.; Yang, J.; Teng, L.; Wang, Z.; Liang, T.; Khan, W.A.; Yang, R.; Qiao, B.; Zhang, Y.; Yang, C. Mechanistic basis for mitigating drought tolerance by selenium application in tobacco (Nicotiana tabacum L.): A multi-omics approach. Front. Plant Sci. 2023, 14, 1255682. [Google Scholar] [CrossRef]
- Gupta, S.; Dong, Y.; Dijkwel, P.P.; Mueller-Roeber, B.; Gechev, T.S. Genome-Wide Analysis of ROS Antioxidant Genes in Resurrection Species Suggest an Involvement of Distinct ROS Detoxification Systems during Desiccation. Int. J. Mol. Sci. 2019, 20, 3101. [Google Scholar] [CrossRef]
- Gallardo, C.; Videm, P.; Serrano-Solano, B. Whole transcriptome analysis of Arabidopsis thaliana. Galaxy Training Materials. Available online: https://training.galaxyproject.org (accessed on 25 November 2024).
- Sablok, G.; Yang, K.; Wen, X. Protocols for miRNA Target Prediction in Plants. Methods Mol. Biol. 2019, 1970, 65–73. [Google Scholar] [CrossRef]
- Cui, S.; Yu, S.; Huang, H.Y.; Lin, Y.C.; Huang, Y.; Zhang, B.; Xiao, J.; Zuo, H.; Wang, J.; Li, Z.; et al. miRTarBase 2025: Updates to the collection of experimentally validated microRNA-target interactions. Nucleic Acids Res. 2024, 53, D147–D156. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Lin, Y.C.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. miRTarBase update 2022: An informative resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2022, 50, D222–D230. [Google Scholar] [CrossRef]
- Tripathi, A.; Goswami, K.; Sanan-Mishra, N. Role of bioinformatics in establishing microRNAs as modulators of abiotic stress responses: The new revolution. Front. Physiol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Pagliarani, C.; Gambino, G. Small RNA mobility: Spread of RNA silencing effectors and its effect on developmental processes and stress adaptation in plants. Int. J. Mol. Sci. 2019, 20, 4306. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Chang, N.W.; Shrestha, S.; Hsu, S.D.; Lin, Y.L.; Lee, W.H.; Yang, C.D.; Hong, H.C.; Wei, T.Y.; Tu, S.J.; et al. miRTarBase 2016: Updates to the experimentally validated miRNA-target interactions database. Nucleic Acids Res. 2016, 44, D239–D247. [Google Scholar] [CrossRef]
- Krüger, J.; Rehmsmeier, M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006, 34, W451–W454. [Google Scholar] [CrossRef]
- Rehmsmeier, M.; Steffen, P.; Hochsmann, M.; Giegerich, R. Fast and effective prediction of microRNA/target duplexes. RNA 2004, 10, 1507–1517. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, L.; Wang, J.; Gu, P.; Chen, M. MTide: An integrated tool for the identification of miRNA–target interaction in plants. Bioinformatics 2015, 31, 290–291. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Moturu, T.R.; Pandey, P.; Baldwin, I.T.; Pandey, S.P. A comparison of performance of plant miRNA target prediction tools and the characterization of features for genome-wide target prediction. BMC Genom. 2014, 15, 348. [Google Scholar] [CrossRef]
- Bonnet, E.; He, Y.; Billiau, K.; Van de Peer, Y. TAPIR: A web server for the prediction of plant microRNA targets, including target mimics. Bioinformatics 2010, 26, 1566–1568. [Google Scholar] [CrossRef]
- Rakhmetullina, A.; Ivashchenko, A.; Pyrkova, A.; Uteulin, K.; Zielenkiewicz, P. In silico analysis of maize and wheat miRNAs as potential regulators of human gene expression. ExRNA 2023, 5, 4. [Google Scholar] [CrossRef]
- Rakhmetullina, A.; Zielenkiewicz, P.; Pyrkova, A.; Uteulin, K.; Ivashchenko, A. Prediction of characteristics of interactions of miRNA with mRNA of GRAS, ERF, C2H2 genes of A. thaliana, O. sativa, and Z. mays. Curr. Plant Biol. 2021, 28, 100224. [Google Scholar] [CrossRef]
- Rakhmetullina, A.K.; Régnier, M.; Ivashchenko, A.T. The characteristics of MiRNA binding sites with mRNA of MYB plant transcription factors. Int. J. Biol. Chem. 2019, 12, 60–67. [Google Scholar] [CrossRef]
- Leontis, N.B.; Stombaugh, J.; Westhof, E. The non-Watson-Crick base pairs and their associated isostericity matrices. Nucleic Acids Res. 2002, 30, 3497–3531. [Google Scholar] [CrossRef]
- Chen, B.; Ding, Z.; Zhou, X.; Wang, Y.; Huang, F.; Sun, J.; Chen, J.; Han, W. Integrated full-length transcriptome and microRNA sequencing approaches provide insights into salt tolerance in mangrove (Sonneratia apetala Buch.-Ham.). Front. Genet. 2022, 13, 932832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Addo-Quaye, C.; Miller, W.; Axtell, M.J. CleaveLand: A pipeline for using degradome data to find cleaved small RNA targets. Bioinformatics 2009, 25, 130–131. [Google Scholar] [CrossRef]
- Wen, M.; Cong, P.; Zhang, Z.; Lu, H.; Li, T. DeepMirTar: A deep-learning approach for predicting human miRNA targets. Bioinformatics 2018, 34, 3781–3787. [Google Scholar] [CrossRef]
- Allakhverdiev, E.S.; Kossalbayev, B.D.; Sadvakasova, A.K.; Bauenova, M.O.; Belkozhayev, A.M.; Rodnenkov, O.V.; Martynyuk, T.V.; Maksimov, G.V.; Allakhverdiev, S.I. Spectral insights: Navigating the frontiers of biomedical and microbiological exploration with Raman spectroscopy. J. Photochem. Photobiol. B 2024, 252, 112870. [Google Scholar] [CrossRef]
- Vera-Hernández, P.F.; de Folter, S.; Rosas-Cárdenas, F.F. Isolation and Detection Methods of Plant miRNAs. Methods Mol. Biol. 2019, 1932, 109–120. [Google Scholar] [CrossRef]
- Bellato, M.; De Marchi, D.; Gualtieri, C.; Sauta, E.; Magni, P.; Macovei, A.; Pasotti, L. A Bioinformatics Approach to Explore MiRNAs as Tools to Bridge Pathways Between Plants and Animals. Is DNA Damage Response (DDR) a Potential Target Process? Front. Plant Sci. 2019, 10, 1535. [Google Scholar] [CrossRef]
- Kalaigar, S.S.; Rajashekar, R.B.; Nataraj, S.M.; Vishwanath, P.; Prashant, A. Bioinformatic Tools for the Identification of MiRNAs Regulating the Transcription Factors in Patients with β-Thalassemia. Bioinform. Biol. Insights 2022, 16, 11779322221115536. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, F.; Fuchs, R.T.; Robb, G.B. Small RNA Expression Profiling by High-Throughput Sequencing: Implications of Enzymatic Manipulation. J. Nucleic Acids 2012, 2012, 360358. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, M.; Cheng, Q. Capturing the Alternative Cleavage and Polyadenylation Sites of 14 NAC Genes in Populus Using a Combination of 3′-RACE and High-Throughput Sequencing. Molecules 2018, 23, 608. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; You, C.; Wang, X.; Gao, L.; Mo, B.; Liu, L.; Chen, X. Widespread Occurrence of miRNA-Mediated Target Cleavage on Membrane-Bound Polysomes. Genome Biol. 2021, 22, 15. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, P.; Chen, J.; Pennerman, K.K.; Liang, X.; Yan, H.; Zhou, S.; Feng, G.; Wang, C.; Yin, G.; et al. Combinations of Small RNA, RNA, and Degradome Sequencing Uncovers the Expression Pattern of miRNA-mRNA Pairs Adapting to Drought Stress in Leaf and Root of Dactylis glomerata L. Int. J. Mol. Sci. 2018, 19, 3114. [Google Scholar] [CrossRef]
- Carpinetti, P.A.; Fioresi, V.S.; Ignez da Cruz, T.; de Almeida, F.A.N.; Canal, D.; Ferreira, A.; Ferreira, M.F.D.S. Efficient Method for Isolation of High-Quality RNA from Psidium guajava L. tissues. PLoS ONE 2021, 16, e0255245. [Google Scholar] [CrossRef]
- Sasi, S.; Krishnan, S.; Kodackattumannil, P.; Shamisi, A.A.; Aldarmaki, M.; Lekshmi, G.; Kottackal, M.; Amiri, K.M.A. DNA-free High-Quality RNA Extraction from 39 Difficult-to-Extract Plant Species (Representing Seasonal Tissues and Tissue Types) of 32 Families, and Its Validation for Downstream Molecular Applications. Plant Methods 2023, 19, 84. [Google Scholar] [CrossRef]
- Fleige, S.; Pfaffl, M.W. RNA Integrity and the Effect on the Real-Time qRT-PCR Performance. Mol. Asp. Med. 2006, 27, 126–139. [Google Scholar] [CrossRef]
- Brown, J.L.; Gierke, T.; Butkovich, L.V.; Swift, C.L.; Singan, V.; Daum, C.; Barry, K.; Grigoriev, I.V.; O’Malley, M.A. High-Quality RNA Extraction and the Regulation of Genes Encoding Cellulosomes Are Correlated with Growth Stage in Anaerobic Fungi. Front. Fungal Biol. 2023, 4, 1171100. [Google Scholar] [CrossRef]
- Xu, W.B.; Cao, F.; Liu, P.; Yan, K.; Guo, Q.H. The multifaceted role of RNA-based regulation in plant stress memory. Front. Plant Sci. 2024, 15, 1387575. [Google Scholar] [CrossRef]
- Lv, D.K.; Bai, X.; Li, Y.; Ding, X.D.; Ge, Y.; Cai, H.; Ji, W.; Wu, N.; Zhu, Y.M. Profiling of Cold-Stress-Responsive miRNAs in Rice by Microarrays. Gene 2010, 459, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, T.; Amjid, M.W.; El-Kereamy, A.; Niu, S.-H.; Wu, H.X. MiRNA and cDNA-Microarray as Potential Targets Against Abiotic Stress Response in Plants: Advances and Prospects. Agronomy 2022, 12, 11. [Google Scholar] [CrossRef]
- Sunkar, R.; Zhu, J.-K. Novel and Stress-Regulated miRNAs and Other Small RNAs from Arabidopsis. Plant Cell 2004, 16, 2001–2019. [Google Scholar] [CrossRef]
- Zhao, B.; Liang, R.; Ge, L.; Li, W.; Xiao, H.; Lin, H.; Ruan, K.; Jin, Y. Identification of Drought-Induced miRNAs in Rice. Biochem. Biophys. Res. Commun. 2007, 354, 585–590. [Google Scholar] [CrossRef]
- Sunkar, R.; Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Small RNAs as Big Players in Plant Abiotic Stress Responses and Nutrient Deprivation. Trends Plant Sci. 2007, 12, 301–309. [Google Scholar] [CrossRef]
- Ma, X.; Tang, Z.; Qin, J.; Meng, Y. The Use of High-Throughput Sequencing Methods for Plant MicroRNA Research. RNA Biol. 2015, 12, 709–719. [Google Scholar] [CrossRef]
- Katiyar, A.; Smita, S.; Muthusamy, S.K.; Chinnusamy, V.; Pandey, D.M.; Bansal, K.C. Identification of Novel Drought-Responsive MicroRNAs and Trans-Acting siRNAs from Sorghum bicolor (L.) Moench by High-Throughput Sequencing Analysis. Front. Plant Sci. 2015, 6, 506. [Google Scholar] [CrossRef]
- Ahmed, W.; Li, R.; Xia, Y.; Bai, G.; Siddique, K.H.M.; Zhang, H.; Zheng, Y.; Yang, X.; Guo, P. Comparative Analysis of miRNA Expression Profiles Between Heat-Tolerant and Heat-Sensitive Genotypes of Flowering Chinese Cabbage Under Heat Stress Using High-Throughput Sequencing. Genes 2020, 11, 264. [Google Scholar] [CrossRef]
- Kutnjak, D.; Tamisier, L.; Adams, I.; Boonham, N.; Candresse, T.; Chiumenti, M.; De Jonghe, K.; Kreuze, J.F.; Lefebvre, M.; Silva, G.; et al. A Primer on the Analysis of High-Throughput Sequencing Data for Detection of Plant Viruses. Microorganisms 2021, 9, 841. [Google Scholar] [CrossRef]
- Diallo, I.; Provost, P. RNA-Sequencing Analyses of Small Bacterial RNAs and Their Emergence as Virulence Factors in Host-Pathogen Interactions. Int. J. Mol. Sci. 2020, 21, 1627. [Google Scholar] [CrossRef]
- Bousios, A.; Gaut, B.S.; Darzentas, N. Considerations and Complications of Mapping Small RNA High-Throughput Data to Transposable Elements. Mob. DNA 2017, 8, 3. [Google Scholar] [CrossRef]
- Stokowy, T.; Eszlinger, M.; Świerniak, M.; Fujarewicz, K.; Jarząb, B.; Paschke, R.; Krohn, K. Analysis Options for High-Throughput Sequencing in miRNA Expression Profiling. BMC Res. 2014, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Maree, H.J.; Fox, A.; Al Rwahnih, M.; Boonham, N.; Candresse, T. Application of HTS for Routine Plant Virus Diagnostics: State of the Art and Challenges. Front. Plant Sci. 2018, 9, 1082. [Google Scholar] [CrossRef]
- He, Z.; Liu, C.; Zhang, Z.; Wang, R.; Chen, Y. Integration of mRNA and miRNA Analysis Reveals the Differentially Regulatory Network in Two Different Camellia oleifera Cultivars under Drought Stress. Front. Plant Sci. 2022, 13, 1001357. [Google Scholar] [CrossRef]
- Liu, M.; Yu, H.; Zhao, G.; Huang, Q.; Lu, Y.; Ouyang, B. Profiling of Drought-Responsive MicroRNA and mRNA in Tomato Using High-Throughput Sequencing. BMC Genom. 2017, 18, 481. [Google Scholar] [CrossRef]
- Thomson, D.W.; Bracken, C.P.; Goodall, G.J. Experimental Strategies for MicroRNA Target Identification. Nucleic Acids Res. 2011, 39, 6845–6853. [Google Scholar] [CrossRef]
- Adkar-Purushothama, C.R.; Bru, P.; Perreault, J.P. 3′ RNA Ligase-Mediated Rapid Amplification of cDNA Ends for Validating Viroid Induced Cleavage at the 3′ Extremity of the Host mRNA. J. Virol. Methods 2017, 250, 29–33. [Google Scholar] [CrossRef]
- Mehdi, S.M.M.; Krishnamoorthy, S.; Szczesniak, M.W.; Ludwików, A. Identification of Novel miRNAs and Their Target Genes in the Response to Abscisic Acid in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 7153. [Google Scholar] [CrossRef]
- Chen, J.; Li, L. Multiple Regression Analysis Reveals MicroRNA Regulatory Networks in Oryza sativa under Drought Stress. Int. J. Genom. 2018, 2018, 9395261. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, X.; Shi, M.; Yu, J.; Guo, C. The miR159-MYB33-ABI5 module regulates seed germination in Arabidopsis. Physiol. Plant. 2022, 174, e13659. [Google Scholar] [CrossRef]
- Gao, S.; Yang, L.; Zeng, H.Q.; Zhou, Z.S.; Yang, Z.M.; Li, H.; Sun, D.; Xie, F.; Zhang, B. A Cotton miRNA Is Involved in Regulation of Plant Response to Salt Stress. Sci. Rep. 2016, 6, 19736. [Google Scholar] [CrossRef] [PubMed]


| Tool | Description | miRNAs Studied for Drought Tolerance | Refs. |
|---|---|---|---|
| psRNATarget | Predicts miRNA-mRNA interactions via sequence complementarity and target accessibility. Used for studying drought pathways like ABA signaling and ROS detoxification | miR159a, miR1119, miR156d-3p, miR160a-5p, miR162a-3p, miR172b-3p, miR398a-5p, Novel_8, Novel_9, Novel_105 | [106,107,108,109,110] |
| TargetFinder | Uses a position-weighted scoring algorithm to evaluate miRNA-mRNA complementarity | miR171, miR319, miR398, miR1432, miR156, miR396 | [111,112] |
| miRTarBase | A database of experimentally validated miRNA–target interactions | miRNAs involved in ABA signaling and stress response pathways | [113,114,115,116,117] |
| RNAhybrid | Predicts energetically favorable miRNA-mRNA duplexes | miR160, miR164, miR166, miR393, miR529, miR169, miR2275 | [118,119] |
| Tapir | Predicts imperfectly matched miRNA-mRNA interactions using FASTA- and RNAhybrid-based algorithms | miR172, miR164, miR160 | [120,121,122] |
| MirTarget | Analyzes miRNA binding sites in coding and untranslated regions of mRNAs | tae-miR1127b-3p, tae-miR159a,b-3p, tae-miR164-5p, tae-miR171a-3p | [123,124,125,126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhakypbek, Y.; Belkozhayev, A.M.; Kerimkulova, A.; Kossalbayev, B.D.; Murat, T.; Tursbekov, S.; Turysbekova, G.; Tursunova, A.; Tastambek, K.T.; Allakhverdiev, S.I. MicroRNAs in Plant Genetic Regulation of Drought Tolerance and Their Function in Enhancing Stress Adaptation. Plants 2025, 14, 410. https://doi.org/10.3390/plants14030410
Zhakypbek Y, Belkozhayev AM, Kerimkulova A, Kossalbayev BD, Murat T, Tursbekov S, Turysbekova G, Tursunova A, Tastambek KT, Allakhverdiev SI. MicroRNAs in Plant Genetic Regulation of Drought Tolerance and Their Function in Enhancing Stress Adaptation. Plants. 2025; 14(3):410. https://doi.org/10.3390/plants14030410
Chicago/Turabian StyleZhakypbek, Yryszhan, Ayaz M. Belkozhayev, Aygul Kerimkulova, Bekzhan D. Kossalbayev, Toktar Murat, Serik Tursbekov, Gaukhar Turysbekova, Alnura Tursunova, Kuanysh T. Tastambek, and Suleyman I. Allakhverdiev. 2025. "MicroRNAs in Plant Genetic Regulation of Drought Tolerance and Their Function in Enhancing Stress Adaptation" Plants 14, no. 3: 410. https://doi.org/10.3390/plants14030410
APA StyleZhakypbek, Y., Belkozhayev, A. M., Kerimkulova, A., Kossalbayev, B. D., Murat, T., Tursbekov, S., Turysbekova, G., Tursunova, A., Tastambek, K. T., & Allakhverdiev, S. I. (2025). MicroRNAs in Plant Genetic Regulation of Drought Tolerance and Their Function in Enhancing Stress Adaptation. Plants, 14(3), 410. https://doi.org/10.3390/plants14030410








