Postharvest Drying and Curing Affect Cannabinoid Contents and Microbial Levels in Industrial Hemp (Cannabis sativa L.)
Abstract
:1. Introduction
2. Results and Discussion
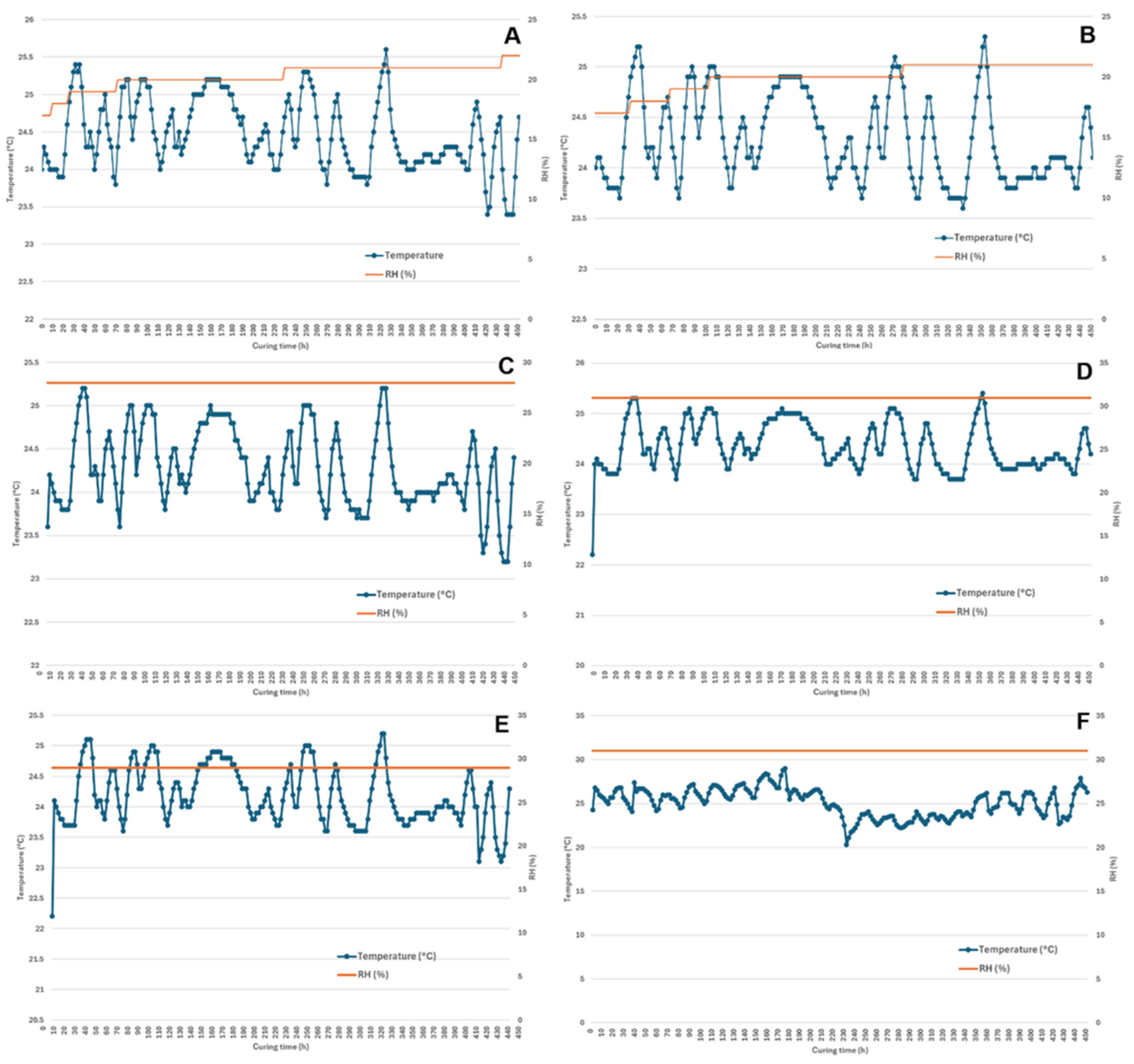
2.1. Moisture Content and Drying Characteristics
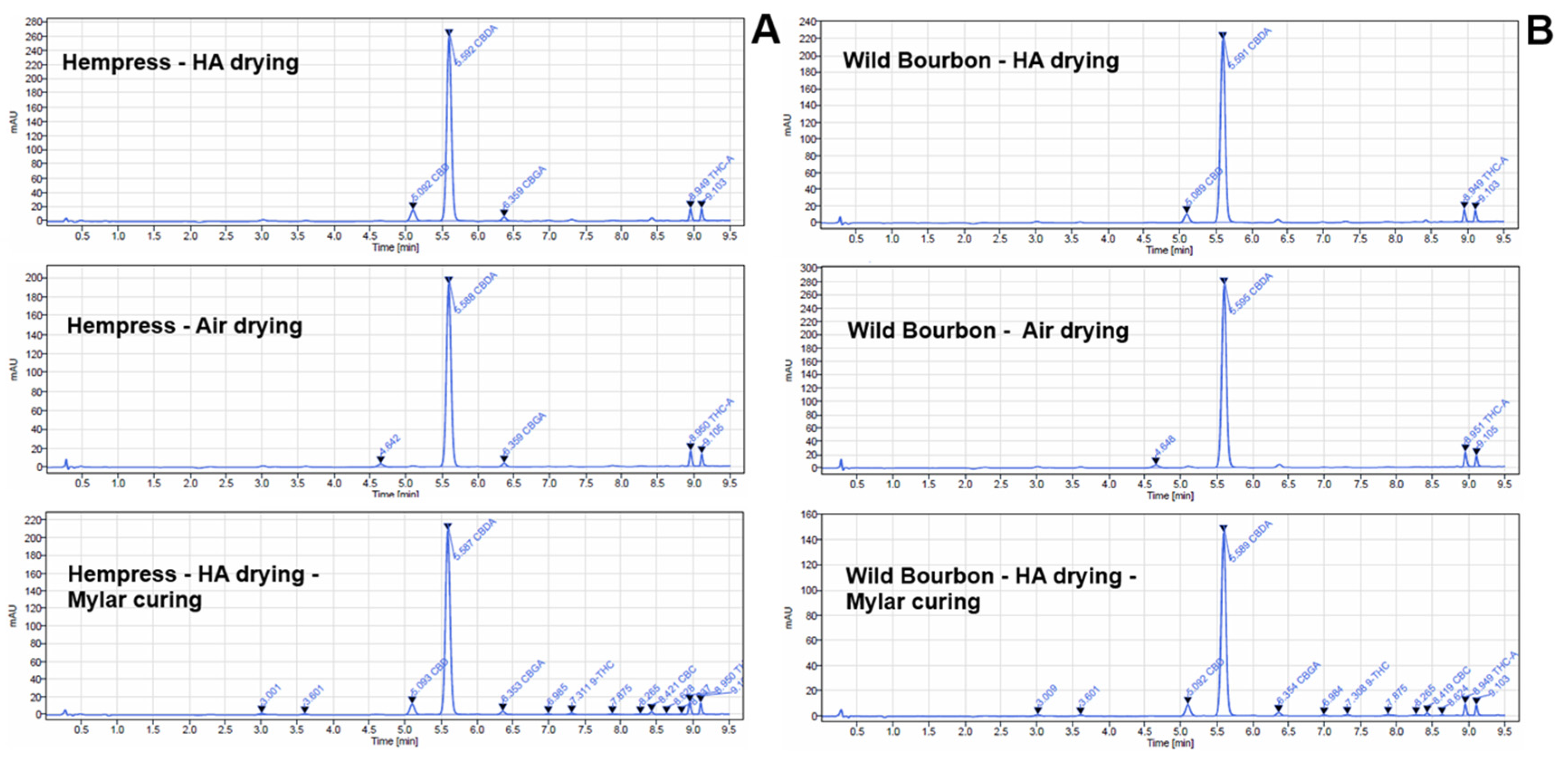
2.2. Cannabinoids Content
2.3. Color Attributes
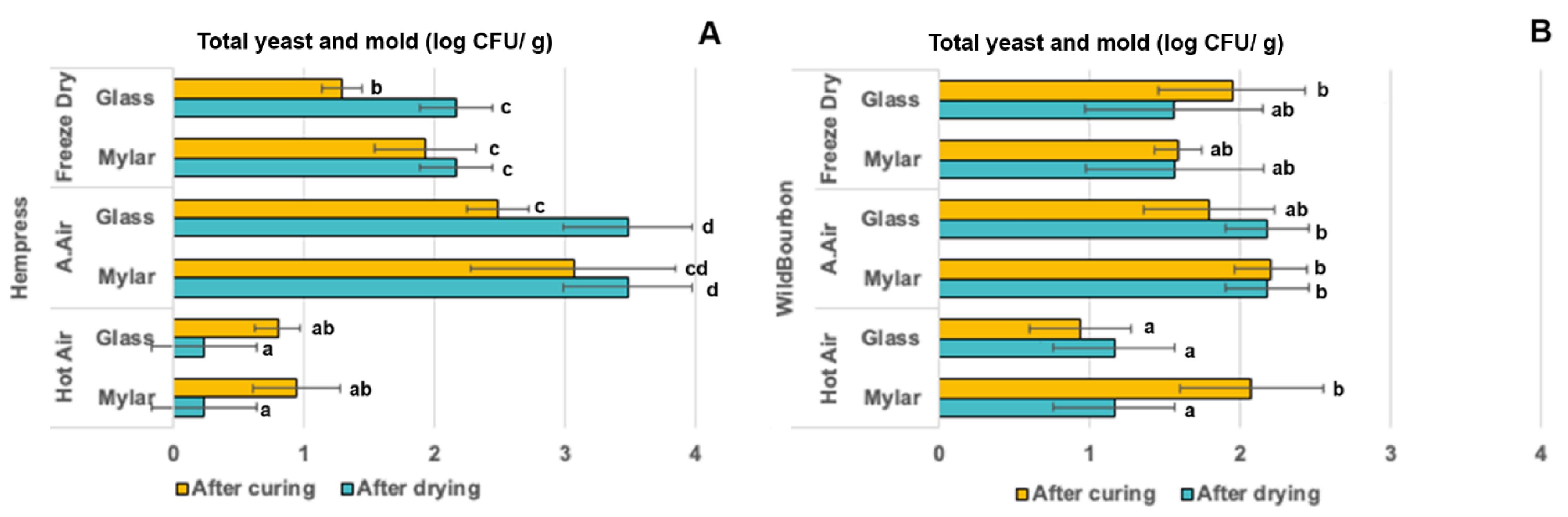
2.4. Microbial Properties
3. Materials and Methods
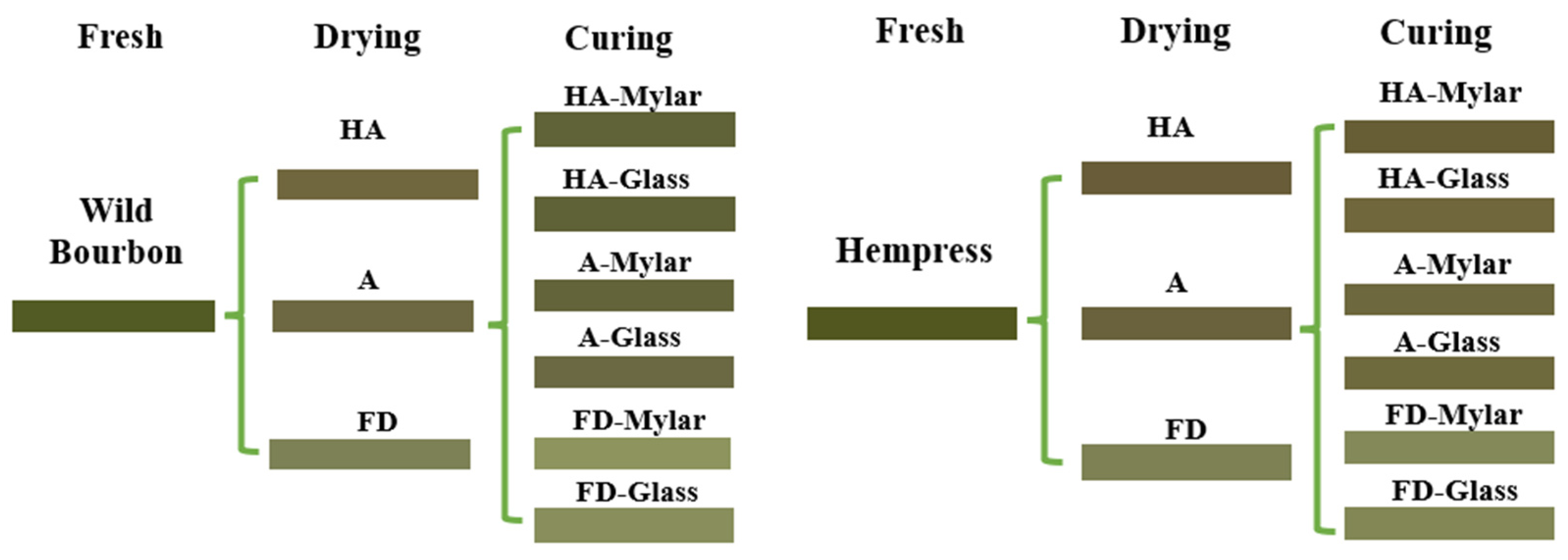
3.1. Material Preparation
3.2. Chemicals and Reagents
3.3. Postharvest Drying and Curing
3.4. Color Measurement
3.5. Cannabinoid Content Measurement
3.6. Microbial Level Measurement
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Small, E. Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human Utilization. Bot. Rev. 2015, 81, 189–294. [Google Scholar] [CrossRef]
- Chen, C.; Pan, Z. Cannabidiol and terpenes from hemp–ingredients for future foods and processing technologies. J. Future Foods 2021, 1, 113–127. [Google Scholar] [CrossRef]
- National Agricultural Statistic Service, United States Department of Agriculture. National Hemp Report. 2023. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/gf06h2430/3t947c84r/mg74s940n/hempan24.pdf (accessed on 12 April 2024).
- Das, P.C.; Vista, A.R.; Tabil, L.G.; Baik, O.D. Postharvest operations of cannabis and their effect on cannabinoid content: A review. Bioengineering 2022, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Challa, S.K.R.; Misra, N.N.; Martynenko, A. Drying of cannabis—State of the practices and future needs. Dry. Technol. 2021, 39, 2055–2064. [Google Scholar] [CrossRef]
- Bruce, D.M.; Hobson, R.N.; Hamer, P.J.C.; White, R.P. Drying of hemp for long fibre production. Biosyst. Eng. 2005, 91, 45–59. [Google Scholar] [CrossRef]
- Chen, C.; Wongso, I.; Putnam, D.; Khir, R.; Pan, Z. Effect of hot air and infrared drying on the retention of cannabidiol and terpenes in industrial hemp (Cannabis sativa L.). Ind. Crops Prod. 2021, 172, 114051. [Google Scholar] [CrossRef]
- Oduola, A.A.; Bruce, R.M.; Shafiekhani, S.; Atungulu, G.G. Impacts of industrial microwave and infrared drying approaches on hemp (Cannabis sativa L.) quality and chemical components. Food Bioprod. Process. 2023, 137, 20–27. [Google Scholar] [CrossRef]
- Chasiotis, V.; Tsakirakis, A.; Termentzi, A.; Machera, K.; Filios, A. Drying and quality characteristics of Cannabis sativa L. inflorescences under constant and time-varying convective drying temperature schemes. Therm. Sci. Eng. Prog. 2022, 28, 101076. [Google Scholar] [CrossRef]
- Chen, C.; Wang, K.; Wongso, I.; Ning, Z.; Khir, R.; Putnam, D.; Donis-González, I.R.; Pan, Z. Postharvest blanching and drying of industrial hemp (Cannabis sativa L.) with infrared and hot air heating for enhanced processing efficiency and microbial inactivation. Dry. Technol. 2023, 41, 1454–1468. [Google Scholar] [CrossRef]
- Addo, P.W.; Chauvin-Bossé, T.; Taylor, N.; MacPherson, S.; Paris, M.; Lefsrud, M. Freeze-drying Cannabis sativa L. using real-time relative humidity monitoring and mathematical modeling for the cannabis industry. Ind. Crops Prod. 2023, 199, 116754. [Google Scholar] [CrossRef]
- Caplan, D.; Matzneller, P.; Gutierrez, J.D. Harvest and post-harvest. In Handbook of Cannabis Production in Controlled Environments; CRC Press: Boca Raton, FL, USA, 2022; pp. 291–311. [Google Scholar]
- Jin, D.; Jin, S.; Chen, J. Cannabis Indoor Growing Conditions, Management Practices, and Post-Harvest Treatment: A Review. Am. J. Plant Sci. 2019, 10, 925–946. [Google Scholar] [CrossRef]
- Lazarjani, M.P.; Young, O.; Kebede, L.; Seyfoddin, A. Processing and Extraction Methods of Medicinal Cannabis: A Narrative Review. J. Cannabis Res. 2021, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Venkitasamy, C.; Zhang, W.; Khir, R.; Upadhyaya, S.; Pan, Z. Effective moisture diffusivity and drying simulation of walnuts under hot air. Int. J. Heat Mass Transf. 2020, 150, 119283. [Google Scholar] [CrossRef]
- Lumu, S. Optimization of Drying and Storage Conditions of Floral Hemp for Cannabinoids Extraction. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2023. [Google Scholar]
- Kwaśnica, A.; Pachura, N.; Masztalerz, K.; Figiel, A.; Zimmer, A.; Kupczyński, R.; Wujcikowska, K.; Carbonell-Barrachina, A.A.; Szumny, A.; Różański, H. Volatile composition and sensory properties as quality attributes of fresh and dried hemp flowers (Cannabis sativa L.). Foods 2020, 9, 1118. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, Z.; Lai, M.; Zhao, M.; Lin, B. Determination of optimum humidity for air-curing of cigar tobacco leaves during the browning period. Ind. Crops Prod. 2022, 183, 114939. [Google Scholar] [CrossRef]
- Ocieczek, A.; Pukszta, T.; Żyłka, K.; Kirieieva, N. The influence of storage conditions on the stability of selected health-promoting properties of tea. LWT 2023, 184, 115029. [Google Scholar] [CrossRef]
- Singh, A.P.; Fathordoobady, F.; Guo, Y.; Singh, A.; Kitts, D.D. Antioxidants Help Favorably Regulate the Kinetics of Lipid Peroxidation, Polyunsaturated Fatty Acids Degradation and Acidic Cannabinoids Decarboxylation in Hempseed Oil. Sci. Rep. 2020, 10, 10567. [Google Scholar]
- Kim, E.S.; Park, S.H.; Kinney, C.A.; Olejar, K.J.; Corredor-Perilla, I.C. Comparison of decarboxylation rates of acidic cannabinoids between secretory cavity contents and air-dried inflorescence extracts in Cannabis sativa cv. ‘Cherry Wine’. Sci. Rep. 2024, 14, 16411. [Google Scholar] [CrossRef]
- Moreno, T.; Dyer, P.; Tallon, S. Cannabinoid decarboxylation: A comparative kinetic study. Ind. Eng. Chem. Res. 2020, 59, 20307–20315. [Google Scholar] [CrossRef]
- Zaharia, L.S.; Trofin, I.; Vaireanu, D.I.; Dabija, G. Influence of temperature and heating time on the decarboxylation of Δ9-THCA and CBDA in the cannabis inflorescences. UPB Sci. Bull. Ser. B 2020, 82, 73–84. [Google Scholar]
- Fellows, P.J. Blanching. In Food Processing Technology, 3rd ed.; Fellows, P.J., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 369–380. [Google Scholar]
- Esfandi, A.; Mehrafarin, A.; Kalateh Jari, S.; Naghdi Badi, H.; Larijani, K. Variability in Color and Phytochemical Properties of Hemp (Cannabis sativa L.) upon Drying Techniques; An Opportunity for Industrial Products. J. Med. Plants By-Prod. 2023, 13, 79–86. [Google Scholar]
- Weemaes, C.A.; Ooms, V.; Van Loey, A.M.; Hendrickx, M.E. Kinetics of chlorophyll degradation and color loss in heated broccoli juice. J. Agric. Food Chem. 1999, 47, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Pan, L.; Liu, J.; Yang, X.; Tang, H.; Zhou, Y.; Huang, S.; Pan, G. Proanthocyanidins alleviate cadmium stress in industrial hemp (Cannabis sativa L.). Plants 2022, 11, 2364. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, A.; Aita, S.E.; Cannazza, G.; Capriotti, A.L.; Cavaliere, C.; Citti, C.; Bosco, C.D.; Gentili, A.; Montone, C.M.; Paris, R.; et al. Evaluation of the carotenoid and fat-soluble vitamin profile of industrial hemp inflorescence by liquid chromatography coupled to mass spectrometry and photodiode-array detection. J. Chromatogr. A 2023, 1692, 463838. [Google Scholar] [CrossRef]
- Punja, Z.K.; Collyer, D.; Scott, C.; Lung, S.; Holmes, J.; Sutton, D. Pathogens and molds affecting production and quality of Cannabis sativa L. Front. Plant Sci. 2019, 10, 1120. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, C.; Pan, Z.; Zheng, Z. Vacuum and Infrared-Assisted Hot Air Impingement Drying for Improving the Processing Performance and Quality of Poria cocos (Schw.) Wolf Cubes. Foods 2021, 10, 992. [Google Scholar] [CrossRef]
- Zhang, W.P.; Chen, C.; Pan, Z.; Xiao, H.W.; Xie, L.; Gao, Z.J.; Zheng, Z.A. Design and performance evaluation of a pilot-scale pulsed vacuum infrared drying (PVID) system for drying of berries. Dry. Technol. 2020, 38, 1340–1355. [Google Scholar] [CrossRef]
- Chen, C.; Espinal-Ruiz, M.; Francavilla, A.; Joye, I.J.; Corradini, M.G. Morphological changes and color development during cookie baking—Kinetic, heat, and mass transfer considerations. J. Food Sci. 2024, 89, 4331–4344. [Google Scholar] [CrossRef]
- Storm, C.; Zumwalt, M.; Macherone, A. Dedicated cannabinoid potency testing in cannabis or hemp products using the Agilent 1220 infinity II lc system. In Agilent Technologies Application Note; Agilent Technologies, Inc.: Santa Clara, CA, USA, 2020; pp. 5991–9285. [Google Scholar]
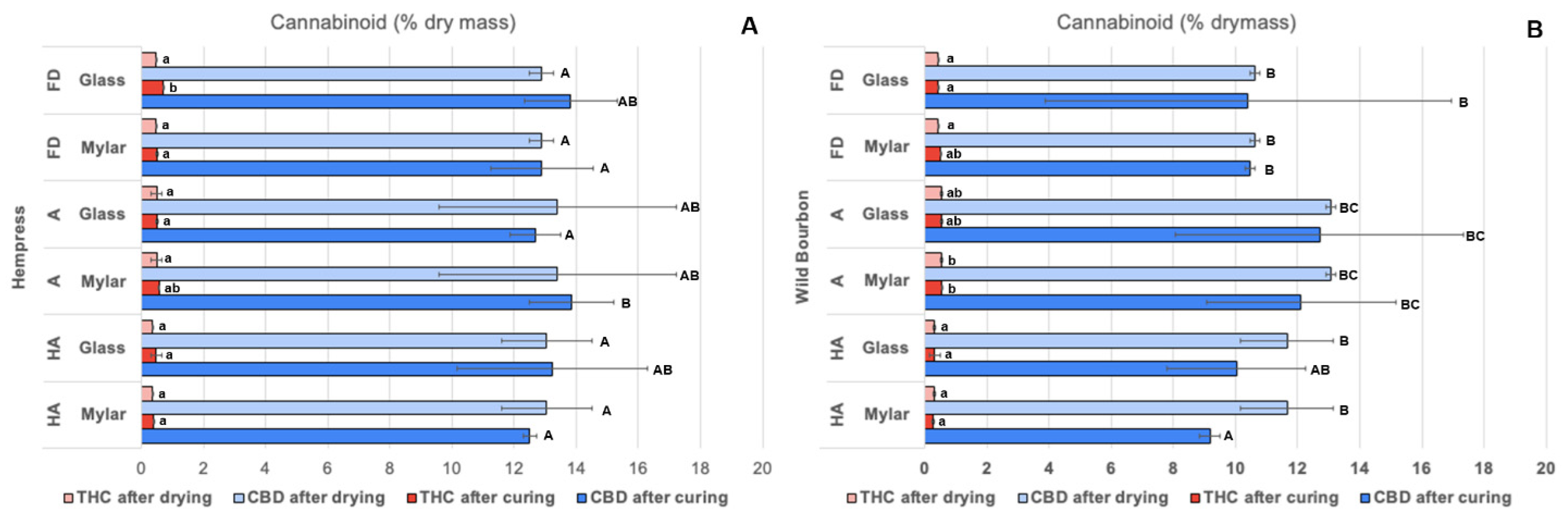

| Cultivar | Drying Method | Curing Method | Initial Moisture | After Drying | After Curing |
|---|---|---|---|---|---|
| Hempress | Hot air | Mylar bag | 75.0 ± 0.5% a | 1.9 ± 0.9% a | 10.2 ± 1.9% b * |
| Glass jar | 5.3 ± 2.8% a | ||||
| Ambient air | Mylar bag | 75.0 ± 4.0% ab | 6.8 ± 1.4% c | 11.3 ± 2.7% b | |
| Glass jar | 11.1 ± 1.9% b | ||||
| Freeze drying | Mylar bag | 74.9 ± 0.6% a | 4.0 ± 1.0% b | 7.4 ± 3.2% a | |
| Glass jar | 11.0 ± 2.4% b | ||||
| Wild Bourbon | Hot air | Mylar bag | 78.9 ± 2.1% b | 5.8 ± 3.9% bc | 12.1 ± 3.7% b |
| Glass jar | 15.8 ± 6.2% bc | ||||
| Ambient air | Mylar bag | 77.4 ± 0.7% b | 2.9 ± 3.3% a | 16.5 ± 3.2% bc | |
| Glass jar | 13.6 ± 5.4% b | ||||
| Freeze drying | Mylar bag | 80.6 ± 4.0% b | 2.8 ± 1.1% a | 6.1 ± 5.2% a | |
| Glass jar | 11.5 ± 5.2% b |
| Cultivar | Status | Acid/Neutral Cannabinoid Ratio | Decarboxylation Percentage † |
|---|---|---|---|
| Initial | >100 * | / | |
| Drying | |||
| HA | 13.7 ± 4.8 | 9.0% ± 3.0% c | |
| Ambient | >100 | <0.1% a | |
| FD | >100 | <0.1% a | |
| Curing | |||
| Hempress | HA-Mylar | 7.5 ± 0.8 | 9.2% ± 1.1% c |
| A-Mylar | 39.8 ± 3.4 | 1.6% ± 0.2% b | |
| FD-Mylar | 60.3 ± 6.7 | 1.0% ± 0.1% b | |
| HA-Glass | 7.9 ± 2.0 | 9.2% ± 2.7% c | |
| A- Glass | 41.0 ± 2.4 | 1.5% ± 0.1% b | |
| FD- Glass | 50.1 ± 9.9 | 1.3% ± 0.3% b | |
| Initial | >100 | / | |
| Drying | |||
| HA | 13.5 ± 1.1 | 8.5% ± 1.0% c | |
| Ambient | >100 | <0.1% a | |
| FD | >100 | <0.1% a | |
| Curing | |||
| Wild Bourbon | HA-Mylar | 8.4 ± 3.0 | 8.7% ± 3.0% c |
| A-Mylar | 35.9 ± 1.9 | 1.8% ± 0.1% b | |
| FD-Mylar | 48.7 ± 3.5 | 1.3% ± 0.1% b | |
| HA-Glass | 8.3 ± 1.3 | 8.3% ± 1.4% c | |
| A-Glass | 47.3 ± 13.3 | 1.7% ± 0.2% b | |
| FD-Glass | 26.3 ± 0.8 | 1.2% ± 0.6% b |
| Cultivar | Status | L* | a* | b* | ΔE | Browning Index |
|---|---|---|---|---|---|---|
| Initial | 35.55 ± 1.38 a | −10.88 ± 0.87 c | 31.37 ± 0.80 c | |||
| Drying | ||||||
| HA | 39.54 ± 2.49 b | −1.09 ± 1.44 a | 22.93 ± 1.10 a | 13.53 ± 1.55 a | 85.49 ± 4.31 c | |
| Ambient | 41.27 ± 2.25 b | −3.04 ± 0.95 a | 22.67 ± 1.23 a | 13.03 ± 1.42 a | 75.41 ± 3.83 b | |
| FD | 52.61 ± 4.29 c | −8.89 ± 1.47 c | 23.77 ± 1.72 a | 18.78 ± 2.33 b | 49.36 ± 2.16 a | |
| Curing | ||||||
| HA-Mylar | 39.71 ± 2.51 b | −2.23 ± 0.50 a | 23.79 ± 0.89 a | 12.23 ± 1.37 a | 87.15 ± 5.22 c | |
| Hempress | A-Mylar | 43.57 ± 1.06 b | −4.78 ± 0.68 b | 24.61 ± 0.82 ab | 12.13 ± 0.87 a | 75.45 ± 4.13 b |
| FD-Mylar | 55.70 ± 1.95 c | −9.71 ± 0.95 c | 24.84 ± 0.70 ab | 21.21 ± 1.05 b | 47.91 ± 3.33 a | |
| HA-Glass | 43.36 ± 1.51 b | −2.84 ± 0.50 a | 25.27 ± 0.43 b | 12.76 ± 0.88 a | 82.92 ± 5.12 bc | |
| A- Glass | 44.12 ± 0.43 b | −4.35 ± 0.35 b | 24.89 ± 0.24 ab | 12.57 ± 0.33 a | 76.26 ± 3.77 b | |
| FD- Glass | 54.91 ± 1.18 c | −10.21 ± 0.49 c | 26.23 ± 0.51 b | 20.04 ± 0.78 b | 52.35 ± 3.01 a | |
| Initial | 36.73 ± 2.44 A | −12.59 ± 1.63 D | 30.42 ± 3.43 CD | |||
| Drying | ||||||
| HA | 43.70 ± 1.92 B | −2.45 ± 1.20 A | 24.02 ± 1.03 AB | 13.87 ± 1.55 B | 75.96 ± 4.43 C | |
| Ambient | 43.48 ± 3.40 B | −3.83 ± 1.19 A | 22.50 ± 1.65 A | 13.60 ± 1.42 B | 68.04 ± 2.73 C | |
| FD | 52.65 ± 3.00 C | −8.73 ± 0.82 C | 23.05 ± 1.21 A | 17.96 ± 2.33 C | 47.28 ± 3.16 A | |
| Wild Bourbon | Curing | |||||
| HA-Mylar | 40.24 ± 0.63 B | −7.82 ± 0.51 C | 23.79 ± 0.14 AB | 8.90 ± 0.35 A | 73.41 ± 5.12 C | |
| A-Mylar | 41.39 ± 0.81 B | −6.65 ± 0.73 B | 23.45 ± 0.32 A | 10.28 ± 0.58 A | 71.34 ± 5.73 C | |
| FD-Mylar | 59.57 ± 0.95 D | −11.46 ± 0.20 D | 28.07 ± 0.18 C | 22.99 ± 0.53 D | 50.65 ± 3.27 A | |
| HA-Glass | 39.93 ± 1.17 AB | −7.93 ± 0.46 C | 23.42 ± 0.24 AB | 9.00 ± 0.75 A | 72.04 ± 4.13 C | |
| A- Glass | 43.77 ± 1.25 B | −6.09 ± 1.10 B | 21.98 ± 0.61 A | 12.77 ± 1.15 AB | 60.83 ± 4.03 B | |
| FD- Glass | 57.54 ± 1.23 D | −10.51 ± 0.66 D | 26.00 ± 0.67 B | 21.38 ± 0.80 CD | 48.18 ± 2.41 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, Y.; Grab, H.; Chen, C. Postharvest Drying and Curing Affect Cannabinoid Contents and Microbial Levels in Industrial Hemp (Cannabis sativa L.). Plants 2025, 14, 414. https://doi.org/10.3390/plants14030414
Baek Y, Grab H, Chen C. Postharvest Drying and Curing Affect Cannabinoid Contents and Microbial Levels in Industrial Hemp (Cannabis sativa L.). Plants. 2025; 14(3):414. https://doi.org/10.3390/plants14030414
Chicago/Turabian StyleBaek, Yousoon, Heather Grab, and Chang Chen. 2025. "Postharvest Drying and Curing Affect Cannabinoid Contents and Microbial Levels in Industrial Hemp (Cannabis sativa L.)" Plants 14, no. 3: 414. https://doi.org/10.3390/plants14030414
APA StyleBaek, Y., Grab, H., & Chen, C. (2025). Postharvest Drying and Curing Affect Cannabinoid Contents and Microbial Levels in Industrial Hemp (Cannabis sativa L.). Plants, 14(3), 414. https://doi.org/10.3390/plants14030414





