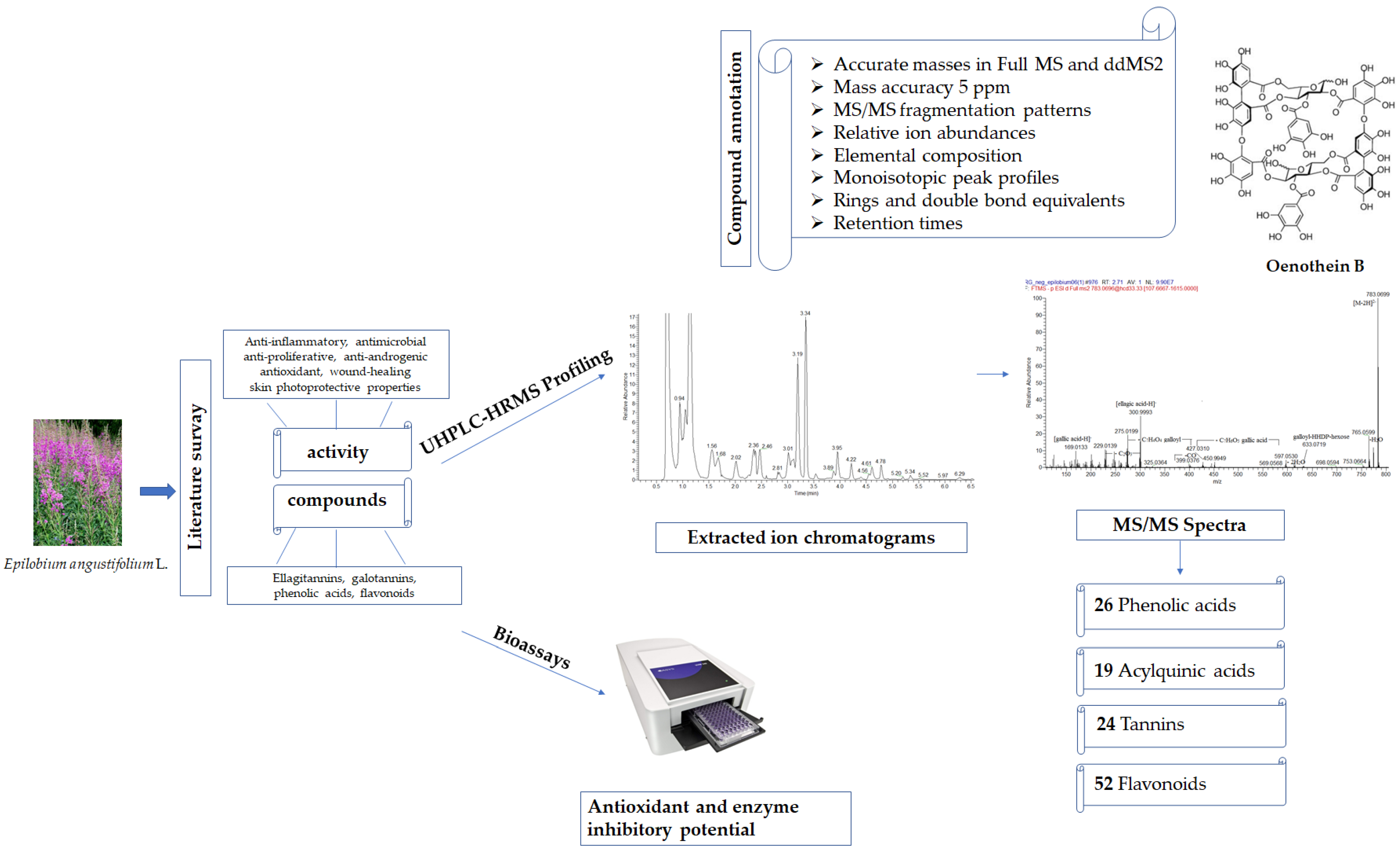
Exploring the Phytochemical Profile and Biological Insights of Epilobium angustifolium L. Herb
Abstract
:1. Introduction
2. Results and Discussion
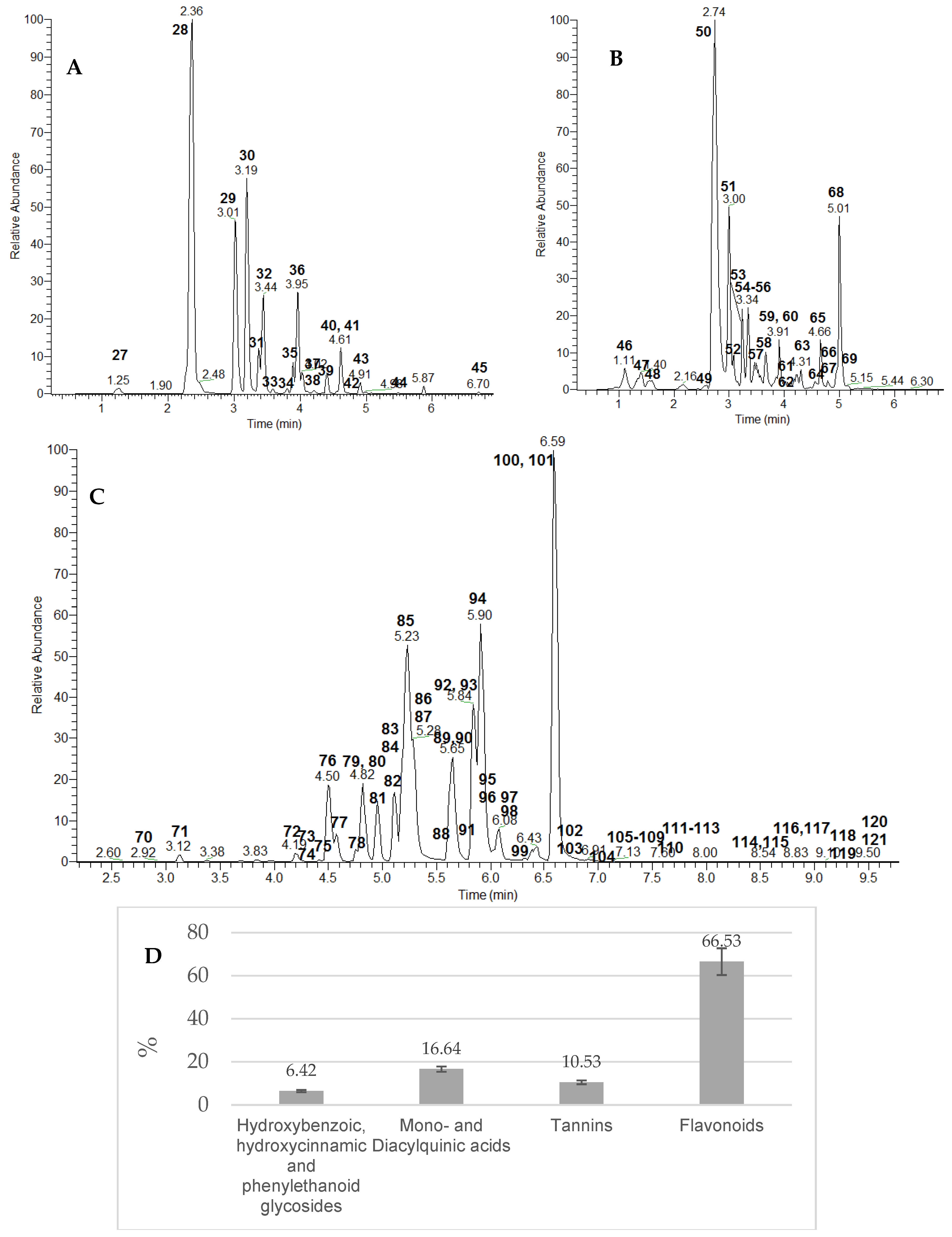
2.1. UHPLC-HRMS Profiling of Secondary Metabolites in E. angustifolium Extract
2.1.1. Phenolic Acids, Their Glycosides, and Coumarins
| No. | Identified/Tentatively Annotated Compound | Molecular Formula | Exact Mass [M-H]− | Fragmentation Pattern in (-) ESI-MS/MS | tR (min) | Δ ppm | References |
|---|---|---|---|---|---|---|---|
| Hydroxybenzoic, Hydroxycinnamic Acids, Phenylethanoid Glycosides, and Coumarins | |||||||
| 1. | galloyl O-hexose | C13H16O10 | 331.0671 | 331.0676 (100), 271.0463 (10.2), 241.0349 (3.1), 211.0244 (10.4), 169.0133 (45.8), 151.0024 (13.4), 125.0230 (17.7), 107.0124 (4.7) | 0.94 | 1.511 | [16,17] |
| 2. | gallic acid a | C7H6O5 | 169.0142 | 169.0132 (33.9), 125.0230 (100), 107.0123 | 1.14 | 6.133 | [10,16,17,22,23] |
| 3. | gallic acid O-hexoside 1 | C13H16O10 | 331.0687 | 331.0676 (100), 271.0465 (0.7), 241.0353 (1.9), 211.0246 (0.6), 169.0131 (16.7), 168.0054 (31.9), 125.0229 (36.7), 107.0123 (2.1) | 1.17 | 1.511 | |
| 4. | hydroxybenzoic acid-O-hexoside | C13H16O8 | 299.0778 | 299.0765 (0.5), 137.0231 (100), 93.0330 (65.2), | 1.26 | −2.543 | |
| 5. | gallic acid O-hexoside 2 | C13H16O10 | 331.0687 | 331.0674 (8.1), 169.0132 (16.7), 125.0236 (38.3) | 1.56 | 1.118 | |
| 6. | protocatechuic acid-O-hexoside | C13H16O9 | 315.0727 | 315.0725 (100), 153.0181 (27.1), 152.0103 (56.8), 123.0072 (2.4), 109.0285 (9.9), 108.0202 (91.8), 81.0332 (0.6) | 1.68 | 1.221 | |
| 7. | vanillic acid-O-hexoside | C14H18O9 | 329.0875 | 329.0903 (2.3),167.0339 (100), 152.0102 (24.0), 123.0437 (14.5), 108.0202 (39.9) | 1.77 | 7.581 | |
| 8. | protocatechuic acid a | C7H6O4 | 153.0181 | 153.0182 (15.4), 109.0280 (100), 91.0174 (1.2), 81.0331 (1.2) | 2.02 | −1.392 | [14,23] |
| 9. | p-hydroxyphenylacetic acid O-hexoside | C14H18O8 | 313.0932 | 313.0915 (1.6), 151.0388 (98.2), 123.0436(1.0), 107.0487 (100) | 2.13 | 0.988 | |
| 10. | hydroxybenzoyl hexose | C13H16O8 | 299.0778 | 299.0774 (26.4), 239.0559 (25.4), 209.0447 (7.4), 179.0339 (28.6), 137.0230 (100), 109.0280 (23.3), 93.0329 (12.4) | 2.16 | 0.600 | |
| 11. | syringic acid-O-hexoside | C15H20O10 | 359.0985 | 359.0991 (7.6), 197.0448 (100), 182.0212 (22.1), 166.9976 (8.3), 153.0545 (16.6), 138.0309 (28.5), 123.0073 (32.9) | 2.26 | 2.311 | |
| 12. | caffeic acid-O-hexoside 1 | C15H18O9 | 341.0871 | 341.0870(4.2), 179.0340 (100), 135.0438 (55.5), 107.0485 (0.3) | 2.40 | −2.332 | [16] |
| 13. | caffeic acid-O-hexoside 2 | C15H18O9 | 341.0871 | 341.0865 (25.7), 281.0658 (2.3), 251.0563 (5.7), 221.0454 (1.9), 179.0341 (26.5), 161.0233 (100), 135.0439 (9.3), 133.0282 (26.4) | 2.61 | −3.827 | |
| 14. | caffeoyl-O-hexose | C15H18O9 | 341.0871 | 341.0859 (19.5), 281.0668 (93.4), 251.0561 (50.7), 221.0452 (47.1), 179.0341 (100), 161.0233 (6.2), 135.0438 (69.4), 133.0281 (20.4) | 2.82 | −5.469 | |
| 15. | 4-hydroxybenzoic acid a | C7H6O3 | 137.0230 | 137.0230 (100), 119.0123 (2.5), 108.0200 (9.8), 93.0329 (13.4), 65.0380 (1.1) | 2.84 | −10.052 | [17] |
| 16. | gentisic acid O-hexoside | C13H16O9 | 315.0727 | 315.0725 (7.9), 153.0181 (100), 125.0230 (5.1), 109.0279 (2.6), 81.0329 (0.4) | 2.84 | 1.221 | |
| 17. | p-coumaric acid a | C9H8O3 | 163.0389 | 163.0390 (6.2), 119.0488 (100), 93.0331 (3.6) | 3.01 | −6.792 | [23] |
| 18. | caffeic acid O-hexoside 3 | C15H18O9 | 341.0871 | 341.0877 (27.3), 281.0663 (0.9), 251.0562 (2.2), 221.0456 (0.9), 179.0340 (100), 135.0438 (71.6), 107.0488 (0.5) | 3.07 | −0.602 | |
| 19. | methylgallate | C8H8O5 | 183.0299 | 183.0289 (100), 168.0053 (11.9), 140.0103 (11.4), 111.0074 (6.2), 83.0122 (0.7) | 3.15 | 0.711 | [14] |
| 20. | quinic acid | C7H12O6 | 191.0549 | 191.0552 (100), 173.0447 (1. 8), 155.0336 (0.2), 127.0387 (4.1), 111.0435 (1.1), 93.0330 (6.6), 85.0278 (18.4) | 3.19 | −5.817 | [16] |
| 21. | umbeliferone | C9H6O3 | 161.0244 | 161.0233 (81.1), 133.0281 (100), 115.0173 (0.7), 105.0331 (1.5), 89.0381 (0.8), 77.0381 (0.8) | 3.19 | −7.250 | |
| 22. | coumaric acid-O-hexoside | C15H18O8 | 325.0930 | 325.0922 (2.3), 163.0389 (100), 145.0284 (0.3), 119.0488 (90.0), 93.0331 (0.3) | 3.34 | −3.355 | |
| 23. | caffeic acid a | C9H8O4 | 179.0339 | 179.0341 (17.8), 135.0438 (100), 107.0488 (1.2) | 3.54 | −6.044 | [23] |
| 24. | o-coumaric acid a | C9H8O3 | 163.0389 | 163.0388 (8.3), 119.0487 (100), 93.0330 (1.3) | 4.55 | −7.467 | [23] |
| 25. | galloyl-(caffeoyl)-hexose | C22H22O13 | 493.0988 | 493.1006 (100), 341.0881 (5.9), 281.0667 (32.9), 251.0560 (24.8), 221.0449 (13.9), 161.0233 (26.0), 135.0438 (31.4), 169.0132 (14.7), 125.0230 (10.4), 107.0123 (1.7) | 4.78 | 3.643 | |
| 26. | salicylic acid a | C7H6O3 | 137.0230 | 137.0230 (8.3), 108.0202 (7.5), 93.0330 (100), 65.0380 (0.7) | 6.29 | −10.125 | |
| Mono- and Diacylquinic Acids | |||||||
| 27. | 3-galloylquinic acid | C14H16O10 | 343.0671 | 343.0675 (38.9), 191.0554 (13.6), 173.0446 (22.2), 169.0132 (100), 125.0230 (32.6), 107.0124 (6.5), 93.0331 (7.8), 85.0279 (4.6) | 1.25 | 1.370 | |
| 28. | neochlorogenic (3-caffeoylquinic) acid a | C16H18O9 | 353.0867 | 353.0883 (39.5), 191.0552 (100), 179.0340 (62.7), 173.0446 (2.7), 161.0230 (4.1), 135.0438 (50.8), 111.0434 (1.6), 93.0331 (3.9), 85.0279 (8.2) | 2.36 | 1.458 | [5,10,23] |
| 29. | 3-p-coumaroylquinic acid | C16H18O8 | 337.0928 | 337.0934 (7.6), 191.0553 (14.4), 163.0390 (100), 135.0437 (0.6), 119.0488 (31.3), 111.0432(1.5), 93.0329 (2.6), 85.0278 (3.0) | 3.01 | 1.096 | [10,16] |
| 30. | chlorogenic (5-caffeoylquinic) acid a | C16H18O9 | 353.0874 | 353.0884 (4.2), 191.0553 (100), 173.0447 (1.1), 161.0235 (1.2), 111.0434 (1.5), 93.0331 (2.8), 85.0278 (2.0) | 3.19 | 0.665 | [10,16,17,23] |
| 31. | 4-caffeoylquinic acid | C16H18O9 | 353.0878 | 353.0882 (33.3), 191.0553 (43.1), 179.0340 (68.6), 173.0445 (100), 135.0438 (57.5), 111.0434 (3.6), 93.0331 (21.0), 85.0279 (7.8) | 3.37 | −0.100 | |
| 32. | 3-feruloylquinic acid | C17H20O9 | 367.1034 | 367.1039 (18.2), 193.0497 (100), 191.0553 (2.6), 173.0445 (4.0), 149.0594 (3.4), 134.0359 (54.0), 111.0435 (1.5), 93.0330 (2.1), 85.0276 (0.4) | 3.44 | 1.157 | [10,16,17] |
| 33. | 1-galloyl-3-caffeoylquinic acid | C23H22O13 | 505.09 | 505.0999 (64.0), 353.0883 (38.8), 191.0553 (100), 179.0341 (52.5), 173.0443 (3.9), 161.0233 (4.2), 135.0438 (52.8), 111.0436 (2.0), 93.0330 (3.8), 85.0280 (8.0) | 3.58 | 2.348 | |
| 34. | 4-p-coumaroylquinic acid | C16H18O8 | 337.0928 | 337.0937 (10.2), 191.0551 (10.8), 173.0444 (100), 163.0390 (19.3), 119.0488 (14.8), 111.0430 (2.3), 93.0330 (18.7), 85.0279 (3.0) | 3.79 | ||
| 35. | 5-caffeoylquinic acid isomer | C16H18O9 | 353.0874 | 353.0884 (6.4), 191.0550 (100), 179.0341 (0.9), 173.0446 (0.9), 161.0232 (1.9), 111.0436 (1.1), 93.0329 (2.5), 85.0279 (8.4) | 3.88 | 1.684 | [16] |
| 36. | 5-p-coumaroylquinic acid | C16H18O8 | 337.0928 | 337.0934 (8.1), 191.0552 (100), 173.0444 (7.1), 163.0389 (6.4), 145.0283 (1.2), 119.0487 (5.3), 93.0330 (19.0), 85.0278 (4.9) | 3.95 | 1.629 | [10] |
| 37. | 1-caffeoyl-3-galloylquinic acid | C23H22O13 | 505.0988 | 505.0999 (100), 353.0883 (5.7), 343.0675 (15.2), 191.0553 (26.5), 179.0342 (18.2), 173.0445 (18.2), 169.0123 (73.3), 161.0230 (6.4), 135.0440 (14.8), 125.0230 (33.1), 107.0124 (4.6), 93.0331 (8.5) | 4.10 | 1.913 | |
| 38. | 1-galloyl-5-caffeoylquinic acid | C23H22O13 | 505.0988 | 505.0998 (35.5), 353.0880 (18.2), 191.0551 (100), 179.0341 (3.1), 173.0447 (1.3), 161.0233 (2.4), 135.0437 (3.0), 111.0434 (0.9), 93.0331 (2.4), 85.0279 (6.9) | 4.19 | 2.032 | |
| 39. | 5-feruloylquinic acid | C17H20O9 | 367.1034 | 367.1040 (20.6), 193.0500 (11.3), 191.0553 (100), 173.0445 (49.6), 149.0598 (0.5), 134.0360 (15.5), 111.0437 (4.1), 93.0330 (29.2), 85.0279 (4.5) | 4.39 | 1.402 | [10] |
| 40. | 3-caffeoyl-5-galloylquinic acid | C23H22O13 | 505.0988 | 505.0996 (54.9), 353.0881 (71.0), 191.0552 (100), 179.0340 (56.8), 173.0447 (4.2), 161.0233 (4.7), 135.0438 (54.2), 111.0434 (0.7), 93.0329 (4.1), 85.0279 (8.4) | 4.56 | 1.735 | |
| 41. | 5-p-coumaroylquinic acid isomer | C16H18O8 | 337.0928 | 337.0935 (6.8), 191.0551 (100), 173.0443 (1.8), 163.0388 (1.8), 145.0279 (0.7), 119.0489 (1.1), 111.0435 (0.9), 93.0329 (4.8), 85.0279 (6.8) | 4.61 | 1.926 | |
| 42. | caffeoyl-hydroxybenzoylquinic acid | C23H24O12 | 491.1196 | 491.1203 (94.4), 447.1305 (16.0), 329.0882 (0.9), 179.0340 (8.3), 161.0232 (100), 137.0235135.0438 (12.0), 133.0281 (40.6), 109.0281 (2.3), 85.0277 (1.5) | 4.89 | 1.691 | |
| 43. | 5-feruloylquinic acid isomer | C17H20O9 | 367.1034 | 367.1038 (9.5), 193.0498 (1.6), 191.0552 (100), 179.0341 (1.6), 173.0444 (2.3), 161.0229 (0.6), 134.0360 (2.8), 111.0434 (1.3), 93.0330 (6.3), 85.0279 (8.0) | 4.91 | 0.939 | |
| 44. | 3-galloyl-5-p-coumaroylquinic acid | C23H22O12 | 489.1038 | 489.1046 (44.3), 337.0936 (16.9), 191.0553 (2.9), 179.0704 (2.9), 173.0443 (1.5), 163.0397 (4.3), 146.0239 (2.7), 119.0488 (1.2), 111.0439 (0.9), 93.0327 (4.9), 85.0279 (6.8) | 5.48 | 1.555 | |
| 45. | 3-feruloyl-5-galloylquinic acid | C26H32O11 | 519.1872 | 519.1877 (100), 193.0498 (37.0), 178.0262 (16.0), 161.0235 (5.1) | 6.70 | 0.954 | |
| Tannins | |||||||
| 46. | galloyl-HHDP-hexose 1 | C27H22O18 | 633.0733 | 633.0746 (100), 300.9993 (73.0), 275.0201 (31.2), 249.0403 (22.4), 257.0088 (4.3), 245.0095 (2.5), 229.0141 (7.9), 185.0236 (4.6), 169.0132 (7.0), 145.0282 (1.9), 125.0231 (9.2), 107.0123 (2.5) | 1.11 | 2.074 | [10] |
| 47. | galloyl-HHDP-hexose 2 | C27H22O18 | 633.0733 | 633.0749 (100), 300.9994 (67.9), 275.0201 (36.5), 249.0405 (27.2), 257.0092 (4.8), 245.0085 (1.9), 229.0139 (8.7), 185.0235 (2.6), 169.0133 (8.5), 145.0282 (0.9), 125.0229 (9.5), 107.0124 (1.4) | 1.40 | 2.264 | |
| 48. | digalloyl-hexose 1 | C20H20O14 | 483.0780 | 483.0786 (100), 331.0677 (16.8), 313.0569 (10.1), 211.0240 (2.9), 169.0131 (81.5), 151.0021 (2.4), 125.0230 (56.8), 107.0124 (7.7) | 1.60 | 1.266 | [23] |
| 49. | digalloyl-hexose 2 | C20H20O14 | 483.0780 | 483.0786 (100), 331.0670 (9.0), 313.0570 (22.0), 271.0458 (7.4), 211.0243 (9.5), 169.0132 (49.9), 151.0026 (3.0), 125.0230 (30.8), 107.0125 (4.3) | 2.60 | 1.204 | |
| 50. | oenothein B 1 | C68H46O44 | 1568.1518 783.0686 [M-2H]2−, | 783.0699 (100), 765.0590 (17.3), 633.0719 (1.0), 597.0530 (4.3), 427.0310 (3.2), 399.0376 (1.9), 300.9992 (27.7), 275.0199 (20.0), 273.0337 (0.4), 249.0405 (4.3), 245.0090 (4.6), 229.0139 (10.3), 217.0137 (3.7), 201.0186 (7.6), 169.0123 (9.5), 145.0282 (4.2), 117.0332 (1.6) | 2.74 | 1.743 | [5,10,16,17] |
| 51. | digalloyl-HHDP-hexose (tellimagrandin I) 1 | C34H26O22 | 785.0843 | 785.0864 (84.5), 300.9993 (100), 275.0200 (45.2), 257.0092 (6.6), 249.0409 (34.2), 245.0078 (4.1), 229.0141 (8.8), 201.0185 (5.5), 185.0232 (5.3), 173.0235 (3.6), 169.0133 (15.4), 145.0286 (3.4), 125.0231 (15.2), 107.0124 (3.2) | 3.01 | 2.630 | [10] |
| 52. | digalloyl-hexose 3 | C20H20O14 | 483.0780 | 483.0788 (100), 331.0680 (5.7), 313.0571 (17.7), 271.0462 (44.6), 211.0242 (13.1), 169.0132 (39.2), 151.0027 (1.9), 125.0231 (29.0), 107.0124 (4.3) | 3.09 | 1.659 | |
| 53. | galloyl-HHDP- hexose 3 | C27H22O18 | 633.0733 | 633.0747 (77.9), 463.0529 (3.4), 300.9992 (100), 275.0202 (11.1), 249.0406 (4.0), 257.0093 (4.7), 245.0095 (1.7), 229.0139 (7.6), 185.0232 (3.4), 169.0133 (2.3), 145.0285 (2.1), 125.0233 (2.9), 107.0121 (0.4) | 3.24 | 2.169 | |
| 54. | oenothein B 2 | C68H46O44 | 1568.1518 783.0686 [M-2H]2−, | 783.0699 (100), 765.0596 (17.3), 633.0742 (1.6), 597.0522 (3.7), 427.0315 (4.5), 399.0354 (1.7), 300.9992 (27.7), 275.0198 (19.7), 273.0337 (0.4), 249.0402 (4.3), 245.0090 (4.6), 229.0139 (10.3), 217.0137 (3.7), 201.0186 (7.7), 185.0235 (2.4), 169.0133 (9.2), 125.0230 (8.2) | 3.33 | 1.590 | [10] |
| 55. | brevifolin carboxylic acid | C13H8O8 | 291.0149 | 291.0151 (13.2), 247.0246 (100), 229.0170 (0.2), 219.0296 (3.1), 203.0341 (0.7), 191.0342 (9.3), 173.0234 (3.7), 145.0282 (3.4), 119.0487 (1.6) | 3.34 | 1.511 | |
| 56. | digalloyl-hexose 4 | C20H20O14 | 483.0780 | 483.0787 (100), 331.0675 (59.0), 313.0568 (10.8), 271.0462 (1.3), 211.0247 (1.6), 169.0133 (13.8), 125.0230 (45.6), 107.0125 (2.8) | 3.35 | 1.390 | |
| 57. | oenothein A1 | C102H70O66 | 2352.2277 1175.6083 [M-2H]2−, | 1175.6090 (100) [M-2H]2−, 785.0854 (4.9), 765.0599 (9.2), 633.0746 (2.9), 597.0533 (3.1), 463.0533 (0.6), 427.0313 (4.0), 399.0364 (2.3), 300.9991 (35.7), 273.0043 (10.9), 257.0092 (4.6), 245.0090 (5.5), 229.0140 (9.9), 217.0138 (3.7), 201.0186 (7.3), 185.0235 (2.5), 173.0235 (4.4) | 3.44 | 2.250 | [5,15] |
| 58. | digalloyl-HHDP-hexose (tellimagrandin I) 2 | C34H26O22 | 785.0843 | 785.0865 (100), 300.9993 (96.9), 275.0199 (38.7), 257.0089 (5.8), 249.0403 (32.8), 245.0088 (3.2), 229.0135 (10.0), 201.0186 (5.7), 185.0236 (2.8), 173.0235 (3.6), 169.0135 (15.4), 145.0282 (3.4), 125.0230 (15.8), 107.0124 (3.2) | 3.66 | 1.776 | [10] |
| 59. | oenothein A2 | C102H70O66 | 2352.2277 1175.6083 [M-2H]2−, | 1175.6084 (100), 785.0846 (3.9), 765.0589 (8.0), 633.0752 (3.3), 597.0527 (3.6), 463.0486 (0.7), 427.0307 (3.5), 399.0361 (2.0), 300.9990 (45.0), 273.0043 (9.6), 257.0092 (4.1), 245.0090 (5.6), 217.0135 (2.8), 201.0186 (7.4), 169.0133 (2.1) | 3.89 | 1.731 | |
| 60. | trigalloyl-hexose 1 | C27H24O18 | 635.0890 | 635.0904 (81.4), 465.0680 (100), 313.0569 (50.4), 253.0372 (0.8), 223.0259 (2.9), 193.0137 (5.5), 169.0132 (80.4), 151.0022 (3.7), 125.0230 (57.1), 107.0123 (12.0) | 3.92 | 2.209 | [10,23] |
| 61. | trigalloyl-hexose 2 | C27H24O18 | 635.0890 | 635.0905 (100), 483.0791 (11.1), 465.0680 (25.5), 313.0568 (27.7), 223.0236 (0.7), 193.0131 (4.9), 169.0131 (84.1), 151.0023 (1.8), 125.0229 (64.1), 107.0122 (7.1) | 4.01 | 2.304 | |
| 62. | trigalloyl-hexose 3 | C27H24O18 | 635.0890 | 635.0902 (100), 483.0786 (21.5), 465.0683 (11.3), 313.0568 (12.6), 253.0346 (0.4), 193.0134 (3.4), 169.0131 (49.2), 151.0024 (2.1), 125.0229 (49.7), 107.0123 (5.4) | 4.11 | 1.926 | |
| 63. | trigalloyl-hexose 4 | C27H24O18 | 635.0890 | 635.0905 (100), 483.0799 (4.8), 465.0681 (15.9), 313.0572 (26.8), 253.0363 (0.7), 193.0139 (1.9), 169.0132 (70.2), 151.0027 (1.8), 125.0230 (57.1), 107.0124 (6.7) | 4.31 | 2.304 | |
| 64. | tellimagradin II 1 | C41H30O26 | 937.0953 | 937.0970 (100), 785.0782 (0.3), 300.9991 (85.3), 275.0199 (18.1), 257.0094 (4.0), 249.0204 (12.32), 245.0090 (3.6), 229.0140 (7.1), 201.0188 (3.9), 169.0132 (13.8), 125.0230 (9.2), 107.0123 (1.9) | 4.57 | 1.796 | |
| 65. | ellagic acid O-pentoside | C19H14O12 | 433.0412 | 433.0416 (100), 300.9990 (69.1), 271.9979 (2.5), 257.0092 (1.4), 245.0090 (0.6), 229.0139 (2.2), 201.0190 (1.5), 185.0237 (1.4), 145.0277 (0.4), | 4.66 | 0.811 | |
| 66. | tellimagradin II 2 | C41H30O26 | 937.0953 | 937.0970 (100), 785.0897 (3.8), 300.9992 (85.0), 275.0200 (19.2), 257.0091 (4.3), 249.0403 (11.3), 245.0087 (3.6), 229.0141 (6.3), 217.0139 (1.6), 201.0186 (3.8), 169.0131 (14.7), 125.0230 (10.4), 107.0124 (1.4) | 4.74 | 1.726 | |
| 67. | tetragalloyl-hexose 1 | C34H28O22 | 787.0999 | 787.1011 (100). 617.0776 (6.3), 465.0681 (32.2), 313.0572 (13.5), 295.0458 (7.8), 193.0135 (4.0), 169.0131 (73.7), 151.0025 (1.8), 125.0230 (70.0), 107.0122 (8.9) | 4.94 | 1.518 | [23] |
| 68. | ellagic acida | C14H6O8 | 300.9991 | 300.9990 (100), 257.0086 (1.6), 229.0143 (3.2), 217.0139 (0.9), 201.0182 (3.2), 185.0235 (2.3), 173.0235 (2.9), 145.0281 (3.1), 129.0332 (0.9), 117.0331 (1.0) | 5.01 | −0.101 | [10] |
| 69. | tetragalloyl-hexose 2 | C34H28O22 | 787.0999 | 787.1019 (81.1). 617.0794 (57.9), 465.0681 (24.1), 313.0571 (10.1), 295.0459 (9.5), 193.0135 (4.2), 169.0123 (100), 151.0023 (3.0), 125.0230 (84.3), 107.0124 (10.4) | 5.05 | 2.521 | |
| Flavonoids | |||||||
| 70. | procyanidin dimer | C30H26O12 | 577.1351 | 577.1367 (100), 425.0887 (80.2), 407.0778 (66.3), 289.0721 (68.6), 245.0816 (4.7), 203.0710 (6.1), 179.0341 (6.0), 137.0232 (17.0), 125.0231 (89.8) | 2.92 | 2.617 | |
| 71. | catechin/epicatechin | C15H14O6 | 289.0718 | 289.0719 (100), 245.0816 (41.3), 203.0707 (15.9), 179.0341 (10.1), 137.0231 (11.4), 109.0280 (34.0) | 3.12 | 0.583 | |
| 72. | myricetin 3-O-galloylhexoside | C28H24O17 | 631.0941 | 631.0953 (100), 479.0839 (27.6), 317.0300 (15.9), 316.0226 (51.7), 299.0195 (3.1), 287.0197 (12.4), 271.0247 (20.5), 259.0253 (4.4), 243.0295 (2.3), 178.9972 (4.0), 169.0130 (4.5), 151.0022 (5.6), 125.0229 (5.7), 107.0125 (3.7) | 4.19 | 1.914 | [10,15,23] |
| 73. | kaempferol 7-O-deoxyhexosylhexoside 1 | C27H30O15 | 593.1512 | 593.1525 (95.1), 447.0926 (8.6), 431.0983 (5.7), 285.0406 (30.5), 255.0298 (30.5), 227.0342 (4.0), 211.0396 (2.9), 151.0025 (1.3), 107.0120 (0.5) | 4.27 | 2.254 | |
| 74. | patuletin 3-O-dihexoside | C28H32O18 | 655.1516 | 655.1529 (100), 331.0439 (14.0), 330.0386 (73.2), 315.0151 (44.7), 287.0201 (16.2), 259.0246 (2.1), 243.0289 (3.9), 231.0300 (4.9), 215.0349 (5.6), 187.0393 (2.8), 165.9897 (4.3) | 4.37 | 1.989 | |
| 75. | myricetin 3-O-hexoside1 | C21H20O13 | 479.0831 | 479.0830 (100), 316.0226 (93.8), 317.0292 (20.8), 287.0199 (17.6), 271.0249 (26.7), 259.0247 (6.4), 243.0292 (3.6), 227.0351 (1.0), 178.9974 (3.4), 151.0025 (4.8), 107.0124 (1.6) | 4.48 | −0.217 | [5,10,15,16,23] |
| 76. | myricetin O-hexuronide | C21H18O14 | 493.0624 | 493.0634 (85.1), 317.0305 (100), 299.0199 (2.7), 271.0248 (3.9), 243.0297 (1.9), 227.0344 (1.3), 199.0391 (1.0), 178.9977 (15.6), 151.0025 (25.1), 137.0231 (16.8), 107.0124 (8.7) | 4.51 | 1.423 | [5,16] |
| 77. | myricetin 3-O-hexoside 2 | C21H20O13 | 479.0831 | 479.0836 (100), 317.0288 (17.9), 316.0226 (90.5), 287.0200 (17.1), 271.0248 (27.4), 259.0244 (7.0), 243.0291 (4.0), 227.0342 (0.9), 178.9975 (3.4), 151.0023 (3.9), 107.0121 (1.8) | 4.57 | 0.994 | [10] |
| 78. | 6-hydroxykaempferol methyl ether O-dihexoside | C28H32O17 | 639.1567 | 639.1581 (100), 459.0948 (0.7), 315.0499 (15.3), 314.0436 (64.2), 299.0199 (49.5), 271.0250 (25.7), 243.0298 (5.4), 227.0351 (3.4), 215.0343 (10.8), 199.0396 (2.4), 183.0445 (2.3), 165.9896 (6.6), 164.9817 (4.2), 136.9872 (0.7), 133.0278 (3.5), 109.9994 (3.0) | 4.77 | 2.233 | |
| 79. | quercetin 3-O-galloylhexoside 1 | C28H24O16 | 615.0992 | 615.1002 (100), 463.0890 (30.7), 301.0354 (30.4), 300.0277 (49.7), 271.0247 (31.7), 255.0299 (14.9), 243.0294 (5.2), 178.9977 (2.3), 169.0134 (9.6), 151.0026 (6.0), 125.0231 (11.8), 121.0283 (1.2), 107.0123 (4.4) | 4.82 | 1.727 | [5,10,16,23] |
| 80. | kaempferol O-dihexoside | C27H30O16 | 609.1461 | 609.1473 (100), 429.0834 (2.2), 285.0395 (26.8), 284.0328 (82.9), 255.0298 (47.9), 227.0346 (32.2), 211.0397 (1.7), 178.9974 (1.6), 163.0030 (0.3), 151.0025 (2.8), 107.0121 (1.7) | 4.85 | 2.023 | |
| 81. | quercetin galloylhexoside 2 | C28H24O16 | 615.0992 | 615.1003 (100), 463.0889 (29.2), 301.0353 (30.4), 300.0274 (37.9), 255.0298 (14.1), 243.0298 (5.2), 178.9977 (2.8), 169.0130 (6.6), 151.0024 (7.2), 125.0231 (7.5), 121.0280 (1.3), 107.0123 (4.3) | 4.96 | 1.825 | [5,10] |
| 82. | myricetin 3-O-deoxyhexoside | C21H20O12 | 463.0882 | 463.0888 (91.6), 317.0291 (26.3), 316.0227 (100), 287.0202 (17.7), 271.0248 (27.0), 259.0248 (5.4), 243.0293 (3.7), 227.0344 (0.6), 178.9974 (4.2) | 5.11 | 1.384 | [5,15] |
| 83. | 6-hydroxykaempferol methyl ether O-deoxyhexosylhexoside | C28H32O16 | 623.1618 | 623.1630 (100), 315.0493 (9.1), 314.0436 (67.1), 299.0200 (51.3), 271.0248 (26.7), 255.0299 (3.9), 243.0294 (5.2), 227.0346 (3.6), 215.0345 (11.8), 165.9897 (7.6), 164.9811 (4.9), 136.9867 (1.8), 133.0284 (4.8) | 5.14 | 1.929 | |
| 84. | Isoquercitrin a | C21H20O12 | 463.0885 | 463.0888 (100), 301.0349 (29.8), 300.0277 (63.8), 271.0249 (36.6), 255.0297 (14.9), 243.0296 (9.4), 227.0344 (2.6), 215.0343 (0.5), 199.0392 (0.9), 178.9978 (2.6), 163.0025 (1.8), 151.0025 (5.7), 121.0278 (1.0), 107.0124 (2.2) | 5.19 | 1.319 | [15,16,23]; |
| 85. | quercetin O-hexuronide | C21H18O13 | 477.0675 | 477.0682 (62.4), 301.0356 (100), 283.0245 (1.8), 255.0296 (3.6), 245.0450 (2.6), 227.0354 (1.3), 211.0396 (1.8), 178.9978 (9.7), 163.0022 (2.6), 151.0024 (21.7), 121.0281 (6.3), 107.0123 (8.2) | 5.22 | 1.543 | [5,10,15,16] |
| 86. | hyperoside a | C21H20O12 | 463.0885 | 463.0891 (100), 301.0351 (40.3), 300.0278 (71.9), 271.0249 (32.3), 255.0298 (13.9), 243.0296 (8.2), 227.0340 (2.7), 215.0343 (0.5), 178.9979 (3.3), 151.0025 (4.9), 121.0278 (1.5), 107.0121 (1.8) | 5.29 | 1.837 | [5,10,15,16] |
| 87. | kaempferol-galloylhexoside 1 | C28H24O15 | 599.1042 | 599.1055 (100), 447.0935 (2.9), 285.0401 (12.6), 284.0327 (12.0), 255.0296 (9.3), 241.0350 (6.6), 227.0350 (5.2), 211.0243 (1.9), 169.0133 (37.2), 151.0027 (4.2), 125.0230 (26.5), 107.0122 (5.6) | 5.29 | 2.148 | [10,16,23] |
| 88. | quercetin O-pentoside | C20H18O11 | 433.0776 | 433.0781 (100), 301.0348 (23.1), 300.0277 (95.0), 271.0248 (49.6), 255.0295 (20.3), 243.0300 (10.6), 227.0353 (3.1), 178.9979 (2.1), 151.0023 (6.1), 107.0119 (2.6) | 5.62 | 0.982 | [5,10,15,16,23] |
| 89. | kaempferol 3-O-glucoside a | C21H20O11 | 447.0934 | 447.0945 (100), 285.0396 (15.6), 284.0328 (53.2), 255.0297 (38.2), 227.0345 (41.5), 211.0402 (1.5), 151.0029 (1.9), 107.0120 (0.5) | 5.65 | 2.607 | [16] |
| 90. | kaempferol-galloylhexoside 2 | C28H24O15 | 599.1042 | 599.1055 (100), 447.0931 (5.2), 285.0403 (17.4), 284.0326 (15.7), 255.0301 (11.9), 241.0349 (4.3), 227.0348 (7.0), 211.0230 (0.7), 169.0132 (31.6), 151.0026 (3.5), 125.0230 (24.5), 107.0124 (6.9) | 5.67 | 2.148 | [16] |
| 91. | myricetin 3-O-caffeoylhexoside 1 | C22H22O12 | 641.1160 | 641.1160 (100), 479.0840 (37.8), 317.0294 (23.6), 316.0227 (78.4), 287.0198 (17.6), 271.0249 (30.0), 259.0243 (2.9), 243.0291 (2.9), 178.9974 (4.6), 151.0022 (4.6), 107.0120 (1.1) | 5.71 | 1.813 | [16] |
| 92. | kaempferol O-hexuronide | C21H18O12 | 461.0725 | 461.0732 (38.4), 285.0405 (100), 257.0450 (4.5), 229.0501 (6.6), 211.0393 (1.3), 175.0236 (5.0), 151.0021 (1.2), 107.01221 (2.1) | 5.83 | 1.368 | [5,10,15,16] |
| 93. | myricetin 3-O-caffeoylhexoside2 | C22H22O12 | 641.1160 | 641.1160 (100), 479.0840 (29.0), 317.0297 (27.7), 316.0227 (70.7), 287.0200 (16.8), 271.0249 (27.3), 259.0245 (4.9), 243.0294 (3.3), 178.9978 (5.1), 151.0024 (6.7), 107.0122 (2.2) | 5.85 | 1.906 | |
| 94. | quercetin 3-O-deoxyhexoside | C21H20O11 | 447.0933 | 447.0940 (100), 301.0352 (71.7), 300.0279 (78.9), 271.0252 (31.7), 255.0297 (17.6), 243.0299 (7.3), 227.0351 (2.3), 178.9972 (4.4), 151.0023 (10.3), 121.0284 (2.9), 107.0123 (4.0) | 5.90 | 1.645 | [5,10,15,23] |
| 95. | isorhamnetin 3-O-glucoside a | C22H22O12 | 477.1038 | 477.1052 (100), 315.0497 (11.9), 314.0436 (54.4), 301.0359 (7.3), 271.0251 (20.5), 257.0454 (4.8), 243.0296 (22.2), 227.0351 (3.6), 199.0396 (3.1), 151.0024 (3.9), 107.0126 (0.7) | 6.02 | 2.831 | |
| 96. | naringenin O-hexoside | C21H22O10 | 433.1140 | 433.1165 (4.5), 271.0613 (100), 227.0711 (0.2), 193.0138 (0.9), 151.0023 (21.0), 119.0487 (22.3), 107.0122 (2.6) | 6.06 | 5.703 | |
| 97. | isorhamnetin O-hexuronide | C22H20O13 | 491.0831 | 491.0838 (49.3), 315.0514 (100), 300.0279 (25.8), 287.0570 (0.9), 271.0249 (26.8), 255.0298 (10.9), 243.0296 (7.7), 227.0340 (1.7), 215.0358 (0.4), 199.0385 (0.4), 175.0237 (5.6), 151.0025 (3.1), 107.0122 (1.3) | 6.08 | 1.397 | |
| 98. | kaempferol 3-O-pentoside | C20H18O10 | 417.0827 | 417.0833 (100), 285.0393 (16.4), 284.0328 (63.3), 255.0298 (41.5), 227.0345 (38.1), 211.0391 (1.0), 151.0031 (0.8) | 6.08 | 0.530 | [15,16] |
| 99. | kaempferol O-deoxyhexosylhexoside 2 | C27H30O15 | 593.1512 | 593.1522 (77.4), 285.0406 (100), 229.0503 (11.8), 211.0393 (2.6), 163.0022 (1.9), 151.0022 (0.3), 135.0076 (1.3), 107.0124 (1.9) | 6.32 | 1.697 | |
| 100. | myricetin O-p-coumaroylhexoside | C30H26O15 | 625.1199 | 625.1210 (85.5), 479.0835 (13.4), 317.0294 (18.9), 316.0227 (100), 287.0200 (15.4), 271.0250 (26.7), 259.0248 (6.3), 243.0301 (3.5), 178.9975 (4.3), 151.0026 (4.0), 107.0123 (1.8) | 6.59 | 1.819 | |
| 101. | kaempferol O-deoxyhexoside | C21H20O10 | 431.0984 | 431.0988 (100), 285.0405 (78.8), 255.0299 (35.6), 227.0346 (30.6), 211.0390 (1.6), 163.0029 (0.9), 151.0012 (0.5), 135.0075 (0.5), 107.0124 (0.9) | 6.59 | 0.380 | [5,10,15,16,23] |
| 102. | myricetin 3-O-feruloylhexoside | C31H28O16 | 655.1305 | 655.1322 (92.2), 479.0842 (8.7), 317.0290 (18.1), 316.0226 (100), 287.0200 (18.4), 271.0250 (26.1), 259.0249 (5.9), 243.0291 (4.7), 178.9978 (3.0), 151.0023 (4.5), 107.0122 (1.7) | 6.74 | 2.659 | |
| 103. | kaempferol 7-O-caffeoylhexoside | C30H26O14 | 609.1250 | 609.1262 (100), 447.0934 (3.1), 285.0407 (75.3), 284.0331 (16.1), 255.0297 (14.6), 227.0345 (11.0), 211.0402 (1.0), 179.0340 (12.9), 161.0233 (37.6), 135.0438 (18.8), 133.0282 (13.6) | 6.81 | 1.940 | |
| 104. | quercetin O-coumaroylhexoside 1 | C30H26O14 | 609.1250 | 609.1260 (100), 463.0891 (22.7), 301.0353 (37.7), 300.0278 (59.7), 271.0250 (33.4), 255.0296 (15.6), 243.0297 (7.1), 227.0347 (3.0), 211.0397 (1.2), 178.9977 (2.6), 151.0025 (6.4), 121.0280 (1.3), 107.0122 (2.6) | 7.05 | 0.643 | [15,23] |
| 105. | quercetin O-coumaroylhexoside 2 | C30H26O14 | 609.1250 | 609.1260 (100), 463.0887 (30.0), 301.0352 (41.2), 300.0277 (78.9), 271.0250 (38.0), 255.0298 (16.6), 243.0295 (8.9), 227.0345 (2.5), 211.0398 (1.8), 178.9977 (2.7), 163.0031 (0.8), 151.0023 (6.6), 121.0281 (0.7), 107.0121 (2.1) | 7.17 | 1.743 | [16] |
| 106. | quercetin 3-O-feruloylhexoside 1 | C31H28O15 | 639.1355 | 639.1367 (100), 463.0887 (16.2), 301.0352 (37.7), 300.0277 (73.2), 271.0250 (36.0), 255.0297 (17.4), 243.0297 (11.1), 227.0351 (2.6), 178.9978 (3.4), 175.0393 (0.9), 151.0026 (8.0), 121.0277 (0.7), 107.0123 (2.8) | 7.20 | 1.841 | [16] |
| 107. | quercetin 3-O-feruloylhexoside 2 | C31H28O15 | 639.1355 | 639.1367 (100), 463.0887 (15.1), 301.0351 (31.9), 300.0278 (72.4), 271.0250 (37.3), 255.0300 (15.5), 243.0295 (9.3), 227.0344 (2.7), 199.0401 (0.6), 193.0502 (0.5), 178.9977 (2.7), 175.0398 (0.5), 161.0231 (1.3), 151.0025 (6.9), 121.0280 (1.4), 107.0121 (3.0) | 7.32 | 1.841 | |
| 108. | quercetin O-coumaroylhexoside 3 | C30H26O14 | 609.1250 | 609.1260 (100), 463.0888 (22.8), 301.0352 (38.1), 300.0278 (77.5), 271.0250 (40.5), 255.0298 (18.5), 243.0295 (10.3), 227.0344 (3.1), 211.0396 (1.2), 178.9980 (2.8), 163.0027 (1.6), 151.0025 (7.4), 121.0281 (1.3), 107.0125 (2.9) | 7.54 | 1.743 | |
| 109. | kaempferol O-coumaroylhexoside 1 | C30H26O13 | 593.1301 | 593.1313 (100), 447.0948 (4.0), 285.0403 (57.7), 284.0328 (68.0), 255.0298 (41.3), 227.0346 (28.0), 211.0396 (2.3), 163.0389 (1.1), 151.0024 (3.1), 145.0283 (11.5), 135.0075 (1.0), 119.0488 (2.7), 107.0125 (2.3) | 7.58 | 2.168 | [16] |
| 110. | quercetin a | C15H10O7 | 301.0354 | 301.0357 (100), 273.0410 (3.2), 257.0463 (0.6), 229.0505 (1.2), 211.0401 (0.4), 178.9978 (22.3), 151.0025 (44.3), 121.0281 (14.5), 107.0124 (15.4) | 7.62 | [17,23] | |
| 111. | kaempferol O-coumaroylhexoside 2 | C30H26O13 | 593.1301 | 593.1313 (100), 447.0934 (2.0), 285.0403 (5.2), 284.0328 (52.4), 255.0298 (33.5), 227.0345 (24.5), 211.0395 (1.0), 163.0388 (2.5), 151.0024 (3.9), 145.0282 (8.0), 119.0488 (2.6), 107.0123 (2.0) | 7.68 | 2.084 | [16] |
| 112. | kaempferol O-feruloylhexoside 1 | C31H28O14 | 623.1406 | 623.1421 (100), 447.0935 (1.7), 323.0779 (2.7), 285.0401 (35.5), 284.0328 (63.0), 255.0298 (39.3), 227.0346 (28.4), 211.0397 (2.0), 193.0503 (1.6), 175.0394 (4.3), 161.0233 (8.1)151.0025 (2.2), 134.0358 (1.6), 107.0124 (1.6) | 7.71 | 2.345 | |
| 113. | kaempferol O-feruloylhexoside 2 | C31H28O14 | 623.1406 | 623.1419 (100), 447.0924 (1.5), 323.0770 (3.7), 285.0403 (61.1), 284.0327 (55.0), 255.0297 (41.4), 227.0344 (28.1), 211.0395 (3.1), 193.0500 (1.9), 175.0388 (4.5), 161.0231 (5.5), 151.0025 (3.6), 134.0359 (1.5), 107.0126 (2.7) | 7.82 | ||
| 114. | 6-hydroxykaempferol methyl ether | C16H12O7 | 315.0510 | 315.0515 (80.4), 300.0279 (100), 272.0326 (8.1), 255.0302 (4.2), 243.0307 (1.2), 227.0345 (1.6), 165.9896 (8.0), 139.0025 (4.4), 136.9865 (4.4), | 8.82 | 1.441 | |
| 115. | naringenin a | C15H12O5 | 271.0612 | 271.0612 (12.6), 151.0020 (12.5), 119.0496 (10.2) | 8.58 | 0.468 | |
| 116. | kaempferol a | C15H9O7 | 285.0406 | 285.0404 (100), 257.0457 (0.9), 239.0352 (1.2), 229.0499 (0.9), 211.0399 (1.0), 187.0390 (1.4), 151.0024 (1.5), 107.0125 (1.2) | 8.83 | −0.215 | [17] |
| 117. | quercetin O-cinnamoylhexoside 1 | C30H26O13 | 593.1301 | 593.1313 (100), 301.0346 (23.2), 300.0278 (80.2), 271.0248 (41.4), 255.0295 (9.6), 243.0295 (14.3), 227.0342 (4.0), 178.9976 (2.7), 151.0024 (5.0), 121.0279 (0.6), 107.0124 (2.3) | 8.85 | 2.067 | |
| 118. | quercetin O-cinnamoylhexoside 2 | C30H26O13 | 593.1301 | 593.1313 (100), 301.0349 (27.3), 300.0278 (73.8), 271.0251 (36.5), 255.0300 (10.0), 243.0297 (10.9), 227.0341 (3.1), 199.0392 (0.6), 178.9971 (2.8), 151.0024 (5.3), 135.0074 (1.0), 121.0283 (0.7), 107.0124 (2.4) | 9.12 | 2.084 | |
| 119. | isorhamnetin a | C16H12O7 | 315.0510 | 315.0515 (100), 300.0279 (47.0), 271.0241 (3.6), 255.0312 (1.2), 227.0346 (1.1), 164.0103 (3.2), 151.0023 (8.3), 107.0122 (7.0) | 9.10 | 1.441 | |
| 120. | kaempferol O-cinnamoylhexoside | C30H26O12 | 577.1351 | 577.1361 (100), 285.0396 (17.2), 284.0328 (71.6), 255.0298 (49.7), 227.0345 (8.1), 211.0393 (1.1), 151.0024 (1.2), | 9.50 | −0.310 | |
| 121. | myricetin a | C15H10O8 | In (+)ESI-MS/MS 319.0448 | 319.0440 (100), 273.0383 (2.6), 245.0440 (4.4), 217.0491 (5.0), 165.0179 (2.5), 153.0180 (13.2) | 9.49 | −0.804 | [15,17] |
2.1.2. Mono- and Diacylquinic Acids
2.1.3. Gallotannins and Ellagitannins
2.1.4. Flavonoids
2.2. Total Phenolic and Flavonoid Content
2.3. Antioxidant Capacity
2.4. Enzyme Inhibitory Activity
3. Materials and Methods
3.1. Plant Material
3.2. Sample Extraction
3.3. Chemicals
3.4. UHPLC-HRMS
3.5. Assay for Total Phenolic and Flavonoid Contents
3.6. Assays for In Vitro Antioxidant Capacity
3.7. Inhibitory Effects Against Some Key Enzymes
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dreger, M.; Adamczak, A.; Foksowicz-Flaczyk, J. Antibacterial and Antimycotic Activity of Epilobium angustifolium L. Extracts: A Review. Pharmaceuticals 2023, 16, 1419. [Google Scholar] [CrossRef]
- Adamczak, A.; Dreger, M.; Seidler-Łożykowska, K.; Wielgus, K. Fireweed (Epilobium angustifolium L.): Botany, phytochemistry and traditional uses. A review. Herba Pol. 2019, 65, 51–63. [Google Scholar] [CrossRef]
- Granica, S.; Piwowarski, J.P.; Czerwińska, M.E.; Kiss, A.K. Phytochemistry, pharmacology and traditional uses of different Epilobium species (Onagraceae): A review. J. Ethnopharmacol. 2014, 156, 316–346. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, K.; Polak-Berecka, M.; Prendecka-Wróbel, M.; Pigoń-Zając, D.; Niedźwiedź, I.; Szwajgier, D.; Baranowska-Wójcik, E.; Waśko, A. Biological activity of an Epilobium angustifolium L.(Fireweed) infusion after in vitro digestion. Molecules 2022, 27, 1006. [Google Scholar] [CrossRef] [PubMed]
- Baert, N.; Kim, J.; Karonen, M.; Salminen, J.-P. Inter-population and inter-organ distribution of the main polyphenolic compounds of Epilobium angustifolium. Phytochemistry 2017, 134, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Baert, N.; Karonen, M.; Salminen, J.-P. Isolation, characterisation and quantification of the main oligomeric macrocyclic ellagitannins in Epilobium angustifolium by ultra-high performance chromatography with diode array detection and electrospray tandem mass spectrometry. J. Chromatogr. A 2015, 1419, 26–36. [Google Scholar] [CrossRef]
- Agnieszka, G.; Mariola, D.; Anna, P.; Piotr, K.; Natalia, W.; Aneta, S.; Marcin, O.; Bogna, O.; Zdzisław, Ł.; Aurelia, P. Qualitative and quantitative analyses of bioactive compounds from ex vitro Chamaenerion angustifolium (L.) (Epilobium augustifolium) herb in different harvest times. Ind. Crops Prod. 2018, 123, 208–220. [Google Scholar] [CrossRef]
- Ducrey, B.; Marston, A.; Göhring, S.; Hartmann, R.; Hostettmann, K. Inhibition of 5α-reductase and aromatase by the ellagitannins oenothein A and oenothein B from Epilobium species. Planta Med. 1997, 63, 111–114. [Google Scholar] [CrossRef]
- Granica, S.; Bazylko, A.; Kiss, A.K. Determination of macrocyclic ellagitannin oenothein B in plant materials by HPLC-DAD-MS: Method development and validation. Phytochem. Anal. 2012, 23, 582–587. [Google Scholar] [CrossRef]
- Stolarczyk, M.; Naruszewicz, M.; Kiss, A.K. Extracts from Epilobium sp. herbs induce apoptosis in human hormone-dependent prostate cancer cells by activating the mitochondrial pathway. J. Pharm. Pharmacol. 2013, 65, 1044–1054. [Google Scholar] [CrossRef]
- Tóth, B.H.; Blazics, B.; Kéry, Á. Polyphenol composition and antioxidant capacity of Epilobium species. J. Pharm. Biomed. Anal. 2009, 49, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.K.; Bazylko, A.; Filipek, A.; Granica, S.; Jaszewska, E.; Kiarszys, U.; Kośmider, A.; Piwowarski, J. Oenothein B’s contribution to the anti-inflammatory and antioxidant activity of Epilobium sp. Phytomedicine 2011, 18, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Ramstead, A.G.; Kirpotina, L.N.; Voyich, J.M.; Jutila, M.A.; Quinn, M.T. Therapeutic potential of polyphenols from Epilobium angustifolium (Fireweed). Phytother. Res. 2016, 30, 1287–1297. [Google Scholar] [CrossRef]
- Deng, L.-Q.; Zhou, S.-Y.; Mao, J.-X.; Liu, S.; Lan, X.-Z.; Liao, Z.-H.; Chen, M. HPLC-ESI-MS/MS analysis of phenolics and in vitro antioxidant activity of Epilobium angustifolium L. Nat. Prod. Res. 2018, 32, 1432–1435. [Google Scholar] [CrossRef] [PubMed]
- Ducrey, B.; Wolfender, J.; Marston, A.; Hostettmann, K. Analysis of flavonol glycosides of thirteen Epilobium species (Onagraceae) by LC-UV and thermospray LC-MS. Phytochemistry 1995, 38, 129–137. [Google Scholar] [CrossRef]
- Dacrema, M.; Sommella, E.; Santarcangelo, C.; Bruno, B.; Marano, M.G.; Insolia, V.; Saviano, A.; Campiglia, P.; Stornaiuolo, M.; Daglia, M. Metabolic profiling, in vitro bioaccessibility and in vivo bioavailability of a commercial bioactive Epilobium angustifolium L. extract. Biomed. Pharmacother. 2020, 131, 110670. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E.; Kukula-Koch, W.; Kowalik, K.; Polak-Berecka, M.; Waśko, A. Evolution of the anticholinesterase, antioxidant, and anti-inflammatory activity of Epilobium angustifolium L. infusion during in vitro digestion. J. Funct. Foods 2021, 85, 104645. [Google Scholar] [CrossRef]
- Vitalone, A.; Allkanjari, O. Epilobium spp: Pharmacology and phytochemistry. Phytother. Res. 2018, 32, 1229–1240. [Google Scholar] [CrossRef]
- Summer, L.; Amberg, A.; Barrett, D.; Beale, M.; Beger, R.; Daykin, C.; Fan, T.; Fiehn, O.; Goodacre, R.; Griffin, J. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Gevrenova, R.; Kostadinova, I.; Stefanova, A.; Balabanova, V.; Zengin, G.; Zheleva-Dimitrova, D.; Momekov, G. Phytochemical Profiling, Antioxidant and Cognitive-Enhancing Effect of Helichrysum italicum ssp. italicum (Roth) G. Don (Asteraceae). Plants 2023, 12, 2755. [Google Scholar] [CrossRef]
- Gevrenova, R.; Zengin, G.; Balabanova, V.; Szakiel, A.; Zheleva-Dimitrova, D. Pelargonium graveolens: Towards In-Depth Metabolite Profiling, Antioxidant and Enzyme-Inhibitory Potential. Plants 2024, 13, 2612. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yoshimura, M.; Amakura, Y. Chemical and biological significance of oenothein B and related ellagitannin oligomers with macrocyclic structure. Molecules 2018, 23, 552. [Google Scholar] [CrossRef] [PubMed]
- Hiermann, A.; Radl, B. Analysis of aromatic plant acids by capillary zone electrophoresis. J. Chromatogr. A 1998, 803, 311–314. [Google Scholar] [CrossRef]
- Sinan, K.I.; Zengin, G.; Zheleva-Dimitrova, D.; Gevrenova, R.; Picot-Allain, M.C.N.; Dall’Acqua, S.; Behl, T.; Goh, B.H.; Ying, P.T.S.; Mahomoodally, M.F. Exploring the chemical profiles and biological values of two Spondias species (S. dulcis and S. mombin): Valuable Sources of Bioactive Natural Products. Antioxidants 2021, 10, 1771. [Google Scholar] [CrossRef] [PubMed]
- Ak, G.; Gevrenova, R.; Sinan, K.I.; Zengin, G.; Zheleva, D.; Mahomoodally, M.F.; Senkardes, I.; Brunetti, L.; Leone, S.; Di Simone, S.C. Tanacetum vulgare L.(Tansy) as an effective bioresource with promising pharmacological effects from natural arsenal. Food Chem. Toxicol. 2021, 153, 112268. [Google Scholar] [CrossRef]
- Bijttebier, S.; Van der Auwera, A.; Voorspoels, S.; Noten, B.; Hermans, N.; Pieters, L.; Apers, S. A first step in the quest for the active constituents in Filipendula ulmaria (Meadowsweet): Comprehensive phytochemical identification by liquid chromatography coupled to quadrupole-orbitrap mass spectrometry. Planta Med. 2016, 82, 559–572. [Google Scholar] [CrossRef]
- Karonen, M.; Parker, J.; Agrawal, A.; Salminen, J.P. First evidence of hexameric and heptameric ellagitannins in plants detected by liquid chromatography/electrospray ionisation mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 3151–3156. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Düüna, K.; Jõul, P.; Vaher, M. Extraction and Determination of Total Phenolic Contents, Flavonoid Contents, and Volatile Compounds in Epilobium angustifolium and Cannabis sativa Varieties. Proceedings 2023, 92, 21. [Google Scholar] [CrossRef]
- Monschein, M.; Jaindl, K.; Buzimkić, S.; Bucar, F. Content of phenolic compounds in wild populations of Epilobium angustifolium growing at different altitudes. Pharm. Biol. 2015, 53, 1576–1582. [Google Scholar] [CrossRef]
- Ak, G.; Zengin, G.; Mahomoodally, M.F.; Llorent-Martínez, E.; Orlando, G.; Chiavaroli, A.; Brunetti, L.; Recinella, L.; Leone, S.; Di Simone, S.C. Shedding light into the connection between chemical components and biological effects of extracts from Epilobium hirsutum: Is it a potent source of bioactive agents from natural treasure? Antioxidants 2021, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Maruška, A.; Akuņeca, I.; Stankevičius, M.; Ragažinskienė, O.; Bartkuvienė, V.; Kornyšova, O.; Briedis, V.; Ugenskienė, R. Screening of antioxidant activity and volatile compounds composition of Chamerion angustifolium (L.) Holub ecotypes grown in Lithuania. Nat. Prod. Res. 2016, 30, 1373–1381. [Google Scholar] [CrossRef]
- Okuyama, S.; Furukawa, Y.; Yoshimura, M.; Amakura, Y.; Nakajima, M.; Yoshida, T. Oenothein B, a Bioactive Ellagitannin, Activates the Extracellular Signal-Regulated Kinase 2 Signaling Pathway in the Mouse Brain. Plants 2021, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Gunny, A.A.N.; Subramanian, P.; Mahmod, S.S.; Al-Rajabi, M.M.; Ahmad, A.A.; Abu Bakar, A.R. Mechanism of inhibition of alpha-amylase by caffeic acid using in-vitro and in-silico techniques. Nat. Prod. Res. 2024, 1–5. [Google Scholar] [CrossRef]
- Rangel-Galván, M.; Pacheco-Hernández, Y.; Lozoya-Gloria, E.; Villa-Ruano, N. Dietary natural products as inhibitors of α-amylase and α-glucosidase: An updated review of ligand-receptor correlations validated by docking studies. Food Biosci. 2024, 62, 105456. [Google Scholar] [CrossRef]
- Zolghadri, S.; Beygi, M.; Mohammad, T.F.; Alijanianzadeh, M.; Pillaiyar, T.; Garcia-Molina, P.; Garcia-Canovas, F.; Munoz-Munoz, J.; Saboury, A.A. Targeting tyrosinase in hyperpigmentation: Current status, limitations and future promises. Biochem. Pharmacol. 2023, 212, 115574. [Google Scholar] [CrossRef]
- Lesuisse, D.; Berjonneau, J.; Ciot, C.; Devaux, P.; Doucet, B.; Gourvest, J.F.; Khemis, B.; Lang, C.; Legrand, R.; Lowinski, M.; et al. Determination of oenothein B as the active 5-alpha-reductase-inhibiting principle of the folk medicine Epilobium parviflorum. J. Nat. Prod. 1996, 59, 490–492. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Ziemlewska, A.; Bujak, T.; Niziol-Lukaszewska, Z.; Hordyjewicz-Baran, Z. Cosmetic and Dermatological Properties of Selected Ayurvedic Plant Extracts. Molecules 2021, 26, 614. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Ara, L.; Moazzeni, H.; Esmaeili, S. Evaluating the antioxidant and acetylcholinesterase inhibitory activities of some plants from Kohgiluyeh va Boyerahmad province, Iran. Res. J. Pharmacogn. 2016, 3, 1–7. [Google Scholar]
- Huang, M.; Xiao, Q.; Li, Y.; Ahmad, M.; Tang, J.; Liao, Q.; Tan, C. Inhibition of α-amylase activity by quercetin via multi-spectroscopic and molecular docking approaches. Food Biosci. 2024, 61, 104951. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, G.; Hu, X.; Xu, X.; Gong, D. Quercetin as a tyrosinase inhibitor: Inhibitory activity, conformational change and mechanism. Food Res. Int. 2017, 100, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E. Cholinesterase inhibitory potential of quercetin towards Alzheimer’s disease-a promising natural molecule or fashion of the day?—A narrowed review. Curr. Neuropharmacol. 2021, 19, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Santa-arthampreecha, S.; Samakthanasan, S.; Kitphati, W.; Pratuangdejkul, J.; Nukoolkam, V. Gallic acid and derivatives as acetylcholinesterase inhibitors. Thai J. Pharm. Sci. 2012, 36, 35–37. [Google Scholar] [CrossRef]
- Huang, Y.; Richardson, S.J.; Brennan, C.S.; Kasapis, S. Mechanistic insights into α-amylase inhibition, binding affinity and structural changes upon interaction with gallic acid. Food Hydrocoll. 2024, 148, 109467. [Google Scholar] [CrossRef]
- Kumar, K.S.; Vani, M.G.; Wang, S.Y.; Liao, J.W.; Hsu, L.S.; Yang, H.L.; Hseu, Y.C. In vitro and in vivo studies disclosed the depigmenting effects of gallic acid: A novel skin lightening agent for hyperpigmentary skin diseases. Biofactors 2013, 39, 259–270. [Google Scholar] [CrossRef]
- Garcia-Jimenez, A.; Munoz-Munoz, J.L.; García-Molina, F.; Teruel-Puche, J.A.; García-Cánovas, F. Spectrophotometric characterization of the action of tyrosinase on p-coumaric and caffeic acids: Characteristics of o-caffeoquinone. J. Agric. Food Chem. 2017, 65, 3378–3386. [Google Scholar] [CrossRef]
- Farasat, A.; Ghorbani, M.; Gheibi, N.; Shariatifar, H. In silico assessment of the inhibitory effect of four flavonoids (Chrysin, Naringin, Quercetin, Kaempferol) on tyrosinase activity using the MD simulation approach. BioTechnol. J. Biotechnol. Comput. Biol. Bionanotechnol. 2020, 101, 193–204. [Google Scholar] [CrossRef]
- Fan, M.; Ding, H.; Zhang, G.; Hu, X.; Gong, D. Relationships of dietary flavonoid structure with its tyrosinase inhibitory activity and affinity. Lwt 2019, 107, 25–34. [Google Scholar] [CrossRef]
- Padure, I.; Mountford, O.; Negrean, G.; Anastasiu, P.; Bita-Nicolae, C. Floristic biodiversity in Vitosha Nature Park, Bulgaria. Acta Horti Bot. Bucur. 2008, 35, 26–35. [Google Scholar]
- Gevrenova, R.; Zheleva-Dimitrova, D.; Balabanova, V.; Voynikov, Y.; Sinan, K.I.; Mahomoodally, M.F.; Zengin, G. Integrated phytochemistry, bio-functional potential and multivariate analysis of Tanacetum macrophyllum (Waldst. & Kit.) Sch. Bip. and Telekia speciosa (Schreb.) Baumg.(Asteraceae). Ind. Crops Prod. 2020, 155, 112817. [Google Scholar]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Nithiyanantham, S.; Locatelli, M.; Ceylan, R.; Uysal, S.; Aktumsek, A.; Selvi, P.K.; Maskovic, P. Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integr. Med. 2016, 8, 286–292. [Google Scholar] [CrossRef]
| Total bioactive compounds | |
| Total phenolic content (mg GAE/g) | 85.04 ± 0.18 |
| Total flavonoid content (mg RE/g) | 27.71 ± 0.74 |
| Antioxidant properties | |
| DPPH scavenging ability (mg TE/g) | 310.74 ± 11.09 |
| ABTS scavenging ability (mg TE/g) | 466.82 ± 23.60 |
| CUPRAC (mg TE/g) | 442.83 ± 12.27 |
| FRAP (mg TE/g) | 291.50 ± 4.32 |
| Metal chelating (mg EDTAE/g) | 48.20 ± 0.44 |
| Phosphomolybdenum (mmol TE/g) | 2.10 ± 0.09 |
| Enzyme inhibitory properties | |
| AChE inhibition (mg GALAE/g) | 2.05 ± 0.04 |
| BChE inhibition (mg GALAE/g) | 1.67 ± 0.07 |
| Tyrosinase inhibition (mg KAE/g) | 61.94 ± 0.05 |
| Amylase inhibition (mmol ACAE/g) | 0.44 ± 0.01 |
| Glucosidase inhibition (mmol ACAE/g) | 3.48 ± 0.08 |
| Lipase inhibition (mg OE/g) | 8.03 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gevrenova, R.; Zengin, G.; Ozturk, G.; Zheleva-Dimitrova, D. Exploring the Phytochemical Profile and Biological Insights of Epilobium angustifolium L. Herb. Plants 2025, 14, 415. https://doi.org/10.3390/plants14030415
Gevrenova R, Zengin G, Ozturk G, Zheleva-Dimitrova D. Exploring the Phytochemical Profile and Biological Insights of Epilobium angustifolium L. Herb. Plants. 2025; 14(3):415. https://doi.org/10.3390/plants14030415
Chicago/Turabian StyleGevrenova, Reneta, Gokhan Zengin, Gulsah Ozturk, and Dimitrina Zheleva-Dimitrova. 2025. "Exploring the Phytochemical Profile and Biological Insights of Epilobium angustifolium L. Herb" Plants 14, no. 3: 415. https://doi.org/10.3390/plants14030415
APA StyleGevrenova, R., Zengin, G., Ozturk, G., & Zheleva-Dimitrova, D. (2025). Exploring the Phytochemical Profile and Biological Insights of Epilobium angustifolium L. Herb. Plants, 14(3), 415. https://doi.org/10.3390/plants14030415








