Transcriptome Analysis Revealed the Regulatory Mechanism of DIMBOA Affecting Early Somatic Embryogenesis in Dimocarpus longan Lour.
Abstract
:1. Introduction
2. Results
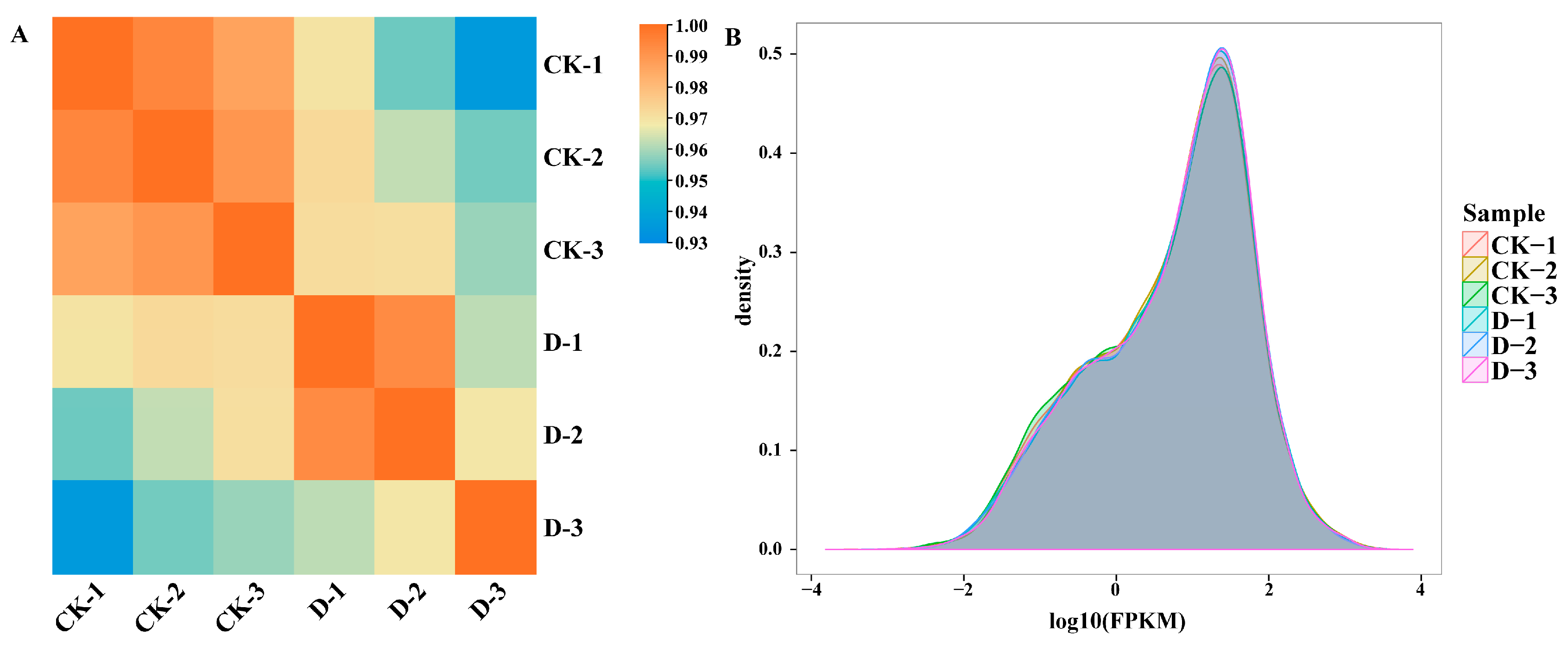
2.1. Transcriptome Sequencing Quality Assessment
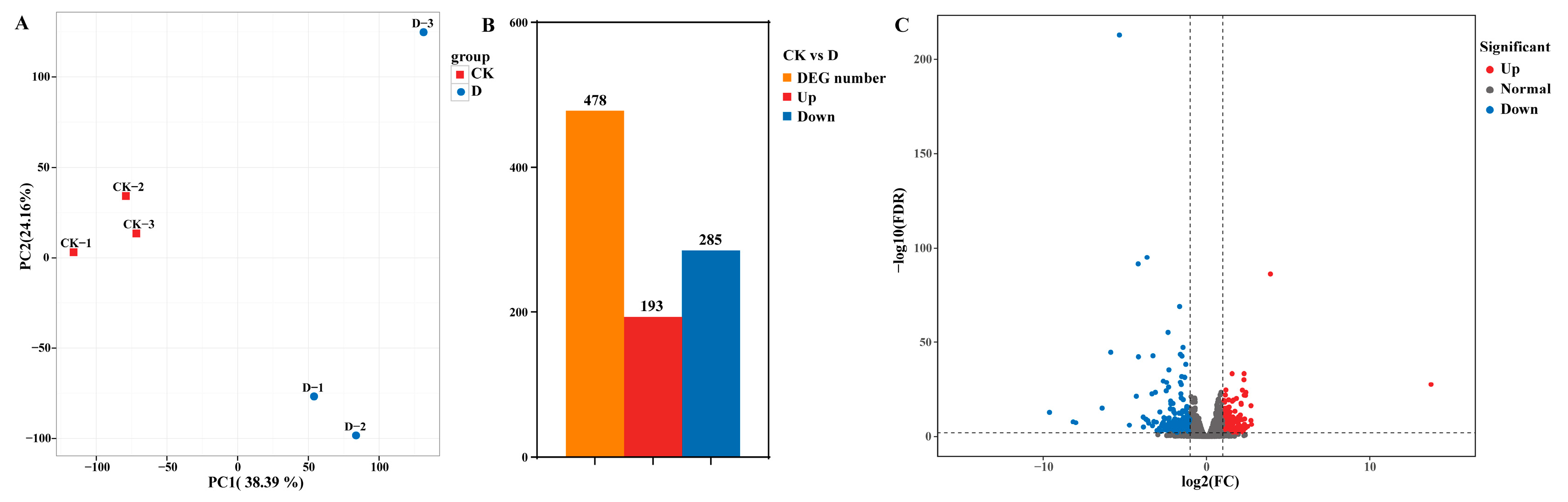
2.2. Data Analysis and Identification of Differentially Expressed Genes Under DIMBOA Treatment
2.3. KEGG Enrichment Analysis
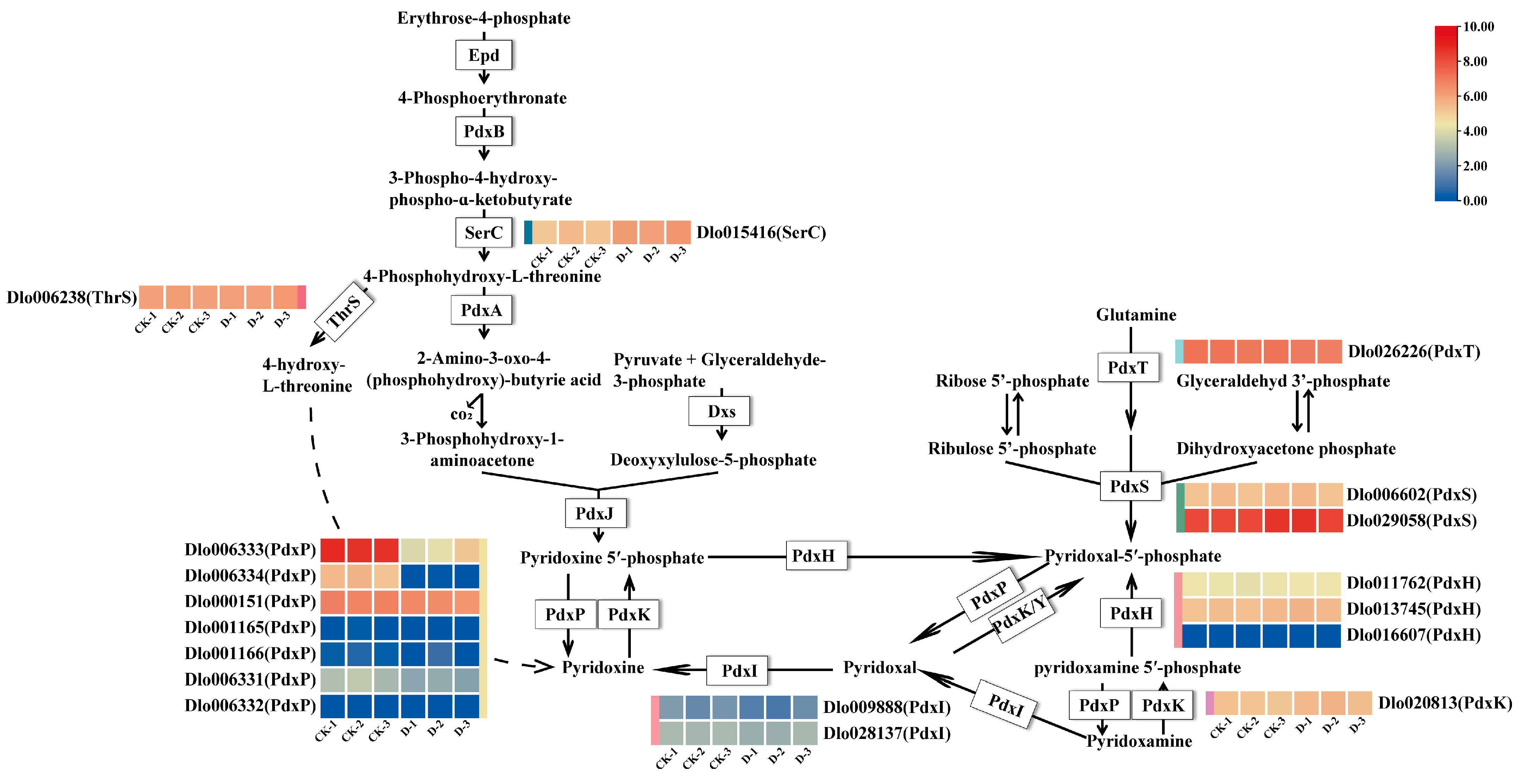
2.4. DEGs Involved in Vitamin B6 Biosynthesis Pathway in Longan Embryogenic Cultures
2.5. DEGs Involved in Phenylpropanoid Metabolism-Related Pathways in Longan Embryogenic Cultures
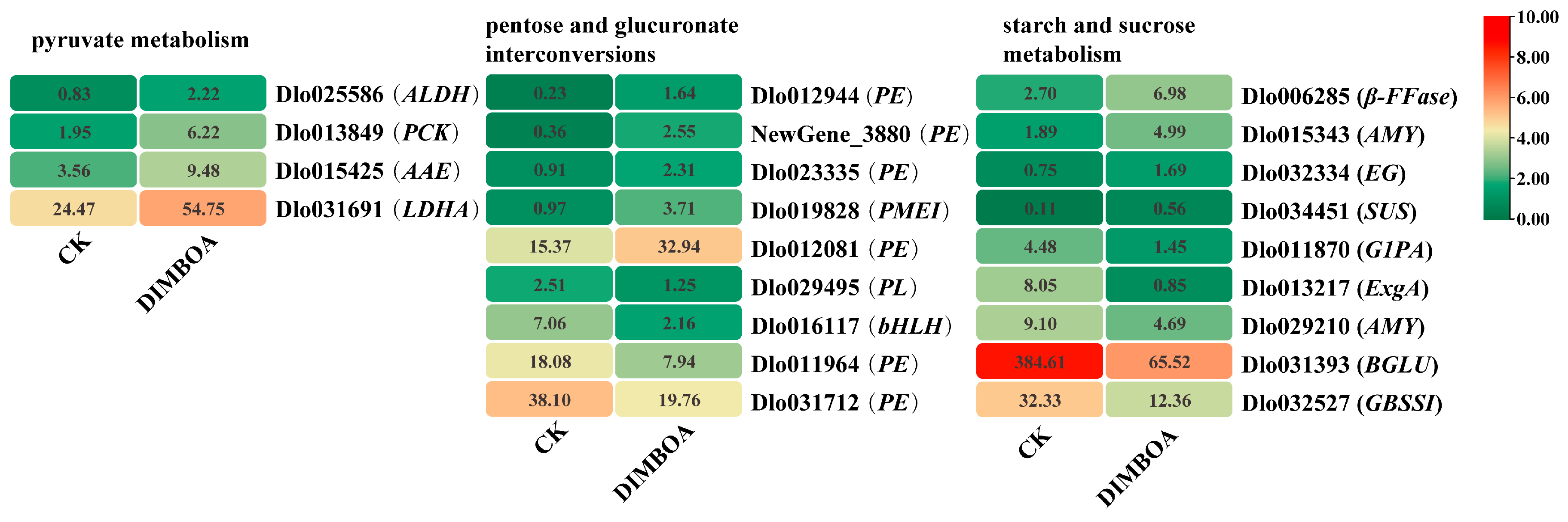
2.6. DEGs Involved in Carbohydrate Metabolism in Longan Embryogenic Cultures
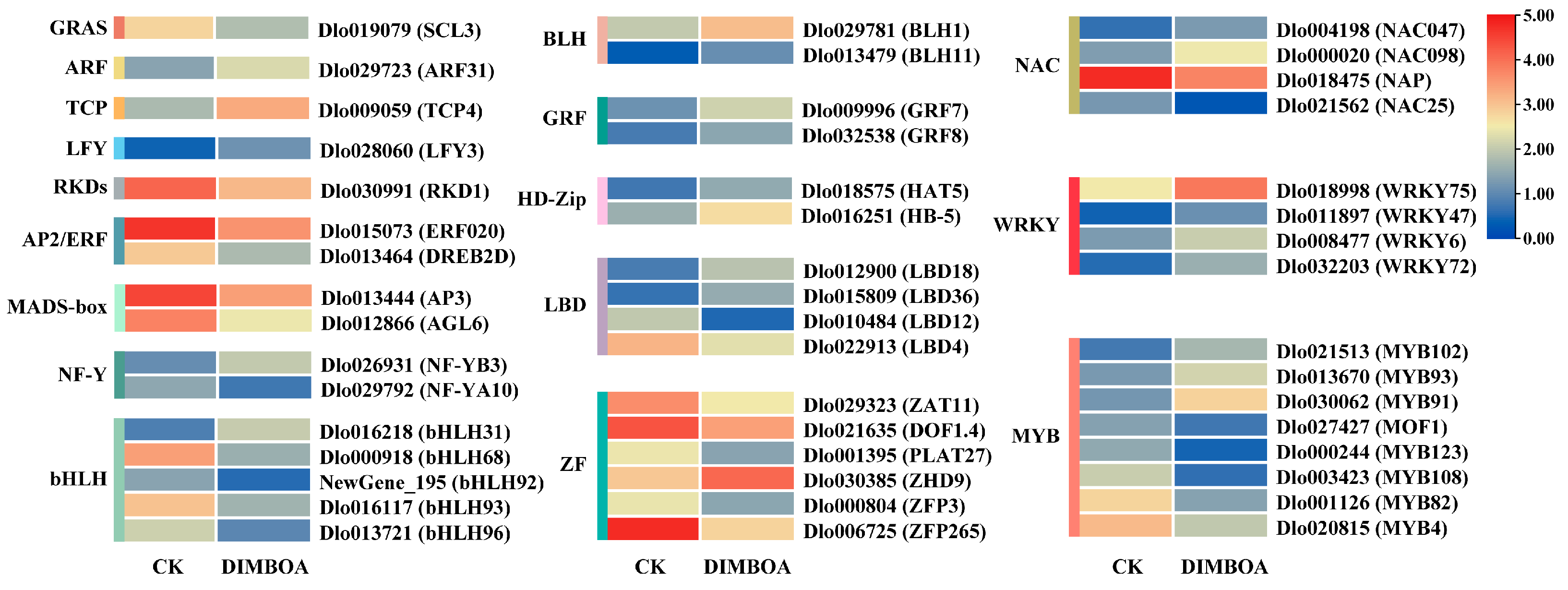
2.7. Response of Differentially Expressed TFs to DIMBOA
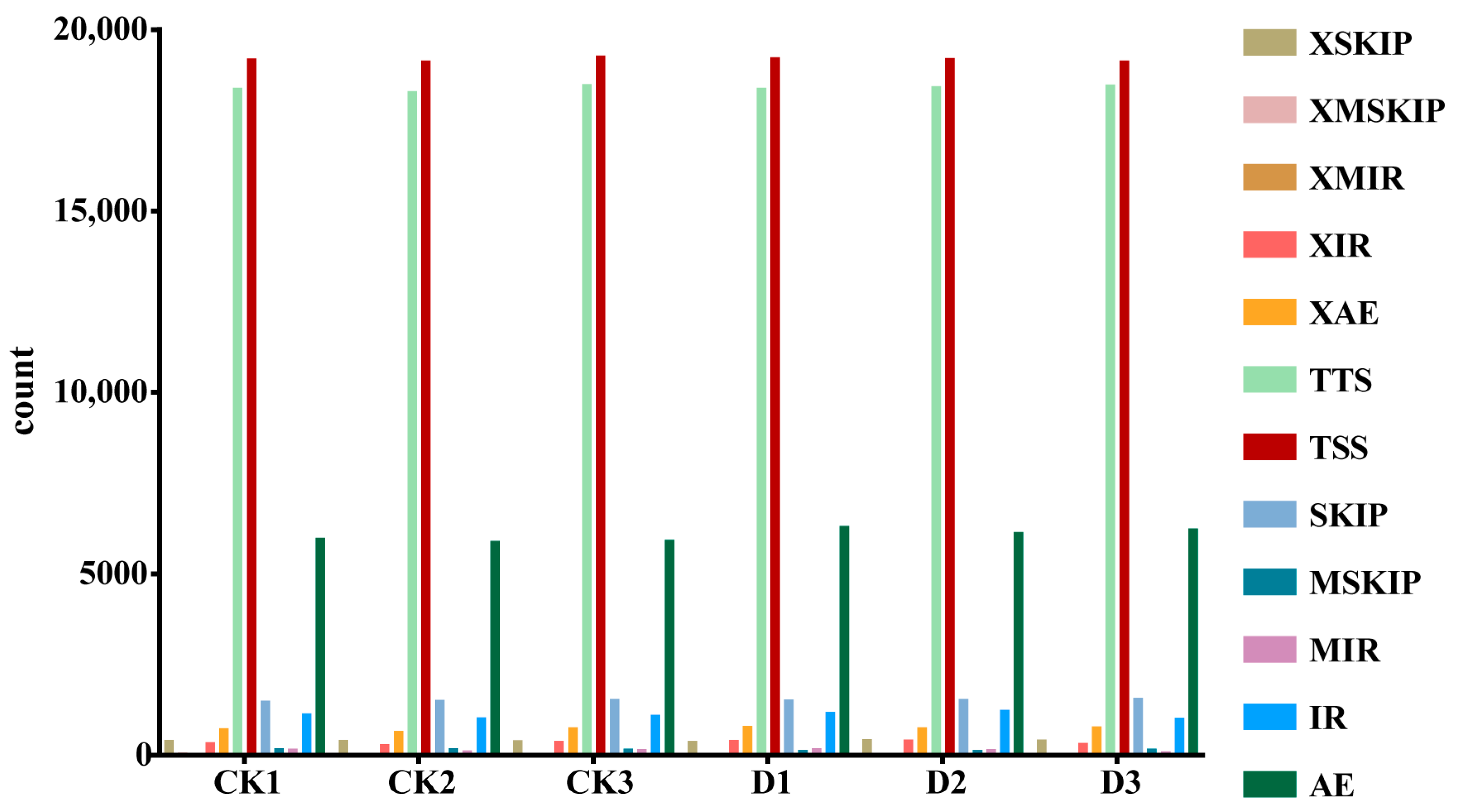
2.8. Alternative Splicing Analysis
2.9. Expression Analysis of Key Genes of Auxin Biosynthesis Under Different Concentrations of DIMBOA
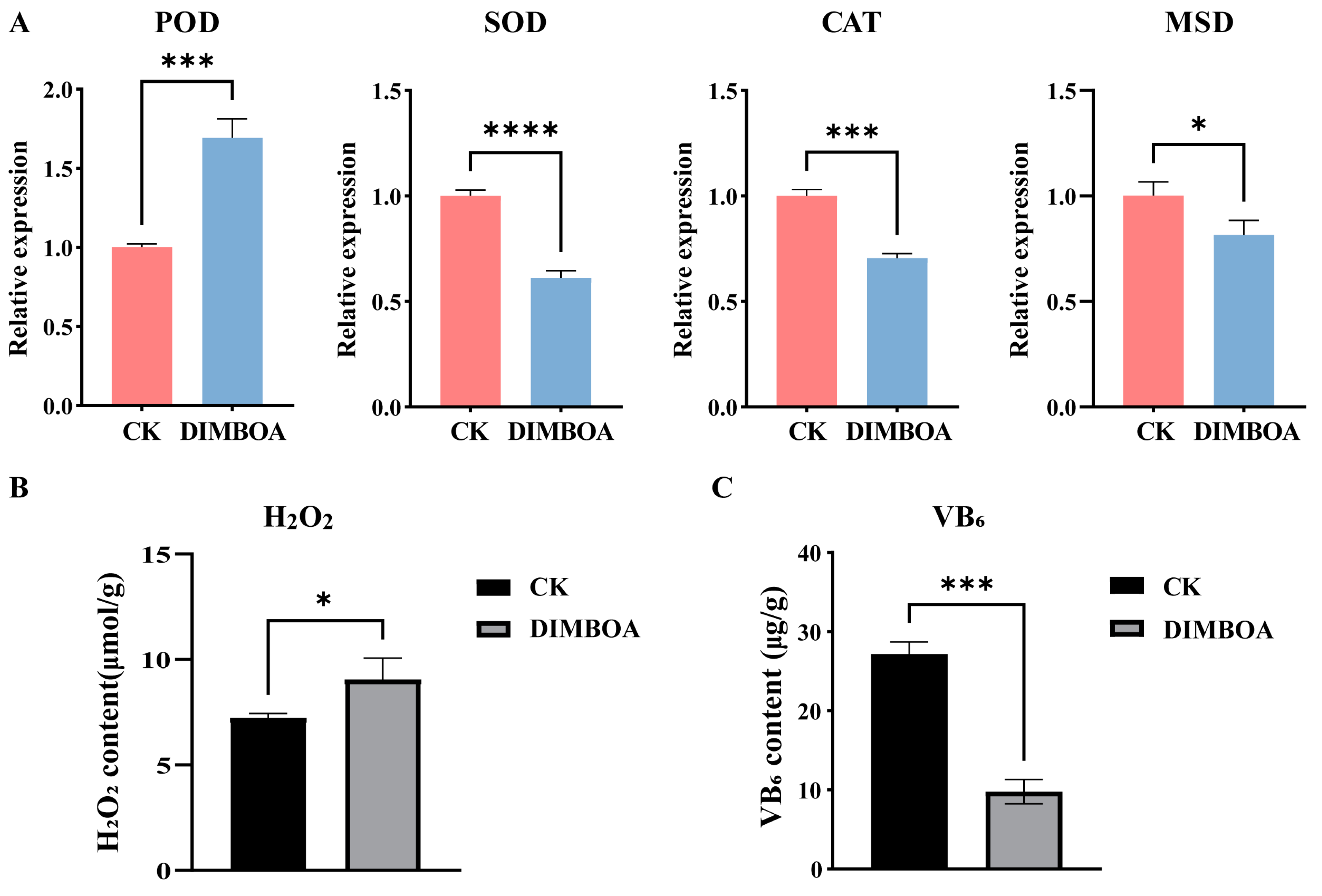
2.10. Determination of H2O2 Content and Vitamin B6 Content
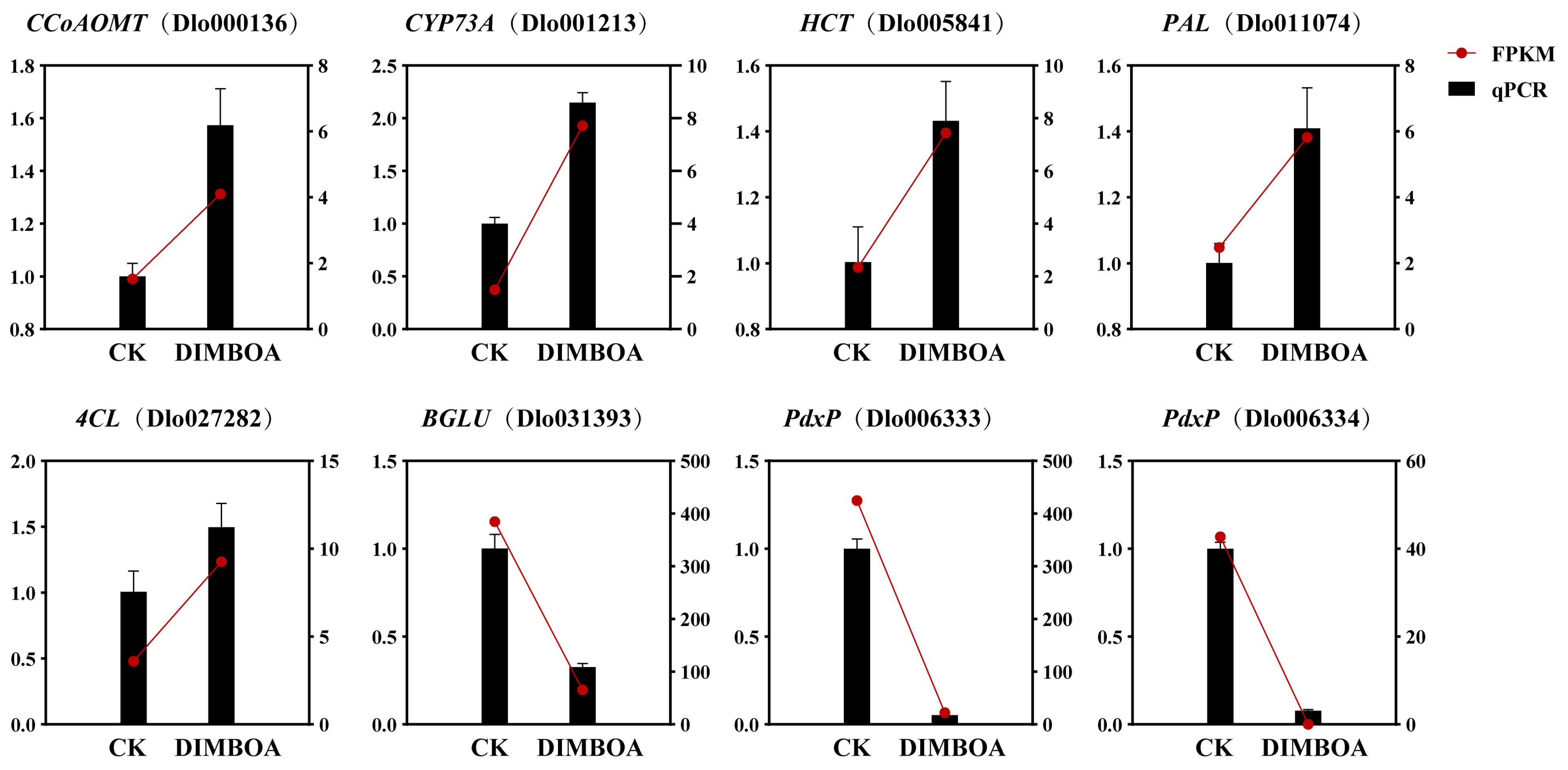
2.11. qRT-PCR Analysis of DEGs
3. Discussion
3.1. DIMBOA May Regulate Early SE of Longan via Various Metabolic Pathways
3.2. TFs in Response to Exogenous DIMBOA Treatment Are Involved in Regulating Early SE Processes in Longan
3.3. DIMBOA May Regulate the Morphogenesis of Early SE in Longan by Affecting the VB6 Metabolic Pathway
4. Materials and Methods
4.1. Plant Material
4.2. Transcriptome Library Construction and Sequencing
4.3. Analysis of Differentially Expressed Genes
4.4. Determination of H2O2 Content in Embryogenic Cultures of Longan
4.5. Determination of VB6 Content in Embryogenic Cultures of Longan
4.6. Quantitative PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheu, S.Y.; Fu, Y.T.; Huang, W.D.; Chen, Y.A.; Lei, Y.C.; Yao, C.H.; Hsu, F.L.; Kuo, T.F. Evaluation of Xanthine Oxidase Inhibitory Potential and In vivo Hypouricemic Activity of Dimocarpus longan Lour. Extracts. Pharmacogn. Mag. 2016, 12, S206–S212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, X.; Liu, Z.; Zhang, Z.; XuHan, X.; Lin, Y.; Lai, Z. Global scale transcriptome analysis reveals differentially expressed genes involve in early somatic embryogenesis in Dimocarpus longan Lour. BMC Genom. 2020, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z. Establishment and maintenance of longan embryonic cell line. J. Fujian Agric. For. Univ. 1997, 26, 160–167. [Google Scholar]
- Lai, Z.; Chen, Z. High-frequency somatic embryogenesis of longan embryonic callus. J. Fujian Agric. Univ. 1997, 26, 6. [Google Scholar]
- Wang, F.X.; Shang, G.D.; Wu, L.Y.; Xu, Z.G.; Zhao, X.Y.; Wang, J.W. Chromatin Accessibility Dynamics and a Hierarchical Transcriptional Regulatory Network Structure for Plant Somatic Embryogenesis. Dev. Cell 2020, 54, 742–757.e8. [Google Scholar] [CrossRef]
- Gaj, M.D. Factors Influencing Somatic Embryogenesis Induction and Plant Regeneration with Particular Reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul. 2004, 43, 27–47. [Google Scholar] [CrossRef]
- Raghavan, V. Role of 2,4-dichlorophenoxyacetic acid (2,4-D) in somatic embryogenesis on cultured zygotic embryos of Arabidopsis: Cell expansion, cell cycling, and morphogenesis during continuous exposure of embryos to 2,4-D. Am. J. Bot. 2004, 91, 1743–1756. [Google Scholar] [CrossRef]
- Borkird, C.; Choi, J.H.; Sung, Z.R. Effect of 2,4-dichlorophenoxyacetic Acid on the expression of embryogenic program in carrot. Plant Physiol. 1986, 81, 1143–1146. [Google Scholar] [CrossRef]
- Asghar, S.; Ghori, N.; Hyat, F.; Li, Y.; Chen, C. Use of auxin and cytokinin for somatic embryogenesis in plant: A story from competence towards completion. Plant Growth Regul. 2023, 99, 413–428. [Google Scholar] [CrossRef]
- Martínez, M.T.; Corredoira, E. Recent Advances in Plant Somatic Embryogenesis: Where We Stand and Where to Go? Int. J. Mol. Sci. 2024, 25, 8912. [Google Scholar] [CrossRef]
- Lai, Z.; Chen, C. Endogenous hormonal changes during somatic embryogenesis of longan. Chin. J. Trop. Crops 2002, 23, 7. [Google Scholar]
- Lai, Z.; Lin, Y. Analysis of the global transcriptome of longan (Dimocarpus longan Lour.) embryogenic callus using Illumina paired-end sequencing. BMC Genom. 2013, 14, 561. [Google Scholar] [CrossRef]
- Massardo, F.; Zúñiga, G.E.; Ptrez, L.M.; Corcuerat, L.J. Effects of hydroxamic acids on electron transport and their cellular location in corn. Phytochemistry 1994, 35, 873–876. [Google Scholar] [CrossRef]
- Zúñiga, G.E.; Copaja, S.V.; Bravo, H.R.; Argandoña, V.H. Hydroxamic acids accumulation by wheat callus. Phytochemistry 1990, 29, 2139–2141. [Google Scholar] [CrossRef]
- He, X.; Gu, Z.; Zhang, G.; Ye, L. Identification of metabolites associated with plant regeneration capacity of barley callus. Plant Growth Regul. 2023, 100, 71–83. [Google Scholar] [CrossRef]
- Lozovaya, V.; Ulanov, A.; Lygin, A.; Duncan, D.; Widholm, J. Biochemical features of maize tissues with different capacities to regenerate plants. Planta 2006, 224, 1385–1399. [Google Scholar] [CrossRef]
- Li, X.; Shi, S.; Zhang, X.; Li, C.; Wang, H.; Kang, W.; Yin, G. Potential Effect of DIMBOA (2,4-Dihydroxy-7-methoxy-1,4-benzoxazin-3-one) on Alleviating the Autotoxic Coumarin Stress in Alfalfa (Medicago sativa) Seedlings. Life 2022, 12, 2140. [Google Scholar] [CrossRef]
- Venis, M.A.; Watson, P.J. Naturally occuring modifiers of auxin-receptor interaction in corn: Identification as benzoxazolinones. Planta 1978, 142, 103–107. [Google Scholar] [CrossRef]
- Hasegawa, K.; Togo, S.; Urashima, M.; Mizutani, J.; Kosemura, S.; Yamamura, S. An auxin-inhibiting substance from light-grown maize shoots. Phytochemistry 1992, 31, 3673–3676. [Google Scholar] [CrossRef]
- González, L.F.; Rojas, M.C. Role of wall peroxidases in oat growth inhibition by DIMBOA. Phytochemistry 1999, 50, 931–937. [Google Scholar] [CrossRef]
- Nakajima, E.; Hasegawa, K.; Yamada, K.; Kosemura, S.; Yamamura, S. Effects of the auxin-inhibiting substances raphanusanin and benzoxazolinone on apical dominance of pea seedlings. Plant Growth Regul. 2001, 35, 11–15. [Google Scholar] [CrossRef]
- Park, W.J.; Schäfer, A.; Prinsen, E.; van Onckelen, H.; Kang, B.G.; Hertel, R. Auxin-induced elongation of short maize coleoptile segments is supported by 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one. Planta 2001, 213, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hasegawa, T.; Miyamoto, K.; Ueda, J.; Hasegawa, K. Isolation and Identification of Phototropism-regulating Sub stances Benzoxazinoids from Maize Coleoptiles. Heterocycles 2004, 63, 2707–2712. [Google Scholar] [CrossRef]
- Hoshi-Sakoda, M.; Usui, K.; Ishizuka, K.; Kosemura, S.; Yamamura, S.; Hasegawa, K. Structure-activity relationships of benzoxazolinones with respect to auxin-induced growth and auxin-binding protein. Phytochemistry 1994, 37, 297–300. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, C.; Lai, C.; Zhang, Z.; Wu, J.; Su, Q.; Gan, Y.; Zhang, Z.; Chen, Y.; Guo, R.; et al. Genome-Wide Identification and Expression Analysis of Bx Involved in Benzoxazinoids Biosynthesis Revealed the Roles of DIMBOA during Early Somatic Embryogenesis in Dimocarpus longan Lour. Plants 2024, 13, 1373. [Google Scholar] [CrossRef]
- Yamada, K.; Jabeen, R.; Hasegawa, T.; Minami, E.; Hasegawa, K. Direct Involvement of Benzoxazinoids in the Growth Suppression Induced by Phototropic Stimulation in Maize Coleoptiles. Heterocycles 2007, 71, 2316–2319. [Google Scholar] [CrossRef]
- Su, C.; Liu, L.; Liu, H.; Ferguson, B.J.; Zou, Y.; Zhao, Y.; Wang, T.; Wang, Y.; Li, X. H2O2 regulates root system architecture by modulating the polar transport and redistribution of auxin. J. Plant Biol. 2016, 59, 260–270. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, M. Effect of hydrogen peroxide on auxin signaling-related proteins in Arabidopsis thaliana. J. South China Norm. Univ. 2017, 49, 64–71. [Google Scholar]
- Wójcikowska, B.; Chwiałkowska, K.; Nowak, K.; Citerne, S.; Morończyk, J.; Wójcik, A.M.; Kiwior-Wesołowska, A.; Francikowski, J.; Kwaśniewski, M.; Gaj, M.D. Transcriptomic profiling reveals histone acetylation-regulated genes involved in somatic embryogenesis in Arabidopsis thaliana. BMC Genom. 2024, 25, 788. [Google Scholar] [CrossRef]
- Gliwicka, M.; Nowak, K.; Balazadeh, S.; Mueller-Roeber, B.; Gaj, M.D. Extensive modulation of the transcription factor transcriptome during somatic embryogenesis in Arabidopsis thaliana. PLoS ONE 2013, 8, e69261. [Google Scholar] [CrossRef]
- Li, H.Z.; Wu, H.; Song, K.K.; Zhao, H.H.; Tang, X.Y.; Zhang, X.H.; Wang, D.; Dong, S.L.; Liu, F.; Wang, J.; et al. Transcriptome analysis revealed enrichment pathways and regulation of gene expression associated with somatic embryogenesis in Camellia sinensis. Sci. Rep. 2023, 13, 15946. [Google Scholar] [CrossRef]
- Awada, R.; Lepelley, M.; Breton, D.; Charpagne, A.; Campa, C.; Berry, V.; Georget, F.; Breitler, J.-C.; Léran, S.; Djerrab, D.; et al. Global transcriptome profiling reveals differential regulatory, metabolic and hormonal networks during somatic embryogenesis in Coffea arabica. BMC Genom. 2023, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.-L.; Zhou, Y.-K.; Guo, D.; Zhu, J.-H.; Peng, S.-Q. Transcriptomes analysis reveals novel insight into the molecular mechanisms of somatic embryogenesis in Hevea brasiliensis. BMC Genom. 2021, 22, 183. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-I.; Lee, C.-B.; Kwon, S.-H.; Park, J.-M.; Kang, K.-S.; Shim, D. Comparative transcriptome analysis during developmental stages of direct somatic embryogenesis in Tilia amurensis Rupr. Sci. Rep. 2021, 11, 6359. [Google Scholar] [CrossRef] [PubMed]
- Chen, R. Genome-Wide Identification, Expression and Function Analysis of AGO Gene Family in Early Somatic Embryogenesis of Longan. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2021. [Google Scholar]
- Drewke, C.; Leistner, E. Biosynthesis of vitamin B6 and structurally related derivatives. In Vitamins & Hormones; Academic Press: Cambridge, MA, USA, 2001; Volume 61, pp. 121–155. [Google Scholar]
- Nowak, K.; Gaj, M.D. Transcription Factors in the Regulation of Somatic Embryogenesis. In Somatic Embryogenesis: Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 53–79. [Google Scholar]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A. Somatic embryogenesis—Stress-induced remodeling of plant cell fate. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2015, 1849, 385–402. [Google Scholar] [CrossRef]
- Reis, E.; Batista, M.T.; Canhoto, J.M. Effect and analysis of phenolic compounds during somatic embryogenesis induction in Feijoa sellowiana Berg. Protoplasma 2008, 232, 193–202. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, P.; Htwe, Y.M.; Li, Z.; Ihase, L.O.; Mason, A.S.; Sun, X.; Xiao, Y.; Wang, Y. Caffeate may play an important role in the somatic embryogenesis of oil palm (Elaeis guineensis Jacq.). Ind. Crops Prod. 2021, 174, 114143. [Google Scholar] [CrossRef]
- Fan, Y.; Yu, X.; Guo, H.; Wei, J.; Guo, H.; Zhang, L.; Zeng, F. Dynamic Transcriptome Analysis Reveals Uncharacterized Complex Regulatory Pathway Underlying Dose IBA-Induced Embryogenic Redifferentiation in Cotton. Int. J. Mol. Sci. 2020, 21, 426. [Google Scholar] [CrossRef]
- Olivares-García, C.A.; Mata-Rosas, M.; Peña-Montes, C.; Quiroz-Figueroa, F.; Segura-Cabrera, A.; Shannon, L.M.; Loyola-Vargas, V.M.; Monribot-Villanueva, J.L.; Elizalde-Contreras, J.M.; Ibarra-Laclette, E.; et al. Phenylpropanoids Are Connected to Cell Wall Fortification and Stress Tolerance in Avocado Somatic Embryogenesis. Int. J. Mol. Sci. 2020, 21, 5679. [Google Scholar] [CrossRef]
- Cvikrová, M.; Malá, J.; Hrubcová, M.; Eder, J.; Zoń, J.; Macháčková, I. Effect of inhibition of biosynthesis of phenylpropanoids on sessile oak somatic embryogenesis. Plant Physiol. Biochem. 2003, 41, 251–259. [Google Scholar] [CrossRef]
- Kurepa, J.; Shull, T.E.; Karunadasa, S.S.; Smalle, J.A. Modulation of auxin and cytokinin responses by early steps of the phenylpropanoid pathway. BMC Plant Biol. 2018, 18, 278. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.E.; Rashotte, A.M.; Murphy, A.S.; Normanly, J.; Tague, B.W.; Peer, W.A.; Taiz, L.; Muday, G.K. Flavonoids act as negative regulators of auxin transport in vivo in arabidopsis. Plant Physiol. 2001, 126, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Steenackers, W.; Cesarino, I.; Klíma, P.; Quareshy, M.; Vanholme, R.; Corneillie, S.; Kumpf, R.P.; Van de Wouwer, D.; Ljung, K.; Goeminne, G.; et al. The Allelochemical MDCA Inhibits Lignification and Affects Auxin Homeostasis. Plant Physiol. 2016, 172, 874–888. [Google Scholar] [CrossRef]
- Guo, H.; Guo, H.; Zhang, L.; Tang, Z.; Yu, X.; Wu, J.; Zeng, F. Metabolome and Transcriptome Association Analysis Reveals Dynamic Regulation of Purine Metabolism and Flavonoid Synthesis in Transdifferentiation during Somatic Embryogenesis in Cotton. Int. J. Mol. Sci. 2019, 20, 2070. [Google Scholar] [CrossRef]
- Maximova, S.N.; Florez, S.; Shen, X.; Niemenak, N.; Zhang, Y.; Curtis, W.; Guiltinan, M.J. Genome-wide analysis reveals divergent patterns of gene expression during zygotic and somatic embryo maturation of Theobroma cacao L., the chocolate tree. BMC Plant Biol. 2014, 14, 185. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Qi, L.; Zhang, S. Integrated transcriptomic and metabolic analyses provide insights into the maintenance of embryogenic potential and the biosynthesis of phenolic acids and flavonoids involving transcription factors in Larix kaempferi (Lamb.) Carr. Front. Plant Sci. 2022, 13, 1056930. [Google Scholar] [CrossRef]
- Peng, C.; Gao, F.; Tretyakova, I.N.; Nosov, A.M.; Shen, H.; Yang, L. Transcriptomic and Metabolomic Analysis of Korean Pine Cell Lines with Different Somatic Embryogenic Potential. Int. J. Mol. Sci. 2022, 23, 13301. [Google Scholar] [CrossRef]
- Navarro, B.V.; Elbl, P.; De Souza, A.P.; Jardim, V.; de Oliveira, L.F.; Macedo, A.F.; Dos Santos, A.L.W.; Buckeridge, M.S.; Floh, E.I.S. Carbohydrate-mediated responses during zygotic and early somatic embryogenesis in the endangered conifer, Araucaria angustifolia. PLoS ONE 2017, 12, e0180051. [Google Scholar] [CrossRef]
- Cheng, W.H.; Zhu, H.G.; Tian, W.G.; Zhu, S.H.; Xiong, X.P.; Sun, Y.Q.; Zhu, Q.H.; Sun, J. De novo transcriptome analysis reveals insights into dynamic homeostasis regulation of somatic embryogenesis in upland cotton (G. hirsutum L.). Plant Mol. Biol. 2016, 92, 279–292. [Google Scholar] [CrossRef]
- Guo, H.; Guo, H.; Zhang, L.; Fan, Y.; Wu, J.; Tang, Z.; Zhang, Y.; Fan, Y.; Zeng, F. Dynamic Transcriptome Analysis Reveals Uncharacterized Complex Regulatory Pathway Underlying Genotype-Recalcitrant Somatic Embryogenesis Transdifferentiation in Cotton. Genes 2020, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.G.; Cheng, W.H.; Tian, W.G.; Li, Y.J.; Liu, F.; Xue, F.; Zhu, Q.H.; Sun, Y.Q.; Sun, J. iTRAQ-based comparative proteomic analysis provides insights into somatic embryogenesis in Gossypium hirsutum L. Plant Mol. Biol. 2018, 96, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shan, X.; Wu, Y.; Su, S.; Li, S.; Liu, H.; Han, J.; Yuan, Y. iTRAQ-Based Quantitative Proteomic Analysis of Embryogenic and Non-embryogenic Calli Derived from a Maize (Zea mays L.) Inbred Line Y423. Int. J. Mol. Sci. 2018, 19, 4004. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Jiang, Z.; Yao, X.; Zhang, Z.; Lin, H.; Zhao, M.; Liu, H.; Peng, H.; Li, S.; Pan, G. Genome expression profile analysis of the immature maize embryo during dedifferentiation. PLoS ONE 2012, 7, e32237. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Morency, F.; Jones-Overton, C.; Cooke, J. Accumulation pattern and identification of seed storage proteins in zygotic embryos of Pinus strobus and in somatic embryos from different maturation treatments. Physiol. Plant 2004, 121, 682–690. [Google Scholar] [CrossRef]
- Schuller, A.; Reuther, G. Response of Abies alba embryonal-suspensor mass to various carbohydrate treatments. Plant Cell Rep. 1993, 12, 199–202. [Google Scholar] [CrossRef]
- Gómez, L.D.; Gilday, A.; Feil, R.; Lunn, J.E.; Graham, I.A. AtTPS1-mediated trehalose 6-phosphate synthesis is essential for embryogenic and vegetative growth and responsiveness to ABA in germinating seeds and stomatal guard cells. Plant J. Cell Mol. Biol. 2010, 64, 1–13. [Google Scholar] [CrossRef]
- Konrádová, H.; Lipavská, H.; Albrechtová, J.; Vreugdenhil, D. Sucrose metabolism during somatic and zygotic embryogeneses in Norway spruce: Content of soluble saccharides and localisation of key enzyme activities. J. Plant Physiol. 2002, 159, 387–396. [Google Scholar] [CrossRef]
- Kubeš, M.; Drážná, N.; Konrádová, H.; Lipavská, H. Robust carbohydrate dynamics based on sucrose resynthesis in developing Norway spruce somatic embryos at variable sugar supply. Vitr. Cell. Dev. Biol. Plant 2014, 50, 45–57. [Google Scholar] [CrossRef]
- Navarro, B.V.; de Oliveira, L.F.; de Oliveira, L.P.; Elbl, P.; Macedo, A.F.; Buckeridge, M.S.; Floh, E.I.S. Starch turnover is stimulated by nitric oxide in embryogenic cultures of Araucaria angustifolia. Plant Cell Tissue Organ Cult. 2021, 147, 583–597. [Google Scholar] [CrossRef]
- Guo, J.; Sun, B.; He, H.; Zhang, Y.; Tian, H.; Wang, B. Current Understanding of bHLH Transcription Factors in Plant Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 4921. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.Y.; Kagale, S.; Ferrie, A.M.R. Multifaceted roles of transcription factors during plant embryogenesis. Front. Plant Sci. 2023, 14, 1322728. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, T.; Ling, J.; Yao, C.; Lu, N.; Kong, L.; Zhang, H.; Wang, J. Integrated transcriptome and physiological analysis reveal the molecular mechanism of the osmotic-responses induced by cryoprotectants in Norway spruce embryogenic tissue. Ind. Crops Prod. 2023, 195, 116440. [Google Scholar] [CrossRef]
- Li, W.; Pang, S.; Lu, Z.; Jin, B. Function and Mechanism of WRKY Transcription Factors in Abiotic Stress Responses of Plants. Plants 2020, 9, 1515. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Q.; Zhang, L.; Zhang, H. Expression Pattern Analysis of Larch WRKY in Response to Abiotic Stress. Forests 2022, 13, 2123. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Wang, R.; Feng, K.; Di, J.; Feng, T.; Cao, F. Transcriptome and physiological analysis highlights the hormone, phenylpropanoid, and photosynthesis effects on early somatic embryogenesis in Ginkgo biloba. Ind. Crops Prod. 2023, 203, 117176. [Google Scholar] [CrossRef]
- Wang, X.; Niu, Q.-W.; Teng, C.; Li, C.; Mu, J.; Chua, N.-H.; Zuo, J. Overexpression of PGA37/MYB118 and MYB115 promotes vegetative-to-embryonic transition in Arabidopsis. Cell Res. 2009, 19, 224–235. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, N.; Zhao, S. Functional characterization of a WRKY family gene involved in somatic embryogenesis in Panax ginseng. Protoplasma 2020, 257, 449–458. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, J.; Li, L.; Zhao, M.; Zhang, M.; Wang, Y. Research Progress on AP2/ERF Transcription Factor Family in Plants. Biotechnol. Bull. 2018, 34, 7. [Google Scholar]
- Piyatrakul, P.; Putranto, R.A.; Martin, F.; Rio, M.; Dessailly, F.; Leclercq, J.; Dufayard, J.F.; Lardet, L.; Montoro, P. Some ethylene biosynthesis and AP2/ERF genes reveal a specific pattern of expression during somatic embryogenesis in Hevea brasiliensis. BMC Plant Biol. 2012, 12, 244. [Google Scholar] [CrossRef]
- Chen, B.; Maas, L.; Figueiredo, D.; Zhong, Y.; Reis, R.; Li, M.; Horstman, A.; Riksen, T.; Weemen, M.; Liu, H.; et al. BABY BOOM regulates early embryo and endosperm development. Proc. Natl. Acad. Sci. USA 2022, 119, e2201761119. [Google Scholar] [CrossRef] [PubMed]
- Banno, H.; Ikeda, Y.; Niu, Q.W.; Chua, N.H. Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell 2001, 13, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- El Ouakfaoui, S.; Schnell, J.; Abdeen, A.; Colville, A.; Labbé, H.; Han, S.; Baum, B.; Laberge, S.; Miki, B. Control of somatic embryogenesis and embryo development by AP2 transcription factors. Plant Mol. Biol. 2010, 74, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Petroni, K.; Kumimoto, R.W.; Gnesutta, N.; Calvenzani, V.; Fornari, M.; Tonelli, C.; Holt, B.F., 3rd; Mantovani, R. The promiscuous life of plant NUCLEAR FACTOR Y transcription factors. Plant Cell 2012, 24, 4777–4792. [Google Scholar] [CrossRef]
- Lee, H.; Fischer, R.L.; Goldberg, R.B.; Harada, J.J. Arabidopsis LEAFY COTYLEDON1 represents a functionally specialized subunit of the CCAAT binding transcription factor. Proc. Natl. Acad. Sci. USA 2003, 100, 2152–2156. [Google Scholar] [CrossRef]
- Fornari, M.; Calvenzani, V.; Masiero, S.; Tonelli, C.; Petroni, K. The Arabidopsis NF-YA3 and NF-YA8 Genes Are Functionally Redundant and Are Required in Early Embryogenesis. PLoS ONE 2013, 8, e82043. [Google Scholar] [CrossRef]
- Zheng, Y.; Ren, N.; Wang, H.; Stromberg, A.J.; Perry, S.E. Global identification of targets of the Arabidopsis MADS domain protein AGAMOUS-Like15. Plant Cell 2009, 21, 2563–2577. [Google Scholar] [CrossRef]
- Fang, J.; He, Y.; Yu, M.; Zheng, B. Research progress on auxin response factor genes in plants. J. Zhejiang AF Univ. 2012, 29, 611–616. [Google Scholar]
- Zhao, L.; Yang, Y.; Chen, J.; Lin, X.; Zhang, H.; Wang, H.; Wang, H.; Bie, X.; Jiang, J.; Feng, X.; et al. Dynamic chromatin regulatory programs during embryogenesis of hexaploid wheat. Genome Biol. 2023, 24, 7. [Google Scholar] [CrossRef]
- Elhiti, M.; Stasolla, C. Transduction of Signals during Somatic Embryogenesis. Plants 2022, 11, 178. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Ksas, B.; Szewczyk, A.; Rumeau, D.; Franck, F.; Caffarri, S.; Triantaphylidès, C. Vitamin B6 deficient plants display increased sensitivity to high light and photo-oxidative stress. BMC Plant Biol. 2009, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Danon, A.; Miersch, O.; Felix, G.; Camp, R.G.; Apel, K. Concurrent activation of cell death-regulating signaling pathways by singlet oxygen in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2005, 41, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiong, L. Pyridoxine is required for post-embryonic root development and tolerance to osmotic and oxidative stresses. Plant J. Cell Mol. Biol. 2005, 44, 396–408. [Google Scholar] [CrossRef]
- Lu, C.; Tian, Y.; Hou, X.; Hou, X.; Jia, Z.; Li, M.; Hao, M.; Jiang, Y.; Wang, Q.; Pu, Q.; et al. Multiple forms of vitamin B(6) regulate salt tolerance by balancing ROS and abscisic acid levels in maize root. Stress Biol. 2022, 2, 39. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, K.; Xiong, H.; Xie, Y.; Li, R.; Wu, X.; Yang, Y.; Wu, H.; Hao, Z.; Sun, X.; et al. H2O2 Significantly Affects Larix kaempferi × Larix olgensis Somatic Embryogenesis. Int. J. Mol. Sci. 2024, 25, 669. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, X.; Gu, M.; Xu, X. Physiological and biochemical study during embryo induction of somatic cells in Ponkan. Bot. Res. 2021, 10, 7. [Google Scholar]
- Ma, L.; Xie, L.; Lin, G.; Jiang, S.; Chen, H.; Li, H.; Takáč, T.; Šamaj, J.; Xu, C. Histological changes and differences in activities of some antioxidant enzymes and hydrogen peroxide content during somatic embryogenesis of Musa AAA cv. Yueyoukang 1. Sci. Hortic. 2012, 144, 87–92. [Google Scholar] [CrossRef]
- Wu, G.; Wei, X.; Wang, X.; Wei, Y. Changes in biochemistry and histochemical characteristics during somatic embryogenesis in Ormosia henryi Prain. Plant Cell Tissue Organ Cult. 2021, 144, 505–517. [Google Scholar] [CrossRef]
- Muhammad, I.; Ullah, S.; Kumar, N.; Bala, H.; Mathur, A. Efficiency of vitamin B6 (Pyridoxine HCl) on production of Endogenous IAA for growth promotion in Zea mays varieties (Azam & Jalal). Int. J. Bot. Stud. 2021, 6, 486–492. [Google Scholar]
- Tao, Y.; Ferrer, J.L.; Ljung, K.; Pojer, F.; Hong, F.; Long, J.A.; Li, L.; Moreno, J.E.; Bowman, M.E.; Ivans, L.J.; et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 2008, 133, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Won, C.; Shen, X.; Mashiguchi, K.; Zheng, Z.; Dai, X.; Cheng, Y.; Kasahara, H.; Kamiya, Y.; Chory, J.; Zhao, Y. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 18518–18523. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiong, L. The short-rooted vitamin B6-deficient mutant pdx1 has impaired local auxin biosynthesis. Planta 2009, 229, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Boycheva, S.; Dominguez, A.; Rolcik, J.; Boller, T.; Fitzpatrick, T.B. Consequences of a deficit in vitamin B6 biosynthesis de novo for hormone homeostasis and root development in Arabidopsis. Plant Physiol. 2015, 167, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Yoo, H.J.; Hwang, I.; Lee, J.S.; Nam, K.H.; Bae, Y.S. Auxin-induced reactive oxygen species production requires the activation of phosphatidylinositol 3-kinase. FEBS Lett. 2005, 579, 1243–1248. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional Regulation of ROS Controls Transition from Proliferation to Differentiation in the Root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Zhang, C.; Tong, N.; Lan, X.; Cui, J.; Muhammad, A.; Zhang, Z.; Zhang, Z.; Chen, Y.; Lin, Y.; et al. Transcriptome Analysis Revealed the Regulatory Mechanism of DIMBOA Affecting Early Somatic Embryogenesis in Dimocarpus longan Lour. Plants 2025, 14, 442. https://doi.org/10.3390/plants14030442
Xu X, Zhang C, Tong N, Lan X, Cui J, Muhammad A, Zhang Z, Zhang Z, Chen Y, Lin Y, et al. Transcriptome Analysis Revealed the Regulatory Mechanism of DIMBOA Affecting Early Somatic Embryogenesis in Dimocarpus longan Lour. Plants. 2025; 14(3):442. https://doi.org/10.3390/plants14030442
Chicago/Turabian StyleXu, Xiaoqiong, Chunyu Zhang, Ning Tong, Xiaoyuan Lan, Jing Cui, Awais Muhammad, Zhilin Zhang, Zihao Zhang, Yukun Chen, Yuling Lin, and et al. 2025. "Transcriptome Analysis Revealed the Regulatory Mechanism of DIMBOA Affecting Early Somatic Embryogenesis in Dimocarpus longan Lour." Plants 14, no. 3: 442. https://doi.org/10.3390/plants14030442
APA StyleXu, X., Zhang, C., Tong, N., Lan, X., Cui, J., Muhammad, A., Zhang, Z., Zhang, Z., Chen, Y., Lin, Y., & Lai, Z. (2025). Transcriptome Analysis Revealed the Regulatory Mechanism of DIMBOA Affecting Early Somatic Embryogenesis in Dimocarpus longan Lour. Plants, 14(3), 442. https://doi.org/10.3390/plants14030442







