L-Theanine Metabolism in Tea Plants: Biological Functions and Stress Tolerance Mechanisms
Abstract
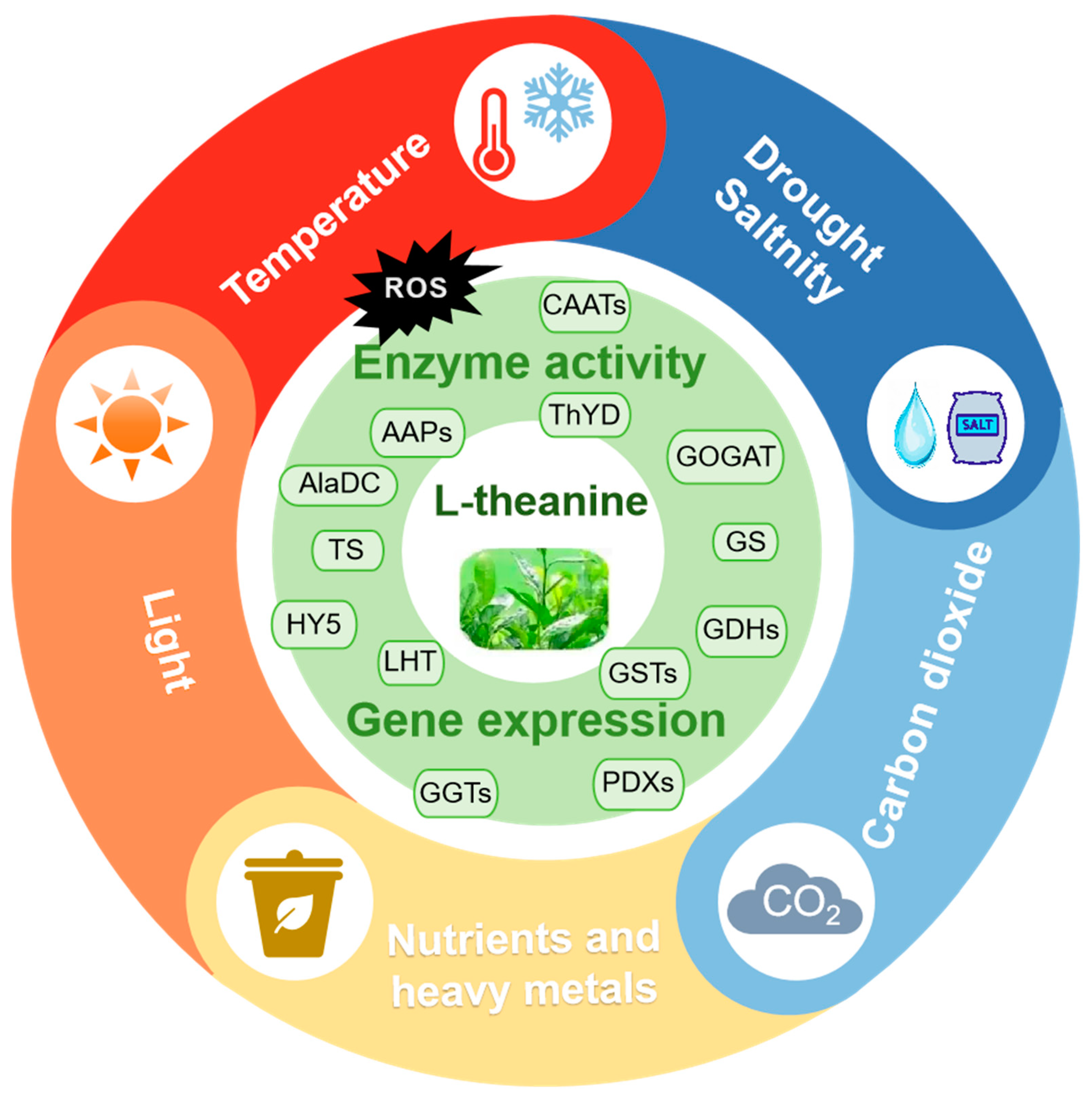
1. Introduction
2. Metabolism and Transport of L-Theanine in Tea Plants
2.1. Spatial Distribution of L-Theanine in Tea Plants
2.2. Biosynthesis of L-Theanine in Tea Plants
2.3. Hydrolysis of L-Theanine in Tea Plants
2.4. Transportation of L-Theanine in Tea Plants
3. Response of L-Theanine Metabolism to Stress in Tea Plants
3.1. Effect of Temperature Stress on L-Theanine Metabolism
3.2. Effect of Light on L-Theanine Metabolism
3.3. Effect of Drought Stress and Salinity on L-Theanine Metabolism
3.4. Effect of Elevated CO2 Concentration on L-Theanine Metabolism
3.5. Effect of Nutrient Elements and Heavy Metal Stress on L-Theanine Metabolism
4. Molecular Mechanism of L-Theanine Metabolism to Stress in Tea Plants
4.1. Effect of Plant Hormones and Growth Regulators on L-Theanine Metabolism
4.2. Effect of Transcription Factors on L-Theanine Metabolism
5. Application of L-Theanine in Abiotic Stress Tolerance
6. Prospects and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Song, C.; Zhao, J.; Xia, E.; Wen, W.; Zeng, L.; Benedito, V.A. Editorial: Secondary Metabolites and Metabolism in Tea Plants. Front. Plant Sci. 2023, 14, 1143022. [Google Scholar] [CrossRef]
- Mahadevan, N.; Sinniah, G.D.; Gunasekaram, P.; Karunajeewa, D.G.N.P. How Tea Plant Defends Against Blister Blight Disease: Facts Revealed and Unexplored Horizons. Plant Dis. 2024, 108, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Sinniah, G.D.; Babu, A.; Tanti, A. How the Global Tea Industry Copes with Fungal Diseases—Challenges and Opportunities. Plant Dis. 2021, 105, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Kaczyński, P.; Iwaniuk, P.; Jankowska, M.; Orywal, K.; Socha, K.; Perkowski, M.; Farhan, J.A.; Lozowicka, B. Pesticide residues in common and herbal teas combined with risk assessment and transfer to the infusion. Chemosphere 2024, 367, 143550. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.S.; Dias, A.P.; Motta, M.H.; Baptista, J.A.B. Phytochemicals and Biological Properties of Azorean Camellia sinensis Black Tea Samples from Different Zones of Tea Plantation. Plants 2025, 14, 103. [Google Scholar] [CrossRef]
- Sakato, Y. The Chemical Constituents of Tea: III. A New Amide Theanine. Nippon Nogkagaku Kaishi 1949, 23, 262–267. [Google Scholar] [CrossRef]
- Chen, S.; Kang, J.; Zhu, H.; Wang, K.; Han, Z.; Wang, L.; Liu, J.; Wu, Y.; He, P.; Tu, Y.; et al. L-Theanine and Immunity: A Review. Molecules 2023, 28, 3846. [Google Scholar] [CrossRef]
- Altinkaynak, Y.; Burenkova, E.; Buket, A. The protective powers of L-theanine against drug-induced kidney damage. Clin. Nephrol. 2025, 103, 116–128. [Google Scholar] [CrossRef]
- Han, W.; Ahmed, S.; Wei, C.; Orians, C.M.; Landi, M. Editorial: Responses of Tea Plants to Climate Change: From Molecules to Ecosystems. Front. Plant Sci. 2020, 11, 594317. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Zhang, Y.; Dong, Y.; Liu, Y.; Liu, L.; Wan, S.; He, J.; Yu, Y. Accumulation of Galactinol and ABA is Involved in Exogenous EBR-Induced Drought Tolerance in Tea Plants. J. Agric. Food Chem. 2022, 70, 13391–13403. [Google Scholar] [CrossRef]
- Ahmed, S.; Griffin, T.S.; Kraner, D.; Schaffner, M.K.; Sharma, D.; Hazel, M.; Leitch, A.R.; Orians, C.M.; Han, W.; Stepp, J.R.; et al. Environmental Factors Variably Impact Tea Secondary Metabolites in the Context of Climate Change. Front. Plant Sci. 2019, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Ogita, S.; Ashihara, H. Distribution and Biosynthesis of Theanine in Theaceae Plants. Plant Physiol. Biochem. 2010, 48, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhou, X.; Zeng, L. How does tea (Camellia sinensis) produce specialized metabolites which determine its unique quality and function: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3751–3767. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xu, Q.; Zhao, S.; Xia, X.; Yan, X.; An, Y.; Mi, X.; Guo, L.; Samarina, L.; Wei, C. Comprehensive Co-Expression Analysis Provides Novel Insights into Temporal Variation of Flavonoids in Fresh Leaves of the Tea Plant (Camellia Sinensis). Plant Sci. Int. J. Exp. Plant Biol. 2020, 290, 110306. [Google Scholar] [CrossRef]
- Gong, A.; Lian, S.; Wu, N.; Zhou, Y.; Zhao, S.; Zhang, L.; Cheng, L.; Yuan, H. Integrated Transcriptomics and Metabolomics Analysis of Catechins, Caffeine and Theanine Biosynthesis in Tea Plant (Camellia Sinensis) over the Course of Seasons. BMC Plant Biol. 2020, 20, 294. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, L.; Lei, Y.; Ju, R.; Miao, S.; Jin, S. Integrated transcriptome and amino acid profile analyses reveal novel insights into differential accumulation of theanine in green and yellow tea cultivars. Tree Physiol. 2022, 42, 1501–1516. [Google Scholar] [CrossRef]
- Wei, C.; Yang, H.; Wang, S.; Zhao, J.; Liu, C.; Gao, L.; Xia, E.; Lu, Y.; Tai, Y.; She, G.; et al. Draft Genome Sequence of Camellia Sinensis Var. Sinensis Provides Insights into the Evolution of the Tea Genome and Tea Quality. Proc. Natl. Acad. Sci. USA 2018, 115, E4151–E4158. [Google Scholar] [CrossRef]
- Fang, K.; Xia, Z.; Li, H.; Jiang, X.; Qin, D.; Wang, Q.; Wang, Q.; Pan, C.; Li, B.; Wu, H. Genome-Wide Association Analysis Identified Molecular Markers Associated with Important Tea Flavor-Related Metabolites. Hortic. Res. 2021, 8, 42. [Google Scholar] [CrossRef]
- Chen, J.; He, W.; Chen, S.; Chen, Q.; Ma, J.; Jin, J.; Ma, C.; Moon, D.; Ercisli, S.; Yao, M.; et al. TeaGVD: A Comprehensive Database of Genomic Variations for Uncovering the Genetic Architecture of Metabolic Traits in Tea Plants. Front. Plant Sci. 2022, 13, 1056891. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, J.; Yu, Y.; Tian, Y.; Li, H.; Chen, X.; Li, W.; Liu, Y.; Lu, T.; He, B.; et al. Root Microbiota of Tea Plants Regulate Nitrogen Homeostasis and Theanine Synthesis to Influence Tea Quality. Current. Biol. 2024, 34, 868–880. [Google Scholar] [CrossRef]
- Ashihara, H. Occurrence, Biosynthesis and Metabolism of Theanine (γ-Glutamyl-L-Ethylamide) in Plants: A Comprehensive Review. Nat. Prod. Commun. 2015, 10, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liao, Y.; Cheng, S.; Xu, X.; Grierson, D.; Yang, Z. Nonaqueous Fractionation and Overexpression of Fluorescent-Tagged Enzymes Reveals the Subcellular Sites of L-Theanine Biosynthesis in Tea. Plant Biotechnol. J. 2021, 19, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Tian, L.; Zheng, D.; Gao, Y.; Sun, C.; Zhang, S.; Zhang, Z.; Wan, X.; Chen, Q. Visualization of Metabolite Distribution Based on Matrix-Assisted Laser Desorption/Ionization-Mass Spectrometry Imaging of Tea Seedlings (Camellia sinensis). Hortic. Res. 2024, 11, uhae218. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, Z.; Chen, T.; Deng, W.; Wan, X.; Zhang, Z. Theanine Metabolism and Transport in Tea Plants (Camellia sinensis L.): Advances and Perspectives. Crit. Rev. Biotechnol. 2023, 43, 327–341. [Google Scholar] [CrossRef]
- Luo, Q.; He, H. Accumulation of theanine in tea plant (Camellia sinensis (L.) O. Kuntze): Biosynthesis, transportation and strategy for improvement. Plant Sci. 2025, 352, 112406. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Z.; Li, H.; Wang, Y.; Zhuang, J. L-Theanine Content and Related Gene Expression: Novel Insights into Theanine Biosynthesis and Hydrolysis among Different Tea Plant (Camellia sinensis L.) Tissues and Cultivars. Front. Plant Sci. 2017, 8, 498. [Google Scholar] [CrossRef]
- Cheng, S.; Fu, X.; Wang, X.; Liao, Y.; Zeng, L.; Dong, F.; Yang, Z. Studies on the Biochemical Formation Pathway of the Amino Acid L-Theanine in Tea (Camellia sinensis) and Other Plants. J. Agric. Food Chem. 2017, 65, 7210–7216. [Google Scholar] [CrossRef]
- She, G.; Yu, S.; Li, Z.; Peng, A.; Li, P.; Li, Y.; Chang, M.; Liu, L.; Chen, Q.; Shi, C.; et al. Characterization of CsTSI in the Biosynthesis of Theanine in Tea Plants (Camellia sinensis). J. Agric. Food Chem. 2022, 70, 826–836. [Google Scholar] [CrossRef]
- Deng, W.; Ashihara, H. Occurrence and de Novo Biosynthesis of Caffeine and Theanine in Seedlings of Tea (Camellia sinensis). Nat. Prod. Commun. 2015, 10, 703–706. [Google Scholar] [CrossRef]
- Dong, C.; Li, F.; Yang, T.; Feng, L.; Zhang, S.; Li, F.; Li, W.; Xu, G.; Bao, S.; Wan, X.; et al. Theanine Transporters Identified in Tea Plants (Camellia sinensis L.). Plant J. Cell Mol. Biol. 2020, 101, 57–70. [Google Scholar] [CrossRef]
- Zhu, B.; Guo, J.; Dong, C.; Li, F.; Qiao, S.; Lin, S.; Yang, T.; Wu, Y.; Bao, S.; Lucas, W.J.; et al. CsAlaDC and CsTSI Work Coordinately to Determine Theanine Biosynthesis in Tea Plants (Camellia sinensis L.) and Confer High Levels of Theanine Accumulation in a Non-tea Plant. Plant Biotechnol. J. 2021, 19, 2395–2397. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Wang, L.; Wei, K.; Ruan, L.; Wu, L.; He, M.; Ni, D.; Cheng, H. Biochemical Characterization of Specific Alanine Decarboxylase (AlaDC) and Its Ancestral Enzyme Serine Decarboxylase (SDC) in Tea Plants (Camellia sinensis). BMC Biotechnol. 2021, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kou, X.; Gao, R.; Chen, X.; Zhao, Z.; Mei, H.; Li, J.; Jeyaraj, A.; Thangaraj, K.; Periakaruppan, R.; et al. Glutamine Synthetases Play a Vital Role in High Accumulation of Theanine in Tender Shoots of Albino Tea Germplasm “Huabai 1”. J. Agric. Food Chem. 2021, 69, 13904–13915. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gulati, A.; Sharma, U. Determination of Theanine and Catechin in Camellia sinensis (Kangra Tea) Leaves by HPTLC and NMR Techniques. Food Anal. Methods 2016, 9, 1666–1674. [Google Scholar] [CrossRef]
- Deng, W.; Ogita, S.; Ashihara, H. Ethylamine Content and Theanine Biosynthesis in Different Organs of Camellia sinensis Seedlings. Z. Fur Naturforschung. C J. Biosci. 2009, 64, 387–390. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; She, G.; Xu, Y.; Peng, A.; Wan, X.; Zhao, J. Molecular Basis of the Distinct Metabolic Features in Shoot Tips and Roots of Tea Plants (Camellia sinensis): Characterization of MYB Regulator for Root Theanine Synthesis. J. Agric. Food Chem. 2021, 69, 3415–3429. [Google Scholar] [CrossRef]
- Fu, X.; Liao, Y.; Cheng, S.; Deng, R.; Yang, Z. Stable Isotope-Labeled Precursor Tracing Reveals That L-Alanine Is Converted to L-Theanine Ala L -Glutamate Not Ethylamine in Tea Plants In Vivo. J. Agric. Food Chem. 2021, 69, 15354–15361. [Google Scholar] [CrossRef]
- Xia, E.; Zhang, H.; Sheng, J.; Li, K.; Zhang, Q.; Kim, C.; Zhang, Y.; Liu, Y.; Zhu, T.; Li, W.; et al. The Tea Tree Genome Provides Insights into Tea Flavor and Independent Evolution of Caffeine Biosynthesis. Mol. Plant 2017, 10, 866–877. [Google Scholar] [CrossRef]
- Tsushida, T.; Takeo, T. An Enzyme Hydrolyzing L-Theanine in Tea Leaves. Agric. Biol. Chem. 1985, 49, 2913–2917. [Google Scholar] [CrossRef][Green Version]
- Chang, M.; Ma, J.; Sun, Y.; Tian, L.; Liu, L.; Chen, Q.; Zhang, Z.; Wan, X.; Sun, J. γ-Glutamyl-transpeptidase CsGGT2 Functions as Light-activated Theanine Hydrolase in Tea Plant (Camellia sinensis L.). Plant Cell Environ. 2023, 46, 1596–1609. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, S.; Liao, Y.; Xu, X.; Wang, X.; Hao, X.; Xu, P.; Dong, F.; Yang, Z. Characterization of l -Theanine Hydrolase in Vitro and Subcellular Distribution of Its Specific Product Ethylamine in Tea (Camellia sinensis). J. Agric. Food Chem. 2020, 68, 10842–10851. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M. Transporters Involved in Source to Sink Partitioning of Amino Acids and Ureides: Opportunities for Crop Improvement. J. Exp. Bot. 2014, 65, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Wang, S.; Chen, Q.; Zhang, Z.; Hu, X. Effect of Salt Treatment on Theanine Biosynthesis in Camellia sinensis Seedlings. Plant Physiol. Biochem. 2012, 56, 35–40. [Google Scholar] [CrossRef]
- Li, F.; Dong, C.; Yang, T.; Ma, J.; Zhang, S.; Wei, C.; Wan, X.; Zhang, Z. Seasonal Theanine Accumulation and Related Gene Expression in the Roots and Leaf Buds of Tea Plants (Camellia sinensis L.). Front. Plant Sci. 2019, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Dong, C.; Yang, T.; Bao, S.; Fang, W.; Lucas, W.J.; Zhang, Z. The Tea Plant CsLHT1 and CsLHT6 Transporters Take up Amino Acids, as a Nitrogen Source, from the Soil of Organic Tea Plantations. Hortic. Res. 2021, 8, 178. [Google Scholar] [CrossRef]
- Li, F.; Lv, C.; Zou, Z.; Duan, Y.; Zhou, J.; Zhu, X.; Ma, Y.; Zhang, Z.; Fang, W. CsAAP7.2 Is Involved in the Uptake of Amino Acids from Soil and the Long-Distance Transport of Theanine in Tea Plants (Camellia sinensis L.). Tree Physiol. 2022, 42, 2369–2381. [Google Scholar] [CrossRef]
- Feng, L.; Yang, T.; Zhang, Z.; Li, F.; Chen, Q.; Sun, J.; Shi, C.; Deng, W.; Tao, M.; Tai, Y.; et al. Identification and Characterization of Cationic Amino Acid Transporters (CATs) in Tea Plant (Camellia sinensis). Plant Growth Regul. 2018, 84, 57–69. [Google Scholar] [CrossRef]
- Feng, L.; Yu, Y.; Lin, S.; Yang, T.; Chen, Q.; Liu, L.; Sun, J.; Zheng, P.; Zhang, Z.; Wan, X. Tonoplast-Localized Theanine Transporter CsCAT2 May Mediate Theanine Storage in the Root of Tea Plants (Camellia sinensis L.). Front. Plant Sci. 2021, 12, 797854. [Google Scholar] [CrossRef]
- Hao, X.; Xia, L.; Zhao, H.; Liu, J.; Guo, F.; Wang, P.; Wang, M.; Wang, Y.; Ni, D.; Zhao, H. CsABCG11.2 Mediates Theanine Uptake to Alleviate Cadmium Toxicity in Tea Plants (Camellia sinensis). Hortic. Adv. 2024, 2, 19. [Google Scholar] [CrossRef]
- Dai, W.; Qi, D.; Yang, T.; Lv, H.; Guo, L.; Zhang, Y.; Zhu, Y.; Peng, Q.; Xie, D.; Tan, J.; et al. Nontargeted Analysis Using Ultraperformance Liquid Chromatography–Quadrupole Time-of-Flight Mass Spectrometry Uncovers the Effects of Harvest Season on the Metabolites and Taste Quality of Tea (Camellia sinensis L.). J. Agric. Food Chem. 2015, 63, 9869–9878. [Google Scholar] [CrossRef]
- Xu, X.; Ye, X.; Xing, A.; Wu, Z.; Li, X.; Shu, Z.; Wang, Y. Camellia sinensis Small GTPase Gene (CsRAC1) Involves in Response to Salt Stress, Drought Stress and ABA Signaling Pathway. Gene 2022, 821, 146318. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Q.; Wang, W.; Ma, R.; Ding, C.; Wei, K.; Wang, L.; Ge, S.; Shi, Y.; Li, X. Transcriptomic and Metabolomic Insights into Temperature-Dependent Changes in Catechin and Anthocyanin Accumulation in Tea Plants with Different Leaf Colors. Plant Stress 2024, 14, 100705. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Liu, M.; Ma, L.; Shi, Y.; Ruan, J. Metabolomic Analyses Reveal Distinct Change of Metabolites and Quality of Green Tea during the Short Duration of a Single Spring Season. J. Agric. Food Chem. 2016, 64, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Wang, W.; Wu, Z.; Cui, X.; Zhuang, J. CsGOGAT Is Important in Dynamic Changes of Theanine Content in Postharvest Tea Plant Leaves Under Different Temperature and Shading Spreadings. J. Agric. Food Chem. 2017, 65, 9693–9702. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Ahammed, G.J.; Zhang, L.; Li, Y.; Yan, P.; Zhang, L.; Han, W. Brassinosteroids Attenuate Moderate High Temperature-Caused Decline in Tea Quality by Enhancing Theanine Biosynthesis in Camellia sinensis L. Front. Plant Sci. 2018, 9, 1016. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Li, J.; Zhou, X.; Xiao, Y.; Liao, Y.; Tang, J.; Dong, F.; Zeng, L. Effects of Temperature and Light on Quality-Related Metabolites in Tea [Camellia sinensis (L.) Kuntze] Leaves. Food Res. Int. 2022, 161, 111882. [Google Scholar] [CrossRef]
- Xie, H.; Chen, Z.; Feng, X.; Wang, M.; Luo, Y.; Wang, Y.; Xu, P. L-Theanine Exuded from Camellia sinensis Roots Regulates Element Cycling in Soil by Shaping the Rhizosphere Microbiome Assembly. Sci. Total Environ. 2022, 837, 155801. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, X.; Mei, X.; Zhou, Y.; Cheng, S.; Zeng, L.; Dong, F.; Yang, Z. Proteolysis of Chloroplast Proteins Is Responsible for Accumulation of Free Amino Acids in Dark-Treated Tea (Camellia sinensis) Leaves. J. Proteom. 2017, 157, 10–17. [Google Scholar] [CrossRef]
- Ruan, J.; Ma, L.; Yang, Y. Magnesium Nutrition on Accumulation and Transport of Amino Acids in Tea Plants. J. Agric. Food Chem. 2011, 92, 1375–1383. [Google Scholar] [CrossRef]
- Cai, J.; Qiu, Z.; Liao, J.; Li, A.; Chen, J.; Wu, Z.; Khan, W.; Sun, B.; Liu, S.; Zheng, P. Comprehensive Analysis of the Yield and Leaf Quality of Fresh Tea (Camellia sinensis cv. Jin Xuan) under Different Nitrogen Fertilization Levels. Foods 2024, 13, 2091. [Google Scholar] [CrossRef]
- Deng, W.; Fei, Y.; Wang, S.; Wan, X.; Zhang, Z.; Hu, X. Effect of shade treatment on theanine biosynthesis in Camellia sinensis seedlings. Plant Growth Regul. 2013, 71, 295–299. [Google Scholar] [CrossRef]
- Deb, S.; Dutta, A.; Phukan, B.C.; Manivasagam, T.; Justin Thenmozhi, A.; Bhattacharya, P.; Paul, R.; Borah, A. Neuroprotective Attributes of L-Theanine, a Bioactive Amino Acid of Tea, and Its Potential Role in Parkinson’s Disease Therapeutics. Neurochem. Int. 2019, 129, 104478. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-G.; Lee, Y.-R.; Lee, M.-S.; Hwang, K.H.; Park, C.Y.; Kim, E.-H.; Park, J.S.; Hong, Y.-S. Diverse Metabolite Variations in Tea (Camellia sinensis L.) Leaves Grown Under Various Shade Conditions Revisited: A Metabolomics Study. J. Agric. Food Chem. 2018, 66, 1889–1897. [Google Scholar] [CrossRef]
- Sano, T.; Horie, H.; Matsunaga, A.; Hirono, Y. Effect of Shading Intensity on Morphological and Color Traits and on Chemical Components of New Tea (Camellia sinensis L.) Shoots under Direct Covering Cultivation: Changes in Morphology, Color, and Chemical Components of Tea Shoots Due to Shade. J. Agric. Food Chem. 2018, 98, 5666–5676. [Google Scholar] [CrossRef]
- Yang, T.; Xie, Y.; Lu, X.; Yan, X.; Wang, Y.; Ma, J.; Cheng, X.; Lin, S.; Bao, S.; Wan, X.; et al. Shading Promoted Theanine Biosynthesis in the Roots and Allocation in the Shoots of the Tea Plant (Camellia sinensis L.) Cultivar Shuchazao. J. Agric. Food Chem. 2021, 69, 4795–4803. [Google Scholar] [CrossRef]
- Feng, L.; Gao, M.-J.; Hou, R.-Y.; Hu, X.-Y.; Zhang, L.; Wan, X.-C.; Wei, S. Determination of Quality Constituents in the Young Leaves of Albino Tea Cultivars. Food Chem. 2014, 155, 98–104. [Google Scholar] [CrossRef]
- Cheng, S.; Fu, X.; Liao, Y.; Xu, X.; Zeng, L.; Tang, J.; Li, J.; Lai, J.; Yang, Z. Differential Accumulation of Specialized Metabolite L-Theanine in Green and Albino-Induced Yellow Tea (Camellia sinensis) Leaves. Food Chem. 2019, 276, 93–100. [Google Scholar] [CrossRef]
- Ma, Q.; Song, L.; Niu, Z.; Li, J.; Wang, Y.; Sun, H.; Ren, Z.; Zhao, H.; Guo, S.; Ding, Z. Red Light Regulates the Metabolite Biosynthesis in the Leaves of “Huangjinya” Through Amino Acid and Phenylpropanoid Metabolisms. Front. Plant Sci. 2022, 12, 810888. [Google Scholar] [CrossRef]
- Hao, X.; Li, L.; Hu, Y.; Zhou, C.; Wang, X.; Wang, L.; Zeng, J.; Yang, Y. Transcriptomic Analysis of the Effects of Three Different Light Treatments on the Biosynthesis of Characteristic Compounds in the Tea Plant by RNA-Seq. Tree Genet. Genomes 2016, 12, 118. [Google Scholar] [CrossRef]
- Hu, Z.; Ban, Q.; Hao, J.; Zhu, X.; Cheng, Y.; Mao, J.; Lin, M.; Xia, E.; Li, Y. Genome-Wide Characterization of the C-Repeat Binding Factor (CBF) Gene Family Involved in the Response to Abiotic Stresses in Tea Plant (Camellia sinensis). Front. Plant Sci. 2020, 11, 921. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; He, C.; Zhou, J.; Chen, Y.; Yu, Z.; Wang, P.; Ni, D. Nonvolatile Metabolism in Postharvest Tea (Camellia sinensis L.) Leaves: Effects of Different Withering Treatments on Nonvolatile Metabolites, Gene Expression Levels, and Enzyme Activity. Food Chem. 2020, 327, 126992. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Sun, Z.; Gao, J.; Peng, J.; Wang, Z.; Zhao, Y.; Lin, Z.; Dai, W. Metabolomics Combined with Proteomics Provides a Novel Interpretation of the Compound Differences among Chinese Tea Cultivars (Camellia sinensis Var. Sinensis) with Different Manufacturing Suitabilities. Food Chem. 2022, 377, 131976. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xin, H.; Wang, M.; Ma, Q.; Wang, L.; Kaleri, N.A.; Wang, Y.; Li, X. Transcriptomic Analysis Reveals the Molecular Mechanisms of Drought-Stress-Induced Decreases in Camellia sinensis Leaf Quality. Front. Plant Sci. 2016, 7, 385. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, M.; Yu, X.; Wang, L.; Guo, C.; Ming, R.; Zhang, J. Transcriptome dynamics of Camellia sinensis in response to continuous salinity and drought stress. Tree Genet. Genomes 2017, 13, 78. [Google Scholar] [CrossRef]
- Cheng, H.; Wu, W.; Liu, X.; Wang, Y.; Xu, P. Transcription Factor CsWRKY40 Regulates L-Theanine Hydrolysis by Activating the CsPDX2.1 Promoter in Tea Leaves during Withering. Hortic. Res. 2022, 9, 025. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, Y.; Zhang, S.; Wei, Y.; Han, M.; Deng, Y.; Guo, J.; Zhu, B.; Yang, T.; Xia, E.; et al. Root-Specific Secondary Metabolism at the Single-Cell Level: A Case Study of Theanine Metabolism and Regulation in the Roots of Tea Plants (Camellia sinensis). eLife 2024, 13, RP95891. [Google Scholar] [CrossRef]
- Li, Z.; Yang, W.; Ahammed, G.J.; Shen, C.; Yan, P.; Li, X.; Han, W. Developmental changes in carbon and nitrogen metabolism affect tea quality in different leaf position. Plant Physiol. Biochem. 2016, 106, 327–335. [Google Scholar] [CrossRef]
- Xu, P.; Yu, J.; Ma, R.; Ji, Y.; Hu, Q.; Mao, Y.; Ding, C.; Li, Z.; Ge, S.; Deng, W.; et al. Chlorophyll and Carotenoid Metabolism Varies with Growth Temperatures among Tea Genotypes with Different Leaf Colors in Camellia sinensis. Int. J. Mol. Sci. 2024, 25, 10772. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Ahammed, G.J.; Li, Z.; Wei, J.; Shen, C.; Yan, P.; Zhang, L.; Han, W. Stimulation in Primary and Secondary Metabolism by Elevated Carbon Dioxide Alters Green Tea Quality in Camellia sinensis L. Sci. Rep. 2017, 7, 7937. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Pokharel, S.S.; Li, C.; Parajulee, M.N.; Chen, F.; Fang, W. Effects of Elevated CO2 on Foliar Soluble Nutrients and Functional Components of Tea, and Population Dynamics of Tea Aphid, Toxoptera Aurantii. Plant Physiol. Biochem. 2019, 145, 84–94. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Physiological and Defense Responses of Tea Plants to Elevated CO2: A Review. Front. Plant Sci. 2020, 11, 305. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Wang, Y.; Lin, S.; Chen, M.; Cheng, P.; Wang, Y.; Du, M.; Jia, X.; Wang, H.; et al. Effects of Magnesium on Transcriptome and Physicochemical Index of Tea Leaves. Plants 2023, 12, 1810. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Chen, Y.; Zeng, L.; Cui, Y.; Li, J.; Tang, H.; Liu, J.; Tang, J. Soil Nutrient Deficiency Decreases the Postharvest Quality-Related Metabolite Contents of Tea (Camellia sinensis (L.) Kuntze) Leaves. Food Chem. 2022, 377, 132003. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Liu, J.; Wang, Y.; Zhuang, J. Integrative transcriptome, proteome, and microRNA analysis reveals the effects of nitrogen sufficiency and deficiency conditions on theanine metabolism in the tea plants (Camellia sinensis). Hortic. Res. 2020, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, F.; Wu, Z.; Jiang, F.; Yu, W.; Yang, J.; Chen, J.; Jian, G.; You, Z.; Zeng, L. Effects of Long-Term Nitrogen Fertilization on the Formation of Metabolites Related to Tea Quality in Subtropical China. Metabolites 2021, 11, 146. [Google Scholar] [CrossRef]
- Qiu, Z.; Liao, J.; Chen, J.; Li, A.; Lin, M.; Liu, H.; Huang, W.; Sun, B.; Liu, J.; Liu, S.; et al. Comprehensive Analysis of Fresh Tea (Camellia sinensis cv. Lingtou Dancong) Leaf Quality under Different Nitrogen Fertilization Regimes. Food Chem. 2024, 439, 138127. [Google Scholar] [CrossRef]
- Gai, S.; Wang, Y.; Li, L.; Liu, S.; Li, Y.; Cheng, X.; Xia, M.; Liu, Z.; Zhou, Z. Research Progress of Tea plants (Camellia sinensis) Growth Under Light Regulation. J. Tea Sci. 2022, 42, 753–767. [Google Scholar]
- Ruan, J.; Haerdter, R.; Gerendás, J. Impact of nitrogen supply on carbon/nitrogen allocation: A case study on amino acids and catechins in green tea [Camellia sinensis (L.) O. Kuntze] plants. Plant Biol. 2010, 12, 724–734. [Google Scholar] [CrossRef]
- Li, F.; Li, H.; Dong, C.; Yang, T.; Zhang, S.; Bao, S.; Wan, X.; Zhang, Z. Theanine Transporters Are Involved in Nitrogen Deficiency Response in Tea Plant (Camellia sinensis L.). Plant Signal. Behav. 2020, 15, 1728109. [Google Scholar] [CrossRef]
- Huang, H.; Yao, Q.; Xia, E.; Gao, L. Metabolomics and Transcriptomics Analyses Reveal Nitrogen Influences on the Accumulation of Flavonoids and Amino Acids in Young Shoots of Tea Plant (Camellia sinensis L.) Associated with Tea Flavor. J. Agric. Food Chem. 2018, 66, 9828–9838. [Google Scholar] [CrossRef]
- Ruan, J.; Gerendás, J.; Härdter, R.; Sattelmacher, B. Effect of root zone pH and form and concentration of nitrogen on accumulation of quality-related components in green tea. J. Agric. Food Chem. 2007, 87, 1505–1516. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Yang, T.; Su, Y.; Lin, S.; Zhang, S.; Zhang, Z. Nitrogen-Regulated Theanine and Flavonoid Biosynthesis in Tea Plant Roots: Protein-Level Regulation Revealed by Multiomics Analyses. J. Agric. Food Chem. 2021, 69, 10002–10016. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Wei, K.; Wang, L.; Cheng, H.; Zhang, F.; Wu, L.; Bai, P.; Zhang, C. Characteristics of NH4+ and NO3− Fluxes in Tea (Camellia sinensis) Roots Measured by Scanning Ion-Selective Electrode Technique. Sci. Rep. 2016, 6, 38370. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Q.; Zhang, X.; Zhang, L.; Zhao, P.; Wen, T.; Zhang, J.; Xu, W.; Guo, F.; Zhao, H.; et al. Exploring the Effects of Magnesium Deficiency on the Quality Constituents of Hydroponic-Cultivated Tea (Camellia sinensis L.) Leaves. J. Agric. Food Chem. 2021, 69, 14278–14286. [Google Scholar] [CrossRef]
- Chen, L.; Liu, M.; Cai, Y.; Wu, L.; Zhang, Q. Magnesium Deficiency Differentiated Effects on the Roots Growth and Shoot Metabolism by Regulating the Distribution of Photosynthetic Products in Tea Plants. Sci. Hortic. 2024, 338, 113748. [Google Scholar] [CrossRef]
- Xu, J.; Wu, L.; Tong, B.; Yin, J.; Huang, Z.; Li, W.; Li, X. Magnesium Supplementation Alters Leaf Metabolic Pathways for Higher Flavor Quality of Oolong Tea. Agriculture 2021, 11, 120. [Google Scholar] [CrossRef]
- Ruan, J.; Ma, L.; Shi, Y. Potassium Management in Tea Plantations: Its Uptake by Field Plants, Status in Soils, and Efficacy on Yields and Quality of Teas in China. J. Plant Nutr. Soil Sci. 2013, 176, 450–459. [Google Scholar] [CrossRef]
- Mao, C.; He, J.; Wen, X.; Xiang, Y.; Feng, J.; Shu, Y. Correlation and Pathway Analysis of the Carbon, Nitrogen, and Phosphorus in Soil-Microorganism-Plant with Main Quality Components of Tea (Camellia sinensis). Phyton 2024, 93, 487–502. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Crit. Rev. Food Sci. Nutr. 2020, 60, 844–858. [Google Scholar] [CrossRef]
- Lin, Z.; Qi, Y.; Chen, R.; Zhang, F.; Chen, L. Effects of Phosphorus Supply on the Quality of Green Tea. Food Chem. 2012, 130, 908–914. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, L.; Geng, S.; Ruan, J. Optimization of Nutrient Management Improves Productivity, Quality and Sustainability of Albino Tea Cultivar Baiye-1. Front. Plant Sci. 2024, 15, 1369015. [Google Scholar] [CrossRef] [PubMed]
- Tolrà, R.; Martos, S.; Hajiboland, R.; Poschenrieder, C. Aluminium Alters Mineral Composition and Polyphenol Metabolism in Leaves of Tea Plants (Camellia sinensis). J. Inorg. Biochem. 2020, 204, 110956. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, Y.; Wu, L.; Liao, H. Comprehensive Analysis Revealed the Close Relationship between N/P/K Status and Secondary Metabolites in Tea Leaves. Adv. Mater. 2019, 4, 176–184. [Google Scholar] [CrossRef]
- Liu, X.; Gao, X.; He, Y.; Gao, X.; Xiao, C.; Wu, G.; Zhou, H.; Yuan, W. Effect of several trace elements on the tea plants physiological and tea quality. Guangdong Agric. Sci. 2010, 37, 4. [Google Scholar]
- Ye, J.; Zhang, Q.; Liu, G.; Lin, L.; Wang, H.; Lin, S.; Wang, Y.; Wang, Y.; Zhang, Q.; Jia, X.; et al. Relationship of Soil pH Value and Soil Pb Bio-Availability and Pb Enrichment in Tea Leaves. J. Agric. Food Chem. 2022, 102, 1137–1145. [Google Scholar] [CrossRef]
- Zhang, C.; He, Q.; Wang, M.; Gao, X.; Chen, J.; Shen, C. Exogenous Indole Acetic Acid Alleviates Cd Toxicity in Tea (Camellia sinensis). Ecotoxicol. Environ. Saf. 2020, 190, 110090. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, Q.; Tang, Q. Effect of Pb on Growth, Accumulation and Quality Component of Tea Plant. Procedia Eng. 2011, 18, 214–219. [Google Scholar]
- Zagoskina, N.V.; Goncharuk, E.A.; Alyavina, A.K. Effect of Cadmium on the Phenolic Compounds Formation in the Callus Cultures Derived from Various Organs of the Tea Plant. Russ. J. Plant Physiol. 2007, 54, 237–243. [Google Scholar] [CrossRef]
- Hao, J.; Peng, A.; Li, Y.; Zuo, H.; Li, P.; Wang, J.; Yu, K.; Liu, C.; Zhao, S.; Wan, X.; et al. Tea plant roots respond to aluminum-induced mineral nutrient imbalances by transcriptional regulation of multiple cation and anion transporters. BMC Plant Biol. 2022, 22, 203. [Google Scholar] [CrossRef]
- Peng, C.; Zhu, X.; Hou, R.; Ge, G.; Hua, R.; Wan, X.; Cai, H. Aluminum and Heavy Metal Accumulation in Tea Leaves: An Interplay of Environmental and Plant Factors and an Assessment of Exposure Risks to Consumers. J. Food Sci. 2018, 83, 1165–1172. [Google Scholar] [CrossRef]
- Peng, A.; Yu, K.; Yu, S.; Li, Y.; Zuo, H.; Li, P.; Li, J.; Huang, J.; Liu, Z.; Zhao, J. Aluminum and Fluoride Stresses Altered Organic Acid and Secondary Metabolism in Tea (Camellia sinensis) Plants: Influences on Plant Tolerance, Tea Quality and Safety. Int. J. Mol. Sci. 2023, 24, 4640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, L.; Luo, S.; Ye, X.; Wen, W. Research advances in aluminum tolerance and accumulation in tea plant (Camellia sinensis). Beverage Plant Res. 2023, 3, 18. [Google Scholar] [CrossRef]
- Liu, S.; Peng, X.; Wang, X.; Zhuang, W. Transcriptome Analysis Reveals Differentially Expressed Genes Involved in Cadmium and Arsenic Accumulation in Tea Plant (Camellia sinensis). Plants 2023, 12, 1182. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chen, H.; Zhang, B.; Lu, L. Transcriptome Analysis Reveals Differentially Expressed Genes Involved in Aluminum, Copper and Cadmium Accumulation in Tea “Qianmei 419” and “Qianfu 4”. Plants 2023, 12, 2580. [Google Scholar] [CrossRef]
- Tan, X.; Huang, J.; Lin, L.; Tang, Q. Exogenous Melatonin Attenuates Cd Toxicity in Tea (Camellia sinensis). Agronomy 2022, 12, 2485. [Google Scholar] [CrossRef]
- Li, H.; Teng, R.; Liu, J.; Yang, R.; Yang, Y.; Lin, S.; Han, M.; Liu, J.; Zhuang, J. Identification and Analysis of Genes Involved in Auxin, Abscisic Acid, Gibberellin, and Brassinosteroid Metabolisms Under Drought Stress in Tender Shoots of Tea Plants. DNA Cell Biol. 2019, 38, 1292–1302. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Li, Z.; Zhang, L.; Wei, J.; Shen, C.; Yan, P.; Zhang, L.; Han, W. Brassinosteroids Improve Quality of Summer Tea (Camellia sinensis L.) by Balancing Biosynthesis of Polyphenols and Amino Acids. Front. Plant Sci. 2016, 7, 1304. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Pal, S.; Yu, J.; Zhou, Y.; Tran, L.-S.P.; Xia, X. The hormonal, metabolic, and environmental regulation of plant shoot branching. New Crops 2024, 1, 100028. [Google Scholar] [CrossRef]
- Huang, S.; Zuo, T.; Xu, W.; Zhang, Y.; Ni, W. Improving Albino Tea Quality by Foliar Application of Glycinebetaine as a Green Regulator under Lower Temperature Conditions. J. Agric. Food Chem. 2021, 69, 1242–1250. [Google Scholar] [CrossRef]
- Li, P.; Xia, E.; Fu, J.; Xu, Y.; Zhao, X.; Tong, W.; Tang, Q.; Tadege, M.; Fernie, A.R.; Zhao, J. Diverse Roles of MYB Transcription Factors in Regulating Secondary Metabolite Biosynthesis, Shoot Development, and Stress Responses in Tea Plants (Camellia sinensis). Plant J. 2022, 110, 1144–1165. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Deng, W.; Ahammed, G.J.; Wei, J.; Yan, P.; Zhang, L.; Fu, J.; Han, W. Exogenous Melatonin Improves Tea Quality Under Moderate High Temperatures by Increasing Epigallocatechin-3-Gallate and Theanine Biosynthesis in Camellia sinensis L. J. Plant Physiol. 2020, 253, 153273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mi, X.; Guo, R.; Xia, X.; Liu, L.; An, Y.; Yan, X.; Wang, S.; Guo, L.; Wei, C. The Biosynthesis of Main Taste Compounds Is Coordinately Regulated by miRNAs and Phytohormones in Tea plants (Camellia sinensis). J. Agric. Food Chem. 2020, 68, 6221–6236. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiang, F.; Su, Y.; Luo, Z.; Luo, W.; Zhou, L.; Liu, H.; Xiao, L. Gibberellin Increases the Bud Yield and Theanine Accumulation in Camellia sinensis (L.) Kuntze. Molecules 2021, 26, 3290. [Google Scholar] [CrossRef]
- Cheng, H.; Pan, Q.; Wu, W.; Jimin, S.; Liu, X.; Shi, Y.; Yin, X.; Xu, P. CsWRKY53 and CsWRKY40 Synergistically Regulate L-Theanine Hydrolysis by Abscisic Acid Signaling Pathway during Tea Withering. J. Exp. Bot. 2024, 13, 460. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, Z.; Wu, W.; Zhou, Z.; Deng, X.; Chen, Z.; Sun, W. Transcriptome and metabolome reveal the effects of ABA promotion and inhibition on flavonoid and amino acid metabolism in tea plant. Tree Physiol. 2024, 44, 065. [Google Scholar] [CrossRef]
- Xu, P.; Su, H.; Zhao, S.; Jin, R.; Cheng, H.; Xu, A.; Lai, W.; Yin, X.; Wang, Y. Transcriptome and Phytochemical Analysis Reveals the Alteration of Plant Hormones, Characteristic Metabolites, and Related Gene Expression in Tea (Camellia sinensis L.) Leaves During Withering. Plants 2020, 9, 204. [Google Scholar] [CrossRef]
- Liu, N.; Li, C.; Wu, F.; Yang, Y.; Yu, A.; Wang, Z.; Zhao, L.; Zhang, X.; Qu, F.; Gao, L.; et al. Genome-Wide Identification and Expression Pattern Analysis of WRKY Transcription Factors in Response to Biotic and Abiotic Stresses in Tea Plants (Camellia sinensis). Plant Physiol. Biochem. 2024, 211, 108670. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; He, S.-P.; Gao, Y.; Wang, N.-N.; Lu, R.; Li, X.-B. A Cotton (Gossypium Hirsutum) WRKY Transcription Factor (GhWRKY22) Participates in Regulating Anther/Pollen Development. Plant Physiol. Biochem. 2019, 141, 231–239. [Google Scholar] [CrossRef]
- Liu, F.; Xi, M.; Liu, T.; Wu, X.; Ju, L.; Wang, D. The central role of transcription factors in bridging biotic and abiotic stress responses for plants’ resilience. New Crops 2024, 1, 100005. [Google Scholar] [CrossRef]
- Chen, X.; Wang, P.; Gu, M.; Lin, X.; Hou, B.; Zheng, Y.; Sun, Y.; Jin, S.; Ye, N. R2R3-MYB Transcription Factor Family in Tea Plant (Camellia sinensis): Genome-Wide Characterization, Phylogeny, Chromosome Location, Structure and Expression Patterns. Genomics 2021, 113, 1565–1578. [Google Scholar] [CrossRef]
- Xie, N.; Huang, X.; Zhou, J.; Song, X.; Lin, J.; Yan, M.; Zhu, M.; Li, J.; Wang, K. The R2R3-MYB Transcription Factor CsMYB42 Regulates Theanine Biosynthesis in Albino Tea Leaves. he R2R3-MYB Transcription Factor CsMYB42 Regulates Theanine Biosynthesis in Albino Tea Leaves. Plant Sci. 2023, 336, 111850. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Sun, Y.; Fang, K.; Fu, M.; Ma, J.; Gao, Y.; Chen, Q.; Liu, L.; Zhang, Z.; Wan, X.; et al. CsMYB73 Negatively Regulates Theanine Accumulation Mediated by CsGGT2 and CsGGT4 in Tea Shoots (Camellia sinensis). Hortic. Res. 2024, 11, uhae012. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Luo, Y.; Liu, D.; Zhang, X.; Peng, Z.; Wang, K.; Li, J.; Huang, J.; Liu, Z. The R2R3-MYB Transcription Factor CsMYB73 Negatively Regulates l-Theanine Biosynthesis in Tea Plants (Camellia sinensis L.). Plant Sci. 2020, 298, 110546. [Google Scholar] [CrossRef]
- Chen, F.; He, Y.; Yao, X.; Zho, B.; Tian, S.; Yin, J.; Lu, L. CsMOF1-Guided Regulation of Drought-Induced Theanine Biosynthesis in Camellia sinensis. Int. J. Biol. Macromol. 2024, 268, 131725. [Google Scholar] [CrossRef]
- Zhou, Z.; Luo, X.; Fu, M.; Li, S.; Cheng, Y.; Li, Y.; Zhang, X. Ethylamine, beyond the Synthetic Precursor of Theanine: CsCBF4-CsAlaDC Module Promoted Ethylamine Synthesis to Enhance Osmotic Tolerance in Tea Plants. Plant J. Cell Mol. Biol. 2024, 120, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Liu, C.; Yu, S.; Yang, H.; Sun, J.; Guo, C.; Huang, B.; Liu, Z.; Yuan, Y.; Xia, E.; et al. Gene Co-Expression Network Analysis Reveals Coordinated Regulation of Three Characteristic Secondary Biosynthetic Pathways in Tea Plant (Camellia sinensis). BMC Genom. 2018, 19, 616. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, S.; Li, J.; Chen, T.; Gu, Q.; Yang, T.; Zhang, Z. Theanine Improves Salt Stress Tolerance via Modulating Redox Homeostasis in Tea Plants (Camellia sinensis L.). Front. Plant Sci. 2021, 12, 770398. [Google Scholar] [CrossRef]
- Shan, D.; Zhang, Q.; Guo, J.; Liu, S.; Chen, Z.; Zhou, T.; Chen, X. Influence of theanine on the growth and physiological indexes of tobacco seedlings. J. Anhui Agric. Univ. 2015, 42, 283–289. [Google Scholar]
- Yan, X.; Wang, Y.; Yang, T.; Wang, F.; Wan, X.; Zhang, Z. Exogenous theanine application improves the fresh leaf yield and quality of an albino green tea Huangjinya. Food Chem. 2024, 467, 142298. [Google Scholar] [CrossRef]
- Lu, M.; Han, J.; Zhu, B.; Jia, H.; Yang, T.; Wang, R.; Deng, W.; Zhang, Z. Significantly Increased Amino Acid Accumulation in a Novel Albino Branch of the Tea Plant (Camellia sinensis). Planta 2019, 249, 363–376. [Google Scholar] [CrossRef]
- Asgher, M.; Sehar, Z.; Rehaman, A.; Rashid, S.; Ahmed, S.; Per, T.S.; Alyemeni, M.N.; Khan, N.A. Exogenously-applied L-glutamic acid protects photosynthetic functions and enhances arsenic tolerance through increased nitrogen assimilation and antioxidant capacity in rice (Oryza Sativa L.). Environ. Pollut. 2022, 301, 1190086. [Google Scholar] [CrossRef] [PubMed]
- The, S.V.; Santiago, J.P.; Pappenberger, C.; Hammes, U.Z.; Tegeder, M. UMAMIT44 is a key player in Glutamate export from Arabidopsis Chloroplasts. Plant Cell 2024, 36, 1119–1139. [Google Scholar] [CrossRef] [PubMed]
- Eprintsev, A.T.; Selivanova, N.V.; Igamberdiev, A.U. Enzymatic Conversions of Glutamate and γ-Aminobutyric Acid as Indicators of Plant Stress Response. Methods Mol. Biol. 2020, 2057, 71–78. [Google Scholar] [PubMed]
- KIm, D.-R.; Kwak, Y.-S. Endophytic Streptomyces Population Induced by L-Glutamic Acid Enhances Plant Resilience to Abiotic Stresses in Tomato. Front. Microbiol. 2023, 14, 1180538. [Google Scholar] [CrossRef]
- Quan, J.; Zheng, W.; Tan, J.; Li, Z.; Wu, M.; Hong, S.; Zhao, Y.; Zhu, Z.; Zang, Y. Glutamic Acid and Poly-γ-Glutamic Acid Enhanced the Heat Resistance of Chinese Cabbage (Brassica rapa L. ssp. pekinensis) by Improving Carotenoid Biosynthesis, Photosynthesis, and ROS Signaling. Int. J. Mol. Sci. 2022, 23, 11671. [Google Scholar] [CrossRef]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alabdallah, N.M.; Alharbi, B.M.; Waseem, M.; Yao, G.; Liu, X.; Abd El-Gawad, H.G.; El-Yazied, A.A.; Ibrahim, M.F.M.; Jahan, M.S.; et al. GABA: A Key Player in Drought Stress Resistance in Plants. Int. J. Mol. Sci. 2021, 22, 10136. [Google Scholar] [CrossRef]
- Bao, H.; Chen, X.; Lv, S.; Jiang, P.; Feng, J.; Fan, P.; Nie, L.; Li, Y. Virus-Induced Gene Silencing Reveals Control of Reactive Oxygen Species Accumulation and Salt Tolerance in Tomato by γ-Aminobutyric Acid Metabolic Pathway. Plant Cell Environ. 2015, 38, 600–613. [Google Scholar] [CrossRef]
- Abdel Razik, E.S.; Alharbi, B.M.; Pirzadah, T.B.; Alnusairi, G.S.H.; Soliman, M.H.; Hakeem, K.R. γ-Aminobutyric Acid (GABA) Mitigates Drought and Heat Stress in Sunflower (Helianthus annuus L.) by Regulating Its Physiological, Biochemical and Molecular Pathways. Physiol. Plant. 2021, 172, 505–527. [Google Scholar] [CrossRef]
- Huang, X.; Jian, S.; Wan, S.; Miao, J.; Zhong, C. Exogenous γ-Aminobutyric Acid (GABA) Alleviates Nitrogen Deficiency by Mediating Nitrate Uptake and Assimilation in Andrographis Paniculata Seedlings. Plant Physiol. Biochem. 2023, 198, 107700. [Google Scholar] [CrossRef]
- Shelp, B.J.; Aghdam, M.S.; Flaherty, E.J. γ-Aminobutyrate (GABA) Regulated Plant Defense: Mechanisms and Opportunities. Plants 2021, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Gahlowt, P.; Singh, S.; Dubey, N.K.; Singh, S.P.; Tripathi, D.K.; Singh, V.P. GABA: A Key Player of Abiotic Stress Regulation. Plant Signal. Behav. 2023, 18, 2163343. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Yu, J.; Lin, W.; Ahammed, G.J.; Wang, W.; Ma, R.; Shi, M.; Ge, S.; Mohamed, A.S.; Wang, L.; et al. L-Theanine Metabolism in Tea Plants: Biological Functions and Stress Tolerance Mechanisms. Plants 2025, 14, 492. https://doi.org/10.3390/plants14030492
Wang Q, Yu J, Lin W, Ahammed GJ, Wang W, Ma R, Shi M, Ge S, Mohamed AS, Wang L, et al. L-Theanine Metabolism in Tea Plants: Biological Functions and Stress Tolerance Mechanisms. Plants. 2025; 14(3):492. https://doi.org/10.3390/plants14030492
Chicago/Turabian StyleWang, Qianying, Jingbo Yu, Wenchao Lin, Golam Jalal Ahammed, Wenli Wang, Ruihong Ma, Mengyao Shi, Shibei Ge, Ahmed S. Mohamed, Liyuan Wang, and et al. 2025. "L-Theanine Metabolism in Tea Plants: Biological Functions and Stress Tolerance Mechanisms" Plants 14, no. 3: 492. https://doi.org/10.3390/plants14030492
APA StyleWang, Q., Yu, J., Lin, W., Ahammed, G. J., Wang, W., Ma, R., Shi, M., Ge, S., Mohamed, A. S., Wang, L., Li, Q., & Li, X. (2025). L-Theanine Metabolism in Tea Plants: Biological Functions and Stress Tolerance Mechanisms. Plants, 14(3), 492. https://doi.org/10.3390/plants14030492






