WRKY36–PIL15 Transcription Factor Complex Negatively Regulates Sheath Blight Resistance and Seed Development in Rice
Abstract
:1. Introduction
2. Results
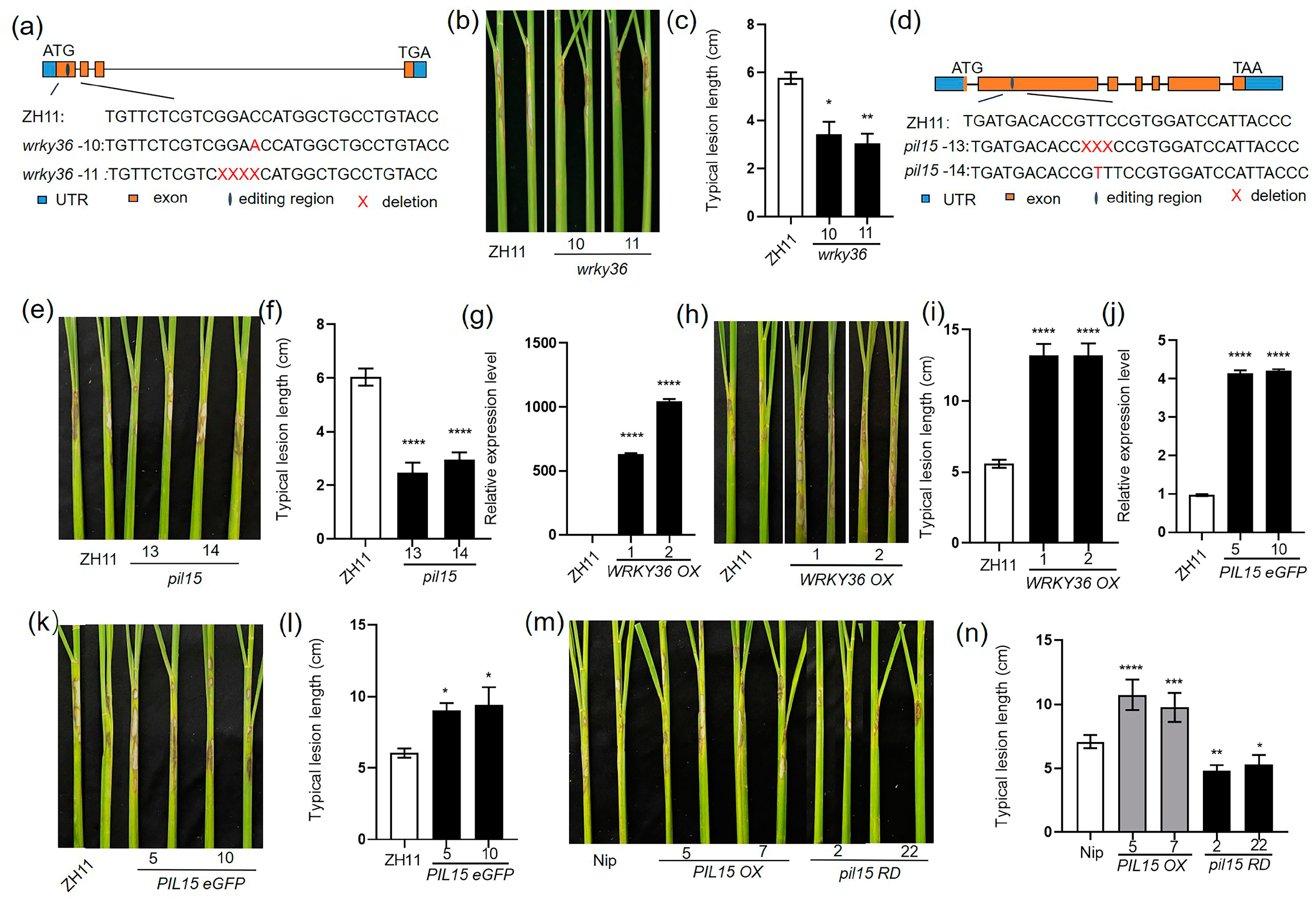
2.1. WRKY36 and PIL15 Negatively Regulate Resistance of Rice to ShB
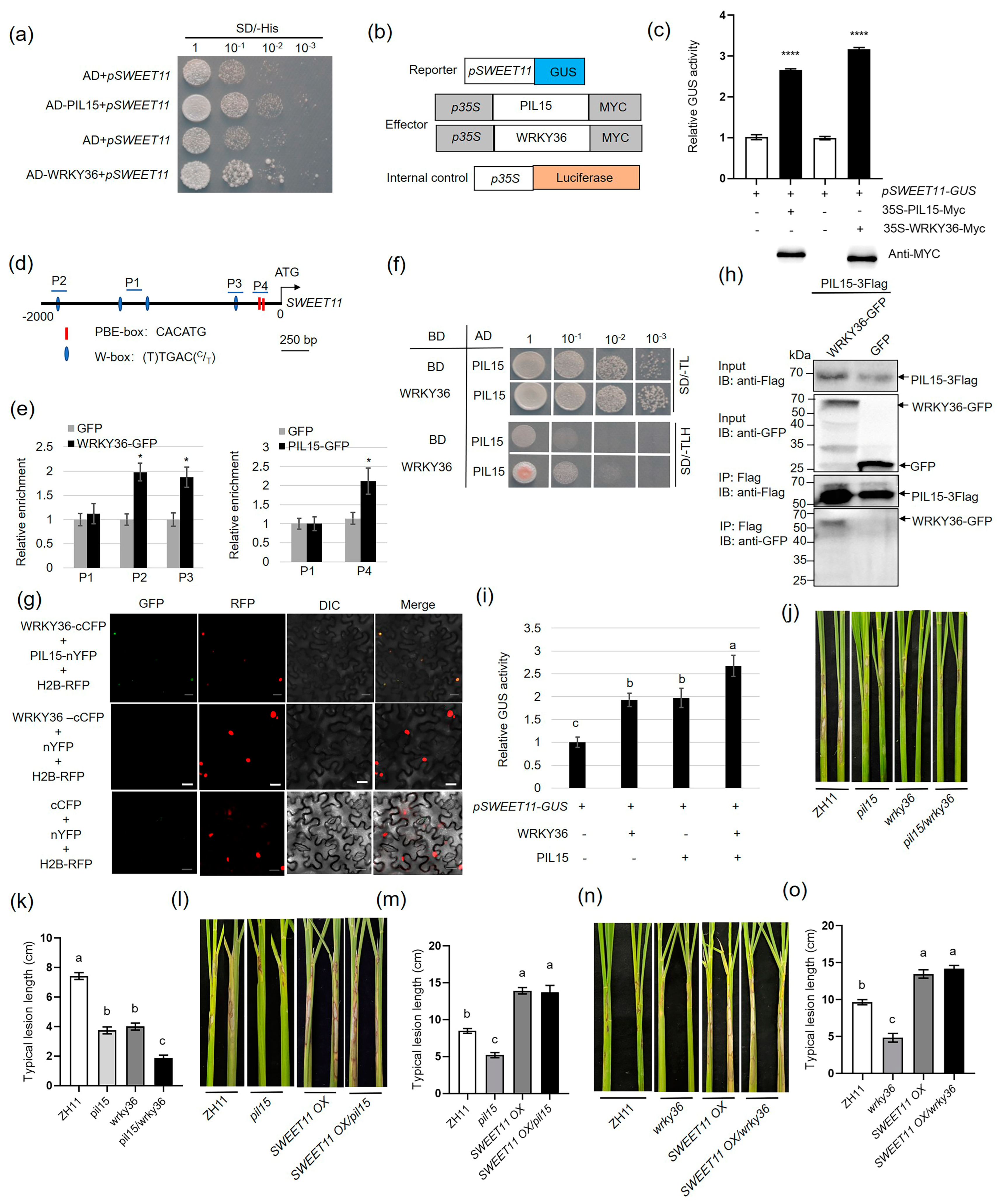
2.2. WRKY36 Interacts with PIL15 to Activate SWEET11
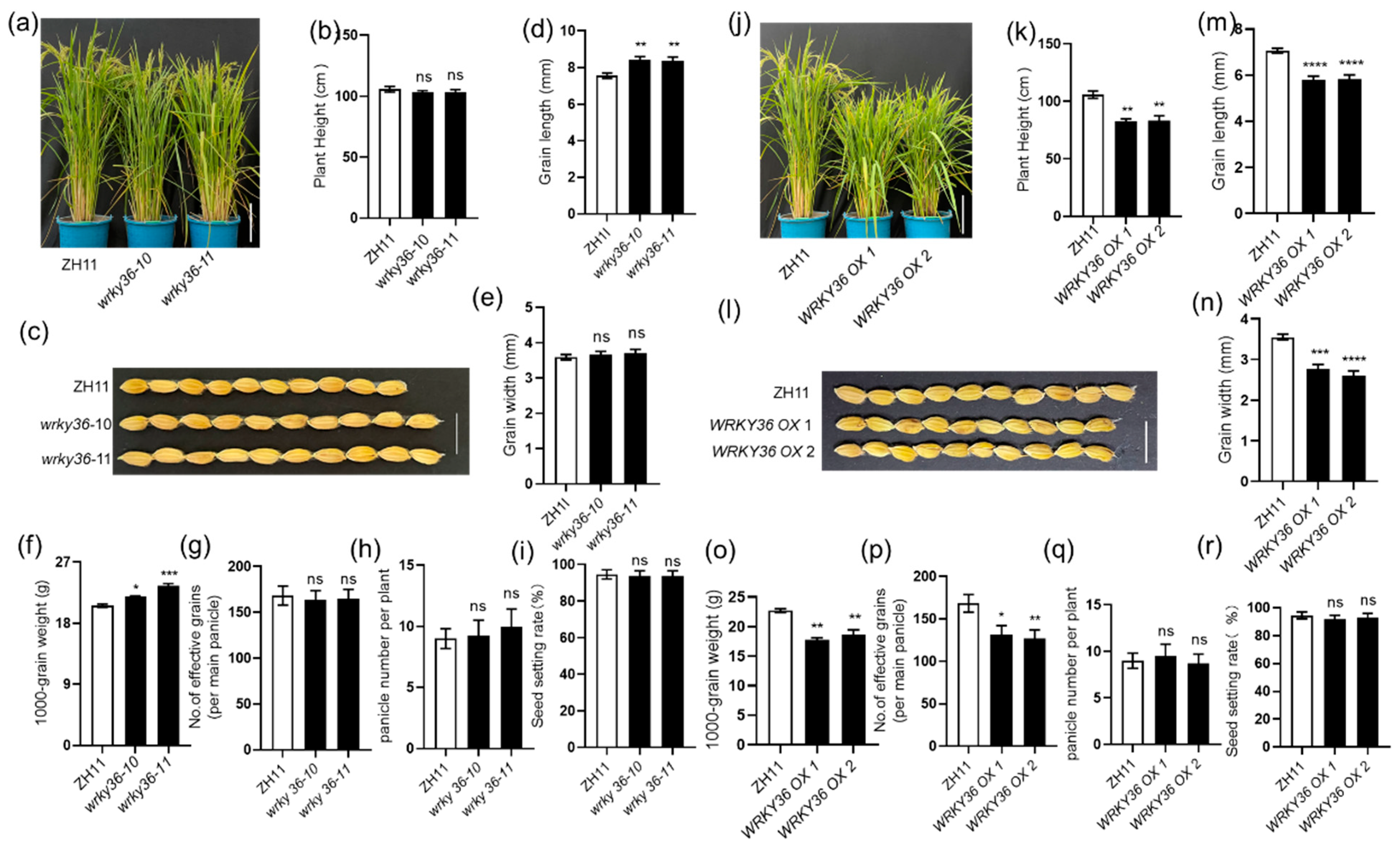
2.3. WRKY36 and PIL15 Negatively Regulate Seed Development
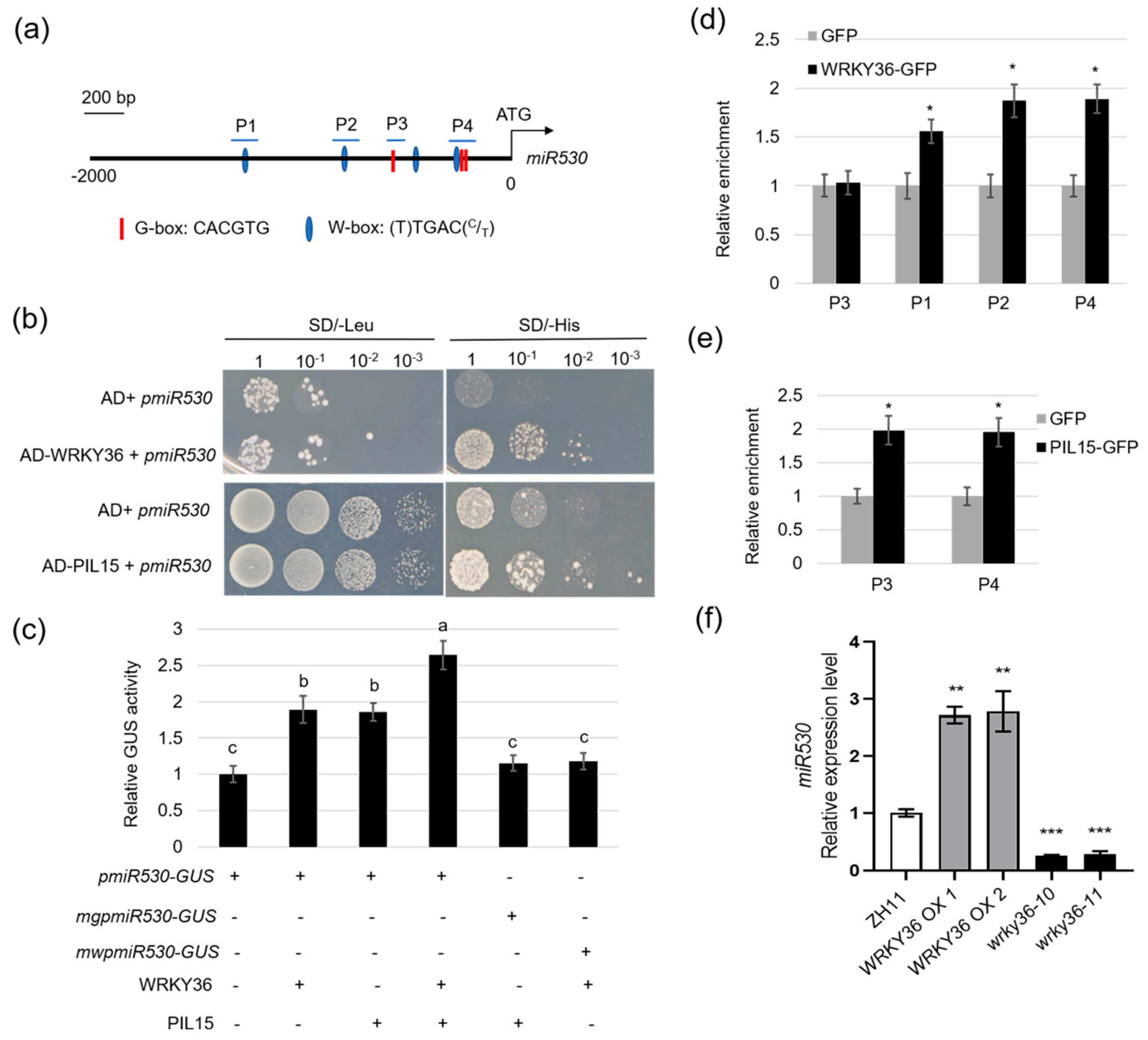
2.4. WRKY36–PIL15 Directly Activates miR530
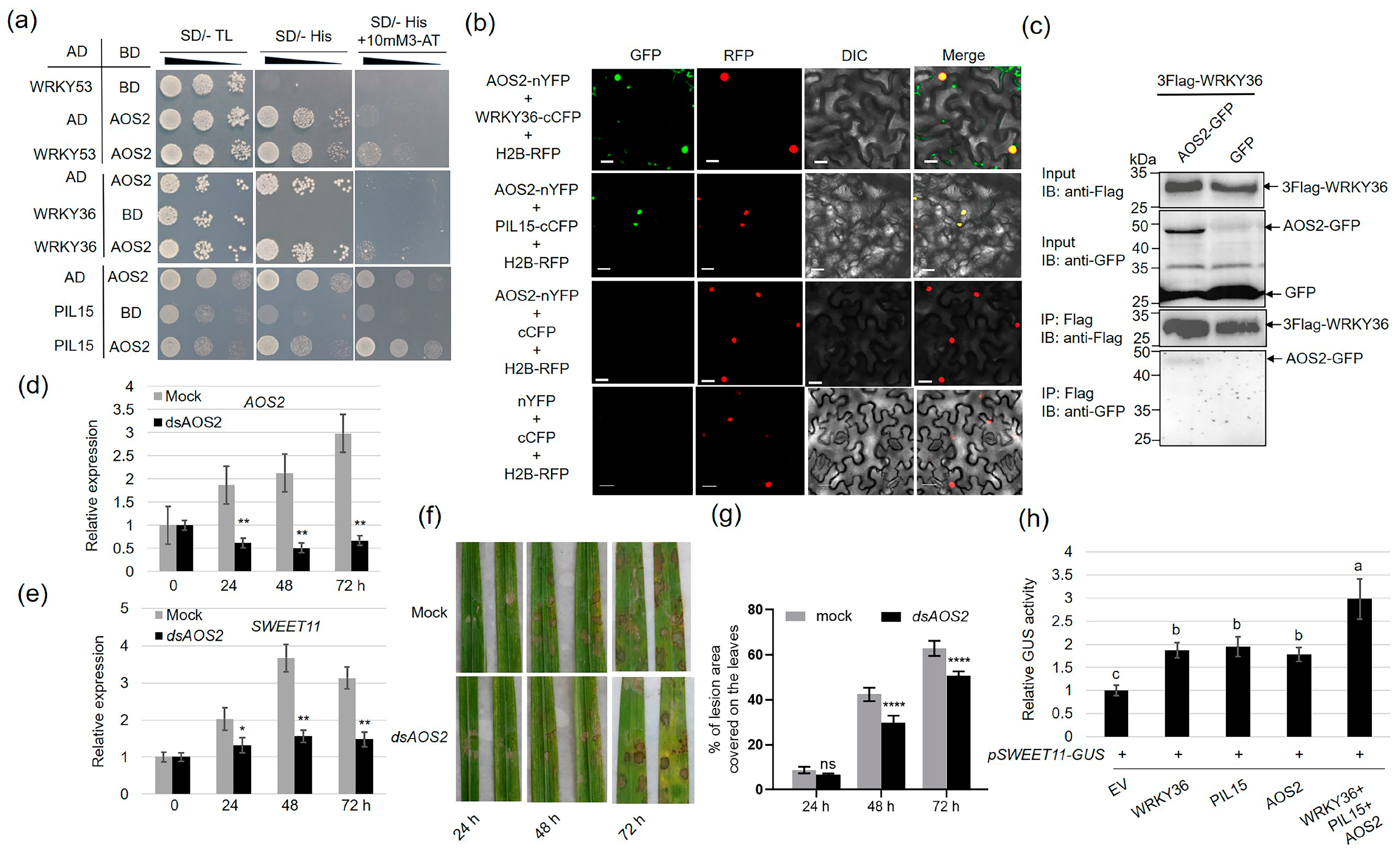
2.5. AOS2 Interacts with WRKY36 and PIL15 to Activate SWEET11
3. Discussion
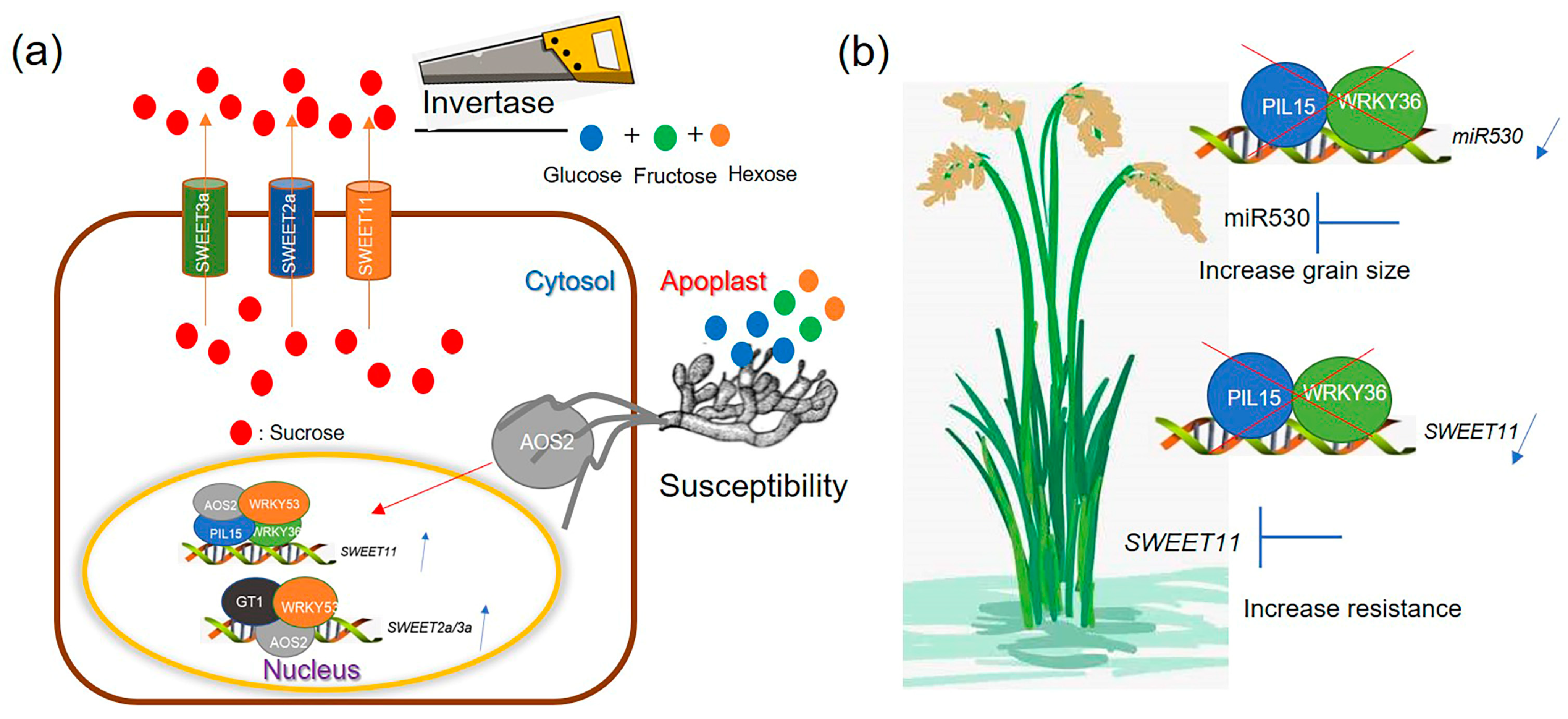
3.1. WRKY36–PIL15–AOS2 Activates SWEET11 to Efflux Sugar
3.2. WRKY36–PIL15–miR530 Signaling Negatively Regulates Grain Size
3.3. The Potential Application of WRKY36–PIL15 in ShB-Resistant Breeding
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Pathogens
4.3. Generation of Transgenic Rice Plants
4.4. Yeast-One Hybrid Assay
4.5. Yeast-Two Hybrid Assay
4.6. Co-IP
4.7. BiFC Assay
4.8. Microscopic Observation
4.9. Transactivation Assay
4.10. RNA Isolation and RT-qPCR
4.11. Chromatin Immunoprecipitation (ChIP)-qPCR Assay
4.12. SIGS Assay
4.13. Lamina Joint Assay
4.14. Production Shape Assay
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.J.; Chen, Y.; Zhang, L.N.; Xu, B.; Zhang, J.H.; Chen, Z.X.; Tong, Y.H.; Zuo, S.M.; Xu, J.Y. Overexpression of OsPGIP1 Enhances Rice Resistance to Sheath Blight. Plant Dis. 2016, 100, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Senapati, M.; Tiwari, A.; Sharma, N.; Chandra, P.; Bashyal, B.M.; Ellur, R.K.; Bhowmick, P.K.; Bollinedi, H.; Vinod, K.K.; Singh, A.K.; et al. Rhizoctonia solani Kühn Pathophysiology: Status and Prospects of Sheath Blight Disease Management in Rice. Front. Plant Sci. 2022, 13, 881116. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Shu, X.; Jing, X.; Jiao, C.; Chen, L.; Zhang, J.; Ma, L.; Jiang, Y.; Yamamoto, N.; Li, S.; et al. Identification of rice (Oryza sativa L.) genes involved in sheath blight resistance via a genome-wide association study. Plant Biotechnol. J. 2021, 19, 1553–1566. [Google Scholar] [CrossRef] [PubMed]
- Kouzai, Y.; Kimura, M.; Watanabe, M.; Kusunoki, K.; Osaka, D.; Suzuki, T.; Matsui, H.; Yamamoto, M.; Ichinose, Y.; Toyoda, K.; et al. Salicylic acid-dependent immunity contributes to resistance against Rhizoctonia solani, a necrotrophic fungal agent of sheath blight, in rice and Brachypodium distachyon. New Phytol. 2018, 217, 771–783. [Google Scholar] [CrossRef]
- Qiao, L.; Zheng, L.; Sheng, C.; Zhao, H.; Jin, H.; Niu, D. Rice siR109944 suppresses plant immunity to sheath blight and impacts multiple agronomic traits by affecting auxin homeostasis. Plant J. Cell Mol. Biol. 2020, 102, 948–964. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Zhang, C.; Wang, Z.Y.; Zhu, X.F.; Xuan, Y.H. RAVL1 Activates Brassinosteroids and Ethylene Signaling to Modulate Response to Sheath Blight Disease in Rice. Phytopathology 2018, 108, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, H.; Jang, J.C.; Xiao, T.; He, H.; Jiang, D.; Tang, X. OsWRKY80-OsWRKY4 Module as a Positive Regulatory Circuit in Rice Resistance Against Rhizoctonia solani. Rice 2016, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xue, C.Y.; Liu, J.M.; He, Y.; Mei, Q.; Wei, S.; Xuan, Y.H. Sheath blight resistance in rice is negatively regulated by WRKY53 via SWEET2a activation. Biochem. Biophys. Res. Commun. 2021, 585, 117–123. [Google Scholar] [CrossRef]
- Mehboob, I.; Baig, S.; Siddique, M.; Shan, X.; Baig, A.; Shah, M.M.; Shahzadi, I.; Zhao, H.; Nawazish, S.; Khalid, S. Deciphering the role of SlWRKY36 and SlWRKY51 in salt stress tolerance via modulating ion homeostasis and proline biosynthesis. Curr. Plant Biol. 2024, 39, 100380. [Google Scholar] [CrossRef]
- Yang, S.; Fu, Y.; Zhang, Y.; Peng Yuan, D.; Li, S.; Kumar, V.; Mei, Q.; Hu Xuan, Y. Rhizoctonia solani transcriptional activator interacts with rice WRKY53 and grassy tiller 1 to activate SWEET transporters for nutrition. J. Adv. Res. 2023, 50, 1–12. [Google Scholar] [CrossRef]
- Li, N.; Lin, B.; Wang, H.; Li, X.; Yang, F.; Ding, X.; Yan, J.; Chu, Z. Natural variation in ZmFBL41 confers banded leaf and sheath blight resistance in maize. Nat. Genet. 2019, 51, 1540–1548. [Google Scholar] [CrossRef]
- Sun, Q.; Li, T.Y.; Li, D.D.; Wang, Z.Y.; Li, S.; Li, D.P.; Han, X.; Liu, J.M.; Xuan, Y.H. Overexpression of Loose Plant Architecture 1 increases planting density and resistance to sheath blight disease via activation of PIN-FORMED 1a in rice. Plant Biotechnol. J. 2019, 17, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Xu, H.; Dong, H.; Xuan, Y.H. Loose Plant Architecture 1-Interacting Kinesin-like Protein KLP Promotes Rice Resistance to Sheath Blight Disease. Rice 2021, 14, 60. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Y.; Wang, Z.Y.; Li, S.; Ye, L.; Xie, J.X.; Zhao, G.Q.; Wang, H.N.; Wang, Y.; Li, S.; et al. Isolation and characterization of genes related to sheath blight resistance via the tagging of mutants in rice. Plant Gene 2019, 19, 100200. [Google Scholar] [CrossRef]
- Miao Liu, J.; Mei, Q.; Yun Xue, C.; Yuan Wang, Z.; Pin Li, D.; Xin Zhang, Y.; Hu Xuan, Y. Mutation of G-protein γ subunit DEP1 increases planting density and resistance to sheath blight disease in rice. Plant Biotechnol. J. 2021, 19, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.J.; Chu, J.; Kumar, V.; Yuan, P.; Li, Z.M.; Mei, Q.; Xuan, Y.H. Protein Phosphatase 2A Catalytic Subunit PP2A-1 Enhances Rice Resistance to Sheath Blight Disease. Front. Genome Ed. 2021, 3, 632136. [Google Scholar] [CrossRef]
- He, Z.; Webster, S.; He, S.Y. Growth-defense trade-offs in plants. Curr. Biol. CB 2022, 32, R634–R639. [Google Scholar] [CrossRef]
- Ning, Y.; Liu, W.; Wang, G.L. Balancing Immunity and Yield in Crop Plants. Trends Plant Sci. 2017, 22, 1069–1079. [Google Scholar] [CrossRef]
- Denancé, N.; Sánchez-Vallet, A.; Goffner, D.; Molina, A. Disease resistance or growth: The role of plant hormones in balancing immune responses and fitness costs. Front. Plant Sci. 2013, 4, 155. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pajerowska-Mukhtar, K.; Culler, A.H.; Dong, X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr. Biol. CB 2007, 17, 1784–1790. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.Y. At the intersection of plant growth and immunity. Cell Host Microbe 2014, 15, 400–402. [Google Scholar] [CrossRef]
- Weyhe, M.; Eschen-Lippold, L.; Pecher, P.; Scheel, D.; Lee, J. Ménage à trois: The complex relationships between mitogen-activated protein kinases, WRKY transcription factors, and VQ-motif-containing proteins. Plant Signal. Behav. 2014, 9, e29519. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Yang, S.; Feng, L.; Chu, J.; Dong, H.; Sun, J.; Chen, H.; Li, Z.; Yamamoto, N.; Zheng, A.; et al. Red-light receptor phytochrome B inhibits BZR1-NAC028-CAD8B signaling to negatively regulate rice resistance to sheath blight. Plant Cell Environ. 2023, 46, 1249–1263. [Google Scholar] [CrossRef]
- Sun, W.; Xu, X.H.; Li, Y.; Xie, L.; He, Y.; Li, W.; Lu, X.; Sun, H.; Xie, X. OsmiR530 acts downstream of OsPIL15 to regulate grain yield in rice. New Phytol. 2020, 226, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Du, Y.; Li, F.; Sun, H.; Zhang, J.; Li, J.; Peng, T.; Xin, Z.; Zhao, Q. The basic helix-loop-helix transcription factor, OsPIL15, regulates grain size via directly targeting a purine permease gene OsPUP7 in rice. Plant Biotechnol. J. 2019, 17, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhai, K.; Xie, Z.; Yang, D.; Zhu, X.; Liu, J.; Wang, X.; Qin, P.; Yang, Y.; Zhang, G.; et al. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 2017, 355, 962–965. [Google Scholar] [CrossRef]
- Hu, K.; Cao, J.; Zhang, J.; Xia, F.; Ke, Y.; Zhang, H.; Xie, W.; Liu, H.; Cui, Y.; Cao, Y.; et al. Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nat. Plants 2017, 3, 17009. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, S. Rice MtN3/saliva/SWEET family genes and their homologs in cellular organisms. Mol. Plant 2013, 6, 665–674. [Google Scholar] [CrossRef]
- Bezrutczyk, M.; Yang, J.; Eom, J.S.; Prior, M.; Sosso, D.; Hartwig, T.; Szurek, B.; Oliva, R.; Vera-Cruz, C.; White, F.F.; et al. Sugar flux and signaling in plant-microbe interactions. Plant J. Cell Mol. Biol. 2018, 93, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Jeena, G.S.; Kumar, S.; Shukla, R.K. Structure, evolution and diverse physiological roles of SWEET sugar transporters in plants. Plant Mol. Biol. 2019, 100, 351–365. [Google Scholar] [CrossRef]
- Kim, P.; Xue, C.Y.; Song, H.D.; Gao, Y.; Feng, L.; Li, Y.; Xuan, Y.H. Tissue-specific activation of DOF11 promotes rice resistance to sheath blight disease and increases grain weight via activation of SWEET14. Plant Biotechnol. J. 2021, 19, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Zhang, H.; Zhang, Q.; Zhai, H.; Liu, Q.; He, S. The Plasma Membrane-Localized Sucrose Transporter IbSWEET10 Contributes to the Resistance of Sweet Potato to Fusarium oxysporum. Front. Plant Sci. 2017, 8, 197. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, M.; Zhou, Y.; Li, X.; Xiao, J.; Wang, S. A paralog of the MtN3/saliva family recessively confers race-specific resistance to Xanthomonas oryzae in rice. Plant Cell Environ. 2011, 34, 1958–1969. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, C.; Han, X.; Wang, Z.Y.; Ma, L.; Yuan, P.; Wu, J.N.; Zhu, X.F.; Liu, J.M.; Li, D.P.; et al. Inhibition of OsSWEET11 function in mesophyll cells improves resistance of rice to sheath blight disease. Mol. Plant Pathol. 2018, 19, 2149–2161. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, D.; Miao, Q.; Yang, J.; Xuan, Y.; Hu, Y. Essential Role of Sugar Transporter OsSWEET11 During the Early Stage of Rice Grain Filling. Plant Cell Physiol. 2017, 58, 863–873. [Google Scholar] [CrossRef] [PubMed]
- John Lilly, J.; Subramanian, B. Gene network mediated by WRKY13 to regulate resistance against sheath infecting fungi in rice (Oryza sativa L.). Plant Sci. Int. J. Exp. Plant Biol. 2019, 280, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhang, G.; An, L.; Chen, X.; Fang, R. Phytochrome-interacting factor-like protein OsPIL15 integrates light and gravitropism to regulate tiller angle in rice. Planta 2019, 250, 105–114. [Google Scholar] [CrossRef]
- Aamir, M.; Singh, V.K.; Meena, M.; Upadhyay, R.S.; Gupta, V.K.; Singh, S. Structural and Functional Insights into WRKY3 and WRKY4 Transcription Factors to Unravel the WRKY-DNA (W-Box) Complex Interaction in Tomato (Solanum lycopersicum L.). A Computational Approach. Front. Plant Sci. 2017, 8, 819. [Google Scholar] [CrossRef]
- Xu, Y.P.; Xu, H.; Wang, B.; Su, X.D. Crystal structures of N-terminal WRKY transcription factors and DNA complexes. Protein Cell 2020, 11, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bai, M.Y.; Chong, K. Brassinosteroid-mediated regulation of agronomic traits in rice. Plant Cell Rep. 2014, 33, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; He, M.; Mei, E.; Zhang, B.; Tang, J.; Xu, M.; Liu, J.; Li, X.; Wang, Z.; Tang, W.; et al. WRKY53 integrates classic brassinosteroid signaling and the mitogen-activated protein kinase pathway to regulate rice architecture and seed size. Plant Cell 2021, 33, 2753–2775. [Google Scholar] [CrossRef]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Breia, R.; Conde, A.; Badim, H.; Fortes, A.M.; Gerós, H.; Granell, A. Plant SWEETs: From sugar transport to plant-pathogen interaction and more unexpected physiological roles. Plant Physiol. 2021, 186, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Balyan, H.S.; Gautam, T. SWEET genes and TAL effectors for disease resistance in plants: Present status and future prospects. Mol. Plant Pathol. 2021, 22, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yang, L.; Fang, Z.; Zhang, Y.; Zhuang, M.; Lv, H.; Wang, Y. Plant SWEET Family of Sugar Transporters: Structure, Evolution and Biological Functions. Biomolecules 2022, 12, 205. [Google Scholar] [CrossRef]
- Li, P.; Wang, L.; Liu, H.; Yuan, M. Impaired SWEET-mediated sugar transportation impacts starch metabolism in developing rice seeds. Crop J. 2022, 10, 98–108. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, X.; Li, Y.; Liu, L.; Wang, Q.; Wang, Y.; Wang, Y.; Yan, J.; Cheng, G.; Zou, L.; et al. Tal6b/AvrXa27A, a Hidden TALE Targeting both the Susceptibility Gene OsSWEET11a and the Resistance Gene Xa27 in Rice. Plant Commun. 2023, 5, 100721. [Google Scholar] [CrossRef]
- Lan, J.; Lin, Q.; Zhou, C.; Ren, Y.; Liu, X.; Miao, R.; Jing, R.; Mou, C.; Nguyen, T.; Zhu, X.; et al. Small grain and semi-dwarf 3, a WRKY transcription factor, negatively regulates plant height and grain size by stabilizing SLR1 expression in rice. Plant Mol. Biol. 2020, 104, 429–450. [Google Scholar] [CrossRef]
- Baldrich, P.; Campo, S.; Wu, M.T.; Liu, T.T.; Hsing, Y.I.; San Segundo, B. MicroRNA-mediated regulation of gene expression in the response of rice plants to fungal elicitors. RNA Biol. 2015, 12, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, N.; Guo, J.; Zhou, X.; Bai, X.; Azeem, M.; Zhu, L.; Chen, L.; Chu, M.; Wang, H.; et al. Integrative Transcriptome Analysis of mRNA and miRNA in Pepper’s Response to Phytophthora capsici Infection. Biology 2024, 13, 186. [Google Scholar] [CrossRef]
- Gu, Z.; Gong, J.; Zhu, Z.; Li, Z.; Feng, Q.; Wang, C.; Zhao, Y.; Zhan, Q.; Zhou, C.; Wang, A.; et al. Structure and function of rice hybrid genomes reveal genetic basis and optimal performance of heterosis. Nat. Genet. 2023, 55, 1745–1756. [Google Scholar] [CrossRef]
- Tian, X.; Li, X.; Zhou, W.; Ren, Y.; Wang, Z.; Liu, Z.; Tang, J.; Tong, H.; Fang, J.; Bu, Q. Transcription Factor OsWRKY53 Positively Regulates Brassinosteroid Signaling and Plant Architecture. Plant Physiol. 2017, 175, 1337–1349. [Google Scholar] [CrossRef]
- Kim, J.G.; Li, X.; Roden, J.A.; Taylor, K.W.; Aakre, C.D.; Su, B.; Lalonde, S.; Kirik, A.; Chen, Y.; Baranage, G.; et al. Xanthomonas T3S Effector XopN Suppresses PAMP-Triggered Immunity and Interacts with a Tomato Atypical Receptor-Like Kinase and TFT1. Plant Cell 2009, 21, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Ohtani, M.; Mitsuda, N.; Kubo, M.; Ohme-Takagi, M.; Fukuda, H.; Demura, T. VND-INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis. Plant Cell 2010, 22, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.H.; Priatama, R.A.; Huang, J.; Je, B.I.; Liu, J.M.; Park, S.J.; Piao, H.L.; Son, D.Y.; Lee, J.J.; Park, S.H.; et al. Indeterminate domain 10 regulates ammonium-mediated gene expression in rice roots. New Phytol. 2013, 197, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhu, C.; Xuan, W.; An, H.; Tian, Y.; Wang, B.; Chi, W.; Chen, G.; Ge, Y.; Li, J.; et al. Genome-wide association studies identify OsWRKY53 as a key regulator of salt tolerance in rice. Nat. Commun. 2023, 14, 3550. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Jin, Y.; Liu, W.; Li, F.; Fang, J.; Yin, Y.; Qian, Q.; Zhu, L.; Chu, C. DWARF AND LOW-TILLERING, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant J. Cell Mol. Biol. 2009, 58, 803–816. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Sun, Q.; Yang, S.; Chen, H.; Yuan, D.; Gan, C.; Chen, H.; Zhi, Y.; Zhu, H.; Gao, Y.; et al. WRKY36–PIL15 Transcription Factor Complex Negatively Regulates Sheath Blight Resistance and Seed Development in Rice. Plants 2025, 14, 518. https://doi.org/10.3390/plants14040518
Wang S, Sun Q, Yang S, Chen H, Yuan D, Gan C, Chen H, Zhi Y, Zhu H, Gao Y, et al. WRKY36–PIL15 Transcription Factor Complex Negatively Regulates Sheath Blight Resistance and Seed Development in Rice. Plants. 2025; 14(4):518. https://doi.org/10.3390/plants14040518
Chicago/Turabian StyleWang, Siting, Qian Sun, Shuo Yang, Huan Chen, Depeng Yuan, Changxi Gan, Haixia Chen, Yongxi Zhi, Hongyao Zhu, Yue Gao, and et al. 2025. "WRKY36–PIL15 Transcription Factor Complex Negatively Regulates Sheath Blight Resistance and Seed Development in Rice" Plants 14, no. 4: 518. https://doi.org/10.3390/plants14040518
APA StyleWang, S., Sun, Q., Yang, S., Chen, H., Yuan, D., Gan, C., Chen, H., Zhi, Y., Zhu, H., Gao, Y., Zhu, X., & Xuan, Y. (2025). WRKY36–PIL15 Transcription Factor Complex Negatively Regulates Sheath Blight Resistance and Seed Development in Rice. Plants, 14(4), 518. https://doi.org/10.3390/plants14040518






