Efficacy of Selected Bacterial Strains in the Protection and Growth Stimulation of Winter Wheat and Maize
Abstract
1. Introduction
2. Results
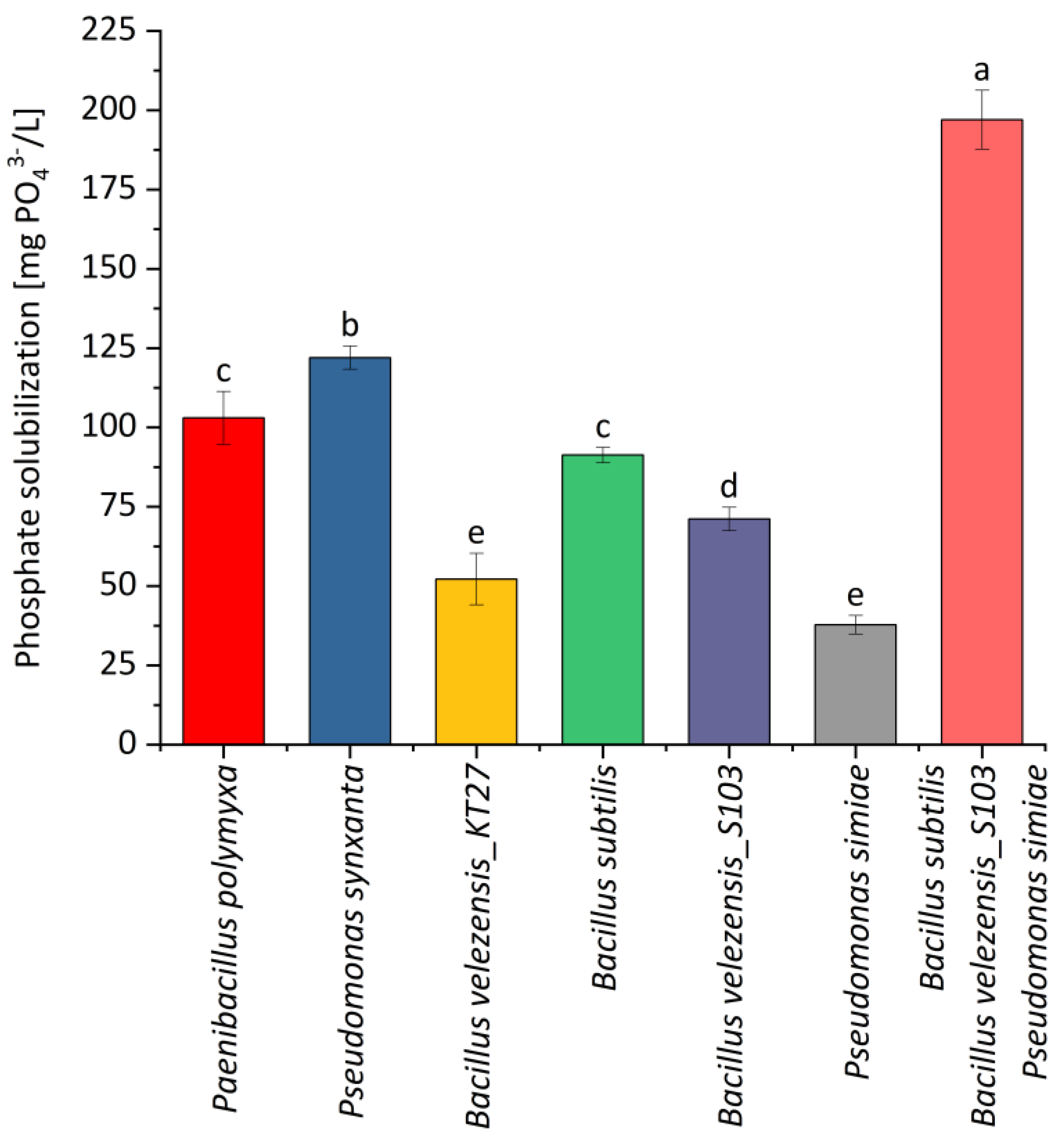
2.1. Phosphate-Dissolving Activity and Fungistatic Activity of Selected Bacterial Strains
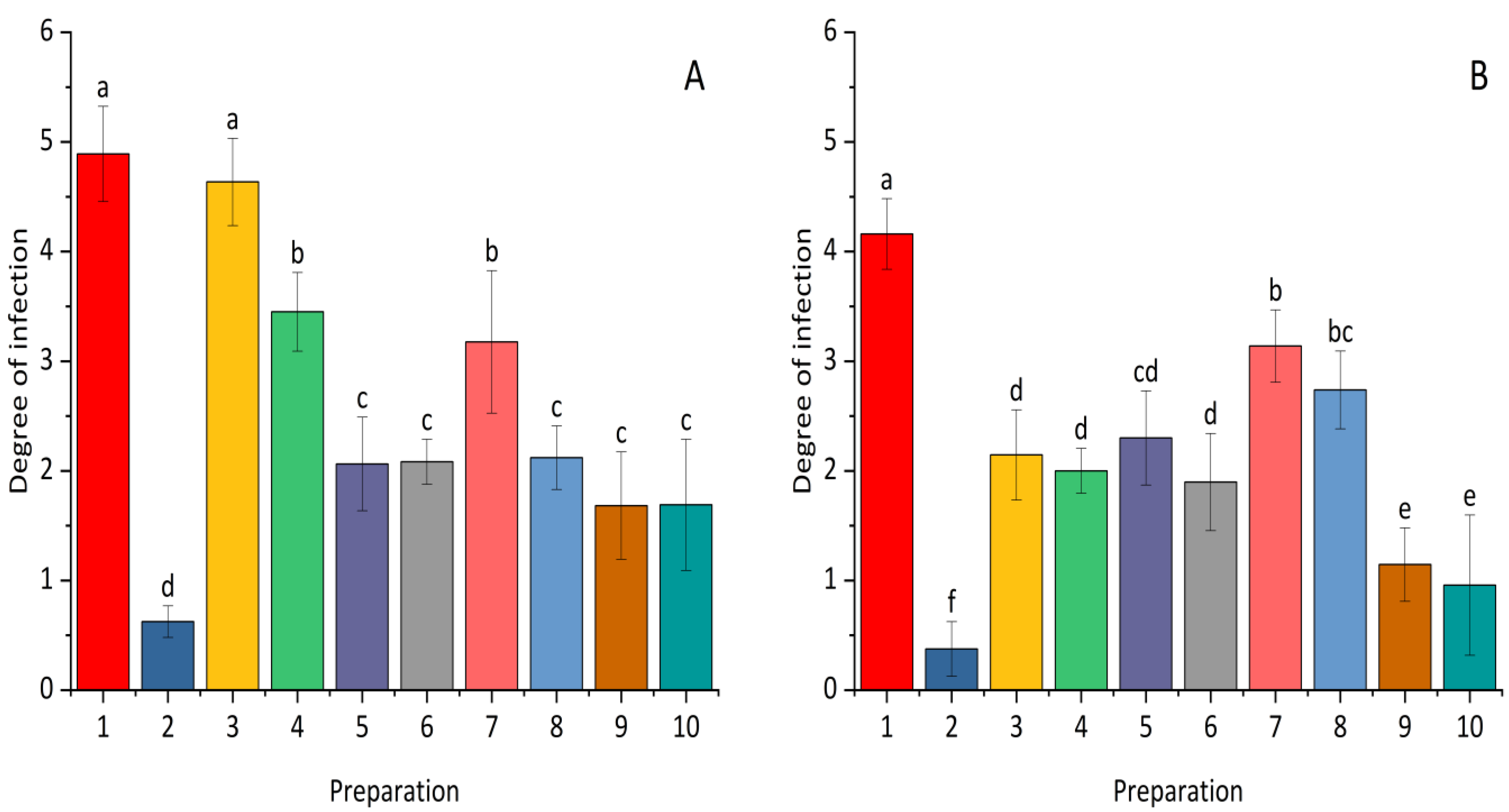
2.2. Seed Germination and Health Parameters of Plant Seedlings (Seeds Inoculated with Fusarium)
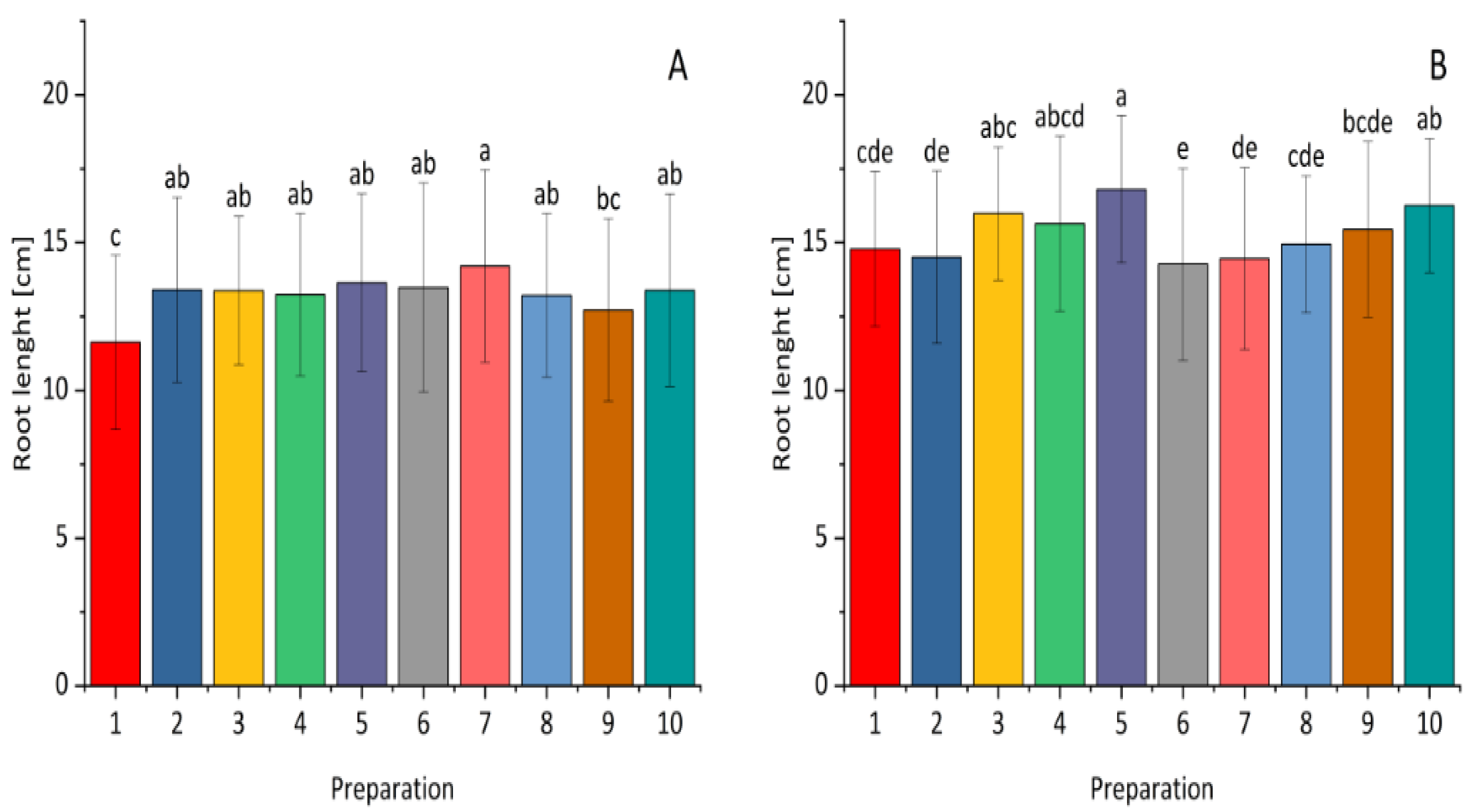
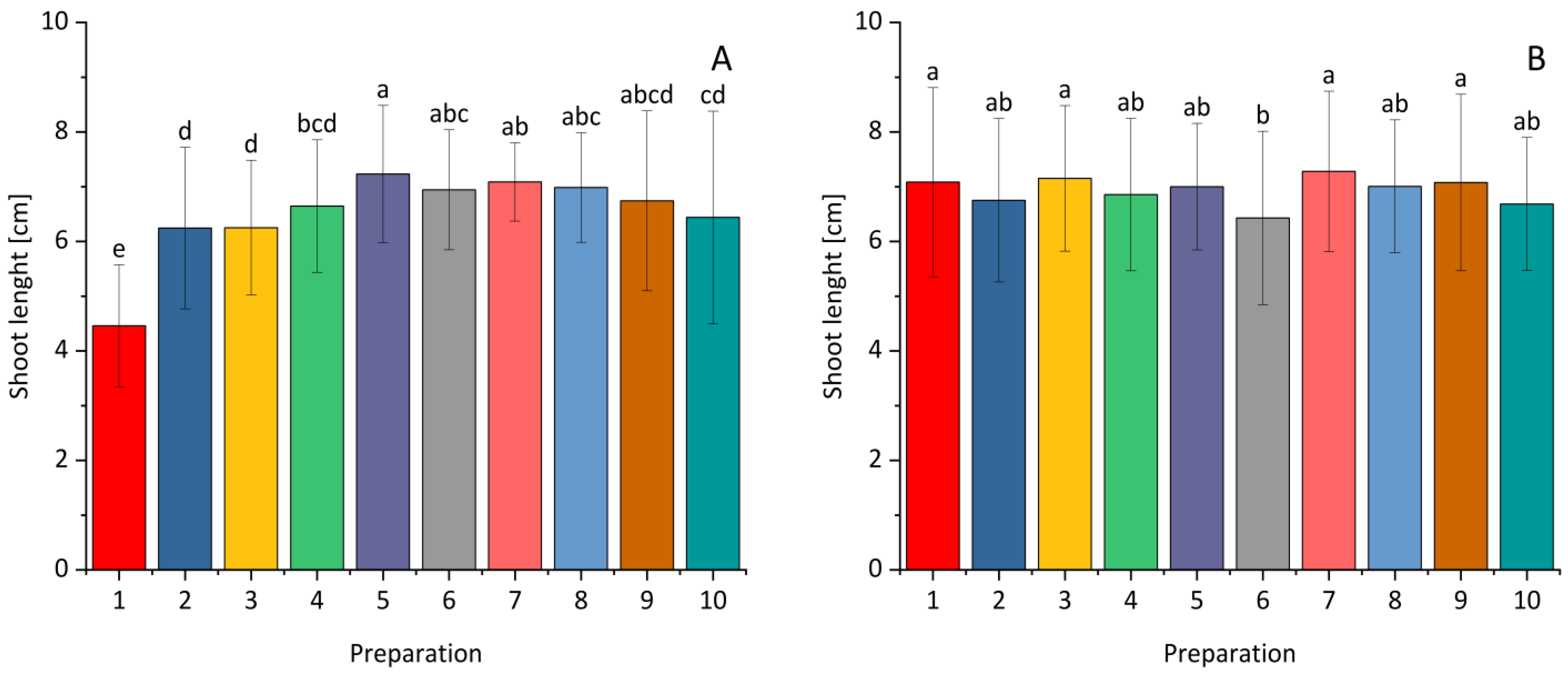
2.3. The Effect of Selected Bacterial Strains on Plant Growth Under Greenhouse Conditions
3. Discussion
4. Materials and Methods
4.1. Sample Collection, Bacteria Isolation, Identification, and Selection
4.2. Production and Standardization of Biopreparations
4.3. Quantitative Assessment of Phosphate Solubilization
4.4. Evaluation of the Antifungal Activity of Bacterial Strains Against Phytopathogenic Fusarium Species
4.5. Seed Germination and Health Parameters of Plant Seedlings (Seed Inoculated with Fusarium)
- I. no symptoms;
- II. less than 50% of seedling attacked;
- III. more than 50% seedling attacked;
- IV. 100% of seedling attacked.
4.6. Effect of Selected Bacterial Strains on Plant Growth Under Greenhouse Conditions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hatfield, J.L.; Dold, C. Agroclimatology and Wheat Production: Coping with Climate Change. Front. Plant Sci. 2018, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Podgórska-Kryszczuk, I.; Solarska, E.; Kordowska-Wiater, M. Biological Control of Fusarium culmorum, Fusarium graminearum and Fusarium poae by Antagonistic Yeasts. Pathogens 2022, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Jakhar, D.S.; Singh, R. Biotic stress response in maize (Zea mays L.). J. Biotechnol. Crop Sci. 2015, 4, 47–51. [Google Scholar]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription Factors Associated with Abiotic and Biotic Stress Tolerance and Their Potential for Crops Improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogenes: A review. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef]
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight from a Microbiome Perspective. Front. Microbiol. 2021, 12, 628373. [Google Scholar] [CrossRef] [PubMed]
- Chandler, E.A.; Simpson, D.R.; Thomsett, M.A.; Nicholson, P. Development of PCR assays to Tri7 and Tri13 trichothecene biosynthetic genes, and characterisation of chemotypes of Fusarium graminearum, Fusarium culmorum and Fusarium cerealis. Physiol. Mol. Plant Pathol. 2003, 62, 355–367. [Google Scholar] [CrossRef]
- Kosiak, B.; Torp, M.; Skjerve, E.; Thrane, U. The Prevalence and Distribution of Fusarium species in Norwegian Cereals: A Survey. Acta Agric. Scand. 2003, 53, 168–176. [Google Scholar] [CrossRef]
- SØrensen, J.N.; Phipps, R.K.; Nielsen, K.F.; Schroers, H.J.; Frank, J.; Thrane, U. Analysis of Fusarium avenaceum Metabolites Produced during Wet Apple Core Rot. J. Agric. Food Chem. 2009, 57, 1632–1639. [Google Scholar] [CrossRef]
- Janevska, S.; Tudzynski, B. Secondary metabolism in Fusarium fujikuroi: Strategies to unravel the function of biosynthetic pathways. Appl. Microbiol. Biotechnol. 2018, 102, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Kardava, K.; Tetz, V.; Vecherkovskaya, M.; Tetz, G. Seed dressing with M451 promotes seedling growth in wheat and reduces root phytopathogenic fungi without affecting endophytes. Front. Plant Sci. 2023, 14, 1176553. [Google Scholar] [CrossRef] [PubMed]
- Tonin, R.B.; Reis, E.M.; Avozani, A. Reduction in the in vitro sensitivity oF Drechslera tritici-repentis, isolated from wheat, to strobilurin and triazole fungicides. Summa Phytopathol. 2017, 43, 20–25. [Google Scholar] [CrossRef][Green Version]
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Alcazar-Fuoli, L.; Mellado, E. Ergosterol biosynthesis in Aspergillus fumigatus: Its relevance as an antifungal target and role in antifungal drug resistance. Front. Microbiol. 2013, 3, 33242. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, H.; Szurek, B.; Van den Ackerveken, G. Stop helping pathogens: Engineering plant susceptibility genes for durable resistance. Curr. Opin. Biotechnol. 2021, 70, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Bainsla, N.K.; Meena, H.P. Breeding for Resistance to Biotic Stresses in Plants. J. Plant Stress Physiol. 2016, 379, 411. Available online: https://www.researchgate.net/publication/321796021 (accessed on 12 November 2024).
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Lengai, G.M.W.; Muthomi, J.W. Biopesticides and Their Role in Sustainable Agricultural Production. J. Biosci. Med. 2018, 6, 7–41. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
- He, D.-C.; He, M.-H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K.-H. Bacillus velezensis: A Valuable Member of Bioactive Molecules within Plant Microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Grantcharova, N.; Wagner, E.G.H. Paenibacillus polymyxa Invades Plant Roots and Forms Biofilms. Appl. Environ. Microbiol. 2005, 71, 7292–7300. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.S.; Din, A.R.J.M.; Rosli, M.A.; Azam, Z.M.; Othman, N.Z.; Sarmidi, M.R. Paenibacillus polymyxa bioactive compounds for agricultural and biotechnological applications. Biocatal. Agric. Biotechnol. 2019, 18, 101092. [Google Scholar] [CrossRef]
- Janakiev, T.; Dimkić, I.; Unković, N.; Ljaljević Grbić, M.; Opsenica, D.; Gašić, U.; Stanković, S.; Berić, T. Phyllosphere Fungal Communities of Plum and Antifungal Activity of Indigenous Phenazine-Producing Pseudomonas synxantha Against Monilinia laxa. Front. Microbiol. 2019, 10, 2287. [Google Scholar] [CrossRef]
- Nithyapriya, S.; Lalitha, S.; Sayyed, R.Z.; Reddy, M.S.; Dailin, D.J.; El Enshasy, H.A.; Luh Suriani, N.; Herlambang, S. Production, Purification, and Characterization of Bacillibactin Siderophore of Bacillus subtilis and Its Application for Improvement in Plant Growth and Oil Content in Sesame. Sustainability 2021, 13, 5394. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Berendsen, R.L.; de Jonge, R.; Stringlis, I.A.; Van Dijken, A.J.H.; Van Pelt, J.A.; Van Wees, S.C.M.; Yu, K.; Zamioudis, C.; Bakker, P.A.H.M. Pseudomonas simiae WCS417: Star track of a model beneficial rhizobacterium. Plant Soil 2021, 461, 245–263. [Google Scholar] [CrossRef]
- Cetner, M.D.; Dąbrowski, P.; Samborska, I.A.; Łukasik, I.; Swoczyna, T.; Pietkiewicz, S.; Bąba, W.; Kalaji, H.M. Application of chlorophyll fluorescence measurements in environmental research. Kosm. Probl. Nauk. Biol. 2016, 65, 197–205. [Google Scholar]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Sobiech, Ł.; Grzanka, M.; Kurasiak-Popowska, D.; Radzikowska, D. Phytotoxic Effect of Herbicides on Various Camelina [Camelina sativa (L.) Crantz] Genotypes and Plant Chlorophyll Fluorescence. Agriculture 2020, 10, 185. [Google Scholar] [CrossRef]
- Huang, S.; Luo, H.; Ashraf, U.; Abrar, M.; He, L.; Zheng, A.; Wang, Z.; Zhang, T.; Tang, X. Seed Treatment with Paclobutrazol Affects Early Growth, Photosynthesis, Chlorophyll Fluorescence and Physiology of Rice. Appl. Ecol. Environ. Res. 2019, 17, 999–1012. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Khaeim, H.M.; Clark, A.; Pearson, T.; Van Sanford, D. Methods of Assessing Fusarium Damage to Wheat Kernels. J. For. Agric. Sci. 2019, 9, 297–308. Available online: https://www.researchgate.net/publication/337658019 (accessed on 25 November 2024). [CrossRef]
- Moumni, M.; Brodal, G.; Romanazzi, G. Recent innovative seed treatment methods in the management of seedborne pathogens. Food Secur. 2023, 15, 1365–1382. [Google Scholar] [CrossRef]
- Ismail, M.A.; Amin, M.A.; Eid, A.M.; Hassan, S.E.D.; Mahgoub, H.A.; Lashin, I.; Abdelwahab, A.T.; Azab, E.; Gobouri, A.A.; Elkelish, A. Comparative Study between Exogenously Applied Plant Growth Hormones versus Metabolites of Microbial Endophytes as Plant Growth-Promoting for Phaseolus vulgaris L. Cells 2021, 10, 1059. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, Y.D.; Chong, X.Y.; Xin, Q.H.; Wang, D.X.; Bian, K. Seed-borne endophytic Bacillus velezensis LHSB1 mediate the biocontrol of peanut stem rot caused by Sclerotium rolfsii. J. Appl. Microbiol. 2020, 128, 803–813. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Shahid, M.A.; Mustafa, A.; Sayyed, R.Z.; Curá, J.A. Insights into the Interactions among Roots, Rhizosphere, and Rhizobacteria for Improving Plant Growth and Tolerance to Abiotic Stresses: A Review. Cells. 2021, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Thakur, D. Phylogenetic and Functional Characterization of Culturable Endophytic Actinobacteria Associated with Camellia spp. for Growth Promotion in Commercial Tea Cultivars. Front. Microbiol. 2020, 11, 318. [Google Scholar] [CrossRef]
- Crowther, T.W.; van den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, I.; Averill, C.; Maynard, D.S. The global soil community and its influence on biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- Phi, Q.-T.; Park, Y.-M.; Seul, K.-J.; Ryu, C.-M.; Park, S.-H.; Kim, J.-G.; Ghim, S.-Y. Assessment of rootassociated Paenibacillus polymyxa groups on growth promotion and induced systemic resistance in pepper. J. Microbiol. Biotechnol. 2010, 20, 1605–1613. [Google Scholar]
- Yerkovich, N.; Cantoro, R.; Palazzini, J.M.; Torres, A.; Chulze, S.N. Fusarium head blight in Argentina: Pathogen aggressiveness, triazole tolerance and biocontrol-cultivar combined strategy to reduce disease and deoxynivalenol in wheat. Crop Prot. 2020, 137, 105300. [Google Scholar] [CrossRef]
- Avozani, A.; Tonin, R.B.; Reis, E.M.; Camera, J.; Ranzi, C. In vitro sensitivity of Fusarium graminearum isolates to fungicides. Summa Phytopathol. 2014, 40, 231–247. [Google Scholar] [CrossRef][Green Version]
- Sooväli, P.; Koppel, M.; Kangor, T. Effectiveness of seed treatment against Fusarium spp. and Cochliobolus sativus of spring barley in different conditions. Agron. Res. 2017, 15, 280–287. [Google Scholar]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The evolution of fungicide resistance. Adv. Appl. Microbiol. 2015, 90, 29–92. [Google Scholar]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Horikoshi, K.; Grant, W.D. Extremophiles: Microbial Life in Extreme Environments; Wiley-Liss: New York, NY, USA, 1998. [Google Scholar]
- Sagova-Mareckova, M.; Boenigk, J.; Bouchez, A.; Cermakova, K.; Chonova, T.; Cordier, T.; Eisendle, U.; Elersek, T.; Fazi, S.; Fleituch, T.; et al. Expanding Ecological Assessment by Integrating Microorganisms into Routine Freshwater Biomonitoring. Water Res. 2021, 191, 116767. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Heng, J.; Qin, S.; Bian, K. A comprehensive understanding of the biocontrol potential of Bacillus velezensis LM2303 against Fusarium head blight. PLoS ONE 2018, 13, e0198560. [Google Scholar] [CrossRef]
- Agha, S.I.; Jahan, N.; Azeem, S.; Parveen, S.; Tabassum, B.; Raheem, A.; Ullah, H.; Khan, A. Research article Characterization of broad-spectrum biocontrol efficacy of Bacillus velezensis against Fusarium oxysporum in Triticum aestivum L. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12590. [Google Scholar] [CrossRef]
- Timmusk, S.; Copolovici, D.; Copolovici, L.; Teder, T.; Nevo, E.; Behers, L. Paenibacillus polymyxa bioflm polysaccharides antagonise Fusarium graminearum. Sci. Rep. 2019, 9, 662. [Google Scholar] [CrossRef]
- Baard, V.; Bakare, O.O.; Daniel, A.I.; Nkomo, M.; Gokul, A.; Keyster, M.; Klein, A. Biocontrol Potential of Bacillus subtilis and Bacillus tequilensis against Four Fusarium Species. Pathogens 2023, 12, 254. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Chen, H.; Yao, J. Characterization of Fusarium graminearum inhibitory lipopeptide from Bacillus subtilis IB. Appl. Microbiol. Biotechnol. 2007, 76, 889–894. [Google Scholar] [CrossRef]
- Gu, Q.; Yang, Y.; Yuan, Q.; Shi, G.; Wu, L.; Lou, Z.; Huo, R.; Wu, H.; Borriss, R.; Gao, X. Bacillomycin D produced by Bacillus amyloliquefaciens is involved in the antagonistic interaction with the plantpathogenic fungus Fusarium graminearum. Appl. Environ. Microbiol. 2017, 83, e01075-17. [Google Scholar] [CrossRef]
- Gong, A.-D.; Li, H.-P.; Yuan, Q.-S.; Song, X.-S.; Yao, W.; He, W.-J.; Zhang, J.-B.; Liao, Y.-C. Antagonistic Mechanism of Iturin A and Plipastatin A from Bacillus amyloliquefaciens S76-3 from Wheat Spikes against Fusarium graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef]
- Sarić-Krsmanović, M.M.; Bozic, D.M.; Radivojevic, L.M.; Gajić Umiljendić, J.S.; Santric, L.; Vrbničanin, S.P. Effects of plant growth promoting rhizobacteria (PGPR) and cover crops on seed germination and early establishment of field dodder (Cuscuta campestris Yunk.) and early establishment of field dodder (Cuscuta campestris Yunk.). Pestic. Fitomed. 2017, 32, 105–111. [Google Scholar] [CrossRef]
- Jha, K.S.; Saraf, M. Effect of Plant Growth Promoting Rhizobacteria on Seed Germination Behaviour and Seedling Vigor of Jatropha Curcas. Int. J. Biotechnol. Biosci. 2011, 1, 161–169. [Google Scholar]
- Meng, Q.; Jiang, H.; Hao, J.J. Effects of Bacillus velezensis strain BAC03 in promoting plant growth. Biol. Control 2016, 98, 18–26. [Google Scholar] [CrossRef]
- Wang, J.; Qu, F.; Liang, J.; Yang, M.; Hu, X. Bacillus velezensis SX13 promoted cucumber growth and production by accelerating the absorption of nutrients and increasing plant photosynthetic metabolism. Sci. Hortic. 2022, 301, 111151. [Google Scholar] [CrossRef]
- Mosela, M.; Andrade, G.; Massucato, L.R.; Almeida, S.R.d.A.; Nogueira, A.F.; Filho, R.B.d.L.; Zeffa, D.M.; Mian, S.; Higashi, A.Y.; Shimizu, G.D.; et al. Bacillus velezensis strain Ag75 as a new multifunctional agent for biocontrol, phosphate solubilization and growth promotion in maize and soybean crops. Sci. Rep. 2022, 12, 15284. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, H.; Singh, B.; Jajoo, A.; Prakash, A. Shielding of Photosynthetic Apparatus by Consortia of Bacterial Endophytes in Tomato Plants Suffering From Fusarium Wilt. Front. Agron. 2022, 4, 831731. [Google Scholar] [CrossRef]
- Padda, K.P.; Puri, A.; Chanway, C.P. Plant growth promotion and nitrogen fixation in canola by an endophytic strain of Paenibacillus polymyxa and its GFPtagged derivative in a long-term study. Botany 2016, 94, 1209–1217. [Google Scholar] [CrossRef]
- Minut, M.; Diaconu, M.; Rosca, M.; Cozma, P.; Bulgariu, L.; Gavrilescu, M. Screening of Azotobacter, Bacillus and Pseudomonas Species as Plant Growth-Promoting Bacteria. Processes 2023, 11, 80. [Google Scholar] [CrossRef]
- Ganesh, J.; Hewitt, K.; Devkota, A.R.; Wilson, T.; Kaundal, A. IAA-producing plant growth promoting rhizobacteria from Ceanothus velutinus enhance cutting propagation efficiency and Arabidopsis biomass. Front. Plant Sci. 2024, 15, 1374877. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zeng, Q.; Han, Y.; Zhou, X.; Xu, H. Effects of Bacillus subtilis on Cucumber Seedling Growth and Photosynthetic System under Different Potassium Ion Levels. Biology 2024, 13, 348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, B.; Wang, X.; Yang, L.; Yang, H.; Zeng, H.; Qiu, Y.; Wang, C.; Yu, J.; Li, J.; Xu, D.; et al. Effects of Bacillus amyloliquefaciens ZM9 on bacterial wilt and rhizosphere microbial communities of tobacco. Appl. Soil Ecol. 2016, 103, 1–12. [Google Scholar] [CrossRef]
- Çelİkten, M.; Bozkurt, İ.A. Determination of efficacies of bacteria isolated from wheat rhizospheres on plant growth. Ziraat Fakültesi Derg. 2018, 23, 33–48. [Google Scholar]
- Schmidt, C.S.; Mrnka, L.; Frantík, T.; Lovecká, P.; Vosátka, M. Plant growth promotion of Miscanthus × giganteus by endophytic bacteria and fungi on non-polluted and polluted soils. World J. Microbiol. Biotechnol. 2018, 34, 48. [Google Scholar] [CrossRef] [PubMed]
- Hawkesford, M.J.; Cakmak, I.; Coskun, D.; De Kok, L.J.; Lambers, H.; Schjoerring, J.K.; White, P.J. Functions of macronutrients. In Marschner’s Mineral Nutrition of Plants; Academic Press: Cambridge, MA, USA, 2023; pp. 201–281. [Google Scholar]
- Che, S.; Xu, Y.; Qin, X.; Tian, S.; Wang, J.; Zhou, X.; Cao, Z.; Wang, D.; Wu, M.; Wu, Z.; et al. Building microbial consortia to enhance straw degradation, phosphorus solubilization, and soil fertility for rice growth. Microb. Cell Factories 2024, 23, 232. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Shi, J.; Li, M.; Chen, J.; Wang, T.; Zhang, Q.; Yang, L.; Zhu, N.; Wang, Y. Consortium of Phosphorus-Solubilizing Bacteria Promotes Maize Growth and Changes the Microbial Community Composition of Rhizosphere Soil. Agronomy 2024, 14, 1535. [Google Scholar] [CrossRef]
- Biostimulants Market Size, Share & Trends Analysis Report By Active Ingredients (Acid Based, Microbial), by Crop Type, by Application (Foliar, Soil Treatment); by Region, and Segment Forecasts, 2023–2030; Report ID: GVR-2-68038-346-1; San Francisco, CA, USA. Available online: https://www.grandviewresearch.com/industry-analysis/biostimulants-market (accessed on 29 December 2024).
- Wita, A.; Białas, W.; Wilk, R.; Szychowska, K.; Czaczyk, K. The Influence of Temperature and Nitrogen Source on Cellulolytic Potential of Microbiota Isolated from Natural Environment. Pol. J. Microbiol. 2019, 68, 105–114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pikovskaya, R.I. Mobilization of Phosphorus in Soil Connection with the Vital Activity of Some Microbial Species. Microbiology 1948, 17, 362–370. [Google Scholar]
- Prasad, S.R. Testing seed for quality. In Seed Science and Technology; Dadlani, D., Yadava, K., Eds.; Springer: Singapore, 2023; pp. 299–334. [Google Scholar]
- ISTA. ISTA Handbook on Seedling Evaluation; ISTA: Bassersdorf, Switzerland, 2006. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]

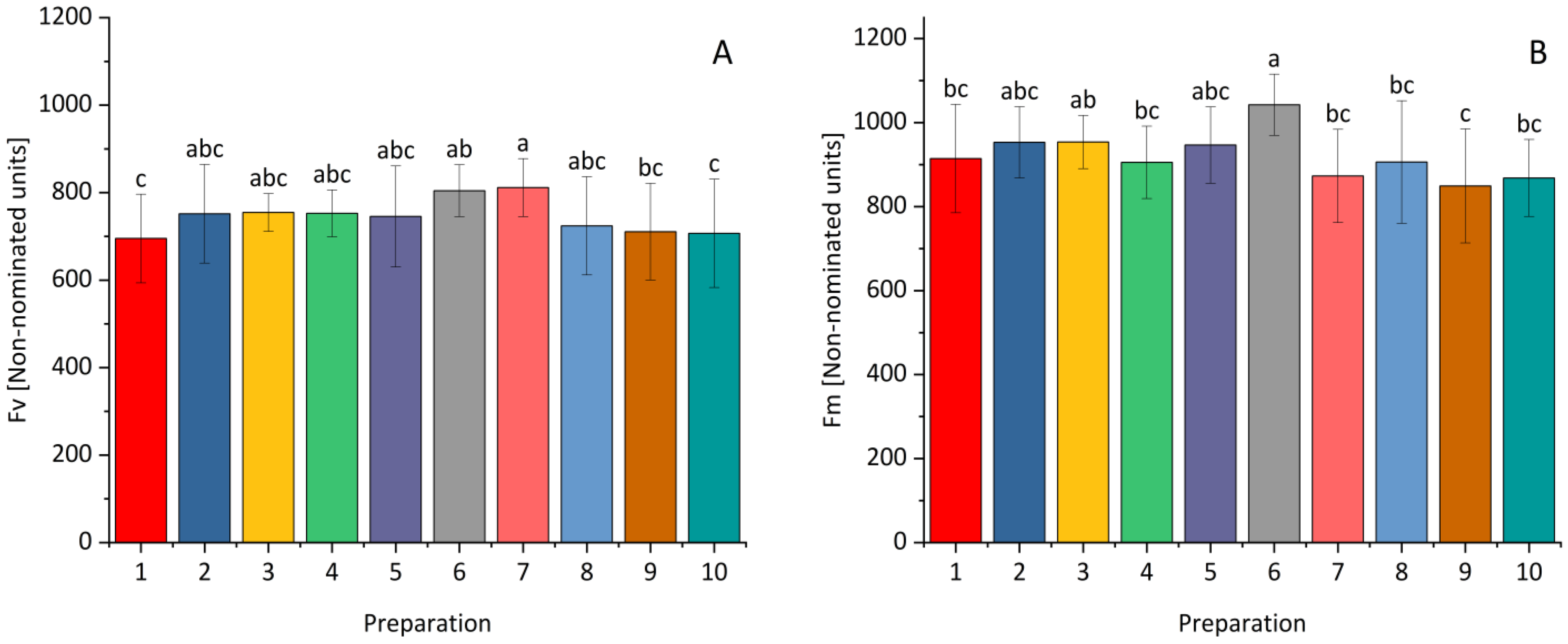
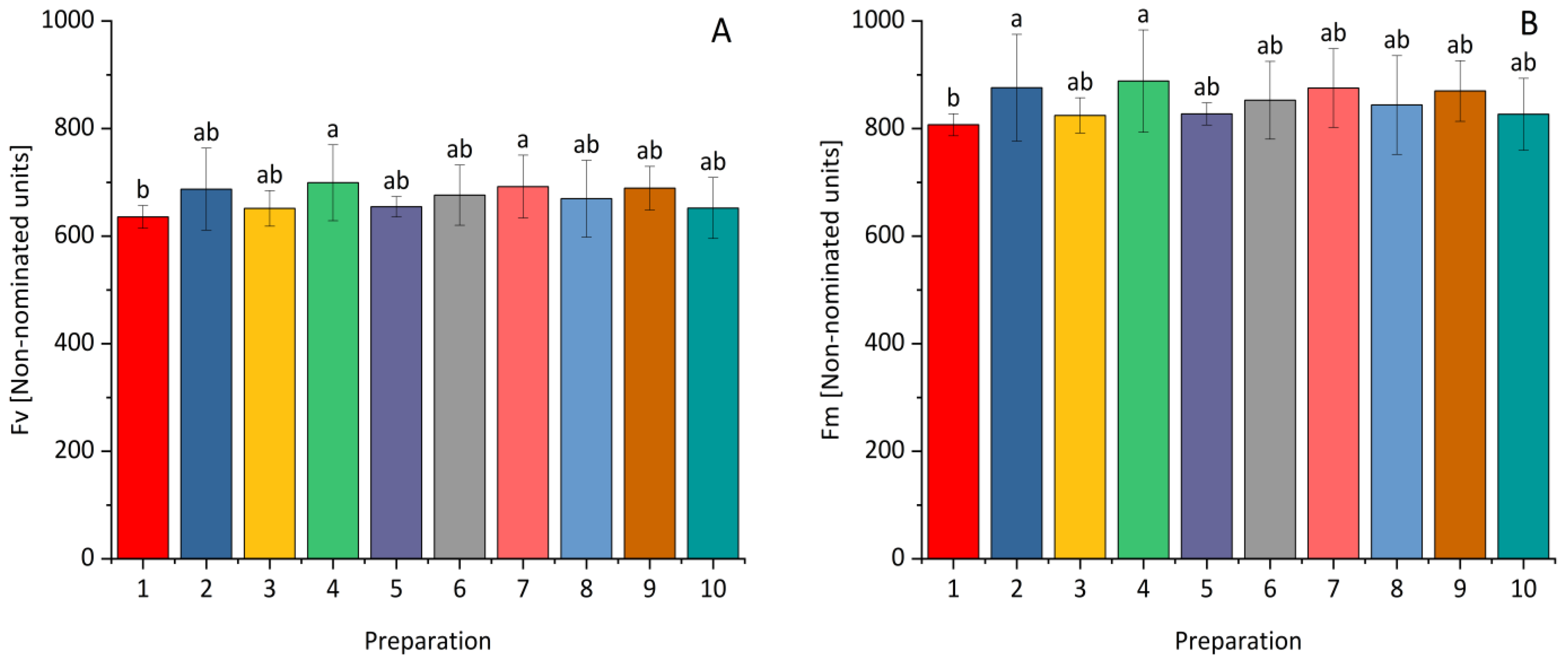
| Fraction | Bacterial Strain | The Diameter of the Clear Zone [mm] | |||
|---|---|---|---|---|---|
| Fusarium culmorum | Fusarium graminearum | Fusarium fujikuori | Fusarium avenaceum | ||
| Culture fluid containing bacterial cells | Bacillus velenensis_KT27 | 0 | 50 | 26 | 14 |
| Paenibacillus polymyxa | 0 | 50 | 50 | 14 | |
| Pseudomonas synxantha | 0 | 0 | 16 | 20 | |
| Bacillus subtilis | 12 | 0 | 24 | 14 | |
| Bacillus velenensis_S103 | 0 | 0 | 22 | 14 | |
| Pseudomonas simiae | 12 | 0 | 18 | 0 | |
| Cell-free supernatant post-centrifugation and post-filtration | Bacillus velenensis | 0 | 50 | 24 | 12 |
| Paenibacillus polymyxa | 0 | 40 | 40 | 12 | |
| Pseudomonas synxantha | 0 | 0 | 14 | 16 | |
| Bacillus subtilis | 0 | 0 | 20 | 12 | |
| Bacillus velenensis_S103 | 0 | 0 | 18 | 12 | |
| Pseudomonas simiae | 0 | 0 | 16 | 0 | |
| No. | Preparation | Dose per 100 kg of Grain (L) | Winter Wheat | Maize | ||
|---|---|---|---|---|---|---|
| Germination Energy (%) | Germination Capacity (%) | Germination Energy (%) | Germination Capacity (%) | |||
| 1 | Control | - | 96 a | 98 a | 95 a | 95 a |
| 2 | Prothioconazole | 0.1 | 96 a | 96 a | 95 a | 96 a |
| 3 | Bacillus velezensis_KT27 | 0.5 | 96 a | 96 a | 95 a | 96 a |
| 4 | Bacillus velezensis_KT27 | 1.0 | 96 a | 97 a | 92 a | 95 a |
| 5 | Paenibacillus polymyxa | 0.5 | 94 ab | 97 a | 95 a | 96 a |
| 6 | Paenibacillus polymyxa | 1.0 | 93 ab | 95 a | 94 a | 95 a |
| 7 | Pseudomonas synxatha | 0.5 | 93 ab | 96 a | 97 a | 98 a |
| 8 | Pseudomonas synxatha | 1.0 | 95 ab | 97 a | 93 a | 95 a |
| 9 | Bacillus subtilis, Pseudomonas simiae, Bacillus velezensis_S103 | 0.5 | 97 a | 97 a | 96 a | 96 a |
| 10 | Bacillus subtilis, Pseudomonas simiae, Bacillus velezensis_S103 | 1.0 | 90.5 b | 94 a | 94 a | 94 a |
| No. | Preparation | Dose per 100 kg of Grain (L) | Winter Wheat Height (cm) | Maize Height (cm) | ||
|---|---|---|---|---|---|---|
| 18 DAS * | 28 DAS * | 18 DAS * | 28 DAS * | |||
| 1 | Control | - | 10.85 bcd | 30.56 ab | 20.69 d | 70.46 c |
| 2 | Prothioconazole | 0.1 | 10.50 d | 30.65 ab | 21.35 cd | 70.71 bc |
| 3 | Bacillus velezensis_KT27 | 0.5 | 11.38 abc | 31.39 a | 23.26 b | 73.61 a |
| 4 | Bacillus velezensis_KT27 | 1.0 | 11.52 ab | 31.05 ab | 22.46 b | 72.28 abc |
| 5 | Paenibacillus polymyxa | 0.5 | 11.55 a | 30.70 ab | 23.02 b | 73.42 ab |
| 6 | Paenibacillus polymyxa | 1.0 | 10.90 a–d | 30.26 b | 23.03 b | 73.53 ab |
| 7 | Pseudomonas synxatha | 0.5 | 10.98 a–d | 29.39 c | 24.28 a | 74.96 a |
| 8 | Pseudomonas synxatha | 1.0 | 11.56 a | 30.45 b | 22.70 b | 72.80 abc |
| 9 | Bacillus subtilis, Pseudomonas simiae, Bacillus velezensis_S103 | 0.5 | 10.82 cd | 30.63 ab | 22.28 bc | 72.68 abc |
| 10 | Bacillus subtilis, Pseudomonas simiae, Bacillus velezensis_S103 | 1.0 | 11.25 abc | 30.49 b | 22.52 b | 72.60 abc |
| No. | Preparation | Dose per 100 kg of Grain (L) | Winter Wheat | Maize |
|---|---|---|---|---|
| Fv/Fm | Fv/Fm | |||
| 1 | Control | - | 0.808 d | 0.787 a |
| 2 | Prothioconazole | 0.1 | 0.810 cd | 0.784 a |
| 3 | Bacillus velezensis_KT27 | 0.5 | 0.810 cd | 0.789 a |
| 4 | Bacillus velezensis_KT27 | 1.0 | 0.813 abc | 0.787 a |
| 5 | Paenibacillus polymyxa | 0.5 | 0.811 bcd | 0.791 a |
| 6 | Paenibacillus polymyxa | 1.0 | 0.813 abc | 0.793 a |
| 7 | Pseudomonas synxatha | 0.5 | 0.816 a | 0.790 a |
| 8 | Pseudomonas synxatha | 1.0 | 0.814 ab | 0.793 a |
| 9 | Bacillus subtilis, Pseudomonas simiae, Bacillus velezensis_S103 | 0.5 | 0.815 a | 0.792 a |
| 10 | Bacillus subtilis, Pseudomonas simiae, Bacillus velezensis_S103 | 1.0 | 0.812 a–d | 0.788 a |
| No. | Preparation | Dose per 100 kg of Grain (L) | Winter Wheat | Maize |
|---|---|---|---|---|
| Plant Fresh Weight (g) | Plant Fresh Weight (g) | |||
| 1 | Control | - | 8.29 d | 40.18 cd |
| 2 | Prothioconazole | 0.1 | 8.75 cd | 40.10 d |
| 3 | Bacillus velezensis_KT27 | 0.5 | 9.81 ab | 45.40 ab |
| 4 | Bacillus velezensis_KT27 | 1.0 | 10.12 a | 44.65 ab |
| 5 | Paenibacillus polymyxa | 0.5 | 9.61 abc | 47.20 ab |
| 6 | Paenibacillus polymyxa | 1.0 | 10.02 ab | 45.05 ab |
| 7 | Pseudomonas synxatha | 0.5 | 9.07 bcd | 47.47 a |
| 8 | Pseudomonas synxatha | 1.0 | 9.26 a–d | 44.96 ab |
| 9 | Bacillus subtilis, Pseudomonas simiae, Bacillus velezensis_S103 | 0.5 | 8.81 cd | 44.55 ab |
| 10 | Bacillus subtilis, Pseudomonas simiae, Bacillus velezensis_S103 | 1.0 | 8.42 d | 43.66 bc |
| No. | Preparation | Dose per 100 kg of Grain (L) |
|---|---|---|
| 1 | Control | - |
| 2 | Prothioconazole | 0.1 |
| 3 | Bacillus velezensis_KT27 | 0.5 |
| 4 | Bacillus velezensis_KT27 | 1.0 |
| 5 | Paenibacillus polymyxa | 0.5 |
| 6 | Paenibacillus polymyxa | 1.0 |
| 7 | Pseudomonas synxatha | 0.5 |
| 8 | Pseudomonas synxatha | 1.0 |
| 9 | Bacillus subtilis, Pseudomonas simiae, Bacillus velezensis_S103 | 0.5 |
| 10 | Bacillus subtilis, Pseudomonas simiae, Bacillus velezensis_S103 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipczak, A.; Sobiech, Ł.; Wita, A.; Marecik, R.; Białas, W.; Drożdżyńska, A.; Grzanka, M.; Danielewicz, J.; Szulc, P. Efficacy of Selected Bacterial Strains in the Protection and Growth Stimulation of Winter Wheat and Maize. Plants 2025, 14, 636. https://doi.org/10.3390/plants14050636
Filipczak A, Sobiech Ł, Wita A, Marecik R, Białas W, Drożdżyńska A, Grzanka M, Danielewicz J, Szulc P. Efficacy of Selected Bacterial Strains in the Protection and Growth Stimulation of Winter Wheat and Maize. Plants. 2025; 14(5):636. https://doi.org/10.3390/plants14050636
Chicago/Turabian StyleFilipczak, Arkadiusz, Łukasz Sobiech, Agnieszka Wita, Roman Marecik, Wojciech Białas, Agnieszka Drożdżyńska, Monika Grzanka, Jakub Danielewicz, and Piotr Szulc. 2025. "Efficacy of Selected Bacterial Strains in the Protection and Growth Stimulation of Winter Wheat and Maize" Plants 14, no. 5: 636. https://doi.org/10.3390/plants14050636
APA StyleFilipczak, A., Sobiech, Ł., Wita, A., Marecik, R., Białas, W., Drożdżyńska, A., Grzanka, M., Danielewicz, J., & Szulc, P. (2025). Efficacy of Selected Bacterial Strains in the Protection and Growth Stimulation of Winter Wheat and Maize. Plants, 14(5), 636. https://doi.org/10.3390/plants14050636








