Exploring the Therapeutic Potential of Medicinal Plants in the Context of Gastrointestinal Health: A Review
Abstract
1. Introduction
2. Research Methodology
3. Gastrointestinal Diseases
4. Microbiota and Gastrointestinal Health
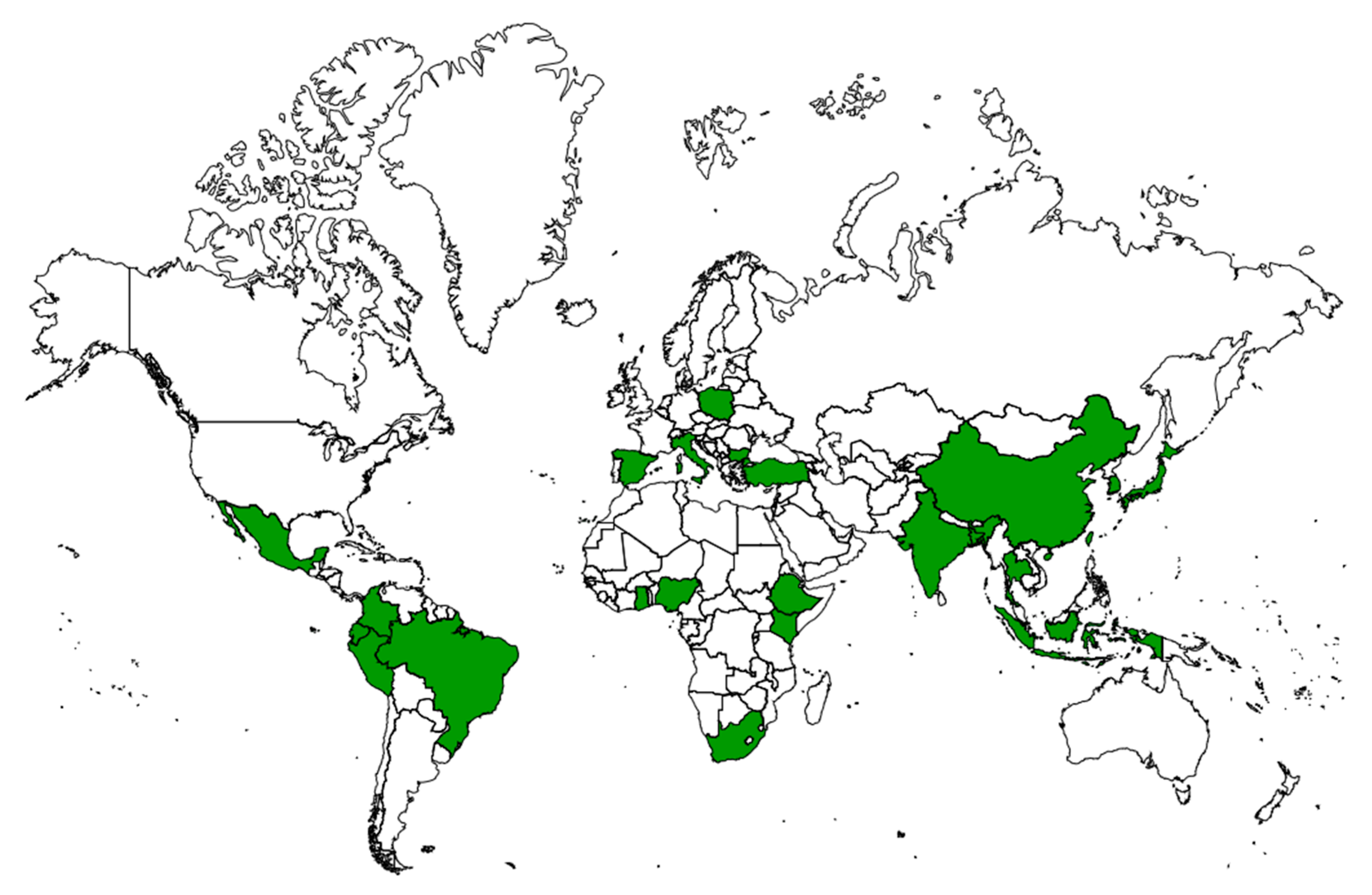
5. Insufficiency of Medical Services and Backwardness
6. Traditional Uses of Plants
7. Plants That Improve Gastrointestinal Health
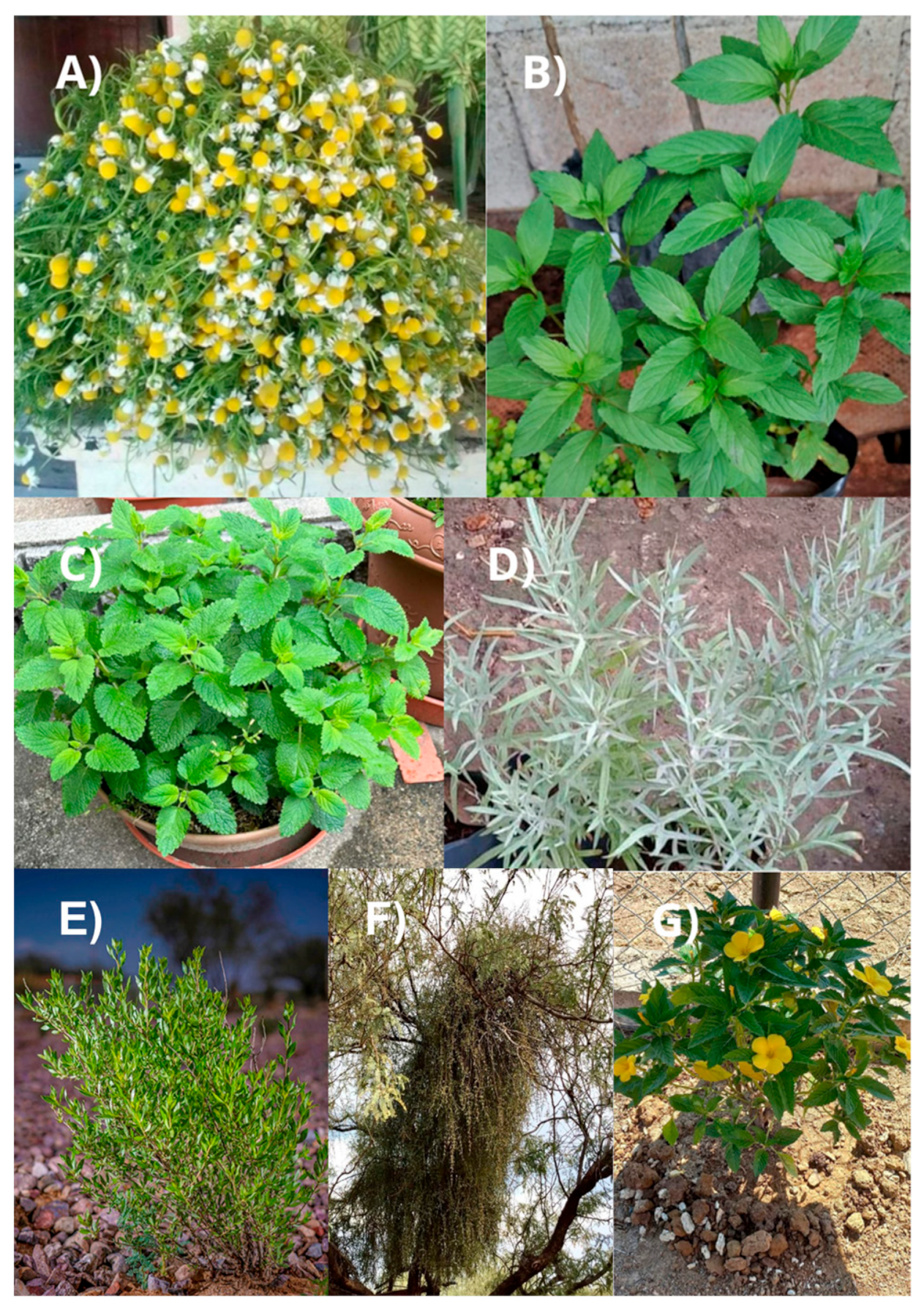
7.1. Matricaria chamomilla
7.2. Mentha spicata
7.3. Melissa officinalis
7.4. Artemisia ludoviciana
7.5. Flourensia cernua
7.6. Phoradendron californicum
7.7. Turnera diffusa
8. Phytochemicals as Treatments for Gastrointestinal Disorders
9. Toxicity of Plants In Vitro and In Vivo
10. Future Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leader, G.; Murray, M.; O’súilleabháin, P.S.; Maher, L.; Naughton, K.; Arndt, S.; White, K.; Traina, I.; Mannion, A. Relationship between parent-reported gastrointestinal symptoms, sleep problems, autism spectrum disorder symptoms, and behavior problems in children and adolescents with 22q11.2 deletion syndrome. Res. Dev. Disabil. 2020, 104, 103698. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106. [Google Scholar] [CrossRef]
- Mazzocchi, S.; Visaggi, P.; Baroni, L. Plant-based diets in gastrointestinal diseases: Which evidence? Best Pract. Res. Clin. Gastroenterol. 2023, 62–63, 101829. [Google Scholar] [CrossRef]
- So, D.; Tuck, C.J. Plant-based diets in gastrointestinal disorders: Something, nothing, or everything? Lancet Gastroenterol. Hepatol. 2021, 6, 992. [Google Scholar] [CrossRef]
- Sharma, A.; Flores-Vallejo, R.d.C.; Cardoso-Taketa, A.; Villarreal, M.L. Antibacterial activities of medicinal plants used in Mexican traditional medicine. J. Ethnopharmacol. 2017, 208, 264–329. [Google Scholar] [CrossRef]
- Sellami, M.; Slimeni, O.; Pokrywka, A.; Kuvačić, G.; Hayes, L.D.; Milic, M.; Padulo, J. Herbal medicine for sports: A review. J. Int. Soc. Sports Nutr. 2018, 15, 14. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Guldiken, B.; Ozkan, G.; Catalkaya, G.; Ceylan, F.D.; Yalcinkaya, I.E.; Capanoglu, E. Phytochemicals of herbs and spices: Health versus toxicological effects. Food Chem. Toxicol. 2018, 119, 37–49. [Google Scholar] [CrossRef]
- Feng, W.; Liu, J.; Ao, H.; Yue, S.; Peng, C. Targeting gut microbiota for precision medicine: Focusing on the efficacy and toxicity of drugs. Theranostics 2020, 10, 11278. [Google Scholar] [CrossRef]
- Contreras-Omaña, R.; Escorcia-Saucedo, A.E.; Velarde-Ruiz Velasco, J.A. Prevalencia e impacto de resistencias a antimicrobianos en infecciones gastrointestinales: Una revisión. Rev. Gastroenterol. Mex. 2021, 86, 265–275. [Google Scholar] [CrossRef]
- Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, P.; Lante, A. Polyphenols: A Comprehensive Review of their Nutritional Properties. Open Biotechnol. J. 2021, 15, 164–172. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional, and national burden of 10 digestive diseases in 204 countries and territories from 1990 to 2019. Front. Public Health 2023, 11, 1061453. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Jensen, E.T.; Kim, H.P.; Egberg, M.D.; Lund, J.L.; Moon, A.M.; Pate, V.; Barnes, E.L.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2022, 162, 621–644. [Google Scholar] [CrossRef]
- Yang, H.; Hu, B. Immunological Perspective: Helicobacter pylori Infection and Gastritis. Mediators Inflamm. 2022, 2022, 2944156. [Google Scholar] [CrossRef]
- Li, Y.; Su, Z.; Li, P.; Li, Y.; Johnson, N.; Zhang, Q.; Du, S.; Zhao, H.; Li, K.; Zhang, C.; et al. Association of Symptoms with Eating Habits and Food Preferences in Chronic Gastritis Patients: A Cross-Sectional Study. Evid.-Based Complement. Altern. Med. 2020, 2020, 5197201. [Google Scholar] [CrossRef]
- Wu, Y.; Murray, G.K.; Byrne, E.M.; Sidorenko, J.; Visscher, P.M.; Wray, N.R. GWAS of peptic ulcer disease implicates Helicobacter pylori infection, other gastrointestinal disorders and depression. Nat. Commun. 2021, 12, 1146. [Google Scholar] [CrossRef]
- Serafim, C.; Araruna, M.E.; Alves Júnior, E.; Diniz, M.; Hiruma-Lima, C.; Batista, L. A Review of the Role of Flavonoids in Peptic Ulcer (2010–2020). Molecules 2020, 25, 5431. [Google Scholar] [CrossRef]
- Katz, P.O.; Dunbar, K.B.; Schnoll-Sussman, F.H.; Greer, K.B.; Yadlapati, R.; Spechler, S.J. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2022, 117, 27–56. [Google Scholar] [CrossRef]
- Delshad, S.D.; Almario, C.V.; Chey, W.D.; Spiegel, B.M.R. Prevalence of Gastroesophageal Reflux Disease and Proton Pump Inhibitor-Refractory Symptoms. Gastroenterology 2019, 158, 1250–1261.e2. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The microbiome and irritable bowel syndrome—A review on the pathophysiology, current research and future therapy. Front. Microbiol. 2019, 10, 424646. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Lembo, A. Microbiome and Its Role in Irritable Bowel Syndrome. Dig. Dis. Sci. 2020, 65, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Guan, Q.A. Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Cocozza, E.; Cemali, Ö.; Bayazıt, A.D.; Nanì, M.F.; Cerqua, I.; Morgillo, F.; Saygılı, S.K.; Berni Canani, R.; Amero, P.; et al. Understanding the role of the gut microbiome in gastrointestinal cancer: A review. Front. Pharmacol. 2023, 14, 1130562. [Google Scholar] [CrossRef]
- Waldum, H.; Fossmark, R. Gastritis, Gastric Polyps and Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 6548. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Wallig, M.A. Digestive System. In Fundamentals of Toxicologic Pathology, 3rd ed.; Wallig, M.A., Bolon, B., Haschek, W.M., Rousseaux, C.G., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 395–442. [Google Scholar] [CrossRef]
- Mirzaei, R.; Dehkhodaie, E.; Bouzari, B.; Rahimi, M.; Gholestani, A.; Hosseini-Fard, S.R.; Keyvani, H.; Teimoori, A.; Karampoor, S. Dual role of microbiota-derived short-chain fatty acids on host and pathogen. Biomed. Pharmacother. 2022, 145, 112352. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the Human Gut Microbiome: An International Review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, 36–44. [Google Scholar] [CrossRef]
- Labanski, A.; Langhorst, J.; Engler, H.; Elsenbruch, S. Stress and the brain-gut axis in functional and chronic-inflammatory gastrointestinal diseases: A transdisciplinary challenge. Psychoneuroendocrinology 2020, 111, 104501. [Google Scholar] [CrossRef]
- Sasso, J.M.; Ammar, R.M.; Tenchov, R.; Lemmel, S.; Kelber, O.; Grieswelle, M.; Zhou, Q.A. Gut Microbiome-Brain Alliance: A Landscape View into Mental and Gastrointestinal Health and Disorders. ACS Chem. Neurosci. 2023, 14, 1717–1763. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2018, 216, 20–40. [Google Scholar] [CrossRef]
- Katzung, B.G. Basic and Clinical Pharmacology, 14th ed.; McGraw-Hill Education: New York, NY, USA, 2017; p. 728. [Google Scholar]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmaco. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Marrelli, M. Medicinal Plants. Plants 2021, 10, 1355. [Google Scholar] [CrossRef]
- Van Wyk, A.S.; Prinsloo, G. Medicinal plant harvesting, sustainability and cultivation in South Africa. Biol. Conserv. 2018, 227, 335–342. [Google Scholar] [CrossRef]
- He, J.; Yang, B.; Dong, M.; Wang, Y. Crossing the roof of the world: Trade in medicinal plants from Nepal to China. J. Ethnopharmacol. 2018, 224, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Jauhari, N.; Bharadvaja, N. Medicinal plants as a potential source of chemopreventive agents. In Anticancer Plants: Natural Products and Biotechnological Implements; Akhtar, M.S., Swamy, M.K., Eds.; Springer: Singapore, 2018; Volume 2, pp. 109–139. [Google Scholar] [CrossRef]
- Torres-León, C.; Rebolledo Ramírez, F.; Aguirre-Joya, J.A.; Ramírez-Moreno, A.; Chávez-González, M.L.; Aguillón-Gutierrez, D.R.; Camacho-Guerra, L.; Ramírez-Guzmán, N.; Hernández Vélez, S.; Aguilar, C.N. Medicinal plants used by rural communities in the arid zone of Viesca and Parras Coahuila in northeast Mexico. Saudi Pharm. J. 2023, 31, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Astutik, S.; Pretzsch, J.; Kimengsi, J.N. Asian Medicinal Plants’ Production and Utilization Potentials: A Review. Sustainability 2019, 11, 5483. [Google Scholar] [CrossRef]
- Spina, D.; Barbieri, C.; Carbone, R.; Hamam, M.; D’Amico, M.; Di Vita, G. Market Trends of Medicinal and Aromatic Plants in Italy: Future Scenarios Based on the Delphi Method. Agronomy 2023, 13, 1703. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J. Inflamm. Res. 2018, 11, 307–317. [Google Scholar] [CrossRef]
- Mata, R.; Figueroa, M.; Navarrete, A.; Rivero-Cruz, I. Chemistry and Biology of Selected Mexican Medicinal Plants. Prog. Chem. Org. Nat. Prod. 2019, 108, 1–142. [Google Scholar] [CrossRef]
- Guzmán Maldonado, H.; Díaz Huacuz, R.; González Chavira, M. Plantas Medicinales la Realidad de una Tradición Ancestral. 2017. Available online: https://vun.inifap.gob.mx/VUN_MEDIA/BibliotecaWeb/_media/_folletoinformativo/1044_4729_Plantas_medicinales_la_realidad_de_una_tradici%C3%B3n_ancestral.pdf (accessed on 19 March 2024).
- Gould, C.V.; Free, R.J.; Bhatnagar, J.; Soto, R.A.; Royer, T.L.; Maley, W.R.; Moss, S.; Berk, M.A.; Craig-Shapiro, R.; Kodiyanplakkal, R.P.L.; et al. Transmission of yellow fever vaccine virus through blood transfusion and organ transplantation in the USA in 2021: Report of an investigation. Lancet Microbe 2023, 4, e711–e721. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Zhang, F.; Abdallah, Y.; Zhang, M.; Wang, Y.; Sun, G.; Qiu, W.; Li, B. Biosynthesis and characterization of magnesium oxide and manganese dioxide nanoparticles using Matricaria chamomilla L. extract and its inhibitory effect on Acidovorax oryzae strain RS-2. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2230–2239. [Google Scholar] [CrossRef]
- Akram, W.; Ahmed, S.; Rihan, M.; Arora, S.; Khalid, M.; Ahmad, S.; Ahmad, F.; Haque, S.; Vashishth, R. An updated comprehensive review of the therapeutic properties of Chamomile (Matricaria chamomilla L.). Int. J. Food Prop. 2024, 27, 133–164. [Google Scholar] [CrossRef]
- El Joumaa, M.M.; Borjac, J.M. Matricaria chamomilla: A valuable insight into recent advances in medicinal uses and pharmacological activities. Phytochem. Rev. 2022, 21, 1913–1940. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Esteves da Silva, J.C.G.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life 2022, 12, 479. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, M. Mentha spicata L. essential oil, phytochemistry and its effectiveness in flatulence. J. Tradit. Med. Complement. 2021, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- el Menyiy, N.; Mrabti, H.N.; el Omari, N.; Bakili, A.E.; Bakrim, S.; Mekkaoui, M.; Balahbib, A.; Amiri-Ardekani, E.; Ullah, R.; Alqahtani, A.S.; et al. Medicinal Uses, Phytochemistry, Pharmacology, and Toxicology of Mentha spicata. Evid.-Based Complement. Altern. Med. 2022, 2022, 7990508. [Google Scholar] [CrossRef]
- Mahendran, G.; Verma, S.K.; Rahman, L.U. The traditional uses, phytochemistry and pharmacology of spearmint (Mentha spicata L.): A review. J. Ethnopharmacol. 2021, 278, 114266. [Google Scholar] [CrossRef]
- Saqib, S.; Ullah, F.; Naeem, M.; Younas, M.; Ayaz, A.; Ali, S.; Zaman, W. Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases. Molecules 2022, 27, 6728. [Google Scholar] [CrossRef]
- Petrisor, G.; Motelica, L.; Craciun, L.N.; Oprea, O.C.; Ficai, D.; Ficai, A. Melissa officinalis: Composition, Pharmacological Effects and Derived Release Systems—A Review. Int. J. Mol. Sci. 2022, 23, 3591. [Google Scholar] [CrossRef]
- Stoyanova, N.; Spasova, M.; Manolova, N.; Rashkov, I.; Kamenova-Nacheva, M.; Staleva, P.; Tavlinova-Kirilova, M. Electrospun PLA-Based Biomaterials Loaded with Melissa officinalis Extract with Strong Antioxidant Activity. Polymers 2023, 15, 1070. [Google Scholar] [CrossRef]
- Ghazizadeh, J.; Hamedeyazdan, S.; Torbati, M.; Farajdokht, F.; Fakhari, A.; Mahmoudi, J.; Araj-khodaei, M.; Sadigh-Eteghad, S. Melissa officinalis L. hydro-alcoholic extract inhibits anxiety and depression through prevention of central oxidative stress and apoptosis. Exp. Physiol. 2020, 105, 707–720. [Google Scholar] [CrossRef]
- Miraj, S.; Rafieian-Kopaei, M.; Kiani, S. Melissa officinalis L: A Review Study With an Antioxidant Prospective. Evid. Based Complement. Alternat Med. 2017, 22, 385–394. [Google Scholar] [CrossRef]
- Swor, K.; Poudel, A.; Satyal, P.; Setzer, W.N. The Essential Oil Compositions of Ambrosia acanthicarpa Hook., Artemisia ludoviciana Nutt., and Gutierrezia sarothrae (Pursh) Britton & Rusby (Asteraceae) from the Owyhee Mountains of Idaho. Molecules 2024, 29, 1383. [Google Scholar] [CrossRef] [PubMed]
- Kamarauskaite, J.; Baniene, R.; Raudone, L.; Vilkickyte, G.; Vainoriene, R.; Motiekaityte, V.; Trumbeckaite, S. Antioxidant and mitochondria-targeted activity of caffeoylquinic-acid-rich fractions of wormwood (Artemisia absinthium L.) and silver wormwood (Artemisia ludoviciana Nutt.). Antioxidants 2021, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Espinosa, J.F.; Núñez-Aragón, P.N.; Gomez-Chang, E.; Linares, E.; Bye, R.; Romero, I. Anti-Helicobacter pylori Activity of Artemisia ludoviciana subsp. mexicana and Two of Its Bioactive Components, Estafiatin and Eupatilin. Molecules 2021, 26, 3654. [Google Scholar] [CrossRef] [PubMed]
- Ezeta-Miranda, A.; Vera-Montenegro, Y.; Avila-Acevedo, J.G.; García-Bores, A.M.; Estrella-Parra, E.A.; Francisco-Marquez, G.; Ibarra-Velarde, F. Efficacy of purified fractions of Artemisia ludoviciana Nutt. mexicana and ultraestructural damage to newly excysted juveniles of Fasciola hepatica in vitro. Vet. Parasitol. 2020, 285, 109184. [Google Scholar] [CrossRef]
- Rivero-Cruz, I.; Anaya-Eugenio, G.; Pérez-Vásquez, A.; Martínez, A.L.; Mata, R. Quantitative Analysis and Pharmacological Effects of Artemisia ludoviciana Aqueous Extract and Compounds. Nat. Prod. Commun. 2017, 12, 1531–1534. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Puente-Romero, G.N.; Díaz-Jiménez, L.; Rodríguez-García, R.; Ramírez-Rodríguez, H.; Villarreal-Quintanilla, J.A.; Flores-López, M.L.; Carrillo-Lomelí, D.A.; Genisheva, Z.A. In vitro gastrointestinal digestion of microencapsulated extracts of Flourensia cernua, F. microphylla, and F. retinophylla. Ind. Crops Prod. 2019, 138, 111444. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Salas-Méndez, E.d.J.; Rodríguez-García, R.; Hernández-Castillo, F.D.; Díaz-Jiménez, M.L.V.; Sáenz-Galindo, A.; González-Morales, S.; Flores-López, M.L.; Villarreal-Quintanilla, J.A.; Peña-Ramos, F.M.; et al. Antifungal activity in vitro of ethanol and aqueous extracts of leaves and branches of Flourensia spp. against postharvest fungi. Ind. Crops Prod. 2017, 107, 499–508. [Google Scholar] [CrossRef]
- Alvarez-Pérez, O.B.; Ventura-Sobrevilla, J.M.; Ascacio-Valdés, J.A.; Rojas, R.; Verma, D.K.; Aguilar, C.N. Valorization of Flourensia cernua DC as source of antioxidants and antifungal bioactives. Ind. Crops Prod. 2020, 152, 112422. [Google Scholar] [CrossRef]
- Assanga, S.B.I.; Luján, L.M.L.; Ruiz, J.C.G.; McCarty, M.F.; Cota-Arce, J.M.; Espinoza, C.L.L.; Salido, A.A.G.; Ángulo, D.F. Comparative analysis of phenolic content and antioxidant power between parasitic Phoradendron californicum (toji) and their hosts from Sonoran Desert. Results Chem. 2020, 2, 100079. [Google Scholar] [CrossRef]
- Iloki-Assanga, S.B.; Lewis-Luján, L.M.; Lara-Espinoza, C.L.; Gil-Salido, A.A.; Fernandez-Angulo, D.; Rubio-Pino, J.L.; Haines, D.D. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum Complementary and Alternative Medicine. BMC Res. Notes 2015, 8, 396. [Google Scholar] [CrossRef]
- Mathiasen, R.L. The classification of California Viscaceae: An alternative perspective. Madroño 2016, 63, 8–33. [Google Scholar] [CrossRef]
- Martínez-Ávila, G.C.G.; Aguilar-Zarate, P.; Rojas, R. Currently Applied Extraction Processes for Secondary Metabolites from Lippia turbinata and Turnera diffusa and Future Perspectives. Separations 2021, 8, 158. [Google Scholar] [CrossRef]
- Chaurasiya, N.D.; Zhao, J.; Pandey, P.; Doerksen, R.J.; Muhammad, I.; Tekwani, B.L. Selective Inhibition of Human Monoamine Oxidase B by Acacetin 7-Methyl Ether Isolated from Turnera diffusa (Damiana). Molecules 2019, 24, 810. [Google Scholar] [CrossRef]
- Tousson, E.; Hafez, E.; Zaki, S.; Gad, A.; Elgharabawy, R.M. Evaluation of the testicular protection conferred by damiana (Turnera diffusa Willd.) against amitriptyline-induced testicular toxicity, DNA damage and apoptosis in rats. Biomed. Pharmacother. 2020, 132, 110819. [Google Scholar] [CrossRef]
- Szewczyk, K.; Zidorn, C. Ethnobotany, phytochemistry, and bioactivity of the genus Turnera (Passifloraceae) with a focus on damiana—Turnera diffusa. J. Ethnopharmacol. 2014, 152, 424–443. [Google Scholar] [CrossRef]
- Urbizu-González, A.L.; Castillo-Ruiz, O.; Martínez-Ávila, G.C.G.; Torres-Castillo, J.A. Natural variability of essential oil and antioxidants in the medicinal plant Turnera diffusa. Asian Pac. J. Trop. Med. 2017, 10, 121–125. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef]
- Feng, W.; Ao, H.; Peng, C. Gut microbiota, short-chain fatty acids, and herbal medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef]
- An, X.; Bao, Q.; Di, S.; Zhao, Y.; Zhao, S.; Zhang, H.; Lian, F.; Tong, X. The interaction between the gut Microbiota and herbal medicines. Biomed. Pharmacother. 2019, 118, 109252. [Google Scholar] [CrossRef]
- Gasaly, N.; Riveros, K.; Gotteland, M. Fitoquímicos: Una nueva clase de prebióticos. Rev. Chil. Nutr. 2020, 47, 317–327. [Google Scholar] [CrossRef]
- Hussein, R.A.; A. El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine; Builders, P.F., Ed.; IntechOpen: London, UK, 2019; pp. 11–30. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant. Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, N.; Rasul, A.; Hussain, G.; Anwar, H.; Shah, M.A.; Sarfraz, I.; Riaz, A.; Batool, R.; Shahbaz, M.; Hussain, A.; et al. Oleandrin: A bioactive phytochemical and potential cancer killer via multiple cellular signaling pathways. Food Chem. Toxicol. 2020, 143, 111570. [Google Scholar] [CrossRef]
- Kandsi, F.; Conte, R.; Marghich, M.; Lafdil, F.Z.; Alajmi, M.F.; Bouhrim, M.; Mechchate, H.; Hano, C.; Aziz, M.; Gseyra, N. Phytochemical Analysis, Antispasmodic, Myorelaxant, and Antioxidant Effect of Dysphania ambrosioides (L.) Mosyakin and Clemants Flower Hydroethanolic Extracts and Its Chloroform and Ethyl Acetate Fractions. Molecules 2021, 26, 7300. [Google Scholar] [CrossRef]
- Hernández-Marín, D.A.; Castro-Rios, R.; Chávez-Montes, A.; Castillo-Hernández, S.L.; Elizondo-Luevano, J.H.; Muñoz-Ortega, M.H.; Sánchez-García, E. Antiparasitic Activity of Isolated Fractions from Parthenium incanum Kunth against the Hemoflagellate Protozoan Trypanosoma cruzi. Antibiotics 2024, 13, 622. [Google Scholar] [CrossRef]
- Jedidi, S.; Sammari, H.; Selmi, H.; Hosni, K.; Rtibi, K.; Aloui, F.; Adouni, O.; Sebai, H. Strong protective effects of Salvia officinalis L. leaves decoction extract against acetic acid-induced ulcerative colitis and metabolic disorders in rat. J. Funct. Foods 2021, 79, 104406. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.E.; Balahbib, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; el Menyiy, N. Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J. Ethnopharmacol. 2021, 265, 113318. [Google Scholar] [CrossRef]
- Khadhri, A.; Bouali, I.; Belkhir, S.; Mokded, R.; Smiti, S.; Falé, P.; Araújo, M.E.M.; Serralheiro, M.L.M. In vitro digestion, antioxidant and antiacetylcholinesterase activities of two species of Ruta: Ruta chalepensis and Ruta montana. Pharm. Biol. 2017, 55, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Castro, P.Y.; García-Baldenegro, C.V.; Santos-Espinosa, A.; Tolano-Villaverde, I.d.J.; Manzanarez-Quin, C.G.; Valdez-Domínguez, R.D.; Ibarra-Zazueta, C.; Osuna-Chávez, R.F.; Rueda-Puente, E.O.; Hernández-Moreno, C.G.; et al. Perfil fitoquímico, actividad antimicrobiana y antioxidante de extractos de Gnaphalium oxyphyllum y Euphorbia maculata nativas de Sonora. Rev. Mex. Cienc. Pecu. 2022, 13, 928–942. [Google Scholar] [CrossRef]
- Salehi, B.; Valussi, M.; Flaviana Bezerra Morais-Braga, M.; Nalyda Pereira Carneiro, J.; Linkoln Alves Borges Leal, A.; Douglas Melo Coutinho, H.; Vitalini, S.; Kręgiel, D.; Antolak, H.; Sharifi-Rad, M.; et al. Tagetes spp. Essential Oils and Other Extracts: Chemical Characterization and Biological Activity. Molecules 2018, 23, 2847. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef]
- Purushothaman, B.; Prasannasrinivasan, R.; Suganthi, P.; Ranganathan, B.; Gimbun, J.; Shanmugam, K. A Comprehensive Review on Ocimum basilicum. J. Nat. Remedies. 2018, 18, 71–85. [Google Scholar] [CrossRef]
- Conde-Hernández, L.A.; Espinosa-Victoria, J.R.; Guerrero-Beltrán, J. Supercritical extraction of essential oils of Piper auritum and Porophyllum ruderale. J. Supercrit. Fluids 2017, 127, 97–102. [Google Scholar] [CrossRef]
- Satyal, P.; Jones, T.H.; Lopez, E.M.; McFeeters, R.L.; Ali, N.A.A.; Mansi, I.; Al-Kaf, A.G.; Setzer, W.N. Chemotypic Characterization and Biological Activity of Rosmarinus officinalis. Foods 2017, 6, 20. [Google Scholar] [CrossRef]
- Rashid, H.M.; Mahmod, A.I.; Afifi, F.U.; Talib, W.H. Antioxidant and Antiproliferation Activities of Lemon Verbena (Aloysia citrodora): An In Vitro and In Vivo Study. Plants 2022, 11, 785. [Google Scholar] [CrossRef]
- Oladeji, O.S.; Adelowo, F.E.; Ayodele, D.T.; Odelade, K.A. Phytochemistry and pharmacological activities of Cymbopogon citratus: A review. Sci. Afr. 2019, 6, e00137. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Olatunde, A.; El-mleeh, A.; Hetta, H.F.; Al-rejaie, S.; Alghamdi, S.; Zahoor, M.; Beshbishy, A.M.; Murata, T.; Zaragoza-bastida, A.; et al. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J.; Fang, H.; Li, Z.; Li, M. Advances and challenges in medicinal plant breeding. Plant Sci. 2020, 298, 110573. [Google Scholar] [CrossRef]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Joshi, V.K.; Joshi, A.; Dhiman, K.S. The Ayurvedic Pharmacopoeia of India, development and perspectives. J. Ethnopharmacol. 2017, 197, 32–38. [Google Scholar] [CrossRef]
- Schifter Aceves, L. Las Farmacopeas Mexicanas en la construcción de la identidad nacional. Rev. Mex. Cienc. Farm. 2014, 45, 43–54. [Google Scholar]
- Iannitti, T.; Morales-Medina, J.C.; Bellavite, P.; Rottigni, V.; Palmieri, B. Effectiveness and Safety of Arnica Montana in Post-Surgical Setting, Pain and Inflammation. Am. J. Ther. 2016, 23, e184–e197. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, N.S.; Megremi, S.F.; Tarantilis, P. Evaluation of antioxidant activity, toxicity, and phenolic profile of aqueous extracts of chamomile (Matricaria chamomilla L.) and sage (Salvia officinalis L.) prepared at different temperatures. Appl. Sci. 2020, 10, 2270. [Google Scholar] [CrossRef]
- Yang, X.; Han, H.; Li, B.; Zhang, D.; Zhang, Z.; Xie, Y. Fumigant toxicity and physiological effects of spearmint (Mentha spicata, Lamiaceae) essential oil and its major constituents against Reticulitermes dabieshanensis. Ind Crop Prod. 2021, 171, 113894. [Google Scholar] [CrossRef]
- Stojanović, N.M.; Randjelović, P.J.; Mladenović, M.Z.; Ilić, I.R.; Petrović, V.; Stojiljković, N.; Ilić, S.; Radulović, N.S. Toxic essential oils, part VI: Acute oral toxicity of lemon balm (Melissa officinalis L.) essential oil in BALB/c mice. Food Chem. Toxicol. 2019, 113, 110794. [Google Scholar] [CrossRef]
- Gálvez Romero, J.L.; Parada Sosa, C.M.; Burgoa, G.L.; Lorenzo Leal, A.C.; El Kassis, E.G.; Bautista Rodríguez, E.; Paredes Juárez, G.A.; Hernández, L.R.; Bach, H.; Juárez, Z.N. Antimycobacterial, cytotoxic, and anti-inflammatory activities of Artemisia ludoviciana. J. Ethnopharmacol. 2022, 293, 115249. [Google Scholar] [CrossRef]
- Jasso de Rodríguez, D.; Torres-Moreno, H.; López-Romero, J.C.; Vidal-Gutiérrez, M.; Villarreal-Quintanilla, J.A.; Carrillo-Lomelí, D.A.; Robles-Zepeda, R.E.; Vilegas, W. Antioxidant, Anti-Inflammatory, and Antiproliferative Activities of Flourensia spp. Biocatal. Agric. Biotechnol. 2023, 47, 102552. [Google Scholar] [CrossRef]
- Mendez-Pfeiffer, P.; Ballesteros-Monrreal, M.G.; Leyva, M.; Ortega-Garcia, J.; Montaño-Leyva, B.; Valencia, D.; Aguilar-Martinez, M. Actividad Antioxidante, Antiproliferativa y Antibacteriana de Extractos de Phoradendron californicum; una Planta Parásita del Noroeste de México. Biotecnia 2024, 26, 401–407. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; Jebur, A.B.; Nasr, H.M.; Hamid, H.M. Modulatory effect of Turnera diffusa against testicular toxicity induced by fenitrothion and/or hexavalent chromium in rats. Environ. Toxicol. 2019, 34, 330–339. [Google Scholar] [CrossRef]


| Gastrointestinal Diseases | Description | Etiologic Agent | Common Symptoms | References |
|---|---|---|---|---|
| Gastritis | Inflammation of the gastric mucosa |
| Stomach pain, abdominal distension, nausea, vomiting, and loss of appetite | [16,17] |
| Peptic ulcer | Lesion in the digestive tract caused by acid, which is usually found in the stomach or proximal duodenum |
| They are nonspecific, but some present with postprandial abdominal pain, nausea, vomiting, and weight loss | [18,19] |
| Gastroesophageal reflux disease | Occurs when stomach contents flow back into the esophagus, causing a series of complications and discomfort |
| Include heartburn and regurgitation, which mainly occur after meals | [20,21] |
| Irritable bowel syndrome | Common functional gastrointestinal disorder characterized by the presence of chronic and recurrent abdominal discomfort |
| Diarrhea, constipation, or an alternation between both | [22,23] |
| Inflammatory bowel disease | Encompasses a range of intestinal disorders characterized by a complex inflammatory response in the small and large intestines | History of ulcerative colitis and Crohn’s disease | Chronic inflammation of the gastrointestinal tract, abdominal pain, diarrhea, presence of blood in the stool, and weight loss | [24,25] |
| Gastrointestinal Cancer | It is a complex disease involving genetic and environmental factors, influenced by the host and its surroundings |
| The luminal growth of tumors most of the time does not cause symptoms due to early-stage luminal obstruction | [26,27,28] |
| Scientific Name | Common Name | Part of the Plant | Associated Compounds | Extraction Method | References |
|---|---|---|---|---|---|
| Nerium oleander | Laurel rosa | Leaves | Oleandrin Digitoxingenin Urosolic acid | Infusion | [88] |
| Dysphania Ambrosioides | Epazote | Aerial parts | Syringic acid Quercetin Hesperetin Luteolin | Maceration | [89] |
| Parthenium incanum | Mariola | Leaves | Parthenin Coronopoline | Maceration | [90] |
| Salvia officinalis | Salvia | Leaves | Rosmarinic acid Salvianolic acid Catechin | Decoction | [91] |
| Origanum majorana | Mejorana | Aerial parts | Carvacrol Thymol Hydroquinone Arbutin | NA | [92] |
| Ruta chalepensis | Ruda | Leaves | Coumarins Hesperidin Acridine | Decoction | [93] |
| Gnaphalium oxyphyllum | Gordolobo | Stems and leaves | Chlorogenic acid Flavones | Maceration | [94] |
| Tagetes erecta | Cempazúchitl | Aerial parts | Dihydrotagetone Tagetones Terpinolene Piperitone | NA | [95] |
| Origanum vulgare | Orégano | Stems, leaves and flowers | Carvacrol Thymol Linalool y-Terpinene | NA | [96] |
| Ocimum basilicum | Albahaca | Leaves | Linalool Estragole Methyl eugenol | Hydrodistillation | [97] |
| Piper auritum | Hierba santa | Leaves | Safrol α-Terpinene | SCE | [98] |
| Rosmarinus officinalis | Romero | Leaves | 1,8-cineole α-pinene Camphor | Hydrodistillation | [99] |
| Aloysia citrodora | Cedrón | Aerial parts | d,l-Limonene γ-Muurolene trans- chrysanthenyl acetate | Hydrodistillation | [100] |
| Cymbopogon citratus | Zacate limón | Leaves | L-linanool Limonene Furfide | Decoction | [101] |
| Artemisia absinthium | Ajenjo | Leaves | Artemisinin α-Thujone 4-Terpineo | NA | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julián-Flores, A.; Aguilar-Zárate, P.; Michel, M.R.; Sepúlveda-Torre, L.; Torres-León, C.; Aguilar, C.N.; Chávez-González, M.L. Exploring the Therapeutic Potential of Medicinal Plants in the Context of Gastrointestinal Health: A Review. Plants 2025, 14, 642. https://doi.org/10.3390/plants14050642
Julián-Flores A, Aguilar-Zárate P, Michel MR, Sepúlveda-Torre L, Torres-León C, Aguilar CN, Chávez-González ML. Exploring the Therapeutic Potential of Medicinal Plants in the Context of Gastrointestinal Health: A Review. Plants. 2025; 14(5):642. https://doi.org/10.3390/plants14050642
Chicago/Turabian StyleJulián-Flores, Antonio, Pedro Aguilar-Zárate, Mariela R. Michel, Leonardo Sepúlveda-Torre, Cristian Torres-León, Cristóbal N. Aguilar, and Mónica L. Chávez-González. 2025. "Exploring the Therapeutic Potential of Medicinal Plants in the Context of Gastrointestinal Health: A Review" Plants 14, no. 5: 642. https://doi.org/10.3390/plants14050642
APA StyleJulián-Flores, A., Aguilar-Zárate, P., Michel, M. R., Sepúlveda-Torre, L., Torres-León, C., Aguilar, C. N., & Chávez-González, M. L. (2025). Exploring the Therapeutic Potential of Medicinal Plants in the Context of Gastrointestinal Health: A Review. Plants, 14(5), 642. https://doi.org/10.3390/plants14050642











