Fungicide Seed Coating Increases Emergence of Bluebunch Wheatgrass (Pseudoroegneria spicata) Under High-Fungal-Biomass Conditions
Abstract
:1. Introduction
2. Results
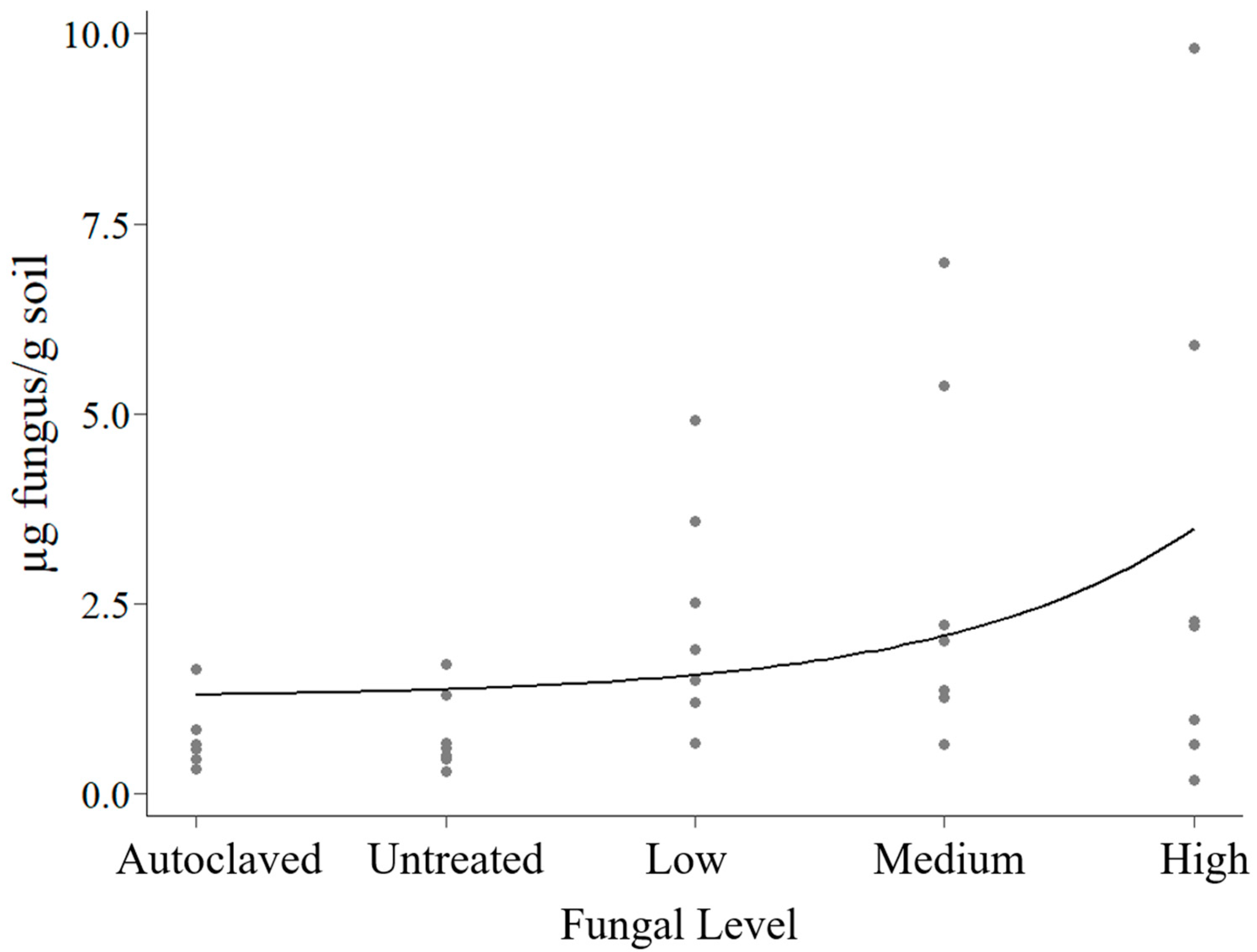
2.1. Fungal Biomass
2.2. Seedling Emergence
2.3. Total Above-Ground Biomass
3. Discussion
4. Materials and Methods
4.1. Model Species
4.2. Seed Treatments
4.3. Soil Inoculation
4.4. Study Design
4.5. Fungal Biomass Quantification
4.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardegree, S.P.; Abatzoglou, J.T.; Brunson, M.W.; Germino, M.J.; Hegewisch, K.C.; Moffet, C.A.; Pilliod, D.S.; Roundy, B.A.; Boehm, A.R.; Meredith, G.R. Weather-centric rangeland revegetation planning. Rangel. Ecol. Manag. 2018, 71, 1–11. [Google Scholar] [CrossRef]
- Arias, M.; Kariyat, R.; Wahl, K.; Mendez, S.; Chavana, J.; Christoffersen, B. Do early-successional weeds facilitate or compete with seedlings in forest restoration? Disentangling abiotic versus biotic factors. Ecol. Solut. Evid. 2021, 2, e12095. [Google Scholar] [CrossRef]
- Coban, O.; De Deyn, G.B.; van der Ploeg, M. Soil microbiota as game-changers in restoration of degraded lands. Science 2022, 375, 0725. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.D.; Davies, K.W.; Boyd, C.S.; Kerby, J.D.; Svejcar, T.J. Emerging seed enhancement technologies for overcoming barriers to restoration. Restor. Ecol. 2016, 24, S77–S84. [Google Scholar] [CrossRef]
- James, J.J.; Sheley, R.L.; Leger, E.A.; Adler, P.B.; Hardegree, S.P.; Gornish, E.S.; Rinella, M.J. Increased soil temperature and decreased precipitation during early life stages constrain grass seedling recruitment in cold desert restoration. J. Appl. Ecol. 2019, 56, 2609–2619. [Google Scholar] [CrossRef]
- Kildisheva, O.A.; Erickson, T.E.; Madsen, M.D.; Dixon, K.W.; Merritt, D.J. Seed germination and dormancy traits of forbs and shrubs important for restoration of North American dryland ecosystems. Plant Biol. 2019, 21, 458–469. [Google Scholar] [CrossRef]
- Pedrini, S.; Balestrazzi, A.; Madsen, M.D.; Bhalsing, K.; Hardegree, S.P.; Dixon, K.W.; Kildisheva, O.A. Seed enhancement: Getting seeds restoration-ready. Restor. Ecol. 2020, 28, S266–S275. [Google Scholar] [CrossRef]
- James, J.J.; Svejcar, T.J.; Rinella, M.J. Demographic processes limiting seedling recruitment in arid grassland restoration. J. Appl. Ecol. 2011, 48, 961–969. [Google Scholar] [CrossRef]
- Gornish, E.S.; Aanderud, Z.T.; Sheley, R.L.; Rinella, M.J.; Svejcar, T.; Englund, S.D.; James, J.J. Altered snowfall and soil disturbance influence the early life stage transitions and recruitment of a native and invasive grass in a cold desert. Oecologia 2015, 177, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Hoose, B.W.; Geary, B.D.; Richardson, W.C.; Petersen, S.L.; Madsen, M.D. Improving dryland seedling recruitment using fungicide seed coatings. Ecol. Solut. Evid. 2022, 3, e12132. [Google Scholar] [CrossRef]
- Wagner, M.; Mitschunas, N. Fungal effects on seed bank persistence and potential applications in weed biocontrol: A review. Basic Appl. Ecol. 2008, 9, 191–203. [Google Scholar] [CrossRef]
- Allen, P.S.; Finch-Boekweg, H.; Meyer, S.E. A proposed mechanism for high pathogen-caused mortality in the seed bank of an invasive annual grass. Fungal Ecol. 2018, 35, 108–115. [Google Scholar] [CrossRef]
- Perkins, L.B.; Bennett, J.R. A field test of commercial soil microbial treatments on native grassland restoration. Restor. Ecol. 2018, 26, 851–857. [Google Scholar] [CrossRef]
- Ehlert, K.A.; Mangold, J.M.; Menalled, F.; Miller, Z.; Dyer, A. Seeding, herbicide, and fungicide impact on perennial grass establishment in cheatgrass infested habitats. Ecol. Restor. 2019, 37, 67–70. [Google Scholar] [CrossRef]
- Kuhnert, R.; Oberkofler, I.; Peintner, U. Fungal growth and biomass development is boosted by plants in snow-covered soil. Microb. Ecol. 2012, 64, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Tilley, D.; Fund, A.; Pickett, T. A Review of techniques and technologies for improving seedling establishment. In Technical Note Plant Materials No. 72; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2018. [Google Scholar]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed coating: Science or marketing spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Baibakova, E.V.; Nefedjeva, E.E.; Suska-Malawska, M.; Wilk, M.; Sevriukova, G.A.; Zheltobriukhov, V.F. Modern fungicides: Mechanisms of action, fungal resistance and phytotoxic effects. Ann. Res. Rev. Biol. 2019, 32, 1–16. [Google Scholar] [CrossRef]
- Crist, T.O.; Friese, C.F. The Impact of fungi on soil seeds: Implications for plants and granivores in a semiarid shrub-steppe. Ecology 1993, 74, 2231–2239. [Google Scholar] [CrossRef]
- Sowards, T.G.; Hamilton, B.T.; Aanderud, Z.T.; Petersen, S.L.; St Clair, S.B.; Madsen, M.D. Improving seedling recruitment and dryland restoration using a targeted fungicide seed coating. Restor. Ecol. 2024, 33, e14312. [Google Scholar] [CrossRef]
- Mordecai, E.A. Soil moisture and fungi affect seed survival in California grassland annual plants. PLoS ONE 2012, 7, e39083. [Google Scholar] [CrossRef] [PubMed]
- Koutzoukis, S.; Madsen, M.D.; Veblen, K.E. Under drought conditions, fungicide coating does not increase emergence of two native grass species in sagebrush stands of the Intermountain West, USA. Restor. Ecol. 2024, 32, e13988. [Google Scholar] [CrossRef]
- Griffin, D.M. Soil moisture and the ecology of soil fungi. Biol. Rev. 1963, 38, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M. A view of fungal ecology. Mycologia 1989, 81, 1–19. [Google Scholar] [CrossRef]
- Krupinsky, J.M.; Bailey, K.L.; McMullen, M.P.; Gossen, B.D.; Turkington, T.K. Managing plant disease risk in diversified cropping systems. Agron. J. 2002, 94, 198–209. [Google Scholar] [CrossRef]
- Rogers, C.H. The relation of moisture and temperature to growth of the cotton root rot fungus. J. Agric. Res. 1939, 58, 701–709. [Google Scholar]
- Shields, J.A.; Paul, E.A.; Lowe, W.E.; Parkinson, D. Turnover of microbial tissue in soil under field conditions. Soil. Biol. Biochem. 1973, 5, 753–764. [Google Scholar] [CrossRef]
- Blaney, C.S.; Kotanen, P.M. Effects of fungal pathogens on seeds of native and exotic plants: A test using congeneric pairs. J. Appl. Ecol. 2001, 38, 1104–1113. [Google Scholar] [CrossRef]
- Connolly, B.M.; Carris, L.M.; Mack, R.N. Soil-borne seed pathogens: Contributors to the naturalization gauntlet in Pacific Northwest (USA) forest and steppe communities? Plant Ecol. 2018, 219, 359–368. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production; Food & Agriculture Organization of the United Nations: Rome, Italy, 2005. [Google Scholar]
- Dumroese, R.K.; Luna, T.; Richardson, B.A.; Kilkenny, F.F.; Runyon, J.B. Conserving and restoring habitat for Greater Sage-Grouse and other sagebrush-obligate wildlife: The crucial link of forbs and sagebrush diversity. Nativ. Plants J. 2015, 16, 276–299. [Google Scholar] [CrossRef]
- Lewandrowski, W.; Erickson, T.E.; Dixon, K.W.; Stevens, J.C. Increasing the germination envelope under water stress improves seedling emergence in two dominant grass species across different pulse rainfall events. J. Appl. Ecol. 2017, 54, 997–1007. [Google Scholar] [CrossRef]
- Mitschunas, N.; Wagner, M.; Filser, J. Evidence for a positive influence of fungivorous soil invertebrates on the seed bank persistence of grassland species. J. Ecol. 2006, 94, 791–800. [Google Scholar] [CrossRef]
- Ogle, D.G.; St John, L.; Jones, T.A. Plant Guide for Bluebunch Wheatgrass (Pseudoroegneria spicata); USDA-Natural Resources Conservation Service, Idaho and Washington Plant Materials Program; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2010; pp. 1–6.
- Munkvold, G.P. Seed pathology progress in academia and industry. Annu. Rev. Phytopathol. 2009, 47, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Halmer, P. Seed technology and seed enhancement. Acta Hortic. 2006, 771, 17–26. [Google Scholar]
- Web Soil Survey. Natural Resources Conservation Service, United States Department of Agriculture 2021. Available online: http://websoilsurvey.nrcs.usda.gov/ (accessed on 6 July 2021).
- Ingham, E.R.; Klein, D.A. Soil fungi: Measurement of hyphal length. Soil. Biol. Biochem. 1984, 16, 279–280. [Google Scholar] [CrossRef]
- Seiter, S.; Ingham, E.R.; William, R.D. Dynamics of soil fungal and bacterial biomass in a temperate climate alley cropping system. Appl. Soil. Ecol. 1999, 12, 139–147. [Google Scholar] [CrossRef]
- Rygiewicz, P.T.; Monleon, V.J.; Ingham, E.R.; Martin, K.J.; Johnson, M.G. Soil life in reconstructed ecosystems: Initial soil food web responses after rebuilding a forest soil profile for a climate change experiment. Appl. Soil. Ecol. 2010, 45, 26–38. [Google Scholar] [CrossRef]
- Scheu, S.; Parkinson, D. Changes in bacterial and fungal biomass C, bacterial and fungal biovolume and ergosterol content after drying, remoistening and incubation of different layers of cool temperate forest soils. Soil. Biol. Biochem. 1994, 26, 1515–1525. [Google Scholar] [CrossRef]
- van Veen, J.A.; Eldor, P.A. Conversion of biovolume measurements of soil organisms, grown under various moisture tensions, to biomass and their nutrient content. Appl. Environ. Microbiol. 1979, 37, 686–692. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Curran-Everett, D. Explorations in statistics: The log transformation. Adv. Physiol. Educ. 2018, 42, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V.; Buerkner, P.; Herve, M.; Love, J.; Miguez, F.; Riebl, H.; Singmann, H. R package, version 1.7.2. Emmeans: Estimated Marginal Means, aka Least-Squares Means. CRAN: Vienna, Austria, 2022.


| Treatment | Agrimer SCP II | Calcium Carbonate | Apron (Mefenoxam) | Dividend (Difenoconazole and Mefenoxam) | Dynasty (Azoxystrobin) | Maxim (Fludioxonil) |
|---|---|---|---|---|---|---|
| ---------------------------------------------------- grams ---------------------------------------------------------- | ||||||
| Fungicide | 130 | 350 | 0.163 | 1.088 | 0.09 | 0.044 |
| Blank | 130 | 350 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, A.J.; Geary, B.; Hulet, A.; Madsen, M.D. Fungicide Seed Coating Increases Emergence of Bluebunch Wheatgrass (Pseudoroegneria spicata) Under High-Fungal-Biomass Conditions. Plants 2025, 14, 679. https://doi.org/10.3390/plants14050679
Johnson AJ, Geary B, Hulet A, Madsen MD. Fungicide Seed Coating Increases Emergence of Bluebunch Wheatgrass (Pseudoroegneria spicata) Under High-Fungal-Biomass Conditions. Plants. 2025; 14(5):679. https://doi.org/10.3390/plants14050679
Chicago/Turabian StyleJohnson, Amber J., Brad Geary, April Hulet, and Matthew D. Madsen. 2025. "Fungicide Seed Coating Increases Emergence of Bluebunch Wheatgrass (Pseudoroegneria spicata) Under High-Fungal-Biomass Conditions" Plants 14, no. 5: 679. https://doi.org/10.3390/plants14050679
APA StyleJohnson, A. J., Geary, B., Hulet, A., & Madsen, M. D. (2025). Fungicide Seed Coating Increases Emergence of Bluebunch Wheatgrass (Pseudoroegneria spicata) Under High-Fungal-Biomass Conditions. Plants, 14(5), 679. https://doi.org/10.3390/plants14050679






