Enhancing Yield, Physiological, and Quality Traits of Strawberry Cultivated Under Organic Management by Applying Different Non-Microbial Biostimulants
Abstract
:1. Introduction
2. Results and Discussion
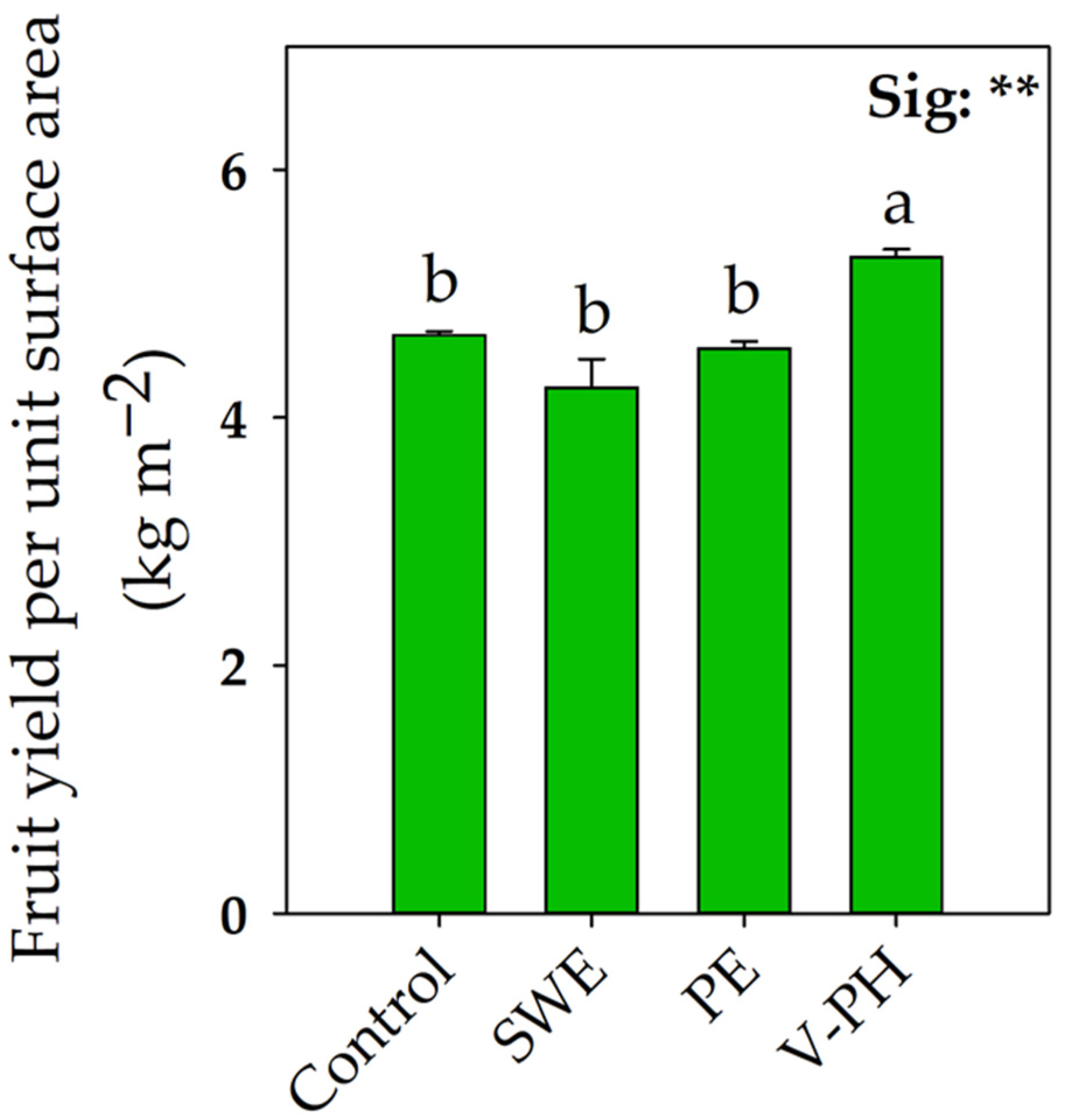
2.1. Strawberry Growth and Fruit Yield Variables as a Function of Biostimulant
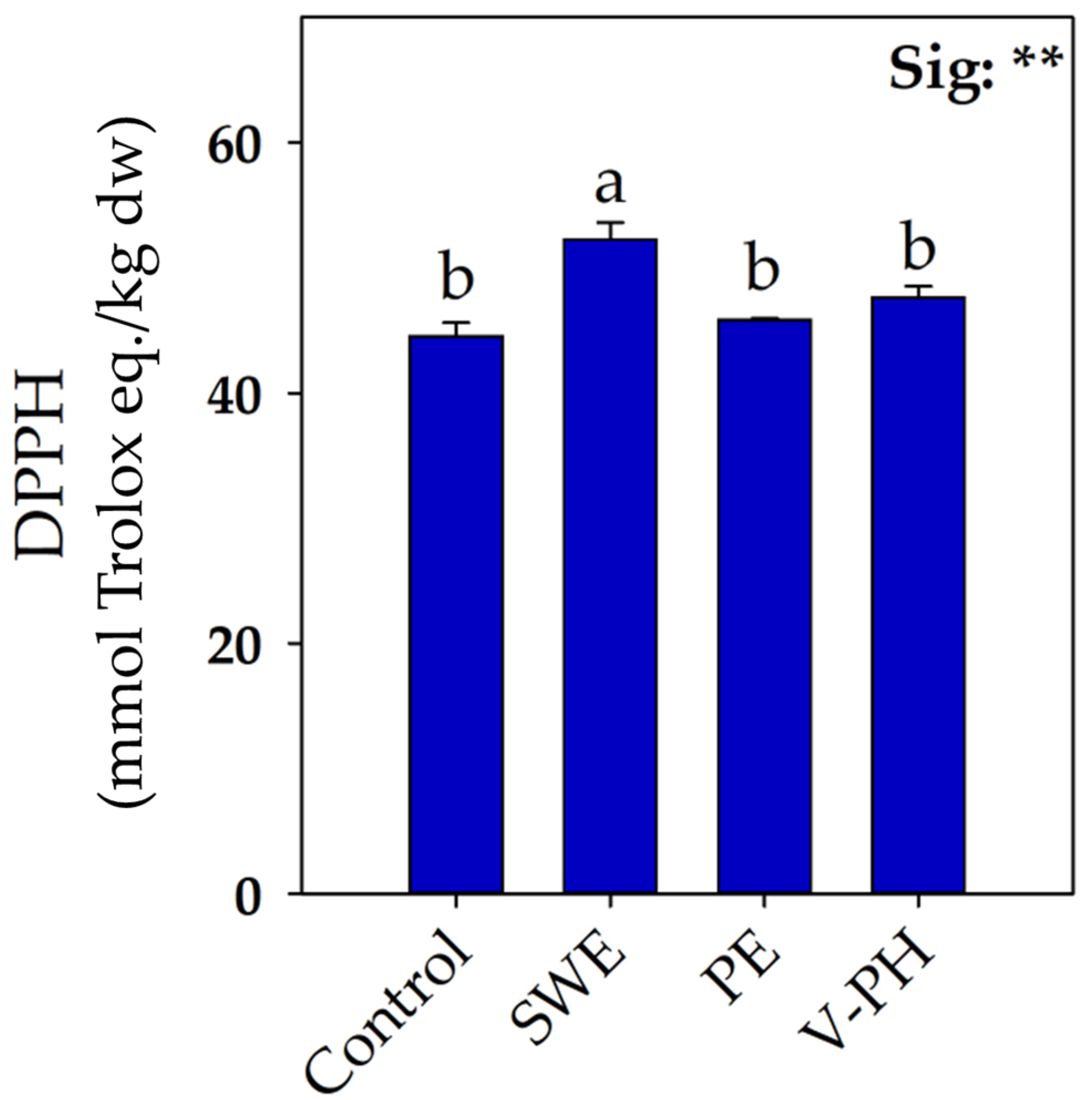
2.2. Strawberry Photosynthetic, Ionic and Antioxidant Response as a Function of Biostimulant
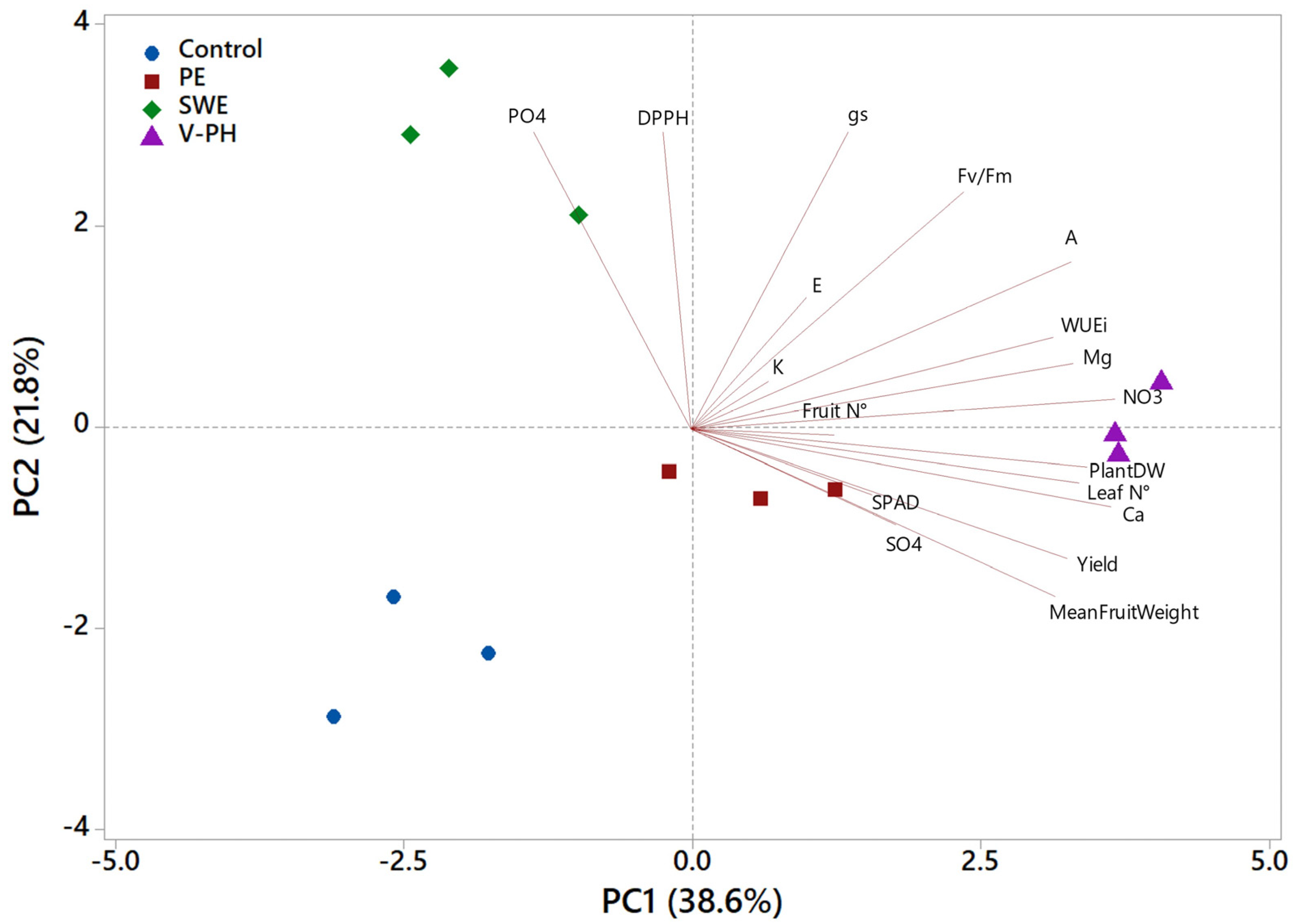
2.3. Principal Component Analysis of Yield Parameters, Ionic Content, Physiological and Antioxidant Activity on Strawberry Treated with Different Non-Microbial Biostimulants
3. Materials and Methods
3.1. Plant Material and Experimental Design
3.2. Fruit Yield, Physiological Parameters, Fruit Quality, and Leaf Ion Content Analyses
3.3. Statical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sani, M.N.H.; Yong, J.W. Harnessing synergistic biostimulatory processes: A plausible approach for enhanced crop growth and resilience in organic farming. Biology 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, M.; El Chami, A.; Rouphael, Y.; Ciriello, M.; Bonini, P.; Erice, G.; Cirino, V.; Basile, B.; Corrado, G.; Choi, S. Plant biostimulants as natural alternatives to synthetic auxins in strawberry production: Physiological and metabolic insights. Front. Plant Sci. 2024, 14, 1337926. [Google Scholar] [CrossRef]
- Wrzaszcz, W. Tendencies and perspectives of organic farming development in the EU–the significance of European Green Deal Strategy. Eur. J. Sustain. Dev. 2023, 12, 143. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Ume, C. The role of improved market access for small-scale organic farming transition: Implications for food security. J. Clean. Prod. 2023, 387, 135889. [Google Scholar] [CrossRef]
- de la Cruz, V.Y.V.; Cheng, W.; Tawaraya, K. Yield gap between organic and conventional farming systems across climate types and sub-types: A meta-analysis. Agric. Syst. 2023, 211, 103732. [Google Scholar] [CrossRef]
- Lesur-Dumoulin, C.; Malézieux, E.; Ben-Ari, T.; Langlais, C.; Makowski, D. Lower average yields but similar yield variability in organic versus conventional horticulture. A meta-analysis. Agron. Sustain. Dev. 2017, 37, 45. [Google Scholar] [CrossRef]
- Ponisio, L.C.; M’Gonigle, L.K.; Mace, K.C.; Palomino, J.; De Valpine, P.; Kremen, C. Diversification practices reduce organic to conventional yield gap. Proc. R. Soc. B Biol. Sci. 2015, 282, 20141396. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Moosa, A.; Ali, H.M.; Bermejo, N.F.; Munné-Bosch, S. Biostimulants: A sufficiently effective tool for sustainable agriculture in the era of climate change? Plant Physiol. Biochem. 2024, 211, 108699. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, T.; Gama, F.; Correia, P.J.; Da Silva, J.P.; Miguel, M.G.; de Varennes, A.; Pestana, M. A novel plant extract as a biostimulant to recover strawberry plants from iron chlorosis. J. Plant Nutr. 2020, 43, 2054–2066. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on crops: Their impact under abiotic stress conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Li, J.; Lardon, R.; Mangelinckx, S.; Geelen, D. Practical guide toward discovery of biomolecules with biostimulant activity. J. Exp. Bot. 2024, 75, 3797–3817. [Google Scholar] [CrossRef] [PubMed]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant biostimulants: A categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Shakya, R.; Capilla, E.; Torres-Pagán, N.; Muñoz, M.; Boscaiu, M.; Lupuţ, I.; Vicente, O.; Verdeguer, M. Effect of two biostimulants, based on Ascophyllum nodosum extracts, on strawberry performance under mild drought stress. Agriculture 2023, 13, 2108. [Google Scholar] [CrossRef]
- Faostat. 2017. Available online: http://www.fao.org/faostat/en/#data (accessed on 29 January 2018).
- Righini, H.; Roberti, R.; Baraldi, E. Use of algae in strawberry management. J. Appl. Phycol. 2018, 30, 3551–3564. [Google Scholar] [CrossRef]
- Soltaniband, V.; Brégard, A.; Gaudreau, L.; Dorais, M. Biostimulants promote plant development, crop productivity, and fruit quality of protected strawberries. Agronomy 2022, 12, 1684. [Google Scholar] [CrossRef]
- Kouam, I.D.; Moungang, S.; Koulagna, H.I.; Ntsoli, G.P.; Titti, R.W.; Yaouba, A. Influence of organic and mineral fertilizers and a foliar biostimulant on the yield and nutritional quality of strawberries (Fragaria × ananassa Duch.) under field conditions. Biochem. Syst. Ecol. 2024, 117, 104917. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Pasković, I.; Popović, L.; Pongrac, P.; Polić Pasković, M.; Kos, T.; Jovanov, P.; Franić, M. Protein Hydrolysates—Production, Effects on Plant Metabolism, and Use in Agriculture. Horticulturae 2024, 10, 1041. [Google Scholar] [CrossRef]
- Malécange, M.; Sergheraert, R.; Teulat, B.; Mounier, E.; Lothier, J.; Sakr, S. Biostimulant properties of protein hydrolysates: Recent advances and future challenges. Int. J. Mol. Sci. 2023, 24, 9714. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of the tricarboxylic acid (TCA) cycle and nitrogen metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef] [PubMed]
- Möller, K. Soil fertility status and nutrient input–output flows of specialised organic cropping systems: A review. Nutr. Cycl. Agroecosystems 2018, 112, 147–164. [Google Scholar] [CrossRef]
- Rajesaheb, K.S.; Subramanian, S.; Boominathan, P.; Thenmozhi, S.; Gnanachitra, M. Bio-stimulant in improving crop yield and soil health. Commun. Soil Sci. Plant Anal. 2024, 56, 458–493. [Google Scholar] [CrossRef]
- Garg, S.; Nain, P.; Kumar, A.; Joshi, S.; Punetha, H.; Sharma, P.K.; Siddiqui, S.; Alshaharni, M.O.; Algopishi, U.B.; Mittal, A. Next generation plant biostimulants & genome sequencing strategies for sustainable agriculture development. Front. Microbiol. 2024, 15, 1439561. [Google Scholar]
- Li, J.; Van Gerrewey, T.; Geelen, D. A meta-analysis of biostimulant yield effectiveness in field trials. Front. Plant Sci. 2022, 13, 836702. [Google Scholar] [CrossRef] [PubMed]
- Masny, A.; Basak, A.; Żurawicz, E. Effects of foliar applications of Kelpak SL and Goëmar BM 86® preparations on yield and fruit quality in two strawberry cultivars. J. Fruit Ornam. Plant Res. 2004, 12, 23–27. [Google Scholar]
- Wise, K.; Selby-Pham, J. Strawberry field trial in Australia demonstrates improvements to fruit yield and quality control conformity, from application of two biostimulant complexes. N. Z. J. Crop Hortic. Sci. 2024, 1–16. [Google Scholar] [CrossRef]
- Mrid, R.B.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 777, 146204. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Cristofano, F.; Cardarelli, M.; Colla, G. Effects of vegetal-versus animal-derived protein hydrolysate on sweet basil morpho-physiological and metabolic traits. Sci. Hortic. 2021, 284, 110123. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Matsumiya, Y.; Kubo, M. Soybean peptide: Novel plant growth promoting peptide from soybean. In Soybean and Nutrition; IntechOpen: London, UK, 2011; Volume 11. [Google Scholar]
- Tabatabaei, S.; Yusefi, M.; Hajiloo, J. Effects of shading and NO3: NH4 ratio on the yield, quality and N metabolism in strawberry. Sci. Hortic. 2008, 116, 264–272. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Fu, G.; Katulanda, P. Induced leaf intercellular CO2, photosynthesis, potassium and nitrate retention and strawberry early fruit formation under macronutrient limitation. Photosynth. Res. 2013, 115, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H.; Kuang, Y.; Wang, N. Amino acids biostimulants and protein hydrolysates in agricultural sciences. Plants 2024, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef] [PubMed]
- Jing, T.; Li, J.; He, Y.; Shankar, A.; Saxena, A.; Tiwari, A.; Maturi, K.C.; Solanki, M.K.; Singh, V.; Eissa, M.A. Role of calcium nutrition in plant Physiology: Advances in research and insights into acidic soil conditions-A comprehensive review. Plant Physiol. Biochem. 2024, 210, 108602. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the regulation of reactive oxygen species metabolism in plants under abiotic stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-Anssary, A.A. Plants secondary metabolites: The key drivers of the pharmacological actions of medicinal plants. Herb. Med. 2019, 1, 11–30. [Google Scholar]
- Iwamoto, K.; Shiraiwa, Y. Salt-regulated mannitol metabolism in algae. Mar. Biotechnol. 2005, 7, 407–415. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Ramya, S.S.; Vijayanand, N.; Rathinavel, S. Foliar application of liquid biofertilizer of brown alga Stoechospermum marginatum on growth, biochemical and yield of Solanum melongena. Int. J. Recycl. Org. Waste Agric. 2015, 4, 167–173. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Nair, P.; Kandasamy, S.; Zhang, J.; Ji, X.; Kirby, C.; Benkel, B.; Hodges, M.D.; Critchley, A.T.; Hiltz, D.; Prithiviraj, B. Transcriptional and metabolomic analysis of Ascophyllum nodosum mediated freezing tolerance in Arabidopsis thaliana. BMC Genom. 2012, 13, 643. [Google Scholar] [CrossRef]
- Drobek, M.; Cybulska, J.; Frąc, M.; Pieczywek, P.; Pertile, G.; Chibrikov, V.; Nosalewicz, A.; Feledyn-Szewczyk, B.; Sas-Paszt, L.; Zdunek, A. Microbial biostimulants affect the development of pathogenic microorganisms and the quality of fresh strawberries (Fragaria ananassa Duch.). Sci. Hortic. 2024, 327, 112793. [Google Scholar] [CrossRef]
- Bremner, J. Total nitrogen. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1965. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- Sunderman, F.W., Jr.; Sunderman, F.W. Studies in Serum Electrolytes XXII. A Rapid, Reliable Method for Serum Potassium Using Tetraphenylboron. Am. J. Clin. Pathol. 1958, 29, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Sicily Region. Regional Integrated Production Regulations: Technical Standards for Integrated Crop Protection and Pest Control. 2020, p. 324. Available online: https://www.regione.sicilia.it/sites/default/files/2023-07/2%20Norme%20tecniche%20Difesa%202023%20del%20DPI%20Sicilia_0.pdf (accessed on 5 January 2025).
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M. A combined phenotypic and metabolomic approach for elucidating the biostimulant action of a plant-derived protein hydrolysate on tomato grown under limited water availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef]
- Ciriello, M.; Campana, E.; Colla, G.; Rouphael, Y. An Appraisal of Nonmicrobial Biostimulants’ Impact on the Productivity and Mineral Content of Wild Rocket (Diplotaxis tenuifolia (L.) DC.) Cultivated under Organic Conditions. Plants 2024, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Formisano, L.; Ciriello, M.; El-Nakhel, C.; De Pascale, S.; Rouphael, Y. Dataset on the effects of anti-insect nets of different porosity on mineral and organic acids profile of Cucurbita pepo L. fruits and leaves. Data 2021, 6, 50. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]

| Treatment | Fruit Number (N°/Plant) | Fruit Fresh Weight (g/Fruit) | Leaf Number (N°/Plant) | Shoot Dry Weight (g/Plant) |
|---|---|---|---|---|
| Control | 35.46 ± 0.23 ab | 20.23 ± 0.21 ab | 55.40 ± 1.24 b | 42.09 ± 3.31 b |
| SWE | 34.56 ± 0.21 b | 18.86 ± 0.85 b | 56.00 ± 2.71 b | 42.21 ± 1.19 b |
| PE | 32.16 ± 0.53 c | 21.80 ± 0.27 a | 71.73 ± 1.95 a | 61.80 ± 1.01 a |
| V-PH | 36.90 ± 0.20 a | 22.07 ± 0.35 a | 69.73 ± 0.40 a | 61.88 ± 1.46 a |
| Significance | *** | ** | *** | *** |
| Treatment | SPAD Index | ACO2 (µmol CO2/m2/s) | E (mol H2O/m2/s) | gs (mol H2O/m2/s) | WUEi (µmol CO2/mol H2O) | Fv/Fm |
|---|---|---|---|---|---|---|
| Control | 45.60 ± 0.23 | 12.05 ± 0.53 b | 2.59 ± 0.18 | 0.15 ± 0.00 b | 4.66 ± 0.18 b | 0.80 ± 0.00 b |
| SWE | 44.37 ± 0.81 | 14.64 ± 0.42 a | 2.83 ± 0.14 | 0.20 ± 0.00 a | 5.18 ± 0.10 ab | 0.81 ± 0.00 a |
| PE | 44.26 ± 0.77 | 15.22 ± 0.40 a | 2.77 ± 0.09 | 0.17 ± 0.00 b | 5.48 ± 0.09 ab | 0.81 ± 0.00 a |
| V-PH | 46.40 ± 0.64 | 16.24 ± 0.11 a | 2.80 ± 0.16 | 0.19 ± 0.00 a | 5.82 ± 0.30 a | 0.81 ± 0.00 a |
| Significance | n.s. | *** | n.s. | *** | * | *** |
| Treatment | NO3− | SO42− | K+ | Ca2+ | Mg2+ | PO43− |
|---|---|---|---|---|---|---|
| (g/kg dw) | ||||||
| Control | 0.91 ± 0.08 b | 0.73 ± 0.04 | 16.99 ± 0.27 | 3.84 ± 0.25 b | 2.15 ± 0.03 b | 2.72 ± 0.18 b |
| SWE | 0.97 ± 0.04 b | 0.64 ± 0.05 | 17.35 ± 0.81 | 3.48 ± 0.30 b | 2.38 ± 0.18 ab | 3.56 ± 0.12 a |
| PE | 1.05 ± 0.05 ab | 0.63 ± 0.09 | 15.39 ± 0.63 | 4.08 ± 0.22 b | 2.20 ± 0.09 b | 2.92 ± 0.10 b |
| V-PH | 1.36 ± 0.10 a | 0.60 ± 0.07 | 17.73 ± 0.60 | 5.62 ± 0.32 a | 2.86 ± 0.02 a | 2.80 ± 0.11 b |
| Significance | * | n.s | n.s. | ** | ** | ** |
| Properties | Unit | Results |
|---|---|---|
| Sand | % | 48.4 |
| Silt | % | 43 |
| Clay | % | 8.6 |
| pH | 7.4 | |
| Electrical conductivity | dS/m | 0.2 |
| Organic Matter | % | 2.4 |
| Total Nitrogen | g/kg | 1.7 |
| C/N | 8.3 | |
| P2O5 (ppm) | mg/kg | 162 |
| K2O (ppm) | mg/kg | 2439 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciriello, M.; Pannico, A.; Rouphael, Y.; Basile, B. Enhancing Yield, Physiological, and Quality Traits of Strawberry Cultivated Under Organic Management by Applying Different Non-Microbial Biostimulants. Plants 2025, 14, 712. https://doi.org/10.3390/plants14050712
Ciriello M, Pannico A, Rouphael Y, Basile B. Enhancing Yield, Physiological, and Quality Traits of Strawberry Cultivated Under Organic Management by Applying Different Non-Microbial Biostimulants. Plants. 2025; 14(5):712. https://doi.org/10.3390/plants14050712
Chicago/Turabian StyleCiriello, Michele, Antonio Pannico, Youssef Rouphael, and Boris Basile. 2025. "Enhancing Yield, Physiological, and Quality Traits of Strawberry Cultivated Under Organic Management by Applying Different Non-Microbial Biostimulants" Plants 14, no. 5: 712. https://doi.org/10.3390/plants14050712
APA StyleCiriello, M., Pannico, A., Rouphael, Y., & Basile, B. (2025). Enhancing Yield, Physiological, and Quality Traits of Strawberry Cultivated Under Organic Management by Applying Different Non-Microbial Biostimulants. Plants, 14(5), 712. https://doi.org/10.3390/plants14050712










