South African Medicinal Plants Traditionally Used for Wound Treatment: An Ethnobotanical Systematic Review
Abstract
1. Introduction
2. Results and Discussion
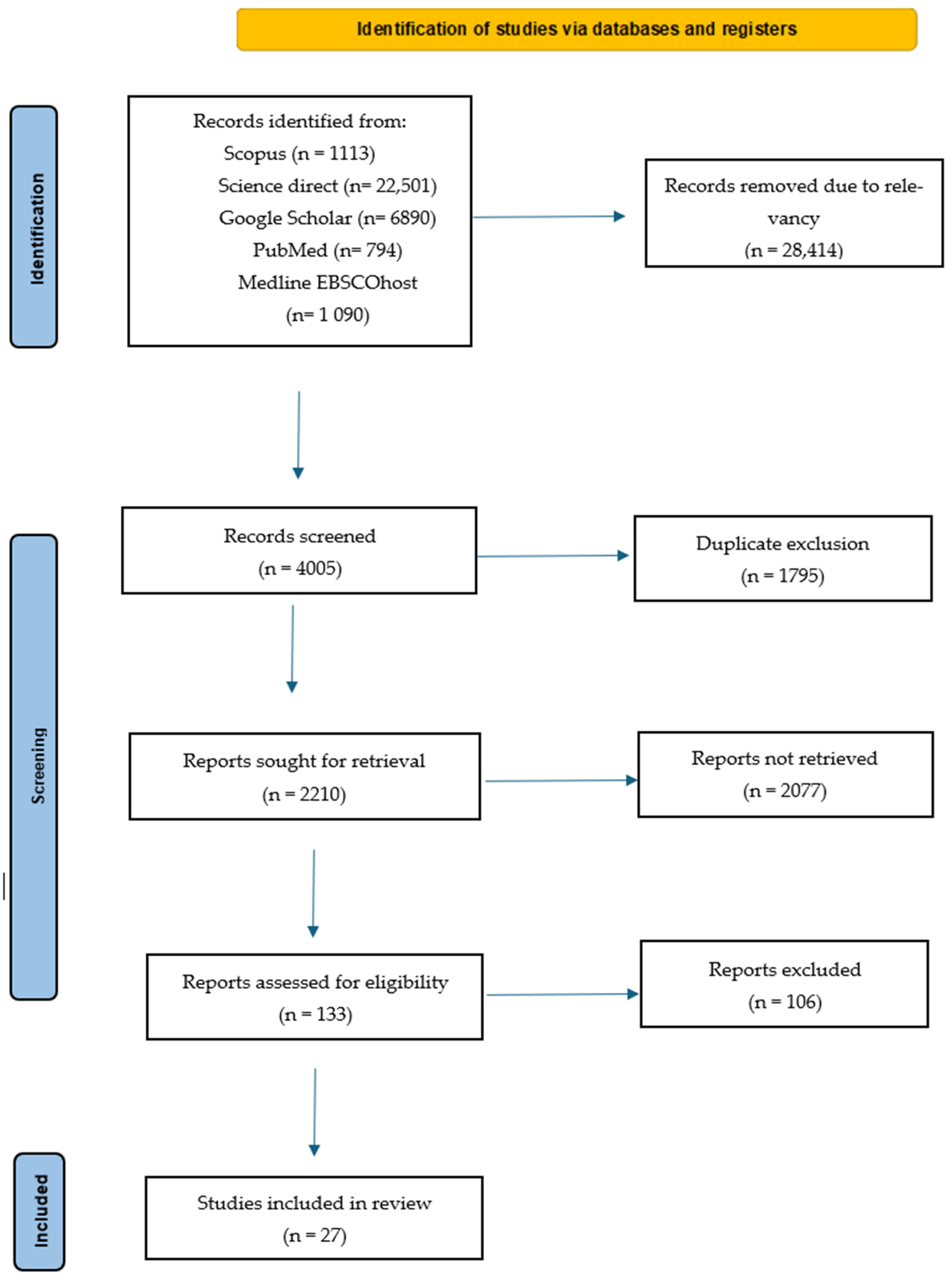
2.1. Literature Search Results
2.2. Characteristics of the Ethnobotanical Studies Documenting the Use of Plants Traditionally Used to Treat Wounds in SA
| Study Reference | Region | Method of Data Collection | Number of Participants | Number of Plant Species | Number of Plant Families |
|---|---|---|---|---|---|
| Zwane et al., 2024 [32] | KwaZulu-Natal | Interview using a structured questionnaire | 55 | 1 | 1 |
| Ndhlovu et al., 2023 [33] | North West | Semi-structured interviews | 101 | 4 | 4 |
| Xaba et al., 2023 [34] | Free State | Interviews using structured questionnaires | 10 | 17 | 13 |
| Setshego et al., 2020 [35] | Limpopo | Semi-structured interviews | 71 | 18 | 14 |
| Asong et al., 2019 [9] | North West | Semi-structured questionnaires with a picture guide | 30 | 13 | 9 |
| Gebashe et al., 2019 [14] | KwaZulu-Natal | Interviews and questionnaires | 60 | 1 | 1 |
| Hulley and Van Wyk, 2019 [36] | Western Cape | Interviews using structured interviews | 70 | 28 | 10 |
| Mhlongo and Van Wyk, 2019 [29] | KwaZulu-Natal | Interviews using matrix method—picture guide | 37 | 42 | 20 |
| Mogale et al., 2019 [37] | Limpopo | Interviews | 27 | 2 | 2 |
| Ndhlovu et al., 2019 [23] | Limpopo | Interviews using semi-structured questionnaires | 79 | 28 | 24 |
| Thibane et al., 2019 [38] | Eastern Cape | Interviews using a structured questionnaire | 50 | 5 | 5 |
| Mongalo and Makhafola, 2018 [39] | Limpopo | Structured questionnaire and interviews. | 40 | 2 | 2 |
| Asowata-Ayodele et al., 2016 [40] | Eastern Cape | Interviews | 74 | 1 | 1 |
| Rankoana, 2016 [41] | Limpopo | Structured interviews | 100 | 2 | 1 |
| Tshikalange et al., 2016 [42] | Mpumalanga | Semi-structured interviews | 15 | 6 | 5 |
| Nortje and Van Wyk, 2015 [43] | Northern Cape | Semi-structured and structured interviews, questionnaires | 24 | 20 | 12 |
| Afolayan et al., 2014 [44] | Eastern Cape | Interviews and discussions | 54 | 29 | 20 |
| De Wet et al., 2013 [15] | KwaZulu-Natal | Interviews using a structured questionnaire | 87 | 2 | 2 |
| Josia, 2013 [8] | Eastern Cape | Interviews and questionnaires | 37 | 31 | 17 |
| Mahwasane et al., 2013 [13] | Limpopo | Interviews and questionnaires | 30 | 1 | 1 |
| Corrigan et al., 2011 [45] | KwaZulu-Natal | Interviews | 5 | 4 | 4 |
| De Beer and Van Wyk, 2011 [30] | Northern Cape | Interviews and questionnaires—Matrix method | 16 | 7 | 5 |
| Philander, 2011 [24] | Western Cape | Interviews | 39 | 9 | 8 |
| Van Wyk et al., 2008 [28] | Areas between Western Cape, Northern Cape, and Eastern Cape | Interviews (Rapid Appraisal approach) | 7 | 6 | 6 |
| Thring and Weitz, 2006 [46] | Western Cape | Interviews using questionnaires | 44 | 4 | 4 |
| Bhat and Jacobs, 1995 [47] | Eastern Cape | Surveys and interviews | Not reported | 6 | 5 |
| Grierson and Afolayan, 1999 [27] | Eastern Cape | General conversations with informants and questionnaires | Not reported | 26 | 19 |
2.3. Medicinal Plants Identified for Wound Healing Purposes
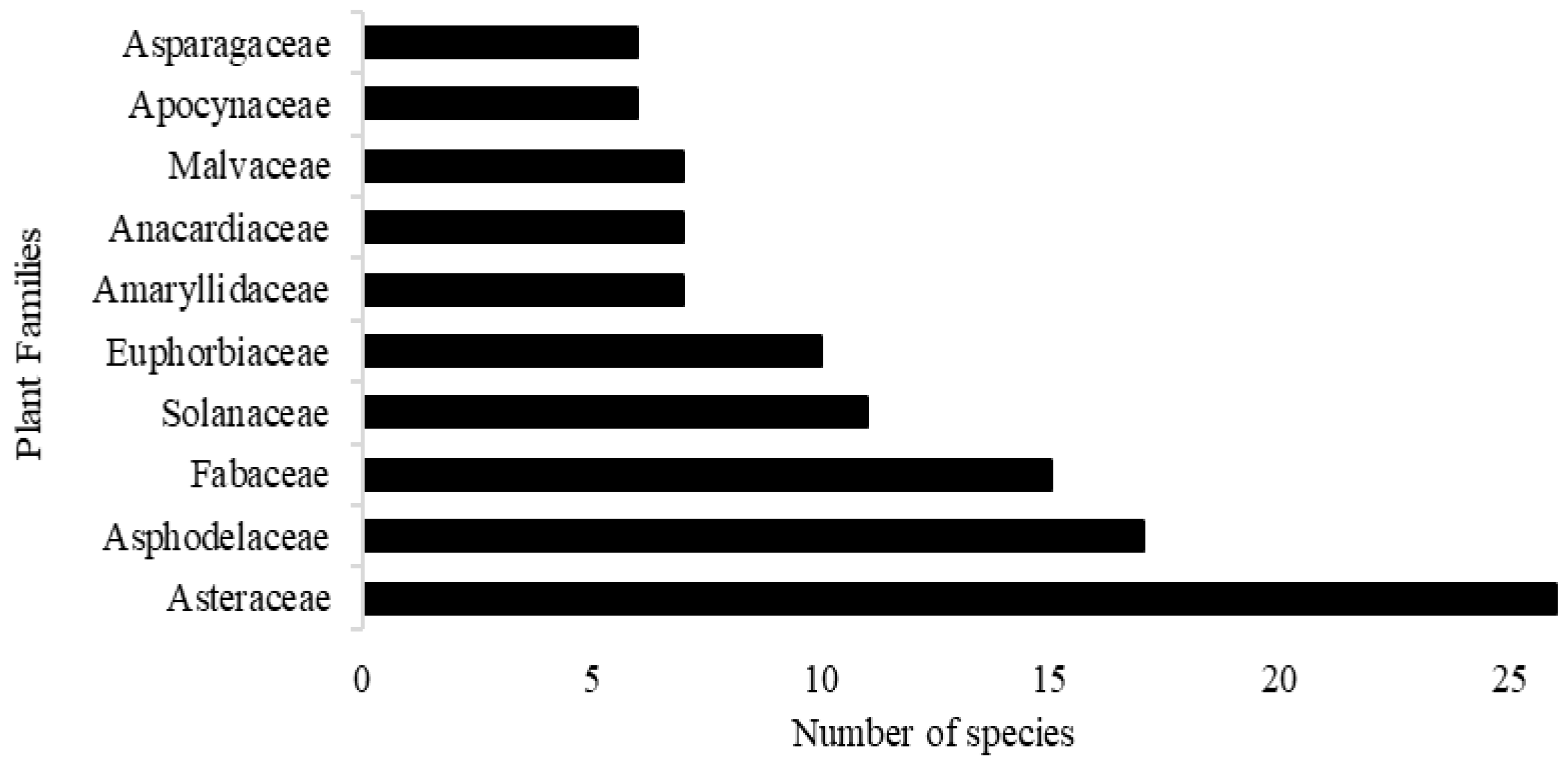
2.4. Biological Activity of Commonly Identified Plant Families and Related Species
2.4.1. Asteraceae
2.4.2. Asphodelaceae
2.4.3. Fabaceae
2.4.4. Amaryllidaceae, Aizoaceae, and Solanaceae
2.5. Plant Parts Reported for Wounds
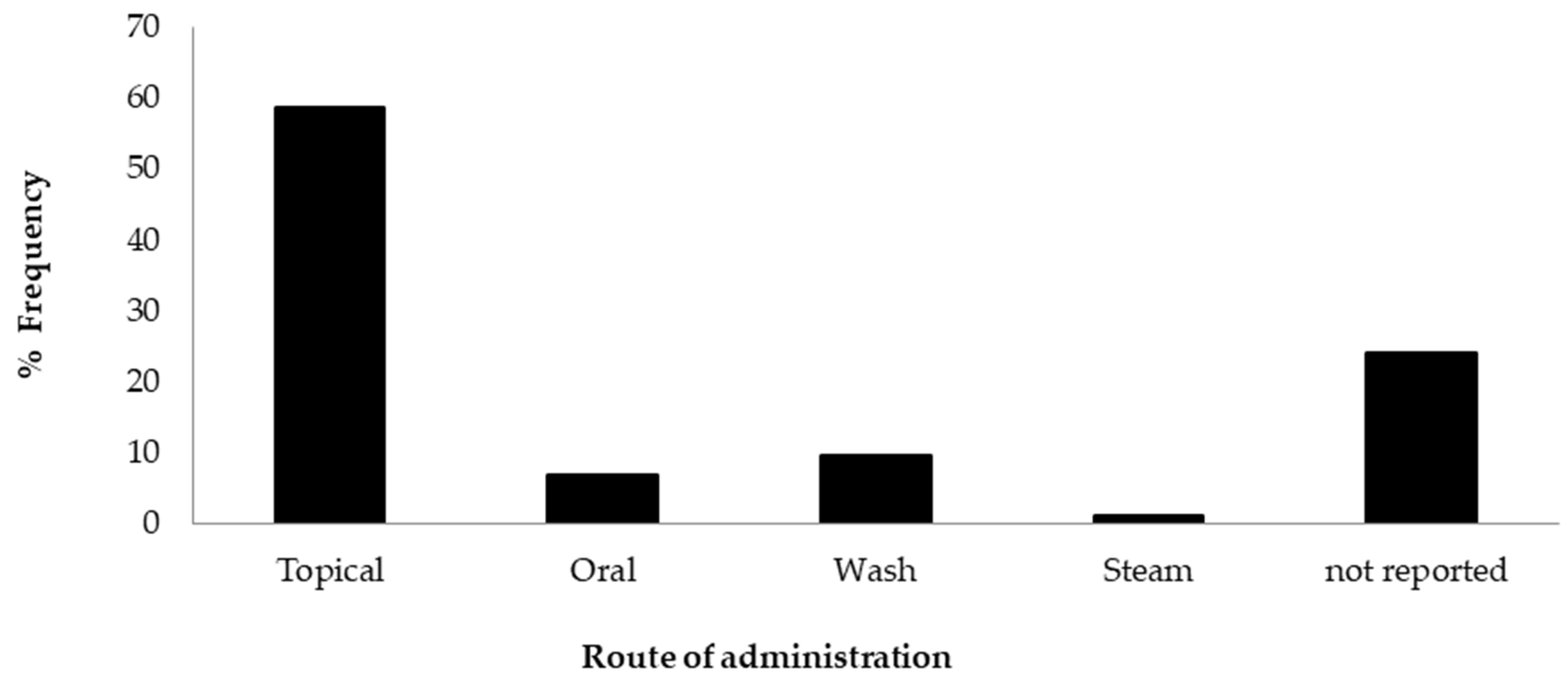
2.6. Methods of Preparation and Route of Administration
2.7. Conservation Status
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anlas, C.; Bakirel, T.; Ustun-Alkan, F.; Celik, B.; Baran, M.Y.; Ustuner, O.; Kuruuzum-Uz, A. In vitro evaluation of the therapeutic potential of Anatolian kermes oak (Quercus coccifera L.) as an alternative wound healing agent. Ind. Crop. Prod. 2019, 137, 24–32. [Google Scholar] [CrossRef]
- Chingwaru, C.; Bagar, T.; Maroyi, A.; Kapewangolo, P.T.; Chingwaru, W. Wound healing potential of selected Southern African medicinal plants: A review. J. Herb. Med. 2019, 17, 100263. [Google Scholar] [CrossRef]
- Gang, R.; Okello, D.; Kang, Y. Medicinal plants used for cutaneous wound healing in Uganda; ethnomedicinal reports and pharmacological evidences. Heliyon 2024, 10, e29717. [Google Scholar] [CrossRef] [PubMed]
- Sagona, R.T.; Sandasi, M.; Ncube, E.; Gouws, C.; Viljoen, A.M. Insights into the wound-healing properties of medicinally important South African Bulbine species—A comparative study. J. Ethnopharmacol. 2024, 337, 118901. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M. The burden of wounds in a resource-constrained tertiary hospital: A cross-sectional study. Wound Heal. South. Afr. 2019, 12, 29–33. [Google Scholar]
- Levy, S.B. The Antibiotic Paradox: How Miracle Drugs Are Destroying the Miracle; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Quave, C.L.; Plano, L.R.; Pantuso, T.; Bennett, B.C. Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2008, 118, 418–428. [Google Scholar] [CrossRef]
- Josia, M. Medicinal Properties of Some Plants Used for the Treatment of Skin Disorders in the OR Tambo and Amathole Municipalities of the Eastern Cape Province. Master’s Dissertation, Walter Sisulu University, Mthatha, South Africa, 2013. [Google Scholar]
- Asong, J.; Ndhlovu, P.; Khosana, N.; Aremu, A.; Otang-Mbeng, W. Medicinal plants used for skin-related diseases among the Batswanas in Ngaka Modiri Molema District Municipality, South Africa. S. Afr. J. Bot. 2019, 126, 11–20. [Google Scholar] [CrossRef]
- Nagoba, B.; Dawale, C.P.; Raju, R.; Wadher, B.; Chidrawar, S.; Selkar, S.; Suryawanshi, N. Citric acid treatment of post operative wound infections in HIV/AIDS patients. J. Tissue Viab. 2014, 23, 24–28. [Google Scholar] [CrossRef]
- Mabona, U.; Van Vuuren, S. Southern African medicinal plants used to treat skin diseases. S. Afr. J. Bot. 2013, 87, 175–193. [Google Scholar] [CrossRef]
- Papo, L.; Van Vuuren, S.; Moteetee, A. The ethnobotany and antimicrobial activity of selected medicinal plants from Ga-Mashashane, Limpopo Province, South Africa. S. Afr. J. Bot. 2022, 149, 196–210. [Google Scholar] [CrossRef]
- Mahwasane, S.; Middleton, L.; Boaduo, N. An ethnobotanical survey of indigenous knowledge on medicinal plants used by the traditional healers of the Lwamondo area, Limpopo province, South Africa. S. Afr. J. Bot. 2013, 88, 69–75. [Google Scholar] [CrossRef]
- Gebashe, F.; Moyo, M.; Aremu, A.O.; Finnie, J.F.; Van Staden, J. Ethnobotanical survey and antibacterial screening of medicinal grasses in KwaZulu-Natal Province, South Africa. S. Afr. J. Bot. 2019, 122, 467–474. [Google Scholar] [CrossRef]
- De Wet, H.; Nciki, S.; van Vuuren, S.F. Medicinal plants used for the treatment of various skin disorders by a rural community in northern Maputaland, South Africa. J. Ethnobiol. Ethnomed. 2013, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Masevhe, N.A.; McGaw, L.J.; Eloff, J.N. The traditional use of plants to manage candidiasis and related infections in Venda, South Africa. J. Ethnopharmacol. 2015, 168, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Agyare, C.; Boakye, Y.D.; Bekoe, E.O.; Hensel, A.; Dapaah, S.O.; Appiah, T. Review: African medicinal plants with wound healing properties. J. Ethnopharmacol. 2016, 177, 85–100. [Google Scholar] [CrossRef]
- Agyare, C.; Dwobeng, A.S.; Agyepong, N.; Boakye, Y.D.; Mensah, K.B.; Ayande, P.G.; Adarkwa-Yiadom, M. Antimicrobial, antioxidant, and wound healing properties of Kigelia africana (Lam.) Beneth. and Strophanthus hispidus DC. Adv. Pharmacol. Pharm. Sci. 2013, 2013, 692613. [Google Scholar]
- Pringle, N.A.; Koekemoer, T.C.; Holzer, A.; Young, C.; Venables, L.; van de Venter, M. Potential therapeutic benefits of green and fermented rooibos (Aspalathus linearis) in dermal wound healing. Planta Medica 2018, 84, 645–652. [Google Scholar] [CrossRef]
- Shikwambana, N.; Mahlo, S.M. A survey of antifungal activity of selected south african plant species used for the treatment of skin infections. Nat. Prod. Commun. 2020, 15, 1934578X20923181. [Google Scholar] [CrossRef]
- Wintola, O.; Afolayan, A. Ethnobotanical survey of plants used for the treatment of constipation within Nkonkobe Municipality of South Africa. S. Afr. J. Bot. 2010, 76, 407. [Google Scholar] [CrossRef][Green Version]
- Chakale, M.V.; Lekhooa, M.; Aremu, A.O. South African medicinal plants used for health conditions affecting males: An ethnobotanical review. J. Herb. Med. 2024, 47, 100931. [Google Scholar] [CrossRef]
- Ndhlovu, P.; Mooki, O.; Mbeng, W.O.; Aremu, A. Plant species used for cosmetic and cosmeceutical purposes by the Vhavenda women in Vhembe District Municipality, Limpopo, South Africa. S. Afr. J. Bot. 2019, 122, 422–431. [Google Scholar] [CrossRef]
- Philander, L.A. An ethnobotany of Western Cape Rasta bush medicine. J. Ethnopharmacol. 2011, 138, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, A.O.; Aremu, A.O. Evaluation of factors influencing the inclusion of indigenous plants for food security among rural households in the North West Province of South Africa. Sustainability 2020, 12, 9562. [Google Scholar] [CrossRef]
- Mongalo, N.I.; Raletsena, M.V. An Inventory of South African Medicinal Plants Used in the Management of Sexually Transmitted and Related Opportunistic Infections: An Appraisal and Some Scientific Evidence (1990–2020). Plants 2022, 11, 3241. [Google Scholar] [CrossRef] [PubMed]
- Grierson, D.; Afolayan, A. An ethnobotanical study of plants used for the treatment of wounds in the Eastern Cape, South Africa. J. Ethnopharmacol. 1999, 67, 327–332. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; de Wet, H.; Van Heerden, F. An ethnobotanical survey of medicinal plants in the southeastern Karoo, South Africa. S. Afr. J. Bot. 2008, 74, 696–704. [Google Scholar] [CrossRef]
- Mhlongo, L.; Van Wyk, B.-E. Zulu medicinal ethnobotany: New records from the Amandawe area of KwaZulu-Natal, South Africa. S. Afr. J. Bot. 2019, 122, 266–290. [Google Scholar] [CrossRef]
- De Beer, J.J.; Van Wyk, B.-E. An ethnobotanical survey of the Agter–Hantam, Northern Cape Province, South Africa. S. Afr. J. Bot. 2011, 77, 741–754. [Google Scholar] [CrossRef]
- Vogl, C.R.; Vogl-Lukasser, B.; Puri, R.K. Tools and methods for data collection in ethnobotanical studies of homegardens. Field Methods 2004, 16, 285–306. [Google Scholar] [CrossRef]
- Zwane, N.; De Wet, H.; Van Vuuren, S. Blood purification practices: Some ethnopharmacological insight from a rural community in KwaZulu-Natal, South Africa. J. Ethnopharmacol. 2024, 324, 117795. [Google Scholar] [CrossRef]
- Ndhlovu, P.T.; Asong, J.A.; Omotayo, A.O.; Otang-Mbeng, W.; Aremu, A.O. Ethnobotanical survey of medicinal plants used by indigenous knowledge holders to manage healthcare needs in children. PLoS ONE 2023, 18, e0282113. [Google Scholar] [CrossRef]
- Xaba, V.M.; Adeniran, A.L.; Lamula, S.Q.N.; Buwa-Komoreng, L.V. In Vitro Bioactivities of Plants Used against Skin Diseases in the Eastern Free State, South Africa. Int. J. Plant Biol. 2023, 15, 13–31. [Google Scholar] [CrossRef]
- Setshego, M.V.; Aremu, A.O.; Mooki, O.; Otang-Mbeng, W. Natural resources used as folk cosmeceuticals among rural communities in Vhembe district municipality, Limpopo province, South Africa. BMC Complement. Med. Ther. 2020, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Hulley, I.; Van Wyk, B.-E. Quantitative medicinal ethnobotany of Kannaland (western Little Karoo, South Africa): Non-homogeneity amongst villages. S. Afr. J. Bot. 2019, 122, 225–265. [Google Scholar] [CrossRef]
- Mogale, M.; Raimondo, D.; VanWyk, B.-E. The ethnobotany of central sekhukhuneland, South Africa. S. Afr. J. Bot. 2019, 122, 90–119. [Google Scholar] [CrossRef]
- Thibane, V.; Ndhlala, A.; Abdelgadir, H.; Finnie, J.; Van Staden, J. The cosmetic potential of plants from the Eastern Cape Province traditionally used for skincare and beauty. S. Afr. J. Bot. 2019, 122, 475–483. [Google Scholar] [CrossRef]
- Mongalo, N.I.; Makhafola, T.J. Ethnobotanical knowledge of the lay people of Blouberg area (Pedi tribe), Limpopo Province, South Africa. J. Ethnobiol. Ethnomed. 2018, 14, 46. [Google Scholar] [CrossRef]
- Asowata-Ayodele, A.M.; Afolayan, A.J.; Otunola, G.A. Ethnobotanical survey of culinary herbs and spices used in the traditional medicinal system of Nkonkobe Municipality, Eastern Cape, South Africa. S. Afr. J. Bot. 2016, 104, 69–75. [Google Scholar] [CrossRef]
- Rankoana, S.A. Curative care through administration of plant-derived medicines in Sekhukhune District Municipality of Limpopo province, South Africa. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 47. [Google Scholar] [CrossRef]
- Tshikalange, T.E.; Mophuting, B.C.; Mahore, J.; Winterboer, S.; Lall, N. An ethnobotanical study of medicinal plants used in villages under Jongilanga tribal council, Mpumalanga, South Africa. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 83–89. [Google Scholar] [CrossRef]
- Nortje, J.; van Wyk, B.-E. Medicinal plants of the kamiesberg, namaqualand, South Africa. J. Ethnopharmacol. 2015, 171, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, A.J.; Grierson, D.S.; Mbeng, W.O. Ethnobotanical survey of medicinal plants used in the management of skin disorders among the Xhosa communities of the Amathole District, Eastern Cape, South Africa. J. Ethnopharmacol. 2014, 153, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, B.; Van Wyk, B.-E.; Geldenhuys, C.; Jardine, J. Ethnobotanical plant uses in the KwaNibela peninsula, St lucia, South Africa. S. Afr. J. Bot. 2010, 77, 346–359. [Google Scholar] [CrossRef]
- Thring, T.; Weitz, F. Medicinal plant use in the Bredasdorp/Elim region of the Southern Overberg in the Western Cape Province of South Africa. J. Ethnopharmacol. 2006, 103, 261–275. [Google Scholar] [CrossRef]
- Bhat, R.; Jacobs, T. Traditional herbal medicine in Transkei. J. Ethnopharmacol. 1995, 48, 7–12. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae family as agents in the protection of human health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Achika, J.I.; Arthur, D.E.; Gerald, I.; Adedayo, A. A review on the phytoconstituents and related medicinal properties of plants in the Asteraceae family. IOSR J. Appl. Chem. 2014, 7, 1–8. [Google Scholar] [CrossRef]
- Makgobole, M.U.; Mpofana, N.; Ajao, A.A.-N. Medicinal Plants for Dermatological Diseases: Ethnopharmacological Significance of Botanicals from West Africa in Skin Care. Cosmetics 2023, 10, 167. [Google Scholar] [CrossRef]
- Akinyede, K.A.; Cupido, C.N.; Hughes, G.D.; Oguntibeju, O.O.; Ekpo, O.E. Medicinal properties and in vitro biological activities of selected Helichrysum species from South Africa: A review. Plants 2021, 10, 1566. [Google Scholar] [CrossRef]
- Olán-Jiménez, K.A.; Cruz-Rodríguez, R.I.; Couder-García, B.D.C.; Jacobo-Herrera, N.; Ruiz-Lau, N.; Hernández-Cruz, M.D.C.; Ruíz-Valdiviezo, V.M. Antibacterial and Wound Healing Activity In Vitro of Individual and Combined Extracts of Tagetes nelsonii Greenm, Agave americana and Aloe vera. Sci. Pharm. 2024, 92, 41. [Google Scholar] [CrossRef]
- Serabele, K.; Chen, W.; Tankeu, S.; Combrinck, S.; Veale, C.G.; van Vuuren, S.; Chaudhary, S.K.; Viljoen, A. Comparative chemical profiling and antimicrobial activity of two interchangeably used ‘Imphepho’ species (Helichrysum odoratissimum and Helichrysum petiolare). S. Afr. J. Bot. 2021, 137, 117–132. [Google Scholar] [CrossRef]
- Cousins, S.; Witkowski, E. African aloe ecology: A review. J. Arid. Environ. 2012, 85, 1–17. [Google Scholar] [CrossRef]
- Bodede, O.; Prinsloo, G. Ethnobotany, phytochemistry and pharmacological significance of the genus Bulbine (Asphodelaceae). J. Ethnopharmacol. 2020, 260, 112986. [Google Scholar] [CrossRef]
- Loggenberg, S.R.; Twilley, D.; De Canha, M.N.; Meyer, D.; Lall, N. The activity of Aloe arborescens Miller varieties on wound-associated pathogens, wound healing and growth factor production. S. Afr. J. Bot. 2022, 147, 1096–1104. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, G.; Jia, J. Preliminary evaluation: The effects of Aloe ferox Miller and Aloe arborescens Miller on wound healing. J. Ethnopharmacol. 2008, 120, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.M.; Sabry, M.M.; Aly, Z.Y.; Hifnawy, M.S. Phytochemical and in-vivo anti-arthritic significance of Aloe thraskii Baker in combined therapy with methotrexate in adjuvant-induced arthritis in rats. Molecules 2021, 26, 3660. [Google Scholar] [CrossRef]
- Fox, L.T.; Mazumder, A.; Dwivedi, A.; Gerber, M.; du Plessis, J.; Hamman, J.H. In vitro wound healing and cytotoxic activity of the gel and whole-leaf materials from selected aloe species. J. Ethnopharmacol. 2017, 200, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pather, N.; Kramer, B. Bulbine natalensis and Bulbine frutescens promote cutaneous wound healing. J. Ethnopharmacol. 2012, 144, 523–532. [Google Scholar] [CrossRef]
- Hattingh, A.; Laux, J.-P.; Willers, C.; Hamman, J.; Steyn, D.; Hamman, H. In vitro wound healing effects of combinations of Aloe vera gel with different extracts of Bulbine frutescens. S. Afr. J. Bot. 2023, 158, 254–264. [Google Scholar] [CrossRef]
- Mongalo, N.I.; Raletsena, M.V. Fabaceae: South African Medicinal Plant Species Used in the Treatment and Management of Sexually Transmitted and Related Opportunistic Infections Associated with HIV-AIDS. Data 2023, 8, 160. [Google Scholar] [CrossRef]
- Jadhavar, P.; Deshpande, D.S. Recent Updates on Medicinal Potentiality of Fabaceae Family: Critical. Int. J. Pharm. Sci. 2022, 13, b32–b41. [Google Scholar] [CrossRef]
- Khumalo, G.P.; Van Wyk, B.E.; Feng, Y.; Cock, I.E. A review of the traditional use of southern African medicinal plants for the treatment of inflammation and inflammatory pain. J. Ethnopharmacol. 2022, 283, 114436. [Google Scholar] [CrossRef] [PubMed]
- Criollo-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Heredia, J.B. Wound healing properties of natural products: Mechanisms of action. Molecules 2023, 28, 598. [Google Scholar] [CrossRef] [PubMed]
- Pitkin, F.; Black, J.; Stedford, K.; Valentine, O.; Knott, J.; Laverdure, E. A comparative study of the antimicrobial effects of the Desmodium incanum and the Moringa oleifera extracts on select microbes. Int. J. Public Health Health Syst. 2019, 4, 27–35. [Google Scholar]
- Dumisa, M.; Aiyegoro, O.A.; Van Vuuren, S. Medicinal plant: Dye combinations—Impact on antimicrobial potency and toxicity. S. Afr. J. Bot. 2020, 135, 188–200. [Google Scholar] [CrossRef]
- Tom, I.M.; Ibrahim, M.M.; Umoru, A.M.; Umar, J.B.; Bukar, M.A.; Haruna, A.B.; Aliyu, A. Infection of wounds by potential bacterial pathogens and their resistogram. OALib 2019, 6, e5528. [Google Scholar] [CrossRef]
- Khumalo, G.P.; Van Wyk, B.-E.; Feng, Y.; Cock, I.E. Immunomodulatory and cytotoxicity properties of selected southern African medicinal plants traditionally used to treat pain and inflammation. S. Afr. J. Bot. 2023, 159, 146–154. [Google Scholar] [CrossRef]
- Wintola, O.A.; Olajuyigbe, A.A.; Afolayan, A.J.; Coopoosamy, R.M.; Olajuyigbe, O.O. Chemical composition, antioxidant activities and antibacterial activities of essential oil from Erythrina caffra Thunb. growing in South Africa. Heliyon 2021, 7, e07244. [Google Scholar] [CrossRef]
- Ateba, S.B.; Njamen, D.; Krenn, L. The genus Eriosema (Fabaceae): From the ethnopharmacology to an evidence-based phytotherapeutic perspective? Front. Pharmacol. 2021, 12, 641225. [Google Scholar] [CrossRef]
- Cock, I.E.; Van Vuuren, S.F. The traditional use of southern African medicinal plants in the treatment of viral respiratory diseases: A review of the ethnobotany and scientific evaluations. J. Ethnopharmacol. 2020, 262, 113194. [Google Scholar] [CrossRef]
- Tonisi, S.; Okaiyeto, K.; Mabinya, L.V.; Okoh, A.I. Evaluation of bioactive compounds, free radical scavenging and anticancer activities of bulb extracts of Boophone disticha from Eastern Cape Province, South Africa. Saudi J. Biol. Sci. 2020, 27, 3559–3569. [Google Scholar] [CrossRef] [PubMed]
- Cordier, W.; Steenkamp, V. Bulb extracts of Boophone disticha induce hepatotoxicity by perturbing growth, without significantly impacting cellular viability. S. Afr. J. Bot. 2018, 114, 1–8. [Google Scholar] [CrossRef]
- Pereira, C.G.; Neng, N.R.; Custódio, L. From threat to opportunity: Harnessing the invasive Carpobrotus edulis (L.) NE Br for nutritional and phytotherapeutic valorization amid seasonal and spatial variability. Marine Drugs 2023, 21, 436. [Google Scholar] [CrossRef]
- Gaire, B.P.; Subedi, L. A review on the pharmacological and toxicological aspects of Datura stramonium L. J. Integr. Med. 2013, 11, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.M.; Khan, M.; Ahmad, M.; Zafar, M.; Jahan, S.; Sultana, S. Ethnopharmacological application of medicinal plants to cure skin diseases and in folk cosmetics among the tribal communities of North-West Frontier Province, Pakistan. J. Ethnopharmacol. 2010, 128, 322–335. [Google Scholar] [CrossRef]
- Sharma, J.; Gairola, S.; Sharma, Y.P.; Gaur, R. Ethnomedicinal plants used to treat skin diseases by Tharu community of district Udham Singh Nagar, Uttarakhand, India. J. Ethnopharmacol. 2014, 158, 140–206. [Google Scholar] [CrossRef]
- Malik, K.; Ahmad, M.; Zafar, M.; Ullah, R.; Mahmood, H.M.; Parveen, B.; Rashid, N.; Sultana, S.; Shah, S.N. Lubna An ethnobotanical study of medicinal plants used to treat skin diseases in northern Pakistan. BMC Complement. Altern. Med. 2019, 19, 210. [Google Scholar] [CrossRef]
- Saikia, A.P.; Ryakala, V.K.; Sharma, P.; Goswami, P.; Bora, U. Ethnobotany of medicinal plants used by Assamese people for various skin ailments and cosmetics. J. Ethnopharmacol. 2006, 106, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.C.; Zuki, R.M.; Milow, P. Traditional knowledge of medicinal plants among the Malay villagers in Kampung Mak Kemas, Terengganu, Malaysia. Stud. Ethno-Med. 2011, 5, 175–185. [Google Scholar] [CrossRef]
- Aumeeruddy, M.Z.; Mahomoodally, M.F. Traditional herbal therapies for hypertension: A systematic review of global ethnobotanical field studies. S. Afr. J. Bot. 2020, 135, 451–464. [Google Scholar] [CrossRef]
- Tugume, P.; Kakudidi, E.K.; Buyinza, M.; Namaalwa, J.; Kamatenesi, M.; Mucunguzi, P.; Kalema, J. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central Forest Reserve, Uganda. J. Ethnobiol. Ethnomed. 2016, 12, 5. [Google Scholar] [CrossRef]
- Mkhonto, C.; Mokgehle, S.N.; Mbeng, W.O.; Ramarumo, L.J.; Ndlhovu, P.T. Review of Mimusops zeyheri Sond.(Milkwood): Distribution, Utilisation, Ecology and Population Genetics. Plants 2024, 13, 2943. [Google Scholar] [CrossRef] [PubMed]
- Meñiza, J.F.; Pasco, M.M.; Alimbon, J.A. A review of ethnobotanical studies reveals over 500 medicinal plants in Mindanao, Philippines. Plant Divers. 2024, 46, 551–564. [Google Scholar] [CrossRef]
- Yadav, S.; Mishra, A.P.; Kumar, S.; Negi, A.; Maurya, V.K. Herbal wound healing agents. In Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier: Amsterdam, The Netherlands, 2021; pp. 169–184. [Google Scholar] [CrossRef]
- Ndhlovu, P.T.; Omotayo, A.O.; Otang-Mbeng, W.; Aremu, A.O. Ethnobotanical review of plants used for the management and treatment of childhood diseases and well-being in South Africa. S. Afr. J. Bot. 2021, 137, 197–215. [Google Scholar] [CrossRef]
- Etheridge, C.J.; Derbyshire, E. Herbal infusions and health: A review of findings from human studies, mechanisms and future research directions. Nutr. Food Sci. 2020, 50, 969–985. [Google Scholar] [CrossRef]
- Gwarzo, I.D.; Bohari, S.P.M.; Wahab, R.A.; Zia, A. Recent advances and future prospects in topical creams from medicinal plants to expedite wound healing: A review. Biotechnol. Biotechnol. Equip. 2022, 36, 82–94. [Google Scholar] [CrossRef]
- Wadagni, A.C.A.; Yao, T.A.K.; Diez, G.; Balle, F.H.; Koffi, A.P.; Aoulou, P.; Zahiri, M.H.; Djossou, P.; Barogui, Y.T.; Assé, H.; et al. Community based integrated wound care: Results of a pilot formative research conducted in Benin and Côte d’Ivoire, West Africa. PLoS Global Public Health 2024, 4, e0002889. [Google Scholar] [CrossRef]
- Bitew, H.; Gebregergs, H.; Tuem, K.B.; Yeshak, M.Y. Ethiopian medicinal plants traditionally used for wound treatment: A systematic review. Ethiop. J. Health Dev. 2019, 33. Available online: https://www.ajol.info/index.php/ejhd/article/view/188853 (accessed on 20 February 2025).
- Alamgeer; Sharif, A.; Asif, H.; Younis, W.; Riaz, H.; Bukhari, I.A.; Assiri, A.M. Indigenous medicinal plants of Pakistan used to treat skin diseases: A review. Chin. Med. 2018, 13, 52. [Google Scholar] [CrossRef]
- Felhaber, T.; Mayeng, I. South African Traditional Healers’ Primary Health Care Handbook; Kagiso Publishers: Johannesburg, South Africa, 1997. [Google Scholar]
- Van Wyk, A.; Prinsloo, G. Medicinal plant harvesting, sustainability and cultivation in South Africa. Biol. Conserv. 2018, 227, 335–342. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, K. UN Meeting Highlights Antimicrobial Resistance “Epiphany”—Lack of Antibiotic Access Is a Key Driver. JAMA 2024, 332, 1776–1778. [Google Scholar] [CrossRef] [PubMed]
- Evelhoch, S.R. Biofilm and Chronic Nonhealing Wound Infections. Surg. Clin. N. Am. 2020, 100, 727–732. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. Traditional therapies for skin wound healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’alessandro, A.M. Phytochemistry and biological activity of medicinal plants in wound healing: An overview of current research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef]
- Ramírez-Zavaleta, C.Y.; García-Barrera, L.J.; Rodríguez-Verástegui, L.L.; Arrieta-Flores, D.; Gregorio-Jorge, J. An overview of PRR-and NLR-mediated immunities: Conserved signaling components across the plant kingdom that communicate both pathwaysys. Int. J. Mol. Sci. 2022, 23, 12974. [Google Scholar] [CrossRef]
- Albahri, G.; Badran, A.; Hijazi, A.; Daou, A.; Baydoun, E.; Nasser, M.; Merah, O. The therapeutic wound healing bioactivities of various medicinal plants. Life 2023, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Khanna, S.; Kaur, G.; Singh, I. Medicinal plants and their components for wound healing applications. Future J. Pharm. Sci. 2021, 7, 53. [Google Scholar] [CrossRef]
- Hekmatpou, D.; Mehrabi, F.; Rahzani, K.; Aminiyan, A. The effect of aloe vera clinical trials on prevention and healing of skin wound: A systematic review. Iran. J. Med. Sci. 2019, 44, 1–9. [Google Scholar]


| Family | Scientific Name of Plant Species | Method of Preparation | Plant Parts Used | Route of Administration | References | |
|---|---|---|---|---|---|---|
| 1 | Asteraceae | Acanthospermum hispidum DC. | Maceration | Leaves | Topical | [23] |
| 2 | Asteraceae | Acmella caulirhiza Delile | Crushed | Leaves | Topical, wash | [35] |
| 3 | Apocynaceae | Acokanthera oppositifolia (Lam.) Codd | Paste Not reported Decoction | Leaves, stem Not reported Leaves | Topical Not reported Topical | [29,44,47] |
| 4 | Lamiaceae | Acrotome inflata Benth. | Not reported | Rhizome | Not reported | [9] |
| 5 | Malvaceae | Adansonia digitata L. | Maceration | Bark | Topical | [23] |
| 6 | Asteraceae | Afroaster hispidus (Thunb.) J.C. Manning & Goldblatt | Not reported | Not reported | Not reported | [29] |
| 7 | Fabaceae | Afzelia quanzensis Welw | Infusion | Bark, root | Oral | [34] |
| 8 | Rosaceae | Agrimonia eupatoria L. | Decoction | Leaves | Topical | [44] |
| 9 | Hyacinthaceae | Albuca bracteata (Thunb.) J.C. Manning & Goldblatt | Not reported | Not reported | Not reported | [29] |
| 10 | Hyacinthaceae | Albuca setosa Jacq. | Paste | Leaves | Topical | [44] |
| 11 | Hyacinthaceae | Albuca virens (Lindl.) J.C. Manning & Goldblatt subsp. virens | Not reported | Not reported | Not reported | [29] |
| 12 | Apiaceae | Alepidea amatymbica Eckl. & Zeyh. | Maceration, decoction Infusion | Roots, rhizome Roots | Oral, topical Not reported | [34] [40] |
| 13 | Asphodelaceae | Aloe aageodonta L.E.Newton | Poultice, paste, gel | Leaves | Topical | [23] |
| 14 | Asphodelaceae | Aloe arborescens Mill. | Paste, gel Not reported | Leaves Not reported | Topical Not reported | [8] [29] |
| 15 | Asphodelaceae | Aloiampelos striatula (Haw.) | Poultice | Leaves | Topical | [27] |
| 16 | Asphodelaceae | Aristaloe aristata (Haw.) Boatwr. & J.C.Manning | Infusion | Leaves | Wash | [34] |
| 17 | Asphodelaceae | Aloe vera (L.) Burm. f. | Sap | Leaves | Topical | [35] |
| 18 | Asphodelaceae | Aloe ferox Mill. | Not reported Gel Not reported Decoction Not reported Juice Not reported | Not reported Leaves Leaves Leaves Not reported Leaves Not reported | Not reported Topical Topical Oral Not reported Topical Not reported | [29] [8] [44] [34] [43] [27] [36] |
| 19 | Asphodelaceae | Aloe maculata All. | Not reported Infusion, maceration | Not reported Leaves, stem, and rhizome | Not reported Oral, topical | [29] [33] |
| 20 | Asphodelaceae | Aloe marlothii A. Berger | Poultice Infusion | Leaves Leaves | Topical Topical | [23] [39] |
| 21 | Asphodelaceae | Aloe microstigma Salm-Dyck | Juice Heated | Leaves Not reported | Topical, wash Topical | [43] [30] |
| 22 | Asphodelaceae | Aloe thraskii Baker | Not reported | Not reported | Not reported | [29] |
| 23 | Anacampserotaceae | Anacampseros papyracea E. Mey. ex Fenzl subsp. papyracea | Not reported | Not reported | Not reported | [36] |
| 24 | Commelinaceae | Aneilema aequinoctiale (P.Beauv.) Loudon | Not reported | Not reported | Not reported | [29] |
| 25 | Scrophulariaceae | Aptosimum indivisum Burch. ex Benth. | Powdered | Leaves | Topical | [36] |
| 26 | Scrophulariaceae | Aptosimum procumbens (Lehm.) Burch. ex Steud. | Powdered ash Not reported Not reported | Whole plant Foliage Not reported | Topical Topical Not reported | [28] [24] [36] |
| 27 | Asteraceae | Arctotis arctotoides (L.f) O.Hoffm | Paste Juice | Leaves Leaves | Topical Topical | [38] [44] |
| 28 | Asteraceae | Artemisia afra Jacq. ex. Willd. | Infusion | Leaves | Wash | [27] |
| 29 | Asparagaceae | Asparagus africanus Lam. | Infusion Decoction | Leaves Roots | Topical Oral | [44] [34] |
| 30 | Asteraceae | Athrixia phylicoides DC. | Crushing | Leaf twigs, roots | Topical | [8] |
| 31 | Iridaceae | Babiana hypogaea Burch | Not reported | Leaves | Not reported | [9] |
| 32 | Lamiaceae | Ballota africana (L.) Benth. | Infusion Infusion | Leaves Leaves | Topical, wash Wash | [43] [36] |
| 33 | Acanthaceae | Barleria obtusa Nees | Paste | Leaves | Topical | [44] |
| 34 | Acanthaceae | Barleria sp. | Not reported | Roots | Not reported | [9] |
| 35 | Acanthaceae | Barleria macrostegia Nees | Not reported | Roots | Not reported | [9] |
| 36 | Fabaceae | Bauhinia thonningii (Schumach.) Milne-Redh. | Juice | Fruit | Topical | [35] |
| 37 | Apiaceae | Berula erecta (Huds.) Coville subsp. thunbergii (DC.) | Not reported | whole plant | Topical | [28] |
| 38 | Asteraceae | Bidens pilosa L. | Poultice Crushed Not reported | Leaves Leaves Not reported | Topical Topical Not reported | [23] [35] [29] |
| 39 | Amaryllidaceae | Boophone disticha (L.f.) Herb. | Crushing Paste Not reported Not reported Poultice Poultice | Bulb and leaves Bulb Bulb Dry bulb Bulb and leaves Bulb and leaves | Topical Topical Topical Topical Topical Topical | [8] [27] [24] [28] [37] [36] |
| 40 | Asteraceae | Brachylaena discolor DC. | Infusion | Roots | Topical | [44] |
| 41 | Ochnaceae | Brackenridgea zanguebarica Oliv. | Maceration | Bark | Topical | [23] |
| 42 | Amaryllidaceae | Brunsvigia grandiflora Lindl. | Crushed | Leaves, Bulb | Topical | [47] |
| 43 | Asphodelaceae | Bulbine asphodeloides (L.) Spreng. | Paste, gel Not reported | Leaves Not reported | Topical Not reported | [8] [29] |
| 44 | Asphodelaceae | Bulbine foleyi E. Phillips | Not reported | Foliage | Topical | [24] |
| 45 | Asphodelaceae | Bulbine frutescens (L.) Willd. | Juice, gel Juice Juice Juice | Leaves Leaves Leaves Leaves | Topical Topical Not reported Topical | [8] [38] [43] [36] |
| 46 | Asphodelaceae | Bulbine lagopus (Thunb.) N.E.Br. | Juice | Leaves | Topical | [46] |
| 47 | Asphodelaceae | Bulbine latifolia (L.f.) Spreng. (=Bulbine natalensis Baker) | Poultice Not reported | Leaves Not reported | Topical Not reported | [27] [29] |
| 48 | Cannaceae | Canna indica L. | Poultice | Leaves | Topical | [36] |
| 49 | Capparaceae | Capparis tomentosa Lam. | Powder | Roots | Topical | [44] |
| 50 | Apocynaceae | Carissa edulis (Forssk.) Vahl. | Infusion | Leaves | Wash | [39] |
| 51 | Aizoaceae | Carpobrotus edulis (L.) L.Bolus subsp. Parviflorus Wisura & Glen. | Lotion Not reported Juice Not reported Not reported Not reported | Leaves Leaves Leaves Foliage Not reported Not reported | Oral, topical Topical Topical Topical Not reported Topical | [34] [44] [8] [24] [43] [30] |
| 52 | Apiaceae | Centella asiatica (L.) Urb | Not reported Not reported Tinctures Poultice, lotion | Whole plant Not reported Leaves Leaves | Not reported Not reported Topical Topical | [9] [29] [44] [27] |
| 53 | Asteraceae | Centaurea benedicta (L.) L. | Infusion | Whole plant | Oral | [8] |
| 54 | Asteraceae | Centaurea scabiosa L. | Infusion | Whole plant | Topical | [33] |
| 55 | Asteraceae | Vernonia spp. | Lotion | Whole plant | Topical | [42] |
| 56 | Celtidaceae | Chaetachme aristata Planch. | Burned ash | Bark | Topical | [45] |
| 57 | Pteridaceae | Cheilanthes viridis (Forssk.) Sw. var. viridis | Powder | Fronds | Topical | [27] |
| 58 | Gentianaceae | Chironia baccifera L. | Infusion | Whole plant | Not reported | [36] |
| 59 | Menispermaceae | Cissampelos capensis L.f. | Poultice | Leaves | Topical | [36] |
| 60 | Euphorbiaceae | Clutia ovalis Sond. | Not reported | Not reported | Not reported | [29] |
| 61 | Nyctaginaceae | Commicarpus pentandrus (Burch) Heimerl | Not reported | Whole plant | Not reported | [9] |
| 62 | Burseraceae | Commiphora mollis (Oliv.) Engl. | Maceration | Bark | Topical | [23] |
| 63 | Burseraceae | Commiphora harveyi (Engl.) Engl. | Not reported | Not reported | Not reported | [29] |
| 64 | Crassulaceae | Cotyledon orbiculata L. | Poultice Ointment Poultice | Leaves and cuticle Leaves Leaves | Topical Topical Topical | [27] [30] [43] |
| 65 | Amaryllidaceae | Crossyne guttata (L.) D.Müll.-Doblies & U.Müll.-Doblies | Not reported | Bulb | Topical | [24] |
| 66 | Poaceae | Cynodon dactylon (L.) Pers. | Burnt, ash powder | Whole plant | Topical | [14] |
| 67 | Fumariaceae | Cysticapnos vesicaria (L.) Fedde subsp. vesicaria | Not reported | Not reported | Wash | [36] |
| 68 | Amaryllidaceae | Cyrtanthus obliquus (L.f.) Aiton | Burned Ash | Dry or fresh roots | Topical | [47] |
| 69 | Thymelaeaceae | Dais cotinifolia L. | Not reported | Not reported | Not reported | [29] |
| 70 | Solanaceae | Datura stramonium L. | Heating Heating Paste Poultice, ointment Poultice | Leaves Leaves Seeds Leaves, seeds Leaves | Topical Topical Topical Topical Topical | [44] [8] [37] [43] [36] |
| 71 | Fabaceae | Desmodium incanum (Sw.) DC. | Not reported | Not reported | Not reported | [29] |
| 72 | Fabaceae | Desmodium setigerum (E.Mey.) Benth. ex Harv. | Not reported | Not reported | Not reported | [29] |
| 73 | Fabaceae | Dichrostachys cinerea (L.) Wight & Arn | Poultice Burned, decoction Decoction, infusion | Leaves Fruit, Bark Roots, Pods | Topical Topical, wash Not reported | [23] [35] [42] |
| 74 | Asteraceae | Dicoma anomala Sond. | Not reported | Roots | Not reported | [9] |
| 75 | Dioscoreaceae | Dioscorea elephantipes (L’Her.) Engl. | Infusion, Decoction, Lotion | Whole plant | Topical | [34] |
| 76 | Dioscoreaceae | Dioscorea sylvatica Eckl. | Infusion | Tuber | Topical | [34] |
| 77 | Ebenaceae | Diospyros lycioides Desf. | Juice | Fruit | Wash | [35] |
| 78 | Asparagaceae | Drimia species | Poultice | Bulb scales | Topical | [46] |
| 79 | Boraginaceae | Ehretia rigida (Thunb.) Druce | Poultice | Leaves | Topical | [23] |
| 80 | Meliaceae | Ekebergia capensis Sparrm. | Maceration, poultice | Leaves | Topical | [23] |
| 81 | Fabaceae | Elephantorrhiza elephantina (Burch) Skeels. | Maceration, poultice Decoction, ointment | Roots Roots | Oral, topical Oral, topical | [33] [34] |
| 82 | Asteraceae | Elytropappus rhinocerotis (L.f.) Less. | Infusion Burned ash | Leaves Aerial parts | Wash Not reported | [46] [36] |
| 83 | Musaceae | Ensete ventricosum (Welw.) E.E. Cheesman | Poultice, lotion | Leaves | Topical | [23] |
| 84 | Fabaceae | Eriosema cordatum E.Mey. | Not reported | Not reported | Not reported | [29] |
| 85 | Fabaceae | Eriosema distinctum N.E.Br. | Not reported | Not reported | Not reported | [29] |
| 86 | Fabaceae | Erythrina caffra Thunb. | Powder | Bark | Topical | [27] |
| 87 | Fabaceae | Erythrina latissima E.Mey. | Not reported | Not reported | Not reported | [29] |
| 88 | Fabaceae | Erythrina lysistemon Hutch. | Powder, Decoction | Bark | Topical, Oral | [44] |
| 89 | Myrtaceae | Eucalyptus globulus Labill. subsp. maidenii (F.Muell.) J.B.Kirkp. | Not reported | Foliage | Wash | [24] |
| 90 | Ebenaceae | Euclea divinorum Hiern | Poultice | Leaves | Topical | [23] |
| 91 | Asparagaceae | Eucomis autumnalis (Mill). Chitt | Decoction Not reported Crushed | Bulbs, roots Not reported Bulb | Oral Not reported Topical | [34] [29] [8] |
| 92 | Asparagaceae | Eucomis bicolor Baker. | Decoctions, infusion | Bulbs | Oral | [34] |
| 93 | Myrtaceae | Eugenia capensis subsp. natalitia (Sond.) F.White | Maceration (Bark) Infusion (Roots) | Bark, roots | Topical, wash | [23] |
| 94 | Euphorbiaceae | Euphorbia bupleurifolia Jacq. | Not reported | Latex | Topical | [44] |
| 95 | Euphorbiaceae | Euphorbia cupularis Boiss. | Not reported | Not reported | Not reported | [29] |
| 96 | Euphorbiaceae | Euphorbia inaequilatera Sond. | Not reported | Roots | Not reported | [9] |
| 97 | Euphorbiaceae | Euphorbia tirucalli L. | Not reported | Leaves | Not reported | [41] |
| 98 | Aizoaceae | Galenia africana L. | Decoction Infusion, ointment | Not reported Leaves, twigs | Wash Wash, topical | [28] [43] |
| 99 | Asteraceae | Gerbera piloselloides (L.) Cass. | Not reported Infusion | Not reported Roots | Not reported Topical | [29] [8] |
| 100 | Colchicaceae | Gloriosa superba L. | Not reported | Not reported | Not reported | [29] |
| 101 | Thymelaeaceae | Gnidia anthylloides (L.f.) Gilg | Burnt and crushed | Roots | Topical | [8] |
| 102 | Thymelaeaceae | Gnidia capitata (L.f.) Burtt Davy | Decoction, burnt, and crushed | Roots | Topical, wash | [8] |
| 103 | Thymelaeaceae | Gnidia kraussiana Meisn. | Not reported | Leaves | Topical | [44] |
| 104 | Apocynaceae | Gomphocarpus physocarpus E.Mey. | Not reported | Not reported | Not reported | [29] |
| 105 | Apocynaceae | Gomphocarpus fruticosus (L) Aiton.f. | Not reported | Whole plant | Not reported | [9] |
| 106 | Asphodelaceae | Gonialoe variegata (L.) Boatwr. & J.C.Manning (=Aloe variegata L.) | Poultice Poultice Poultice Not reported | Leaves Leaves Leaves Not reported | Topical Topical Topical Not reported | [43] [36] [30] [28] |
| 107 | Malvaceae | Grewia occidentalis L. | Infusion, lotion | Small twigs and leaves | Topical | [27] |
| 108 | Gunneraceae | Gunnera perpensa L. | Poultice Decoction | Leaves Rhizomes | Topical Oral | [27] [8] |
| 109 | Celastraceae | Gymnosporia buxifolia (L.) Szyszyl. | Infusion | Leaves, Roots | Not reported | [42] |
| 110 | Celastraceae | Gymnosporia rubra (Harv.) Loes. | Not reported | Not reported | Not reported | [29] |
| 111 | Amaryllidaceae | Haemanthus albiflos Jacq. | Paste Infusion Decoction | Leaves Roots Bulb | Topical Oral Oral | [44] [34] [38] |
| 112 | Amaryllidaceae | Haemanthus coccineus L. | Poultice | Leaves | Topical | [27] |
| 113 | Asteraceae | Haplocarpha scaposa Harv. | Paste | Leaves | Topical, wash | [44] |
| 114 | Anacardiaceae | Harpephyllum caffrum Bernh. | Decoction Decoction | Bark Bark | Topical Oral, wash | [44] [8] |
| 115 | Asteraceae | Helichrysum pedunculatum Hillard & B.LBurtt | Not reported Juice | Leaves Leaves | Topical Topical | [47] [27] |
| 116 | Asteraceae | Helichrysum appendiculatum (L.f.) Less. | Poultice and infusion | Leaves | Topical | [27] |
| 117 | Asteraceae | Helichrysum aureonitens Sch.Bip | Infusion, lotion | Leaves | Topical, wash | [27] |
| 118 | Asteraceae | Helichrysum odoratissimum (L.) Sweet. | Infusion Poultice | Leaves Not reported | Topical Topical | [44] [43] |
| 119 | Asteraceae | Helichrysum petiolare Hilliard & B.L.Burtt | Decoction | Leaves | Oral, steam | [8] |
| 120 | Asteraceae | Helichrysum nudifolium (L.) Less. | Poultice, powder | Leaves, Twigs | Topical, steam | [8] |
| 121 | Malvaceae | Hermannia cuneifolia Jacq. | Poultice | Not reported | Wash | [36] |
| 122 | Malvaceae | Hermannia depressa N.E.Br | Not reported | Root | Topical | [34] |
| 123 | Apiaceae | Heteromorpha arborescens (Spreng.) Cham. & Schltdl. | Paste | Leaves | Topical | [35] |
| 124 | Hypoxidaceae | Hypoxis hemerocallidea Fisch., C.A.Mey. & Avé-Lall. | Not reported Not reported Juice, lotion, powder, infusion | Not reported Bulb Leaves and corms | Not reported Not reported Topical, wash | [29] [9] [27] |
| 125 | Hypoxidaceae | Hypoxis rigidula Baker | Not reported | Not reported | Not reported | [29] |
| 126 | Euphorbiaceae | Jatropha curcas L. | Infusion, Crushed | Roots | Topical, wash | [35] |
| 127 | Euphorbiaceae | Jatropha zeyheri Sond. | Paste | leaves | Topical | [35] |
| 128 | Juncaceae | Juncus lomatophyllus L. | Decoction | Leaves | Topical | [47] |
| 129 | Asphodelaceae | Kniphofia drepanophylla (Baker) | Powder, infusion | Rhizomes | Topical | [8] |
| 130 | Anacardiaceae | Lannea schweinfurthii var. stuhlmannii (Engl.) Kokwaro | Crushed | Leaves | Topical | [35] |
| 131 | Lamiaceae | Leonotis leonurus (L.) R.Br. | Infusion Not reported | Leaves Not reported | Oral Not reported | [8] [36] |
| 132 | Fabaceae | Lessertia frutescens (L.) Goldblatt & J.C.Manning subsp. Frutescens = Sutherlandia frutescens (L.) R. Br | Infusion Infusion | Leaves Leaves | Topical Topical | [8] [43] |
| 133 | Verbenaceae | Lippia javanica (Burm.f.) Spreng. | Poultice, maceration Infusion Not reported | Leaves Leaves Not reported | Topical Topical Not reported | [23] [44] [29] |
| 134 | Boraginaceae | Lobostemon paniculatus (Thunb.) H.Buek | Powder, poultice | Leaves | Topical | [43] |
| 135 | Lycopodiaceae | Lycopodium clavatum L. | Powder, decoction | Whole plant | Topical, oral | [34] |
| 136 | Euphorbiaceae | Macaranga capensis (Baill.) Sim | Paste, decoction | Bark | Topical, oral | [8] |
| 137 | Malvaceae | Malva parviflora L.var. parviflora | Paste Poultice | Leaves Not reported | Topical Not reported | [44] [36] |
| 138 | Melianthaceae | Melianthus comosus Vahl. | Decoction Not reported | Leaves Not reported | Wash Wash | [46] [36] |
| 139 | Melianthaceae | Melianthus major L. | Poultice | Foliage | Topical | [24] |
| 140 | Melianthaceae | Melianthus pectinatus Harv. | Poultice | Leaves | Topical | [43] |
| 141 | Lamiaceae | Mentha longifolia (L) L. | Poultice, lotion, infusion | Leaves | Topical | [27] |
| 142 | Hyacinthaceae | Merwilla plumbea (Lindl.) Speta | Powder, decoction, infusion | Bulbs | Topical, oral | [34] |
| 143 | Poaceae | Miscanthus capensis (Nees) Andersson | Decoction | Roots | Steam | [8] |
| 144 | Musaceae | Musa acuminata Colla | Not reported Not reported | Flowers, leaves Not reported | Not reported Not reported | [35] [29] |
| 145 | Musaceae | Musa x paradisiaca L. | Poultice | Leaves | Topical | [23] |
| 146 | Solanaceae | Nicotiana glauca Graham | Poultice Poultice | Leaves Not reported | Topical Topical | [43] [36] |
| 147 | Solanaceae | Nicotiana tabacum L. | Paste | Leaves | Topical | [44] |
| 148 | Ranunculaceae | Nigella sativa L. | Poultice | Whole plant | Oral, Topical | [33] |
| 149 | Scrophulariaceae | Nemesia fruticans (Thumb.) Benth. | Not reported | Not reported | Not reported | [36] |
| 150 | Cactaceae | Opuntia vulgaris Mill. | Poultice | Stems | Topical | [47] |
| 151 | Asteraceae | Osteospermum calendulaceum L.f. | Ointment | Not reported | Wash | [36] |
| 152 | Asteraceae | Osteospermum herbaceum L.f. | Poultice, infusion | Not reported | Topical | [28] |
| 153 | Anacardiaceae | Ozoroa sphaerocarpa R.Fern. & A.Fern. | Decoction, infusion | Whole plant | Not reported | [42] |
| 154 | Chrysobalanaceae | Parinari curatellifolia Planch. ex Benth. | Decoction, poultice | Leaves | Topical | [23] |
| 155 | Parmeliaceae | Parmelia species | Poultice Ointment | Not reported Not reported | Topical Topical | [43] [30] |
| 156 | Geraniaceae | Pelargonium antidysentericum (Eckl. & Zeyh.) Kostel. | Not reported | Not reported | Not reported | [30] |
| 157 | Geraniaceae | Pelargonium grossularioides (L.) L’Hér. | Powdered | Leaves | Topical | [36] |
| 158 | Geraniaceae | Pelargonium luridum (Andrews) Sweet | Not reported | Roots | Not reported | [9] |
| 159 | Geraniaceae | Pelargonium peltatum (L.) L’Hér. | Poultice | Leaves | Topical | [27] |
| 160 | Rubiaceae | Pentanisia prunelloides (Klotzsch ex Eckl. & Zeyh.) Walp. | Decoction Decoction | Root Root | Topical Wash | [34] [8] |
| 161 | Polygonaceae | Persicaria lapathifolia (L.) Delarbre | Not reported | Not reported | Not reported | [29] |
| 162 | Solanaceae | Physalis angulata L. | Paste | Leaves | Topical | [44] |
| 163 | Piperaceae | Piper capense L.f. | Maceration | Bark | Topical, Oral | [23] |
| 164 | Plantaginaceae | Plantago lanceolata L. | Decoction Poultice | Leaves Not reported | Topical Topical | [38] [36] |
| 165 | Polypodiaceae | Polystichum pungens (Kaulf.) C.Presl | Powder | Fronds | Topical | [27] |
| 166 | Didiereaceae | Portulacaria afra Jacq. | Poultice | Not reported | Not reported | [36] |
| 167 | Urticaceae | Pouzolzia mixta Solms | Powder | Roots | Topical | [35] |
| 168 | Anacardiaceae | Protorhus longifolia (Bernh.) Engl. | Decoction | Bark | Wash | [8] |
| 169 | Rosaceae | Prunus persica (L.) Batsch | Maceration | Bark | Topical | [23] |
| 170 | Rutaceae | Ptaeroxylon obliquum (Thunb.) Radlk. | Not reported | Not reported | Not reported | [29] |
| 171 | Icacinaceae | Pyrenacantha kaurabassana Baill. | Powder | Root tuber | Topical | [32] |
| 172 | Malvaceae | Radyera urens (L.f.) Bullock | Poultice | Leaves | Topical | [43] |
| 173 | Euphorbiaceae | Ricinus communis L. | Ointment, poultice Poultice Not reported Poultice | Seeds Roots and leaves Leaves Leaves | Topical Topical Not reported Topical | [43] [27] [41] [36] |
| 174 | Polygonaceae | Rumex crispus L. | Poultice | Leaves | Topical | [36] |
| 175 | Polygonaceae | Rumex lanceolatus Thunb. | Decoction | Roots, leaves | Wash | [8] |
| 176 | Celastraceae | Salacia rehmannii Schinz | Maceration | Bark | Topical | [23] |
| 177 | Asparagaceae | Sansevieria cylindrica Bojer ex Hook. | Not reported | Not reported | Not reported | [29] |
| 178 | Caprifoliaceae | Scabiosa columbaria L. | Ointment Powder | Roots Roots, leaves | Topical Topical | [27] [8] |
| 179 | Amaryllidaceae | Scadoxus multiflorus (Martyn) Raf. subsp. katharinae | Infusion | Bulb | Topical | [44] |
| 180 | Anacardiaceae | Schinus molle L. | Poultice Not reported | Leaves Foliage | Topical Topical | [43] [24] |
| 181 | Asparagaceae | Scilla nervosa (Burch.) Van der Merwe | Ointment | Bulb | Topical | [44] |
| 182 | Anacardiaceae | Sclerocarya birrea (A. Rich.) Hochst. | Burned Not reported | Stem Bark | Topical Not reported | [35] [15] |
| 183 | Anacardiaceae | Searsia lancea (L.f.) F.A. Barkley | Poultice, paste | Leaves | Topical | [23] |
| 184 | Asteraceae | Senecio cinerascens Aiton | Poultice | Leaves | Topical | [43] |
| 185 | Asteraceae | Senecio deltoideus Less. | Lotion | Leaves | Topical | [44] |
| 186 | Asteraceae | Senecio speciosus Willd. | Decoction, paste | Leaves, stems | Topical, steam | [8] |
| 187 | Fabaceae | Senna obtusifolia (L.) H.S.Irwin & Barneby | Poultice | Leaves | Topical, wash | [23] |
| 188 | Fabaceae | Senna occidentalis (L.) Link | Paste | Leaves | Topical | [35] |
| 189 | Malvaceae | Sida cordifolia L. | Burned ash | Roots | Topical | [35] |
| 190 | Solanaceae | Solanum aculeastrum Dunal | Not reported | Not reported | Not reported | [29] |
| 191 | Solanaceae | Solanum incanum L. | Paste Not reported | Roots Not reported | Topical Not reported | [8] [29] |
| 192 | Solanaceae | Solanum lichtensteinii Willd. | Not reported | Whole plant | Not reported | [9] |
| 193 | Solanaceae | Solanum nigrum L. | Infusion | Leaves | Wash | [27] |
| 194 | Solanaceae | Solanum panduriforme Drège ex Dunal | Juice | Fruit | Topical | [35] |
| 195 | Solanaceae | Solanum tomentosum L. | Not reported | Not reported | Not reported | [30] |
| 196 | Euphorbiaceae | Spirostachys africana Sond. | Infusion Not reported Powder (solution) | Bark Not reported Bark | Not reported Not reported Wash | [45] [29] [8] |
| 197 | Orobanchaceae | Striga asiatica (L) Kuntze | Maceration, paste Burned Burned | Roots Whole plant Whole plant | Topical Topical Topical | [23] [13] [35] |
| 198 | Loganiaceae | Strychnos aculeata Soler. | Maceration, lotion | Roots | Topical | [23] |
| 199 | Loganiaceae | Strychnos decussata (Pappe) Gilg | Not reported | Not reported | Not reported | [29] |
| 200 | Loganiaceae | Strychnos henningsii Gilg | Not reported | Not reported | Not reported | [29] |
| 201 | Myrtaceae | Syzygium cordatum Hochst. ex Krauss | Not reported Decoction | Bark Leaves, stems | Not reported Topical | [15] [45] |
| 202 | Apocynaceae | Tabernaemontana elegans Stapf | Maceration, lotion Not reported | Roots Latex (leaf) | Topical Topical | [23] [45] |
| 203 | Combretaceae | Terminalia sericea Burch. ex DC | Poultice, maceration | Leaves | Topical | [23] |
| 204 | Lamiaceae | Tetradenia riparia (Hochst.) Codd | Not reported | Not reported | Not reported | [29] |
| 205 | Santalaceae | Thesium strictum P.J.Bergius | Paste | Leaves | Topical | [44] |
| 206 | Commelinaceae | Tradescantia pallida (Rose) D.R. Hunt | Not reported | Not reported | Not reported | [29] |
| 207 | Meliaceae | Trichilia emetica Vahl | Poultice | Leaves | Topical | [23] |
| 208 | Alliaceae | Tulbaghia alliacea L.f. | Juice | Bulb | Topical | [8] |
| 209 | Crassulaceae | Tylecodon wallichii (Harv.) Toelken | Poultice | Leaves | Topical | [43] |
| 210 | Typhaceae | Typha capensis (Rohrb.) N.E.Br. | Infusion | Root and lower stem | Wash | [27] |
| 211 | Urticaceae | Urtica urens L. | Infusion | Leaves | Topical | [38] |
| 212 | Fabaceae | Vachellia nilotica subsp. kraussiana (Benth.) Kyal. & Boatwr. | Decoction | Roots | Not reported | [42] |
| 213 | Rubiaceae | Vangueria infausta Burch. | Decoction | Roots | Not reported | [42] |
| 214 | Rutaceae | Vepris lanceolata (Lam) G.Don | Not reported | Not reported | Not reported | [29] |
| 215 | Asteraceae | Vernonia oligocephala | Infusion, lotion | Leaves and stems | Topical | [27] |
| 216 | Solanaceae | Withania somnifera (L.) Dunal | Paste Poultice Poultice | Leaves, roots Leaves Not reported | Topical Topical Not reported | [8] [27] [36] |
| 217 | Olacaceae | Ximenia caffra Sond. | Maceration, poultice | Roots | Topical | [23] |
| 218 | Apocynaceae | Xysmalobium undulatum (L.) Aiton f. | Powder | Roots | Topical | [34] |
| 219 | Araceae | Zantedeschia aethiopica (L.) Spreng. | Poultice, heated Powder Poultice | Leaves Rhizome Leaves | Topical Topical Topical | [27] [44] [36] |
| 220 | Rutaceae | Zanthoxylum davyi (I.Verd.) P.G.Waterman | Crushed | Roots, leaves | Topical | [35] |
| 221 | Poaceae | Zea mays L. | Paste | Leaves | Topical | [44] |
| 222 | Rhamnaceae | Ziziphus mucronata Willd. subsp. mucronata | Poultice | Leaves | Topical | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher, F.; Africa, C.; Klaasen, J.; Fisher, R. South African Medicinal Plants Traditionally Used for Wound Treatment: An Ethnobotanical Systematic Review. Plants 2025, 14, 818. https://doi.org/10.3390/plants14050818
Fisher F, Africa C, Klaasen J, Fisher R. South African Medicinal Plants Traditionally Used for Wound Treatment: An Ethnobotanical Systematic Review. Plants. 2025; 14(5):818. https://doi.org/10.3390/plants14050818
Chicago/Turabian StyleFisher (née Rahiman), Farzana, Charlene Africa, Jeremy Klaasen, and Randall Fisher. 2025. "South African Medicinal Plants Traditionally Used for Wound Treatment: An Ethnobotanical Systematic Review" Plants 14, no. 5: 818. https://doi.org/10.3390/plants14050818
APA StyleFisher, F., Africa, C., Klaasen, J., & Fisher, R. (2025). South African Medicinal Plants Traditionally Used for Wound Treatment: An Ethnobotanical Systematic Review. Plants, 14(5), 818. https://doi.org/10.3390/plants14050818







