Abstract
Medicago ruthenica is a forage legume crop that is widely used as fodder and for ecological restoration in arid and semi-arid areas in Northcentral Asia. During the seedling stage, weeds challenge the growth and development of M. ruthenica, especially in fields sown for seed production. However, strategies to effectively control weeds in crops of M. ruthenica using herbicides have not been investigated. We evaluate the efficacy of different herbicides that control pre- and post-emergence of weeds in M. ruthenica. The results indicated that the most effective pre-emergence herbicides, imazethapyr (1530 mL ha−1) and flumetsulam (120 mL ha−1), resulted in crop safety and soil microbial community equivalent to a weed-free check. The most effective post-emergence herbicides are imazethapyr + haloxyfop-P (1800 + 600 mL ha−1) and 2,4-DB + haloxyfop-P (2250 + 600 mL ha−1). These herbicide treatments demonstrate effective control of most weeds (A. retroflexus, C. album, and grasses) while ensuring crop safety. Application of these herbicides to control weeds in M. ruthenica prior to or after their emergence represents a viable strategy for their control and also improve agricultural viability and crop yield and quality. Our research contributes to sustainable agriculture and ecological restoration in arid regions.
1. Introduction
Medicago ruthenica, a diploid (2n = 2x = 16) perennial legume [1], is an important forage species that is widely distributed in areas of northern China, Siberia, and Mongolia [2,3]. M. ruthenica is a perennial forage species in natural grasslands and is also used for ecological modification because of its tolerance to cold and drought stress, especially on the Mongolian plateau [4,5]. Continued demand for increased livestock production and environmental protection in China has increased the demand for commercial M. ruthenica seed. However, seed production in the field is significantly restricted by weed competition [6] (about 70% to 80% decrease in M. ruthenica), and also, there is a high cost to purify seed due to weed seed contamination [6]. Weeds competition is a major limiting factor for seed production of M. ruthenica.
Weeds are a global problem that influences crop growth and development [7]. With the increased mechanization of modern agriculture production, controlling weeds is also becoming increasingly important [7]. The use of herbicides to control weeds is one strategy [8] that is more efficient, reliable, and cost-effective than traditional manual and mechanical weeding [9]. Herbicides are typically applied to act on weeds prior to or following their emergence [10]. The application of pre-emergence herbicides to soil reduces weed populations by inhibiting seed germination for 0–30 d [11]. Post-emergence herbicides are applied to secondary weed infestations at the 2–4 leaf stages [12]. To obtain desired results, accurate weed identification and an understanding of their life histories are essential [13]. Effective control is best achieved through the application of an appropriate herbicide at an appropriate time and dosage, using an appropriate application method [14]. Because legume forage develops slowly after sowing, weed grasses and broadleaf weeds can outcompete it [15]. This reduces legume yield and quality [16], mainly through failed crop establishment, reduced production, and increased costs associated with harvesting and purifying [17,18]. For example, weed infestation can reduce alfalfa (Medicago sativa) seed yield by 34–48% [19] and decrease forage yield by about 45% [20]. Pre- and post-emergent herbicidal control of weeds can positively affect legume crops [21]. Saflufenacil temporarily injured broadleaf plantain (Plantago major) and ribwort plantain (Plantago lanceolata) and had few negative impacts on alfalfa in the field [22]. The use of imazethapyr as a pre- and post-emergent herbicide effectively controlled weeds and improved the yield of Egyptian clover (Trifolium alexandrinum) [23]. The efficacy of and degree to which pre- and post-emergence herbicides injure legume crop species varies greatly [21].
Compared with alfalfa, M. ruthenica is slower growing, indicating that weeds can outcompete it for soil water, nutrients, or light resources, especially during early growth stages (0–60 days). This also poses challenges for seed field establishment. However, no information is available on chemical weed control in M. ruthenica. In this study, we evaluated the selectivity and sensitivity of pre- and post-emergent herbicides in controlling weeds in M. ruthenica experimental fields. To our knowledge, this is the first study on chemical weed selectivity and sensitivity for M. ruthenica.
2. Results
2.1. Weed Species and Their Density
Prevalent annual broadleaf and grass weed species in M. ruthenica control plots are detailed in Supplementary Table S1. Seven annual weed species in five families occurred in experimental plots over the two years (Supplementary Table S1); most were annual broad-leafed species, of which Chenopodium album (40.64–44.60%) and Amaranthus retroflexus (27.23–28.40%) dominated.
2.2. Pre-Emergence Herbicide Weed Control Efficiency
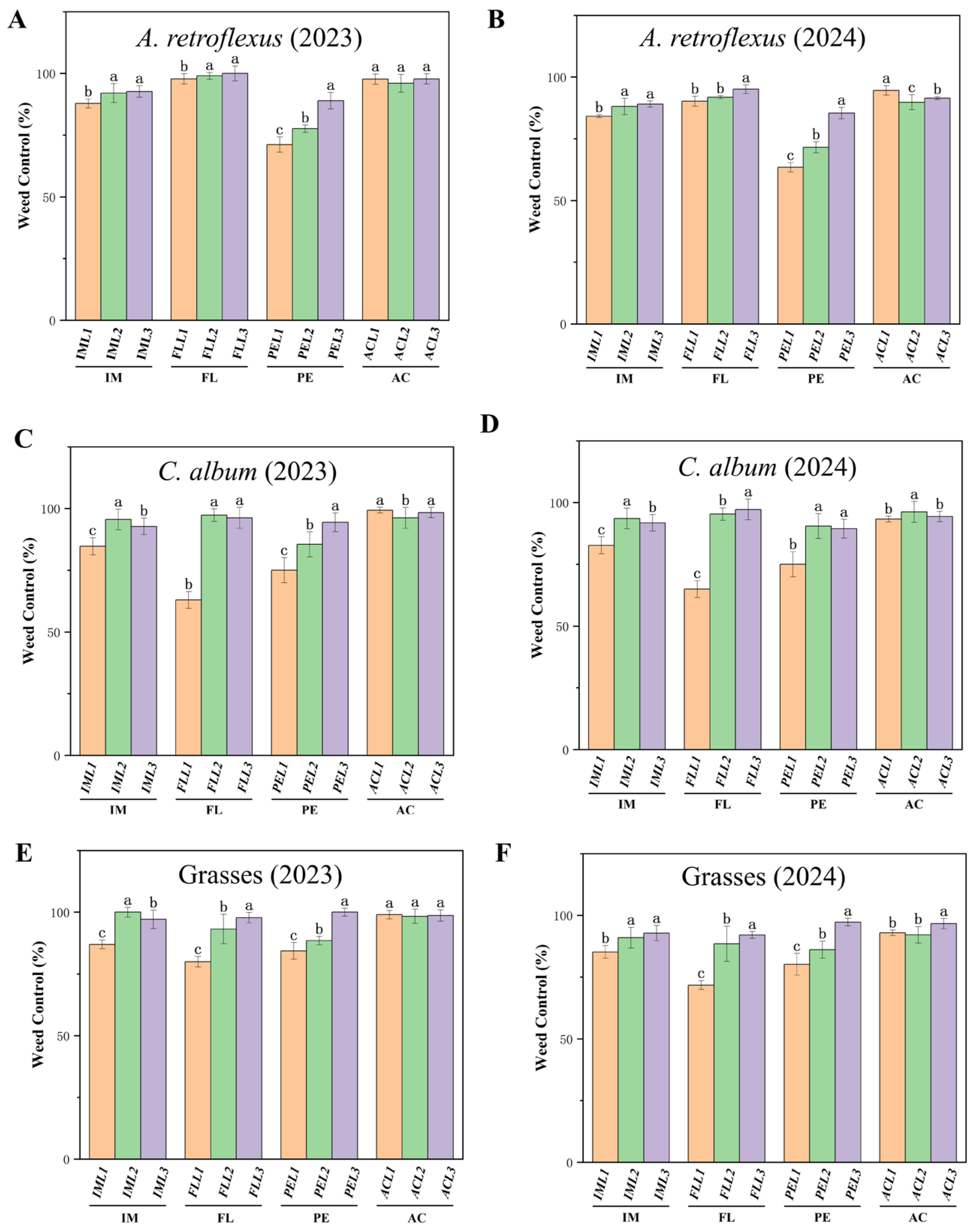
All pre-emergence herbicides significantly reduced weed density compared with the weed control (Figure 1). For example, in IML1-treated plots, control of A. retroflexus (Figure 1A,B) and C. album (Figure 1C,D) had a control efficiency of over 82%, and that of grass had a control efficiency of 85% (Figure 1E,F). In IML2 and IML3-treated plots, control of A. retroflexus (Figure 1A,B), C. album (Figure 1C,D), and grass (Figure 1E,F) had a high efficacy (>90%). In FLL1 treatments, control of A. retroflexus (Figure 1A,B) was 90%, and that for C. album (Figure 1C,D) and grass (Figure 1E,F) was 62–79%. For FLL2 and FLL3 treatments, these weed species showed high efficacy (>90%). All applications of acetochlor were highly effective at controlling weeds (>90%).
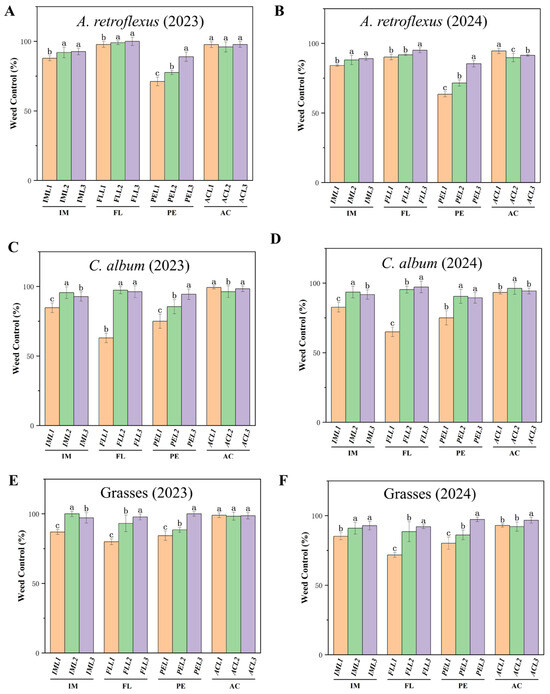
Figure 1.
Effect of pre-emergence herbicides on weed control (%) in different treatments at 30 DAS for A. retroflexus in (A) 2023 and (B) 2024; C. album in (C) 2023 and (D) 2024; and grasses in (E) 2023 and 2024 (F). Pre-emergence herbicides imazethapyr (IM, IML1, IML2 and IML3 denote different concentrations), flumetsulam (FL, FLL1, FLL2, FLL3 denote different concentrations), acetochlor (AC, ACL1, ACL2, ACL3 denote different concentrations), and pendimethalin (PE, PEL1, PEL2, PEL3 denote different concentrations). All treatments were measured with three replications. Different letters indicate significant differences between treatments (Duncan test at p < 0.05).
2.3. Post-Emergence Herbicide Weed Control Efficiency
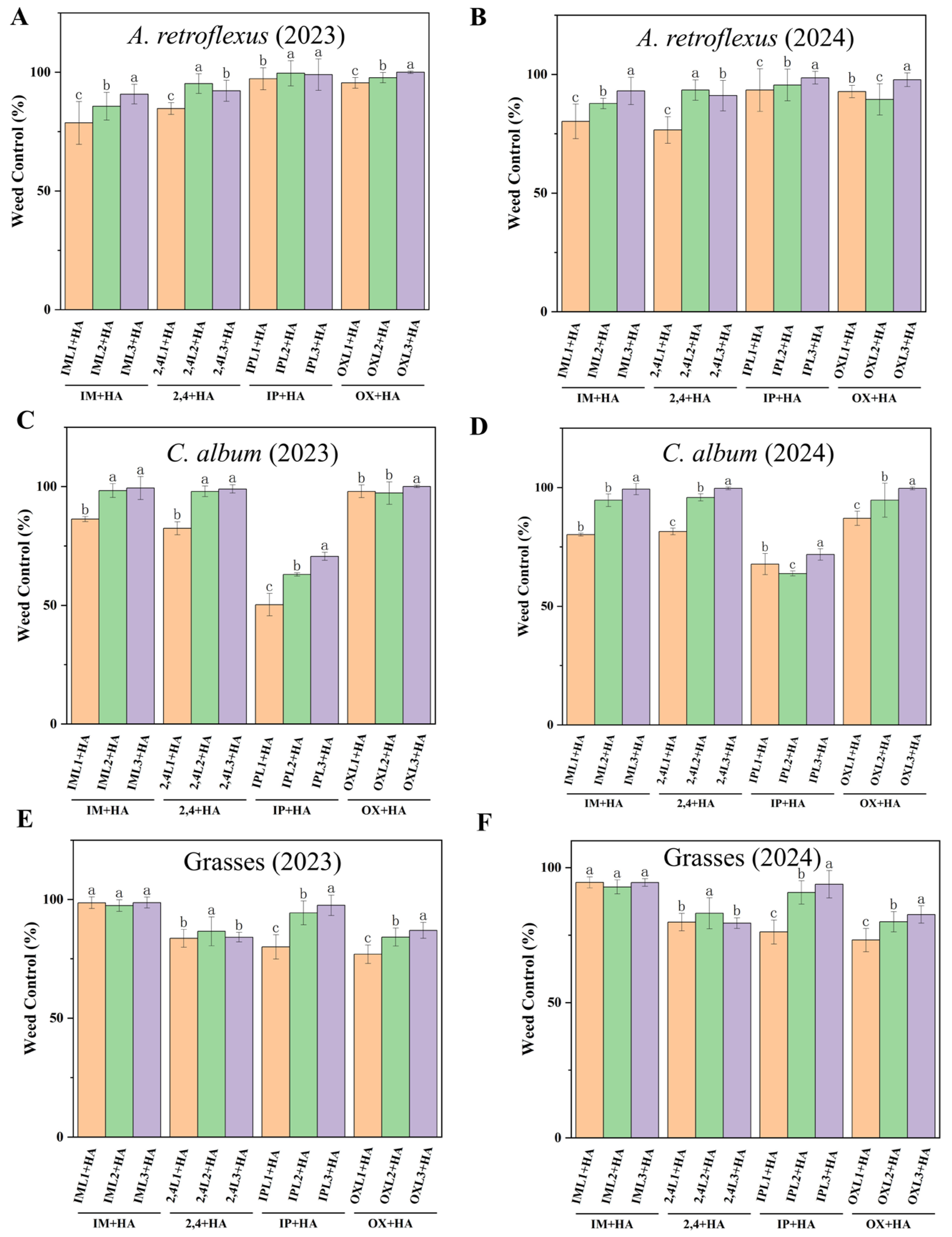
Haloxyfop-P was tank-mixed with herbicides to evaluate weed control efficiency. At 60 DAS, weed densities were substantially reduced in all post-emergence herbicide treatments. Each of 2,4L2 + HA, 2,4L3 + HA, OXL1 + HA, OXL2 + HA, and OXL3 + HA effectively controlled (>89%) A. retroflexus (Figure 2A,B). Excellent control of C. album (Figure 2C,D) was achieved in IM + HA, 2,4 + HA, and OX + HA treatments (>80%). Each of the IM + HA, 2,4 + HA, and IP + HA treatments controlled grasses (Figure 2E,F) with a control efficiency of over 76%.
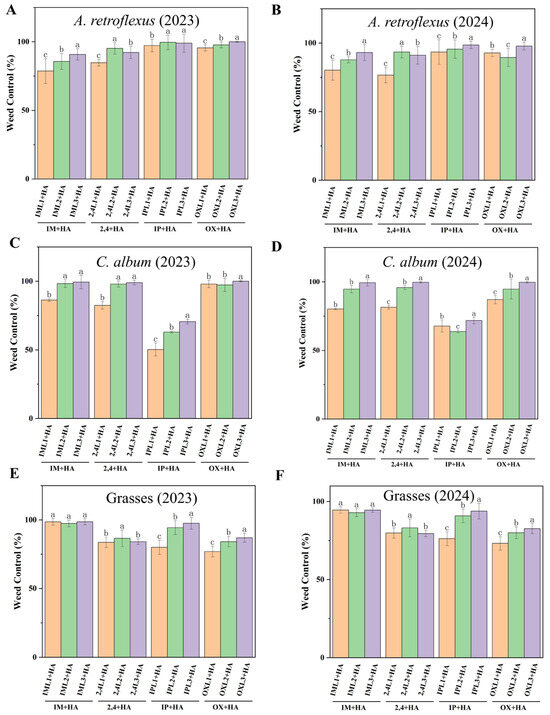
Figure 2.
Effect of post-emergence herbicides on weed control (%) in different treatments at 60 DAS for A. retroflexus in (A) 2023 and (B) 2024; C. album in (C) 2023 and (D) 2024; and grasses in (E) 2023 and (F) 2024. Post-emergence herbicides of imazethapyr + haloxyfop-P (IM + HA, IML1 + HA, IML2 + HA, IML3 + HA denote different concentrations), imazapic + haloxyfop-P (IP + HA, IPL1 + HA, IPL2 + HA, IPL3 + HA denote different concentrations), 2,4-DB + haloxyfop-P (2,4 + HA, 2,4L1 + HA, 2,4L2 + HA, 2,4L3 + HA denote different concentrations), and oxyfluorfen + haloxyfop-P (OX + HA, OXL1 + HA, OXL2 + HA, OXL3 + HA denote different concentrations). All treatments were measured with three replications. Different letters indicate significant differences between treatments (Duncan test at p < 0.05).
2.4. Effects of Herbicides on M. ruthenica Growth and Injury
The toxicity of different herbicide treatments was evaluated in M. ruthenica. At 30 DAS, different levels of injury occurred following the application of each pre-emergence herbicide (Table 1). Crop injury was consistently 1 in IML2 and FLL3 treatments. Compared with weed-free control, M. ruthenica dry matter and plant height were not significantly reduced in IML2 and FLL3 treatments. Crop injuries in acetochlor and pendimethalin treats were rated 4, respectively, with both plant height and dry matter significantly inhibited. Post-emergence herbicide crop injuries are detailed in Table 2. Injury from imazethapyr + haloxyfop-P tank mix (IML1 + HA, IML2 + HA, IML3 + HA) rated 1, as did (excepting the 2,4L3 + HA treatment) application of 2,4-DB + haloxyfop-P tank mix (2,4L1 + HA, 2,4L2 + HA). Conversely, imazapic and haloxyfop-P tank mix (IPL1 + HA, IPL2 + HA, IPL3 + HA) and oxyfluorfen and haloxyfop-P tank mix (OXL1 + HA, OXL2 + HA, OXL3 + HA) had crop injury ratings of 3 or 4. Application of different post-emergence herbicide combinations affected plant height and dry matter. Compared with the weed-free samples, M. ruthenica dry matter and plant height were not significantly reduced in IML3 + HA and 2,4L2 + HA treatments.

Table 1.
Effect of pre-emergence herbicide treatments on M. ruthenica crop injury.

Table 2.
Effect of post-emergence herbicide treatments on M. ruthenica crop injury.
2.5. Effects of Pre-Emergence Herbicides on Soil Microorganisms
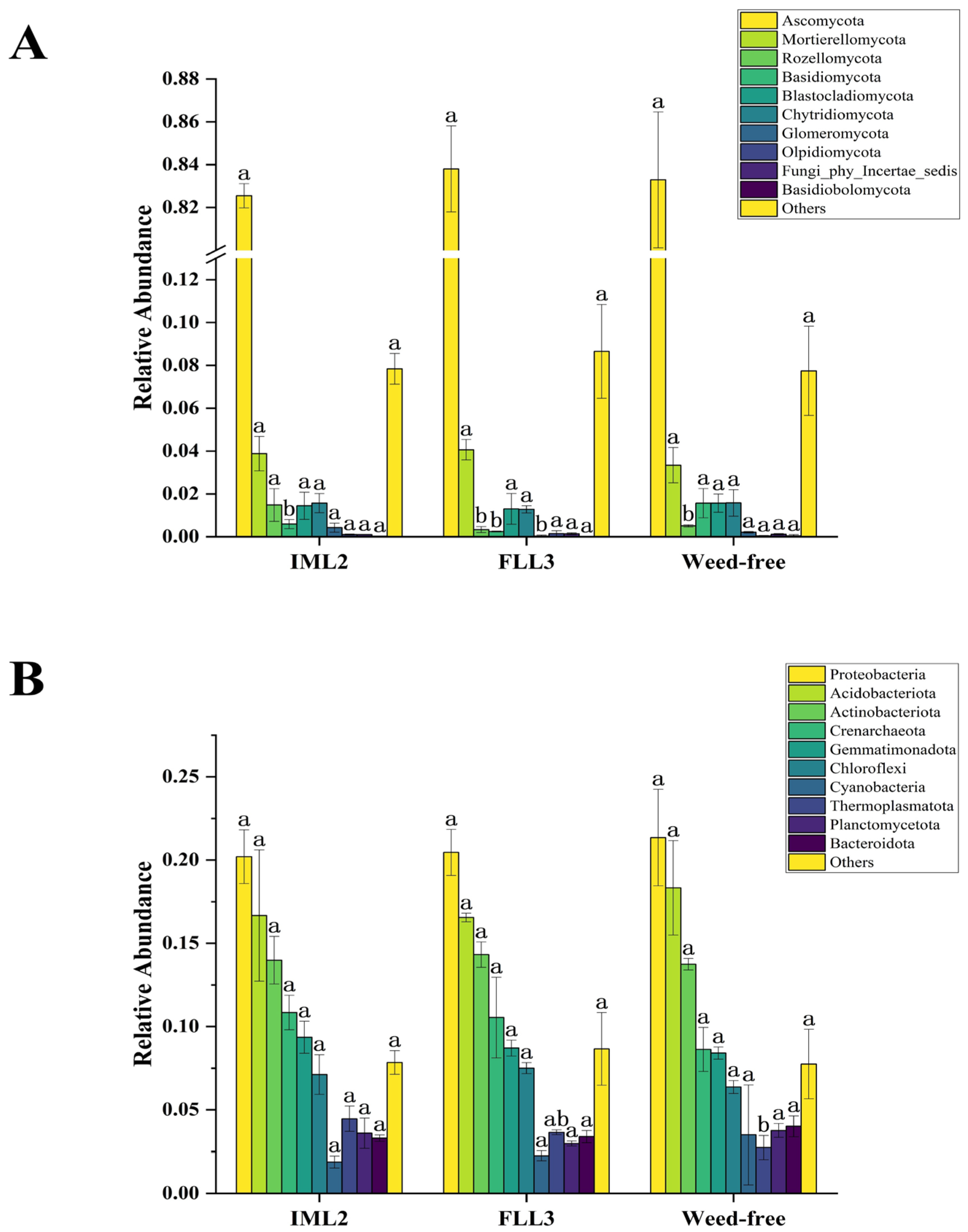
Microorganisms are important in soil ecosystems and agroecosystems. As with many agrochemicals, herbicides can also cause negative environmental impacts. Thus, a better understanding of the interactive responses of soil microbes toward herbicides is essential. We assessed soil microorganisms following pre-herbicide treatment at 30 DAS. Analysis of the relative abundances of fungal communities in each treatment (Figure 3A) revealed fungal taxa to include the phyla Ascomycota, Mortierellomycota, Rozellomycota, Basidiomycota, Blastocladiomycota, Chytridiomycota, Glomeromycota, Olpidiomycota, Fungi-phy-Incertae-sedis, and Basidiobolomycota. Of these, Ascomycota was universally most abundant, and that of Mortierellomycota was relatively stable. Compared with the weed-free (control) samples, there were no significant differences in soil fungal abundances between the IML2 and FLL3 treatments. Analysis of the relative abundances of bacterial communities across samples (Figure 3B) revealed the phyla Bacteroidota, Planctomycetota, Thermoplasmatota, Cyanobacteria, Chloroflexi, Gemmatimonadota, Crenarchaeota, Actinobacteriota, Acidobacteriota, and Proteobacteria. Among these, Proteobacteria, Actinobacteriota, and Acidobacteriota were highly abundant, whereas Chloroflexi, Gemmatimonadota, and Crenarchaeota occurred at moderate levels. As for fungal analyses, compared with the weed-free samples, there were no significant differences in the abundance of soil bacteria between IML2 and FLL3 treatments.
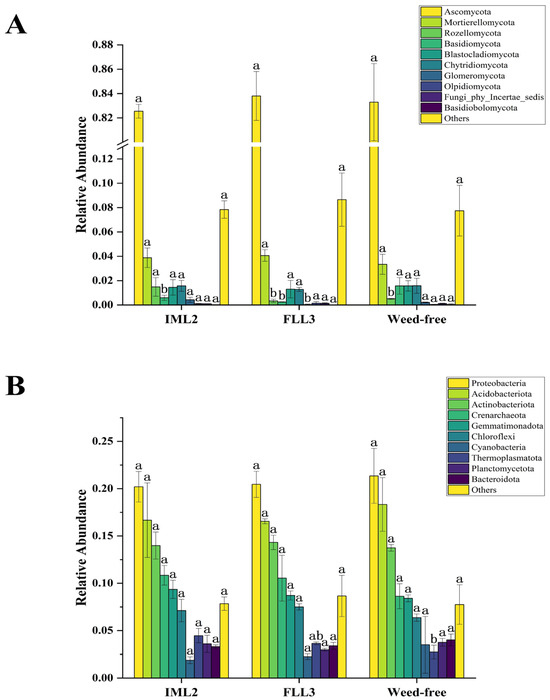
Figure 3.
Column chart of relative abundances (%) of fungi (A) and bacteria (B) at the phylum level in the weed-free (control), IML2, and FLL3 treated soil samples. All treatments were measured with three replications. Different letters indicate significant differences between treatments (Duncan test at p < 0.05).
3. Discussion
M. ruthenica is an important forage legume in Northern China, especially in Inner Mongolia areas [24]. Due to overgrazing, only shrub and grass species were left in large parts of grasslands in Inner Mongolia. Ecological restoration of overgrazed grasslands requires forage species that can tolerate drought and cold winters. The candidate forage species can not only provide high forage yield and quality to the local beef and milk industry but also can adapt well to climatic conditions in this area. M. ruthenica is the best option, and it has been extensively used in this area due to its superior characteristics. However, its seed production is significantly challenged by weeds. Weeds pose many challenges in M. ruthenica seed production, including higher weeding costs and seed yield reduction, specifically at the time of its seedling establishment [25]. The use of selective herbicides is a more feasible option to control weeds on a large scale. However, there was no information about weed control by herbicides for M. ruthenica.
Pre-emergence herbicides are important for modern agricultural production to manage weeds [26]; determining the most appropriate herbicide to use requires an understanding of their effectiveness at controlling specific weeds, as well as possible crop injury and soil safety. We evaluate the effectiveness of weed control, crop injury, and soil safety of several pre-emergent herbicides in M. ruthenica (Table 1, Figure 1 and Figure 4A). Of them, acetochlor and pendimethalin are highly effective at controlling weeds, but they also significantly inhibit M. ruthenica plant height and dry matter accumulation. Imazethapyr and flumetsulam, particularly in IML2 and FLL3 treatments, also effectively suppressed weeds and exerted minimal impact on M. ruthenica plants. Additionally, we report that IML2 and FLL3 treatments do not significantly affect soil bacterial and fungal communities (Figure 3). Imazethapyr, when applied at 104 g ae ha−1 in alfalfa [22], and flumetsulam when applied at rates of 70 g ha−1 [27] and 0.05 to 0.14 kg a.i ha−1 [28] in soybean (Glycine max), significantly reduced weed emergence and legume biomass, which was consistent with our research. The efficiency of weed control and crop safety is affected by herbicide type and concentration [21], indicating that both factors influenced M. ruthenica growth and development.
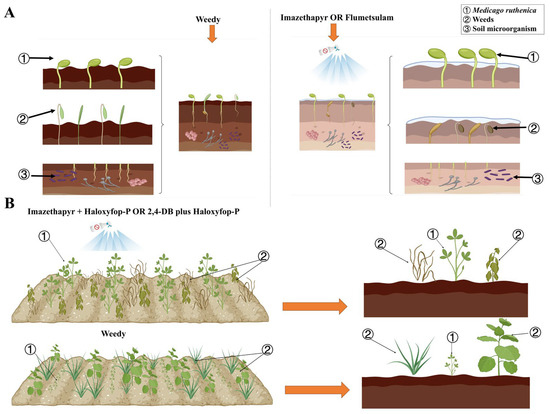
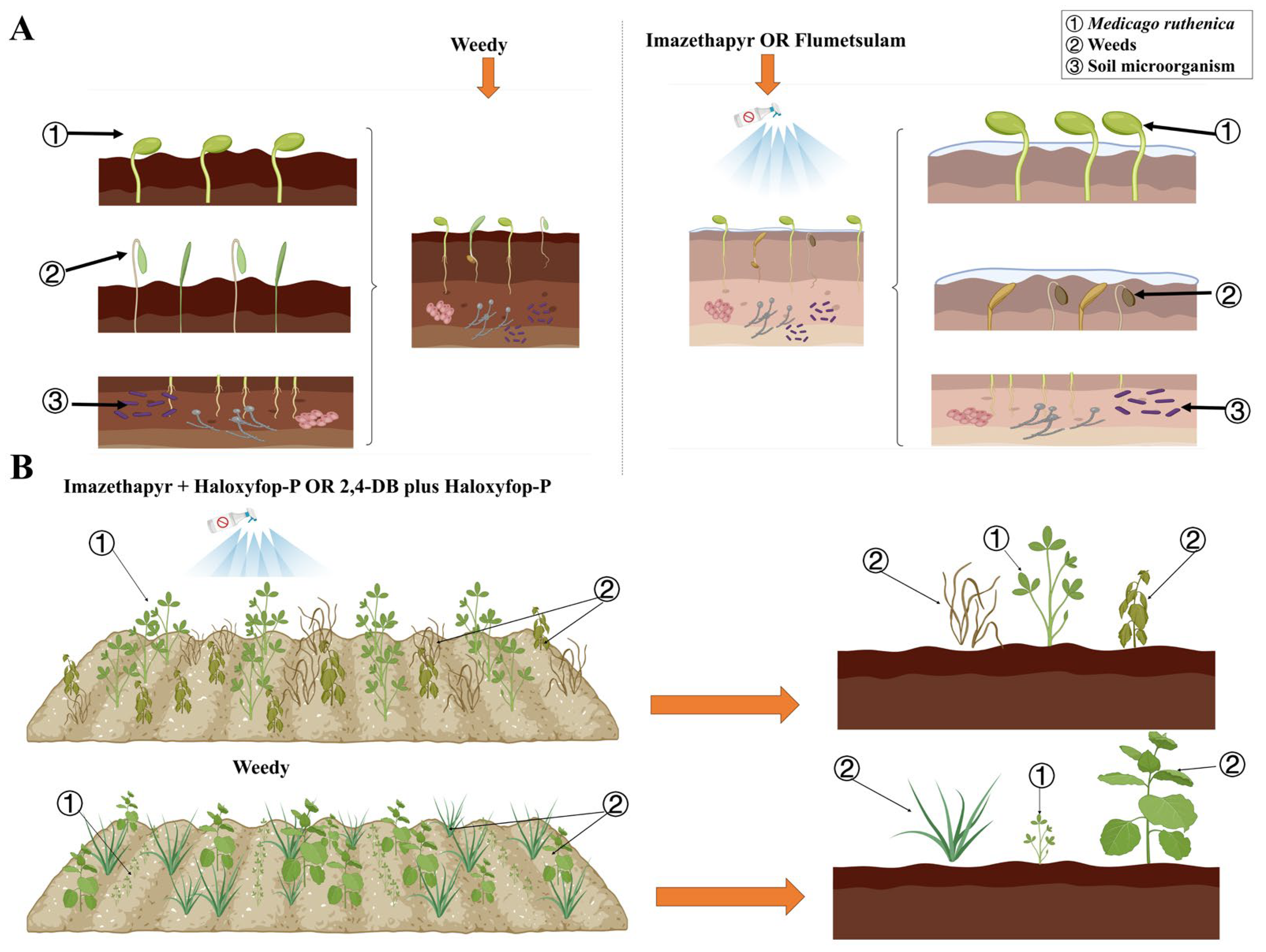
Figure 4.
(A) Effects of pre-emergence herbicide application on M. ruthenica, weeds, and soil microorganisms. Compared with the control, applications of imazethapyr (1530 mL ha−1) and flumetsulam (120 mL ha−1) effectively reduce weed populations and do not negatively affect M. ruthenica crops and soil bacterial and fungal communities. (B) Effects of post-emergence herbicide application on M. ruthenica and weeds. Compared with the control, imazethapyr + haloxyfop-P (1800 + 600 mL ha−1) and 2,4-DB + haloxyfop-P (2250 + 600 mL ha−1) significantly reduce secondary weed infestation and promote M. ruthenica crop health (created in https://BioRender.com, accessed on 8 February 2025).
Post-emergence herbicides play a crucial role in modern agriculture, particularly in the management of actively growing weeds [29]. Based on previous research, post-emergence herbicides are mainly used to control infestation weeds [30]. Because weed community composition and density vary after planting [31], no one herbicide may effectively control all weeds [32]. The tank mix of herbicides can effectively reduce the required dosage of individual herbicides and maintain or enhance weed control efficacy [33]. Haloxyfop-P effectively controls grasses [34]; thus, we mixed it with four post-emergence herbicides used to control broadleaf weeds. We report that imazapic + haloxyfop-P and oxyfluorfen + haloxyfop-P effectively control all weed species but significantly affect M. ruthenica plant height and dry matter. In contrast, the treatments with imazethapyr + haloxyfop-P and 2,4-DB + haloxyfop-P demonstrated relatively high effective control of all weeds without adversely affecting M. ruthenica plant height or dry matter (especially in IML3 + HA and 2,4L2 + HA treatments) (Table 2, Figure 2 and Figure 4B). Carvalho et al. reported that application of 2,4-D + haloxyfop-P (1005 g a.i ha−1 + 62.4 g a.i ha−1) effectively controlled approximately 50–60% of weeds [35]. However, we report that the weed-control efficiency of 2,4L2 + HA exceeds 86%. Combinations of haloxyfop-p-methyl at 135 g ha−1 + imazethapyr at 75 g ha−1 were recommended to control weeds in green gram (Vigna radiata) [36]. In our study, these combinations of post-emergence herbicides effectively control a wide range of weed species in M. ruthenica, which not only effectively manages a wider range of weed species but also contributes to the sustainability of agricultural practices by minimizing the dosage of herbicides.
Compared with other forage legume crops, weeds can outcompete M. ruthenica because it grows slowly as a seedling. Autumn sowing has been considered a method to conduct seed fields in the establishment year with reduced pressure from weeds. Although autumn sowing is a viable, effective strategy for weed control, it still has some issues: (1) Autumn sowing can negatively affect the hardiness and winter survivability of M. ruthenica. (2) Autumn sowing can lead to periods of idle land in spring and summer due to the absence of suitable crops with reproductive periods in northern China. (3) Autumn sowing reduces root and canopy growth and development before winter, resulting in a decreased number of stems in the following year and a subsequent decline in seed yield. Instead, chemical weed control has many advantages in M. ruthenica due to their flexible sowing, efficient weed control, and reduced costs associated with seed production in M. ruthenica. We demonstrate the potential of chemical control of weeds in M. ruthenica, which will enhance seed production and contribute to the longer-term viability of agricultural systems.
4. Materials and Methods
4.1. Location and Experimental Soil Properties
Experimental crops were planted in 2023 and 2024 at Saihan District, Hohhot City, Inner Mongolia Autonomous Region, China (111°53.40′ N, 40°43.70′ E). The soils had a pH of 8.1 and organic matter, nitrogen, phosphorus, and potassium contents of 1.29%, 0.084%, 10 ppm, and 260 ppm, respectively.
4.2. Weed Data Recording
Herbicides were applied to crops in accordance with the standards of the Ministry of Agriculture and Forestry, General Directorate of Agricultural Research and Policies (TAGEM) [12]. Weed species and densities were observed before experimentation. Within each experimental treatment, three replicate 1 m2 frames were randomly placed, and weed species and their numbers therein were recorded [12].
4.3. Plant Materials and Experimental Design
Plants (M. ruthenica (ND-3)) were sourced from the College of Life Sciences, Inner Mongolia University (Hohhot, Inner Mongolia Autonomous Region, China), and planted with a 15 cm spacing. The distance between treatments and each replicate was 1 m. For both years, experimental plots (5 × 4 m2) were arranged in a randomized complete block design with pre- and post-emergence herbicide treatments, each replicated thrice. Based on the previous research, pre- and post-emergence herbicides with potential for application were selected (Supplementary Table S2). To evaluate the selectivity and sensitivity of herbicides at controlling weeds in M. ruthenica, four pre-emergence and four post-emergence herbicide treatments were established. Pre-emergence herbicides imazethapyr (IML1, IML2, IML3), flumetsulam (FLL1, FLL2, FLL3), acetochlor (ACL1, ACL2, ACL3), and pendimethalin (PEL1, PEL2, PEL3) were applied 1 day after the sowing date of M. ruthenica seeds (DAS). Post-emergence herbicides of imazethapyr + haloxyfop-P (IML1 + HA, IML2 + HA, IML3 + HA), imazapic + haloxyfop-P (IPL1 + HA, IPL2 + HA, IPL3 + HA), 2,4-DB + haloxyfop-P (2,4L1 + HA, 2,4L2 + HA, 2,4L3 + HA), and oxyfluorfen + haloxyfop-P (OXL1 + HA, OXL2 + HA, OXL3 + HA) were applied 30 days after M. ruthenica seeds were sown. Herbicide details are summarized in Table 3 and Table 4.

Table 3.
Characteristics of experimental pre-emergence herbicides.

Table 4.
Characteristics of experimental post-emergence herbicides.
4.4. Effects of Herbicides on Weeds
Weed population and dry matter data were measured at 30 DAS (pre) and 60 DAS (post). Weeds were sampled within three 1 m2 quadrants located on the diagonal in each plot. Aboveground weed biomass was harvested, identified, counted, and stored separately in paper bags, then oven-dried at 70 °C for 24 h to constant weight. The effect of herbicides on the total number of weeds within quadrats was determined using Equation (1) [12].
4.5. Effects of Herbicides on M. ruthenica Growth and Injury
Effects of herbicide treatment on M. ruthenica height and dry matter were determined at 30 and 60 DAS. At each harvest, M. ruthenica samples were collected from three 1 m2 quadrats; stems and leaves of each plant were removed and stored separately in paper bags, then oven-dried (70 °C for 24 h) to determine total dry matter. The condition of M. ruthenica plants in each treatment was assessed visually on a 4-point scale by comparing them with control plants, where 1 = very slight injury; 2 = slight injury; 3 = phytotoxic, and 4 = severely phytotoxic [23].
4.6. Effects of Pre-Herbicides on Soil Microbial Communities in M. ruthenica
Five rhizosphere soil samples (depth 0–20 cm) were randomly collected from each replicate pre-herbicide treatment plot 30 DAS. Plant tissues, debris, and gravel were cleaned from samples (passed through a 2 mm sieve). The five samples from each plot were mixed to form a mixed soil sample to produce three biological replicates for each treatment. After sample collection, the soils were stored at −20 °C for metagenomic analysis [37]. Significant differences between treatments were evaluated using Duncan’s multiple-range tests with a significance threshold of p < 0.05.
4.7. Statistical Analysis
Data were analyzed using SAS (Version 9.1) and RStudio (Version 3.4.1). Significant differences between treatments were evaluated using Duncan’s multiple-range tests with a significance threshold of p < 0.05. Figures were created using OriginPro 2021b.
5. Conclusions
Weed control is important for M. ruthenica cultivation. In this study, we first report the effects of various pre- and post-herbicides on weed control and crop safety in M. ruthenica. The results indicated that the most effective pre-emergence herbicides are imazethapyr (1530 mL ha−1) and flumetsulam (120 mL ha−1). Post-emergence herbicides that effectively control weeds and do not damage crops are imazethapyr + haloxyfop-P (1800 + 600 mL ha−1) and 2,4-DB + haloxyfop-P (2250 + 600 mL ha−1). The application of these herbicides can improve M. ruthenica seed production and the use of M. ruthenica in artificial grassland establishment and ecological restoration.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants14060864/s1, Table S1: Dominant annual weeds and density (% m2); Table S2: Effect of pre- and post-emergence herbicides on weed control and crop injury.
Author Contributions
Conceptualization, J.L.; methodology, J.L., Q.L., L.Y., F.H., Y.Z. and W.W.; software, Z.R.; formal analysis, Q.L., Z.R. and H.X.; investigation, Q.L., Z.R. and H.X.; writing—original draft preparation, Q.L.; writing—review and editing, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (No. 32371768; No. 32160325), Key Projects in Science and Technology of Inner Mongolia (No. 2023YFHH0029; No. 2022JBGS0020), National Center of Pratacultural Technology Innovation (underway) Special Fund for Innovation Platform Construction (CCPTZX2024QN 09).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The author thanks BioRender (https://BioRender.com, accessed on 8 February 2025) for its helpful role in this experiment. The authors would like to acknowledge Hu Wang from Grasslands Research Centre, AgResearch Ltd. for kindly providing suggestions.
Conflicts of Interest
Author Yarong Zhang is employed by the company Inner Mongolia Pratacultural Technology Innovation Center Co. Ltd. All the Authors declare no conflicts of interest.
References
- Balabaev, G. Yellow lucernes of Siberia: Medicago ruthenica (L.) Ledb. and M. platycarpa (L.) Ledb. Bull. App. Bot. Genet. Plant Breed. Serv. 1934, 7, 113–123. [Google Scholar]
- Li, J.; Li, H.; Chi, E.; Huang, F.; Liu, L.; Ding, Z.; Shi, W.; Mi, F.; Li, Z. Development of simple sequence repeat (SSR) markers in Medicago ruthenica and their application for evaluating outcrossing fertility under open-pollination conditions. Mol. Breed. 2018, 38, 1–7. [Google Scholar] [CrossRef]
- Small, E.; Jomphe, M. A synopsis of the genus Medicago (Leguminosae). Can. J. Bot. 1989, 67, 3260–3294. [Google Scholar] [CrossRef]
- Campbell, T.A. Molecular analysis of genetic variation among alfalfa (Medicago sativa L.) and Medicago ruthenica clones. Can. J. Plant Sci. 2000, 80, 773–779. [Google Scholar] [CrossRef]
- Campbell, T.A.; Bao, G.; Xia, Z. Agronomic evaluation of Medicago ruthenica collected in Inner Mongolia. Crop Sci. 1997, 37, 599–604. [Google Scholar] [CrossRef]
- Buhler, D.D. 50th Anniversary—Invited Article: Challenges and opportunities for integrated weed management. Weed Sci. 2002, 50, 273–280. [Google Scholar] [CrossRef]
- Boström, U.; Fogelfors, H. Response of weeds and crop yield to herbicide dose decision-support guidelines. Weed Sci. 2002, 50, 186–195. [Google Scholar] [CrossRef]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest Manage. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, Y. Breeding herbicide-resistant rice (Oryza sativa) using CRISPR/Cas gene editing and other technologies. Plant Commun. 2024, 6, 101172. [Google Scholar] [CrossRef]
- Riaz, S.; Basharat, S.; Ahmad, F.; Hameed, M.; Fatima, S.; Ahmad, M.S.A.; Kaushik, P. Dactyloctenium aegyptium (L.) Willd. (Poaceae) differentially responds to pre- and post-emergence herbicides through micro-structural alterations. Agriculture 2022, 12, 1831. [Google Scholar] [CrossRef]
- Pornprom, T.; Sukcharoenvipharat, W.; Sansiriphun, D. Weed control with pre-emergence herbicides in vegetable soybean (Glycine max L. Merrill). Crop Prot. 2010, 29, 684–690. [Google Scholar] [CrossRef]
- Alptekin, H.; Ozkan, A.; Gurbuz, R.; Kulak, M. Management of weeds in maize by sequential or individual applications of pre-and post-emergence herbicides. Agriculture 2023, 13, 421. [Google Scholar] [CrossRef]
- Bhowmik, P.C. Weed biology: Importance to weed management. Weed Sci. 1997, 45, 349–356. [Google Scholar] [CrossRef]
- Landau, C.A.; Hager, A.G.; Tranel, P.J.; Davis, A.S.; Martin, N.F.; Williams, M.M. Future efficacy of pre-emergence herbicides in corn (Zea mays) is threatened by more variable weather. Pest Manage. Sci. 2021, 77, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.A.; Brink, G.; Ruth, L.; Stout, R. Grass–legume mixtures suppress weeds during establishment better than monocultures. Agron. J. 2012, 104, 36–42. [Google Scholar] [CrossRef]
- Cong, W.F.; Suter, M.; Lüscher, A.; Eriksen, J. Species interactions between forbs and grass-clover contribute to yield gains and weed suppression in forage grassland mixtures. Agric. Ecosyst. Environ. 2018, 268, 154–161. [Google Scholar] [CrossRef]
- Hoy, M.D.; Moore, K.J.; George, J.R.; Brummer, E.C. Alfalfa yield and quality as influenced by establishment method. Agron. J. 2002, 94, 65–71. [Google Scholar] [CrossRef]
- Mesbah, A.O.; Miller, S.D. Canada thistle (Cirsium arvense) control in established alfalfa (Medicago sativa) grown for seed production. Weed Technol. 2005, 19, 1025–1029. [Google Scholar] [CrossRef]
- Moyer, J.R.; Schaalje, G.B.; Bergen, P. Alfalfa (Medicago sativa) seed yield loss due to Canada thistle (Cirsium arvense). Weed Technol. 1991, 5, 723–728. [Google Scholar] [CrossRef]
- Cosgrove, D.R.; Barrett, M. Effects of weed control in established alfalfa (Medicago sativa) on forage yield and quality. Weed Sci. 1987, 35, 564–567. [Google Scholar] [CrossRef]
- Kousta, A.; Katsis, C.; Tsekoura, A.; Chachalis, D. Effectiveness and selectivity of pre-and post-emergence herbicides for weed control in grain legumes. Plants 2024, 13, 211. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.; Marsalis, M.; Lauriault, L.; Serena, M. Efficacy of various herbicides for the control of perennial Plantago spp. and effects on alfalfa damage and yield. Agronomy 2020, 10, 1710. [Google Scholar] [CrossRef]
- Govindasamy, P.; Singh, V.; Palsaniya, D.; Srinivasan, R.; Chaudhary, M.; Kantwa, S. Herbicide effect on weed control, soil health parameters and yield of egyptian clover (Trifolium alexandrinum L.). Crop Prot. 2021, 139, 105389. [Google Scholar] [CrossRef]
- Guo, M.; Li, H.; Zhu, L.; Wu, Z.; Li, J.; Li, Z. Genome-wide identification of microRNAs associated with osmotic stress and elucidation of the role of miR319 in Medicago ruthenica seedlings. Plant Physiol. Biochem. 2021, 168, 53–61. [Google Scholar] [CrossRef]
- Melander, B.; Rasmussen, G. Effects of cultural methods and physical weed control on intrarow weed numbers, manual weeding and marketable yield in direct-sown leek and bulb onion. Weed Res. 2001, 41, 491–508. [Google Scholar] [CrossRef]
- Witcher, A.L.; Poudel, I. Pre-emergence herbicides and mulches for weed control in cutting propagation. Agronomy 2020, 10, 1249. [Google Scholar] [CrossRef]
- Shaw, D.R.; Bennett, A.C.; Grant, D.L. Weed control in soybean (Glycine max) with flumetsulam, cloransulam, and diclosulam. Weed Technol. 1999, 13, 791–798. [Google Scholar] [CrossRef]
- Cantwell, J.R.; Liebl, R.A.; Slife, F.W. Imazethapyr for weed control in soybean (Glycine max). Weed Technol. 1989, 3, 596–601. [Google Scholar] [CrossRef]
- Flessner, M.L.; Burke, I.C.; Dille, J.A.; Everman, W.J.; VanGessel, M.J.; Tidemann, B.; Manuchehri, M.R.; Soltani, N.; Sikkema, P.H. Potential wheat yield loss due to weeds in the United States and Canada. Weed Technol. 2021, 35, 916–923. [Google Scholar] [CrossRef]
- Vasilakoglou, I.; Vlachostergios, D.; Dhima, K.; Lithourgidis, A. Response of Vetch, Lentil, Chickpea and Red Pea to Pre- or Post-Emergence Applied Herbicides. Span. J. Agric. Res. 2013, 11, 1101. [Google Scholar] [CrossRef]
- Hock, S.M.; Knezevic, S.Z.; Martin, A.R.; Lindquist, J.L. Soybean row spacing and weed emergence time influence weed competitiveness and competitive indices. Weed Sci. 2006, 54, 38–46. [Google Scholar] [CrossRef]
- Giannopolitis, C.; Strouthopoulos, T.G. Herbicide tank-mixing for post-emergence weed control in sugar beets. Weed Res. 1979, 19, 213–217. [Google Scholar] [CrossRef]
- Jhala, A.J.; Ramirez, A.H.; Singh, M. Tank mixing saflufenacil, glufosinate, and indaziflam improved burndown and residual weed control. Weed Technol. 2013, 27, 422–429. [Google Scholar] [CrossRef]
- Leal, J.F.L.; Souza, A.D.S.; Ribeiro, S.R.D.S.; Oliveira, G.F.P.B.; Araujo, A.L.S.; Borella, J.; Langaro, A.C.; Machado, A.F.L.; Pinho, C.F. 2,4-Dichlorophenoxyacetic-N-methylmethanamine and haloxyfop-P-methyl interaction: Sequential and interval applications to effectively control sourgrass and fleabane. Agron. J. 2020, 112, 1216–1226. [Google Scholar] [CrossRef]
- Carvalho, G.S.D.; Leal, J.F.L.; Souza, A.D.S.; Oliveira Junior, F.F.D.; Langaro, A.C.; Pinho, C.F.D. Cytochrome P450 enzymes inhibitor in the control of digitaria insularis. Cienc. Agrotecnol. 2021, 45, e024520. [Google Scholar] [CrossRef]
- Poornima, S.; Siva Lakshmi, Y.; Ram Prakash, T.; Srinivas, A.; Venkata Krishnan, L. Nodulation, leghemoglobin content and yield of green gram as influenced by new generation early post emergence herbicide combinations. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2134–2137. [Google Scholar] [CrossRef]
- Bhardwaj, L.; Kumar, D.; Singh, U.P.; Joshi, C.G.; Dubey, S.K. Herbicide application impacted soil microbial community composition and biochemical properties in a flooded rice field. Sci. Total Environ. 2024, 914, 169911. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).