Bottom Cooling During Culture Initiation Increases Survival and Reduces Hyperhydricity in Micropropagated Cannabis Plants
Abstract
1. Introduction
2. Results
2.1. Qualitative Observation
2.2. Detached Leaf Assay and Leaf Dry Biomass Assessment
2.3. Chlorophyll Content
2.4. Quantitative Morphological Assessment
3. Materials and Methods
3.1. Explants Resources, Disinfection Protocol, and Culture Condition
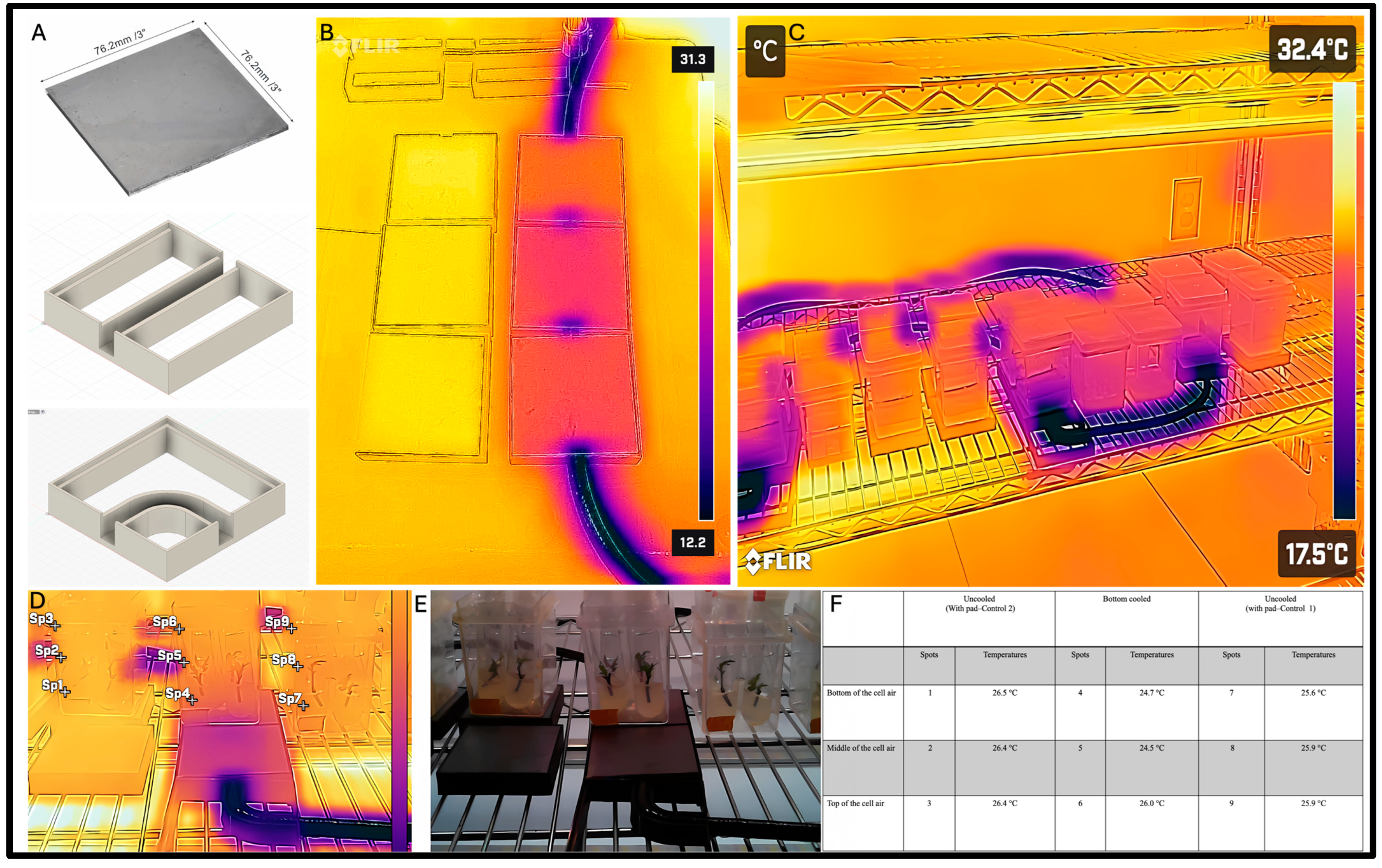
3.2. Bottom Cooling System
3.3. Evaluation of Morpho- and Physiological Traits
3.4. Estimating Chlorophyll Content
3.5. Detached Leaf Water Loss Assay and Leaf Dry Biomass Assessment
3.6. Morphological Assessment
3.7. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, T.; Jeena, G.; Pitakbut, T.; Vasilev, N.; Kayser, O. Cannabis sativa research trends, challenges, and new-age perspectives. Iscience 2021, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Behdarvandi, B.; Tavakouli Dinani, E.; Maccarone, J. Cannabis sativa L. photoautotrophic micropropagation: A powerful tool for industrial scale in vitro propagation. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 932–941. [Google Scholar] [CrossRef]
- Monthony, A.S.; Bagheri, S.; Zheng, Y.; Jones, A.M.P. Flower power: Floral reversion as a viable alternative to nodal micropropagation in Cannabis sativa. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 1018–1030. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; Khan, I.A.; ElSohly, M.A. Cannabis sativa L.: Botany and horticulture. In Cannabis sativa L.—Botany and Biotechnology; Springer: Cham, Switzerland, 2017; pp. 79–100. [Google Scholar]
- Monthony, A.S.; Page, S.R.; Hesami, M.; Jones, A.M.P. The past, present and future of Cannabis sativa tissue culture. Plants 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Punja, Z.K. Emerging diseases of Cannabis sativa and sustainable management. Pest Manag. Sci. 2021, 77, 3857–3870. [Google Scholar] [CrossRef]
- Abiri, R.; Maziah, M.; Shaharuddin, N.; Yusof, Z.; Atabaki, N.; Hanafi, M.; Sahebi, M.; Azizi, P.; Kalhori, N.; Valdiani, A. Enhancing somatic embryogenesis of Malaysian rice cultivar MR219 using adjuvant materials in a high-efficiency protocol. Int. J. Environ. Sci. Technol. 2017, 14, 1091–1108. [Google Scholar] [CrossRef]
- Wróbel, T.; Dreger, M.; Wielgus, K.; Słomski, R. Modified nodal cuttings and shoot tips protocol for rapid regeneration of Cannabis sativa L. J. Nat. Fibers 2022, 19, 536–545. [Google Scholar] [CrossRef]
- Page, S.; Monthony, A.; Jones, A. Basal media optimization for the micropropagation and callogenesis of Cannabis sativa L. BioRxiv 2020, 1, 1–23. [Google Scholar]
- Mestinšek-Mubi, Š.; Svetik, S.; Flajšman, M.; Murovec, J. In vitro tissue culture and genetic analysis of two high-CBD medical cannabis (Cannabis sativa L.) breeding lines. Genetika 2020, 52, 925–941. [Google Scholar] [CrossRef]
- Galán-Ávila, A.; García-Fortea, E.; Prohens, J.; Herraiz, F.J. Development of a direct in vitro plant regeneration protocol from Cannabis sativa L. seedling explants: Developmental morphology of shoot regeneration and ploidy level of regenerated plants. Front. Plant Sci. 2020, 11, 645. [Google Scholar] [CrossRef]
- Pain, S. A potted history. Nature 2015, 525, S10–S11. [Google Scholar] [CrossRef]
- Monthony, A.S.; Kyne, S.T.; Grainger, C.M.; Jones, A.M.P. Recalcitrance of Cannabis sativa to de novo regeneration; a multi-genotype replication study. PLoS ONE 2021, 16, e0235525. [Google Scholar] [CrossRef] [PubMed]
- Piunno, K.F.; Golenia, G.; Boudko, E.A.; Downey, C.; Jones, A.M.P. Regeneration of shoots from immature and mature inflorescences of Cannabis sativa. Can. J. Plant Sci. 2019, 99, 556–559. [Google Scholar] [CrossRef]
- Aliaga-Franco, N.; Zhang, C.; Presa, S.; Srivastava, A.K.; Granell, A.; Alabadí, D.; Sadanandom, A.; Blázquez, M.A.; Minguet, E.G. Identification of transgene-free CRISPR-edited plants of rice, tomato, and Arabidopsis by monitoring DsRED fluorescence in dry seeds. Front. Plant Sci. 2019, 10, 1150. [Google Scholar] [CrossRef] [PubMed]
- Shew, A.M.; Nalley, L.L.; Snell, H.A.; Nayga, R.M., Jr.; Dixon, B.L. CRISPR versus GMOs: Public acceptance and valuation. Glob. Food Secur. 2018, 19, 71–80. [Google Scholar] [CrossRef]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and nonpsychoactive cannabinoids: Their chemistry and role against oxidative stress, inflammation, and cancer. BioMed Res. Int. 2018, 2018, 1691428. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Kareem, F.; El-Mahrouk, M.E.; Fuller, M.P. Artificial seeds (principle, aspects and applications). Agronomy 2017, 7, 71. [Google Scholar] [CrossRef]
- Ren, J.; Wu, P.; Trampe, B.; Tian, X.; Lübberstedt, T.; Chen, S. Novel technologies in doubled haploid line development. Plant Biotechnol. J. 2017, 15, 1361–1370. [Google Scholar] [CrossRef]
- Page, S.R.; Monthony, A.S.; Jones, A.M.P. DKW basal salts improve micropropagation and callogenesis compared with MS basal salts in multiple commercial cultivars of Cannabis sativa. Botany 2021, 99, 269–279. [Google Scholar] [CrossRef]
- Picoli, E.A.; Otoni, W.C.; Figueira, M.L.; Carolino, S.M.; Almeida, R.S.; Silva, E.A.; Carvalho, C.R.; Fontes, E.P. Hyperhydricity in in vitro eggplant regenerated plants: Structural characteristics and involvement of BiP (Binding Protein). Plant Sci. 2001, 160, 857–868. [Google Scholar] [CrossRef]
- Saher, S.; Piqueras, A.; Hellin, E.; Olmos, E. Hyperhydricity in micropropagated carnation shoots: The role of oxidative stress. Physiol. Plant. 2004, 120, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Kevers, C.; Franck, T.; Strasser, R.J.; Dommes, J.; Gaspar, T. Hyperhydricity of micropropagated shoots: A typically stress-induced change of physiological state. Plant Cell Tissue Organ Cult. 2004, 77, 181–191. [Google Scholar] [CrossRef]
- Park, S.; Jeon, J.H.; Kim, H.S.; Park, Y.; Aswath, C.; Joung, H. Effect of sealed and vented gaseous microenvironments on the hyperhydricity of potato shoots in vitro. Sci. Hortic. 2004, 99, 199–205. [Google Scholar] [CrossRef]
- Sreedhar, R.V.; Venkatachalam, L.; Neelwarne, B. Hyperhydricity-related morphologic and biochemical changes in Vanilla (Vanilla planifolia). J. Plant Growth Regul. 2009, 28, 46–57. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Park, S.; Ali, M.B.; Shin, K.; Paek, K. Hyperhydricity in apple: Ultrastuctural and physiological aspects. Tree Physiol. 2006, 26, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Changes in the biochemical parameters of albino, hyperhydric and normal green leaves of Caladium bicolor cv.“Bleeding hearts” in vitro long-term cultures. J. Photochem. Photobiol. B Biol. 2019, 191, 88–98. [Google Scholar] [CrossRef]
- Hazarika, B. Morpho-physiological disorders in in vitro culture of plants. Sci. Hortic. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Ziv, M. Quality of micropropagated plants—Vitrification. Vitr. Cell. Dev. Biol. Plant 1991, 27, 64–69. [Google Scholar] [CrossRef]
- Phillips, D.J.; Matthews, G.J. Growth and development of carnation shoot tips in vitro. Bot. Gaz. 1964, 125, 7–12. [Google Scholar] [CrossRef]
- Zhou, T.-S. In vitro culture of Doritaenopsis: Comparison between formation of the hyperhydric protocorm-like-body (PLB) and the normal PLB. Plant Cell Rep. 1995, 15, 181–185. [Google Scholar] [CrossRef]
- Mayor, M.; Nestares, G.; Zorzoli, R.; Picardi, L. Reduction of hyperhydricity in sunflower tissue culture. Plant Cell Tissue Organ Cult. 2003, 72, 99–103. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, L.; Long, Y. Analysis of ultrastructure and reactive oxygen species of hyperhydric garlic (Allium sativum L.) shoots. Vitr. Cell. Dev. Biol. Plant 2009, 45, 483–490. [Google Scholar] [CrossRef]
- Bakir, Y.; Eldem, V.; Zararsiz, G.; Unver, T. Global transcriptome analysis reveals differences in gene expression patterns between nonhyperhydric and hyperhydric peach leaves. Plant Genome 2016, 9. [Google Scholar] [CrossRef]
- Gao, H.; Xu, D.; Zhang, H.; Cheng, X.; Yang, Q. Effects of culture medium composition and PEG on hyperhydricity in Dendrobium officinale. Vitr. Cell. Dev. Biol. Plant 2020, 56, 143–149. [Google Scholar] [CrossRef]
- Casanova, E.; Moysset, L.; Trillas, M. Effects of agar concentration and vessel closure on the organogenesis and hyperhydricity of adventitious carnation shoots. Biol. Plant. 2008, 52, 1–8. [Google Scholar] [CrossRef]
- Fernandez-Garcia, N.; Piqueras, A.; Olmos, E. Sub-cellular location of H2O2, peroxidases and pectin epitopes in control and hyperhydric shoots of carnation. Environ. Exp. Bot. 2008, 62, 168–175. [Google Scholar] [CrossRef]
- Sen, A.; Alikamanoglu, S. Antioxidant enzyme activities, malondialdehyde, and total phenolic content of PEG-induced hyperhydric leaves in sugar beet tissue culture. Vitr. Cell. Dev. Biol. Plant 2013, 49, 396–404. [Google Scholar] [CrossRef]
- van den Dries, N.; Giannì, S.; Czerednik, A.; Krens, F.A.; de Klerk, G.-J.M. Flooding of the apoplast is a key factor in the development of hyperhydricity. J. Exp. Bot. 2013, 64, 5221–5230. [Google Scholar] [CrossRef]
- Polivanova, O.B.; Bedarev, V.A. Hyperhydricity in plant tissue culture. Plants 2022, 11, 3313. [Google Scholar] [CrossRef]
- Gao, H.; Li, J.; Ji, H.; An, L.; Xia, X. Hyperhydricity-induced ultrastructural and physiological changes in blueberry (Vaccinium spp.). Plant Cell Tissue Organ Cult. 2018, 133, 65–76. [Google Scholar] [CrossRef]
- Ziv, M.; Schwartz, A.; Fleminger, D. Malfunctioning stomata in vitreous leaves of carnation (Dianthus caryophyllus) plants propagated in vitro; implications for hardening. Plant Sci. 1987, 52, 127–134. [Google Scholar] [CrossRef]
- Bhatia, N.; Runions, A.; Tsiantis, M. Leaf shape diversity: From genetic modules to computational models. Annu. Rev. Plant Biol. 2021, 72, 325–356. [Google Scholar] [CrossRef] [PubMed]
- Balant, M.; Garnatje, T.; Vitales, D.; Hidalgo, O.; Chitwood, D.H. Intra-leaf modeling of Cannabis leaflet shape produces synthetic leaves that predict genetic and developmental identities. bioRxiv 2008. [Google Scholar]
- Zarei, A.; Davis, B.; Feyissa, B.A.; Dinani, E.T.; Simons, B. Improvement of mineral nutrition and rooting efficiency of Cannabis sativa L. for in vitro large-scale propagation. Vitr. Cell. Dev. Biol. Plant 2023, 59, 95–105. [Google Scholar] [CrossRef]
- Kevers, C.; Gaspar, T. Vitrification of carnation in vitro: Changes in water contents, extracellular space, air volume, and ion levels. Physiol. Végétale 1987, 24, 647–653. [Google Scholar]
- MlllerI, R.G.; Tate, C.; Mallinson, E.; Scherrer, J. Xylose-lysine-tergitol 4: An improved selective agar medium for the isolation of Salmonella. Poult. Sci. 1991, 70, 2429–2432. [Google Scholar] [CrossRef]
- Purohit, S.D.; Teixeira da Silva, J.; Habibi, N. Current approaches for cheaper and better micropropagation technologies. Int. J. Plant Dev. Biol. 2011, 5, 1–36. [Google Scholar]
- Soundararajan, P.; Manivannan, A.; Cho, Y.S.; Jeong, B.R. Exogenous supplementation of silicon improved the recovery of hyperhydric shoots in Dianthus caryophyllus L. by stabilizing the physiology and protein expression. Front. Plant Sci. 2017, 8, 738. [Google Scholar] [CrossRef]
- Wu, H.; Yu, X.; Teixeira da Silva, J.A.; Lu, G. Direct shoot induction of Paeonia lactiflora ‘Zhong Sheng Fen’and rejuvenation of hyperhydric shoots. J. Crop Hortic. Sci. 2011, 39, 271–278. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, F.; Kong, X.; Tian, J.; Wu, Z.; Wu, Z. Effects of multiple factors on hyperhydricity of Allium sativum L. Sci. Hortic. 2017, 217, 285–296. [Google Scholar] [CrossRef]
- Pérez-Tornero, O.; Egea, J.; Olmos, E.; Burgos, L. Control of hyperhydricity in micropropagated apricot cultivars. Vitr. Cell. Dev. Biol.-Plant 2001, 37, 250–254. [Google Scholar] [CrossRef]
- Saher, S.; Piqueras, A.; Hellin, E.; Olmos, E. Prevention of hyperhydricity in micropropagated carnation shoots by bottom cooling: Implications of oxidative stress. Plant Cell Tissue Organ Cult. 2005, 81, 149–158. [Google Scholar] [CrossRef]
- Piqueras, A.; Cortina, M.; Serna, M.; Casas, J. Polyamines and hyperhydricity in micropropagated carnation plants. Plant Sci. 2002, 162, 671–678. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Jones, A.M.P. Morphological characterization of Cannabis sativa L. throughout its complete life cycle. Plants 2023, 12, 3646. [Google Scholar] [CrossRef]
- Piqueras, A.; Han, B.; Van Huylenbroeck, J.; Debergh, P. Effect of different environmental conditions in vitro on sucrose metabolism and antioxidant enzymatic activities in cultured shoots of Nicotiana tabacum L. Plant Growth Regul. 1998, 25, 5–10. [Google Scholar] [CrossRef]
- Caplan, D.; Stemeroff, J.; Dixon, M.; Zheng, Y. Vegetative propagation of cannabis by stem cuttings: Effects of leaf number, cutting position, rooting hormone, and leaf tip removal. Can. J. Plant Sci. 2018, 98, 1126–1132. [Google Scholar] [CrossRef]
- Coffman, C.; Gentner, W. Greenhouse propagation of Cannabis sativa L. by vegetative cuttings. Econ. Bot. 1979, 33, 124–127. [Google Scholar] [CrossRef]
- Punja, Z.K.; Collyer, D.; Scott, C.; Lung, S.; Holmes, J.; Sutton, D. Pathogens and molds affecting production and quality of Cannabis sativa L. Front. Plant Sci. 2019, 10, 1120. [Google Scholar] [CrossRef]
- El-Esawi, M.A. Micropropagation technology and its applications for crop improvement. In Plant Tissue Culture: Propagation, Conservation and Crop Improvement; Springer: Singapore, 2016; pp. 523–545. [Google Scholar]
- Debergh, P.; Aitken-Christie, J.; Cohen, D.; Grout, B.; Von Arnold, S.; Zimmerman, R.; Ziv, M. Reconsideration of the term ‘vitrification’as used in micropropagation. Plant Cell Tissue Organ Cult. 1992, 30, 135–140. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Debergh, P.; Maene, L.; Paques, M.; Boxus, P. Vitrification: Morphological, physiological, and ecological aspects. In Cell and Tissue Culture in Forestry: General Principles and Biotechnology; Springer: Dordrecht, The Netherlands, 1987; pp. 152–166. [Google Scholar]
- Jan, T.; Gul, S.; Khan, A.; Pervez, S.; Noor, A.; Amin, H.; Bibi, S.; Nawaz, M.; Rahim, A.; Ahmad, M. Range of factors in the reduction of hyperhydricity associated with in vitro shoots of Salvia santolinifolia Bioss. Braz. J. Biol. 2021, 83, e246904. [Google Scholar] [CrossRef]
- Leshem, B. Growth of carnation meristems in vitro: Anatomical structure of abnormal plantlets and the effect of agar concentration in the medium on their formation. Ann. Bot. 1983, 52, 413–415. [Google Scholar] [CrossRef]
- Vieitez, A.; Ballester, A.; San-José, M.; Vieitez, E. Anatomical and chemical studies of vitrified shoots of chestnut regenerated in vitro. Physiol. Plant. 1985, 65, 177–184. [Google Scholar] [CrossRef]
- Werker, E.; Leshem, B. Structural changes during vitrification of carnation plantlets. Ann. Bot. 1987, 59, 377–385. [Google Scholar] [CrossRef]
- Fei, L.; Weathers, P. From leaf explants to rooted plantlets in a mist reactor. Vitr. Cell. Dev. Biol.-Plant 2015, 51, 669–681. [Google Scholar] [CrossRef]
- Ivanova, M.; Van Staden, J. Natural ventilation effectively reduces hyperhydricity in shoot cultures of Aloe polyphylla Schönland ex Pillans. Plant Growth Regul. 2010, 60, 143–150. [Google Scholar] [CrossRef]
- Ivanova, M.; Van Staden, J. Influence of gelling agent and cytokinins on the control of hyperhydricity in Aloe polyphylla. Plant Cell Tissue Organ Cult. 2011, 104, 13–21. [Google Scholar] [CrossRef]
- Nikolov, L.A.; Runions, A.; Gupta, M.D.; Tsiantis, M. Leaf development and evolution. Curr. Top. Dev. Biol. 2019, 131, 109–139. [Google Scholar] [PubMed]
- Tsay, H.-S.; Lee, C.-Y.; Agrawal, D.C.; Basker, S. Influence of ventilation closure, gelling agent and explant type on shoot bud proliferation and hyperhydricity in Scrophularia yoshimurae—A medicinal plant. Vitr. Cell. Dev. Biol. Plant 2006, 42, 445–449. [Google Scholar] [CrossRef]
- Tsukaya, H. A consideration of leaf shape evolution in the context of the primary function of the leaf as a photosynthetic organ. Leaf A Platf. Perform. Photosynth. 2018, 44, 1–26. [Google Scholar]
- Vahdati, K.; Aliniaeifard, S. Investigation of physiological components involved in low water conservation capacity of in vitro walnut plants. Sci. Hortic. 2017, 224, 1–7. [Google Scholar]
- Vasudevan, R.; Van Staden, J. Cytokinin and explant types influence in vitro plant regeneration of Leopard Orchid (Ansellia africana Lindl.). Plant Cell Tissue Organ Cult. 2011, 107, 123–129. [Google Scholar] [CrossRef]
- Bhattacharjee, S. Sites of generation and physicochemical basis of formation of reactive oxygen species in plant cell. React. Oxyg. Species Antioxid. High. Plants 2010, 1, 1–30. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef]
- Balen, B.; Tkalec, M.; Pavoković, D.; Pevalek-Kozlina, B.; Krsnik-Rasol, M. Growth conditions in in vitro culture can induce oxidative stress in Mammillaria gracilis tissues. J. Plant Growth Regul. 2009, 28, 36–45. [Google Scholar] [CrossRef]
- Dewir, Y.; Chakrabarty, D.; Ali, M.; Hahn, E.; Paek, K. Lipid peroxidation and antioxidant enzyme activities of Euphorbia millii hyperhydric shoots. Environ. Exp. Bot. 2006, 58, 93–99. [Google Scholar] [CrossRef]
- Jaspers, P.; Kangasjärvi, J. Reactive oxygen species in abiotic stress signaling. Physiol. Plant. 2010, 138, 405–413. [Google Scholar] [CrossRef]
- Muneer, S.; Soundararajan, P.; Jeong, B.R. Proteomic and antioxidant analysis elucidates the underlying mechanism of tolerance to hyperhydricity stress in in vitro shoot cultures of Dianthus caryophyllus. J. Plant Growth Regul. 2016, 35, 667–679. [Google Scholar] [CrossRef]
- Tian, J.; Cheng, Y.; Kong, X.; Liu, M.; Jiang, F.; Wu, Z. Induction of reactive oxygen species and the potential role of NADPH oxidase in hyperhydricity of garlic plantlets in vitro. Protoplasma 2017, 254, 379–388. [Google Scholar] [CrossRef]
- Li, T.; Yun, Z.; Zhang, D.; Yang, C.; Zhu, H.; Jiang, Y.; Duan, X. Proteomic analysis of differentially expressed proteins involved in ethylene-induced chilling tolerance in harvested banana fruit. Front. Plant Sci. 2015, 6, 845. [Google Scholar] [CrossRef]
- Wi, S.J.; Jang, S.J.; Park, K.Y. Inhibition of biphasic ethylene production enhances tolerance to abiotic stress by reducing the accumulation of reactive oxygen species in Nicotiana tabacum. Mol. Cells 2010, 30, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Doubnerová, V.; Ryšlavá, H. What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Sci. 2011, 180, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Vanderschaeghe, A.; Debergh, P. Technical aspects of the control of the relative humidity in tissue culture containers. Meded. Fac. Landbouwwetenschappen. Rijksuniv. Gent 1987, 52, 1429–1437. [Google Scholar]
- De Riek, J.; Piqueras, A.; Debergh, P.C. Sucrose uptake and metabolism in a double layer system for micropropagation of Rosa multiflora. Plant Cell Tissue Organ Cult. 1997, 47, 269–278. [Google Scholar] [CrossRef]
- Alabarrán, J.; Bertrand, B.; Lartaud, M.; Etienne, H. Cycle characteristics in a temporary immersion bioreactor affect regeneration, morphology, water and mineral status of coffee (Coffea arabica) somatic embryos. Plant. Ceii. Tissue Organ. Cult. 2005, 81, 27–36. [Google Scholar] [CrossRef]
- Orozco-Ortiz, C.; Sánchez, L.; Araya-Mattey, J.; Vargas-Solórzano, I.; Araya-Valverde, E. BIT® bioreactor increases in vitro multiplication of quality shoots in sugarcane (Saccharum spp. variety LAICA 04-809). Plant Cell Tissue Organ Cult. 2023, 152, 115–128. [Google Scholar] [CrossRef]
- Brand, M.H. Agar and ammonium nitrate influence hyperhydricity, tissue nitrate and total nitrogen content of serviceberry (Amelanchier arborea) shoots in vitro. Plant Cell Tissue Organ Cult. 1993, 35, 203–209. [Google Scholar] [CrossRef]
- Daguin, F.; Letouze, R. Ammonium-induced vitrification in cultured tissues. Physiol. Plant. 1986, 66, 94–98. [Google Scholar] [CrossRef]
- Ivanova, M.; van Staden, J. Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoots of Aloe polyphylla. Plant Cell Tissue Organ Cult. 2008, 92, 227–231. [Google Scholar] [CrossRef]
- El-Dawayati, M.M.; Zayed, Z.E. Controlling hyperhydricity in date palm in vitro culture by reduced concentration of nitrate nutrients. Date Palm Biotechnol. Protoc. Vol. I Tissue Cult. Appl. 2017, 1637, 175–183. [Google Scholar]
- Nikam, T.D.; Mulye, K.V.; Chambhare, M.R.; Nikule, H.A.; Ahire, M.L. Reduction in hyperhydricity and improvement in in vitro propagation of commercial hard fibre and medicinal glycoside yielding Agave sisalana Perr. ex Engelm by NaCl and polyethylene glycol. Plant Cell Tissue Organ Cult. 2019, 138, 67–78. [Google Scholar] [CrossRef]
- Gantait, S.; Mahanta, M. Hyperhydricity-induced changes among in vitro regenerants of gerbera. S. Afr. J. Bot. 2022, 149, 496–501. [Google Scholar] [CrossRef]
- Fauguel, C.; Vega, T.; Nestares, G.; Zorzoli, R.; Picardi, L. Anatomy of Normal and Hyperhydric Sunflower Shoots Regenerated in vitro. Helia 2008, 31, 17–26. [Google Scholar] [CrossRef]
- Thomas, P.; Mythili, J.; Stumman, B.; Shivashankar, K. Explant, medium and vessel aeration affect the incidence of hyperhydricity and recovery of normal plantlets in triploid watermelon. J. Hortic. Sci. Biotechnol. 2000, 75, 19–25. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, Z.; Lin, Y.; Liu, W.; Guo, H.; Zhang, W.; Zhang, C. Comparative study on antioxidative system in normal and vitrified shoots of Populus suaveolens in tissue culture. For. Stud. China 2004, 6, 1–8. [Google Scholar] [CrossRef]
- Ibrahim, R.; Debergh, P.C. Factors controlling high efficiency adventitious bud formation and plant regeneration from in vitro leaf explants of roses (Rosa hybrida L.). Sci. Hortic. 2001, 88, 41–57. [Google Scholar] [CrossRef]
- Maene, L.; Debergh, P. Optimalisation of the transfer of tissue cultured shoots to in vivo conditions. In Proceedings of Symposium on In Vitro Problems Related to Mass Propagation of Horticultural Plants; ISHS Acta Horticulturae 212: Gembloux, Belgium; pp. 335–348.

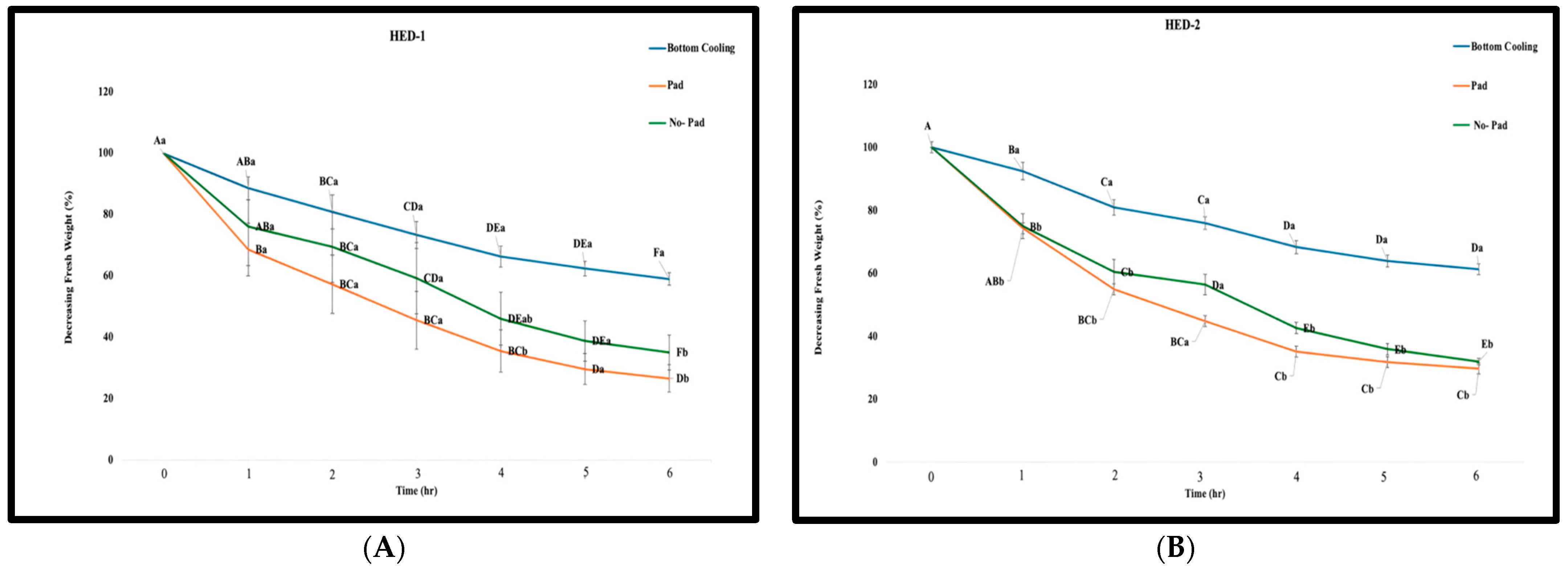

| HED-1 | HED-2 | |||
|---|---|---|---|---|
| Chlorophyll Fluorescence Ratio | Chlorophyll Content (μg cm−2) | Chlorophyll Fluorescence Ratio | Chlorophyll Content (μg cm−2) | |
| Bottom Cooling | 1.38 ± 0.057 a | 488.7 ± 35.16 a | 1.30 ± 0.028 a | 441.0 ± 30.02 a |
| Pad | 1.28 ± 0.054 b | 423.0 ± 35.13 b | 1.18 ± 0.059 b | 369.3 ± 36.31 b |
| No-Pad | 1.20 ± 0.077 b | 374.7 ± 49.57 b | 1.13 ± 0.046 b | 330.3 ± 30.75 b |
| Correlation coefficient | 0.999 | 0.998 | ||
| HED-1 | HED-2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Leaflets | No. of Primary Serrations of the Central Leaflet | Plant Length (mm) | Length of the C. L. (mm) | Survival Rate | No. of Leaflets | No. of Primary Serrations of the Central Leaflet | Plant Length (mm) | Length of the C.L. (mm) | Survival Rate | |
| Bottom Cooling | 7.0 ± 2.0 a | 10.4 ± 1.0 a | 50.7 ± 4.7 b | 37.4 ± 4.0 a | 83.33 ± 6.45 a | 12.0 ± 1.0 a | 9.00 ± 2.0 a | 52.7 ± 6.6 a | 34.4 ± 2.0 a | 91.66 ± 4.68 a |
| Pad | 6.0 ± 3.0 a | 8 ± 1.00 a | 78.3 ± 3.6 a | 24.2 ± 2.4 ab | 58.33 ± 5.76 b | 8 ± 1.00 b | 7 ± 1.00 a | 52.4 ± 3.2 a | 22.2 ± 2.6 a | 66.66 ± 3.43 b |
| No-Pad | 6.0 ± 1.0 a | 7 ± 3.00 a | 68.02 ± 4.6 ab | 28.9 ± 1.7 b | 58.33 ± 5.98 b | 7 ± 2.00 c | 8 ± 2.00 a | 52.01 ± 3.8 a | 25.3 ± 1.9 a | 66.66 ± 5.46 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abiri, R.; O’Reilly, D.; Jones, A.M.P. Bottom Cooling During Culture Initiation Increases Survival and Reduces Hyperhydricity in Micropropagated Cannabis Plants. Plants 2025, 14, 886. https://doi.org/10.3390/plants14060886
Abiri R, O’Reilly D, Jones AMP. Bottom Cooling During Culture Initiation Increases Survival and Reduces Hyperhydricity in Micropropagated Cannabis Plants. Plants. 2025; 14(6):886. https://doi.org/10.3390/plants14060886
Chicago/Turabian StyleAbiri, Rambod, Declan O’Reilly, and Andrew Maxwell Phineas Jones. 2025. "Bottom Cooling During Culture Initiation Increases Survival and Reduces Hyperhydricity in Micropropagated Cannabis Plants" Plants 14, no. 6: 886. https://doi.org/10.3390/plants14060886
APA StyleAbiri, R., O’Reilly, D., & Jones, A. M. P. (2025). Bottom Cooling During Culture Initiation Increases Survival and Reduces Hyperhydricity in Micropropagated Cannabis Plants. Plants, 14(6), 886. https://doi.org/10.3390/plants14060886







