Identification of the UGT Family and Functional Validation of MwUGT2 in Meconopsis wilsonii
Abstract
:1. Introduction
2. Results
2.1. Identification of MwUGTs in M. wilsonii
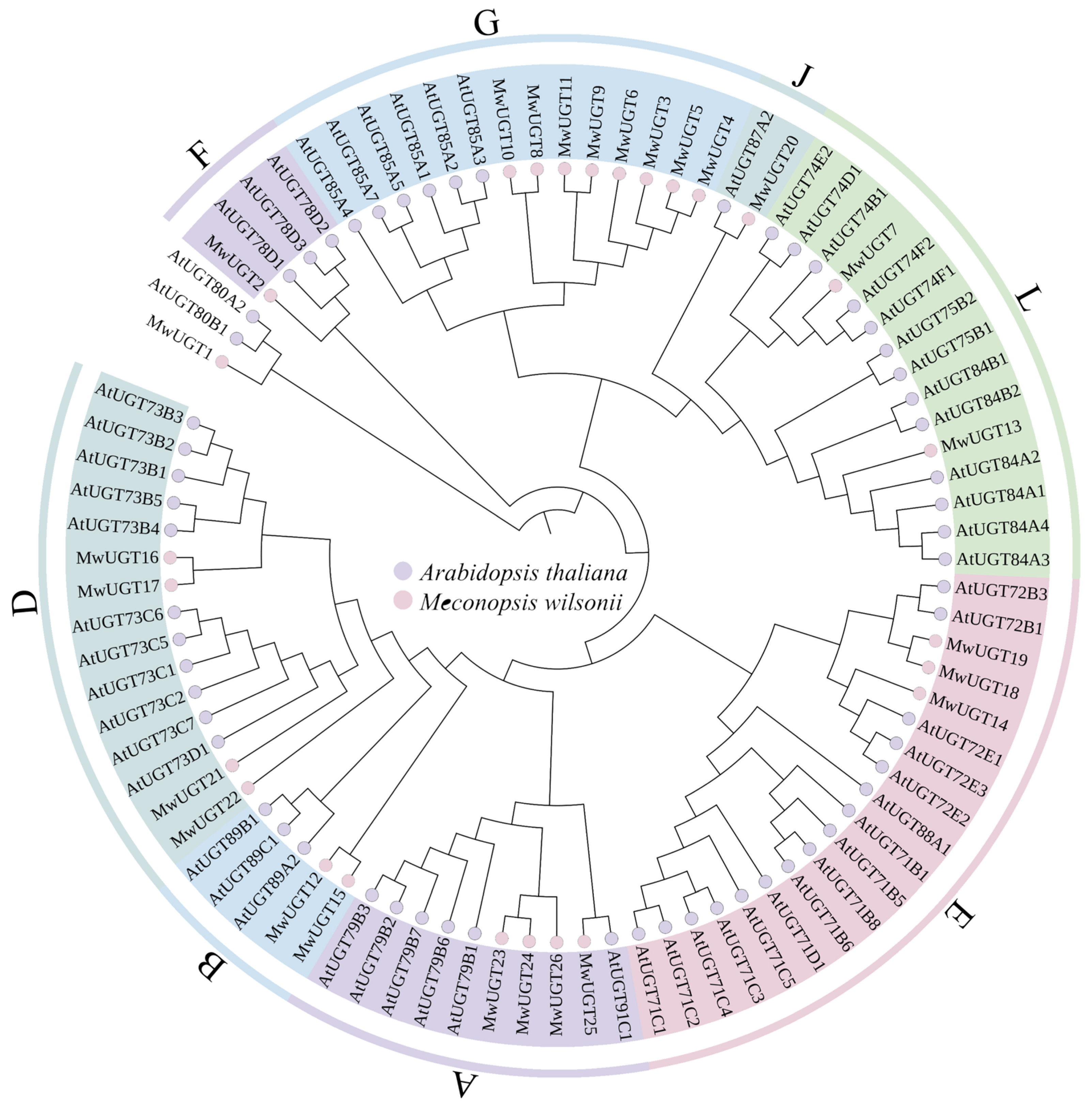
2.2. Phylogenetic Analysis of MwUGTs
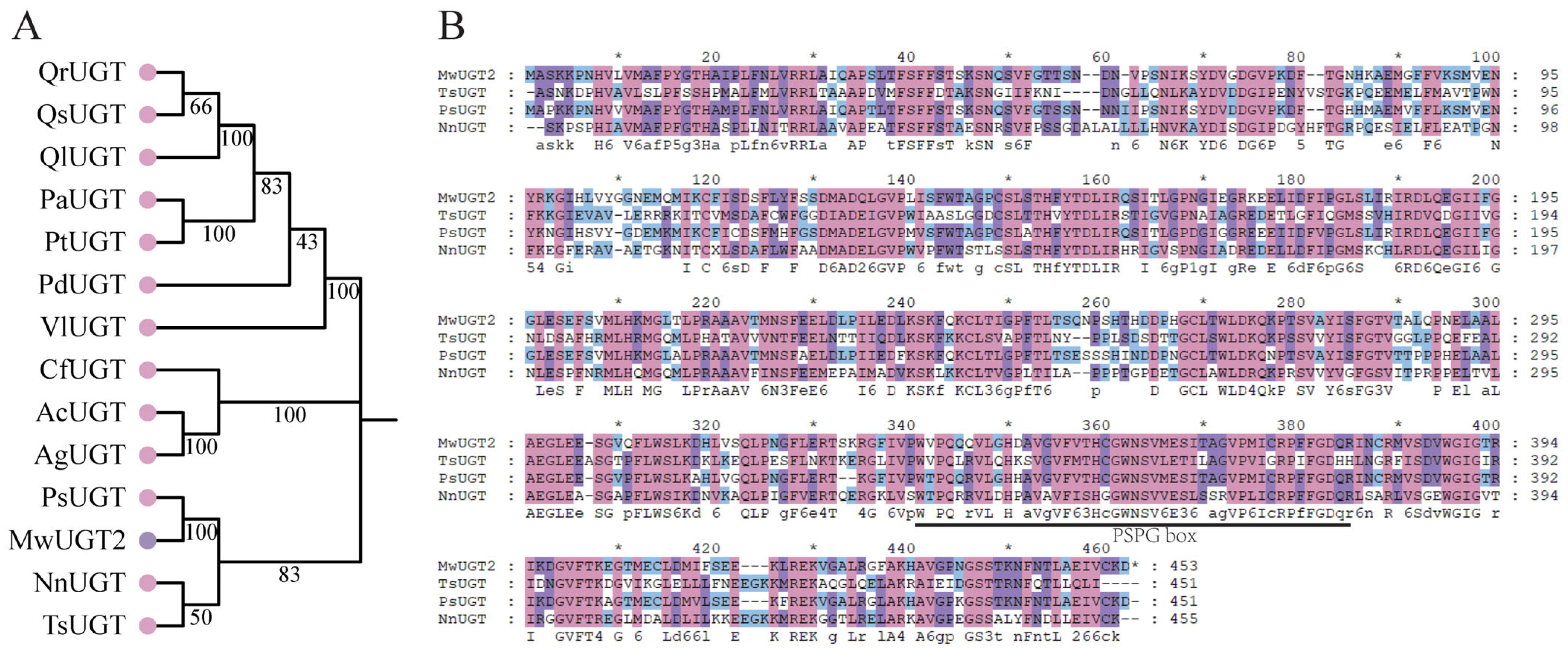
2.3. Cloning and Sequence Analysis of MwUGT2 from M. wilsonii
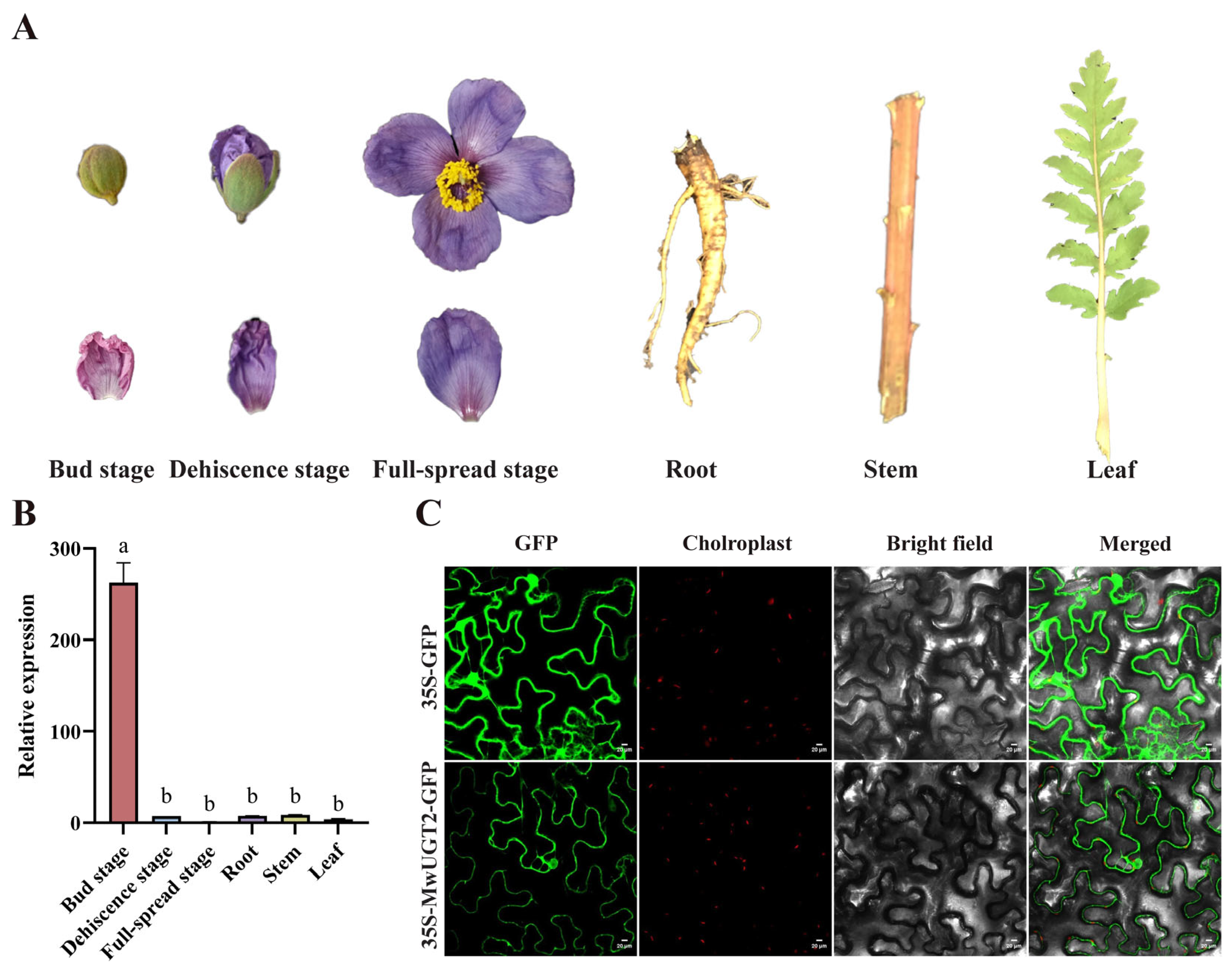
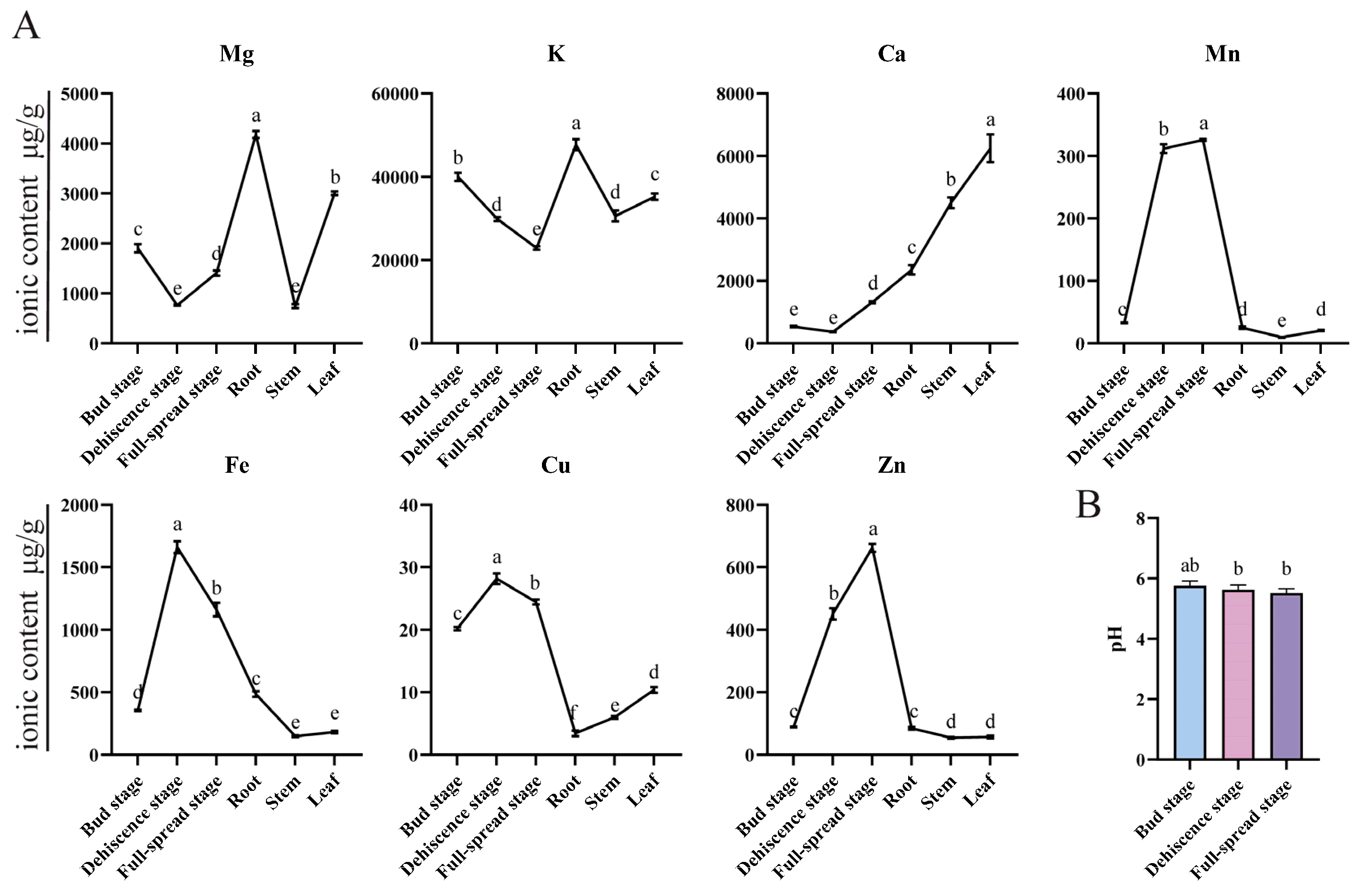
2.4. Analysis of MwUGT2 Expression Patterns and Subcellular Localization
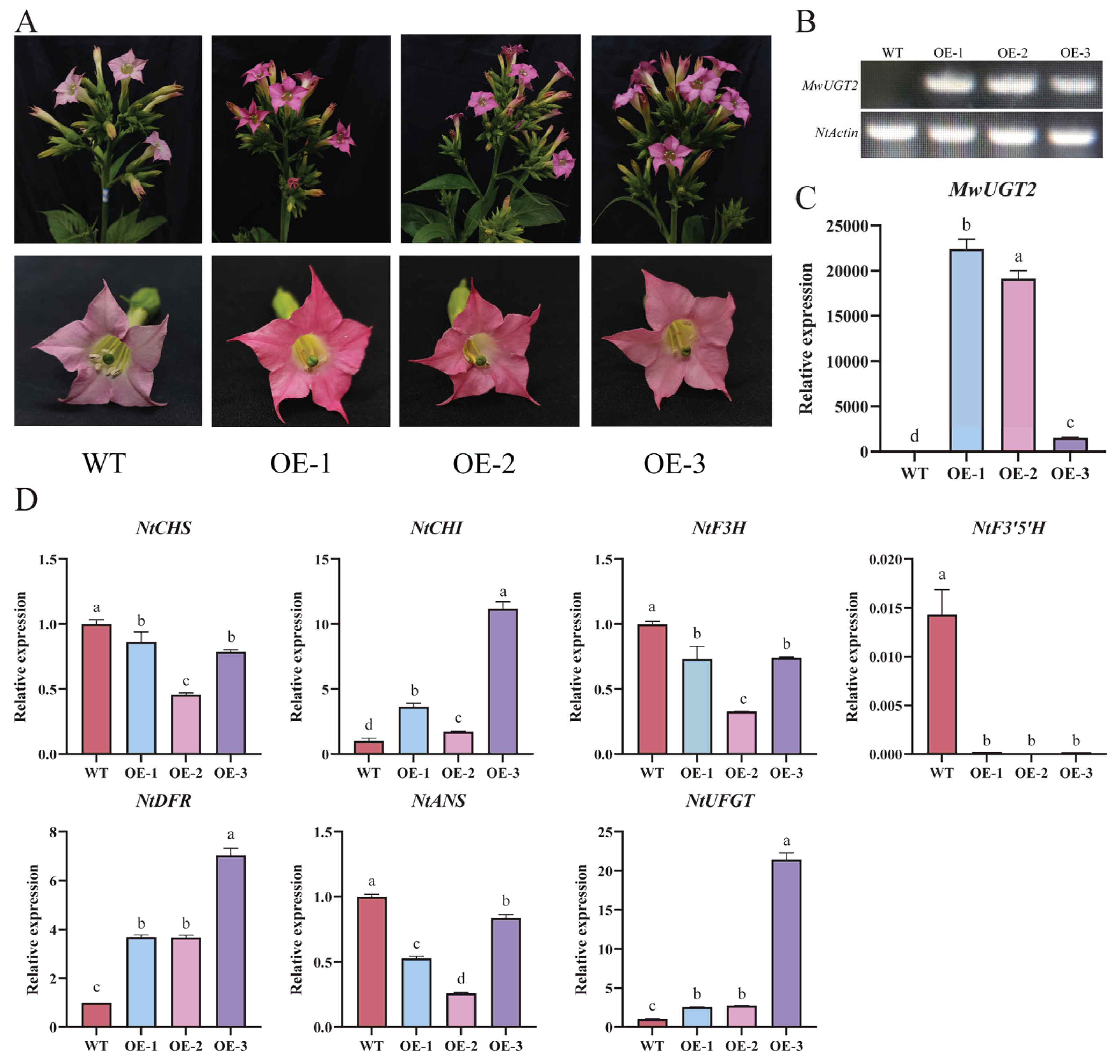
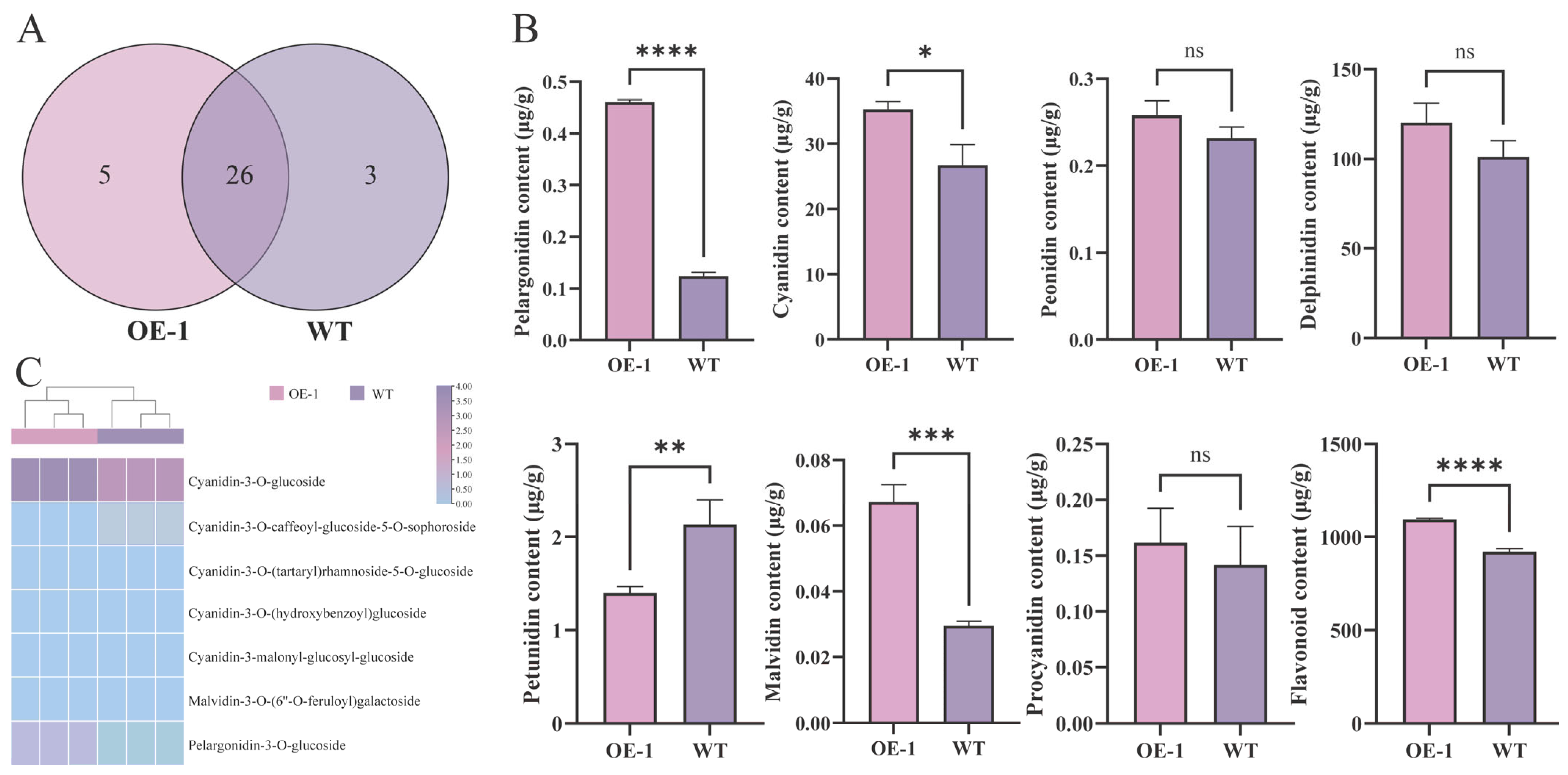
2.5. MwUGT2 Promotes Anthocyanin Accumulation in Transgenic Tobacco
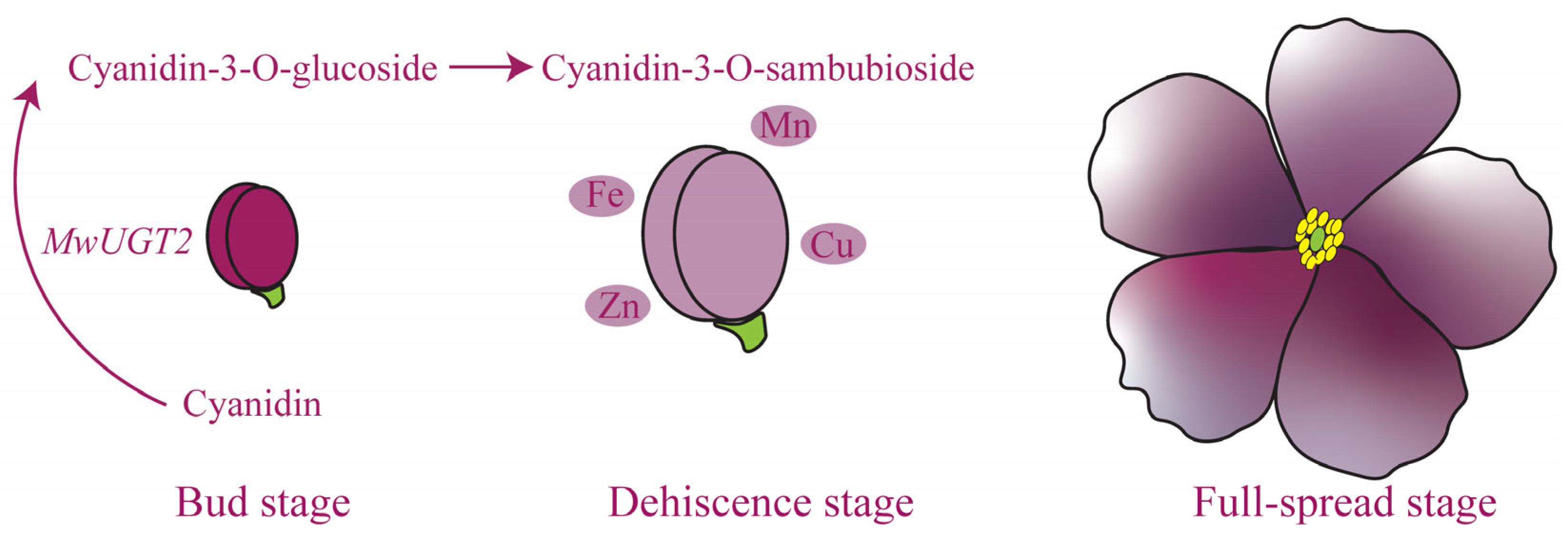
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Identification of MwUGTs
4.3. Phylogenetic Analysis of MwUGTs
4.4. Cloning MwUGT2
4.5. Construction of Overexpression Vectors
4.6. Agrobacterium-Mediated Infiltration Tobacco
4.7. Quantitative Real-Time PCR Analysis
4.8. Targeted Metabolome Testing and Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chai, Q.; Wang, X.; Gao, M.; Zhao, X.; Chen, Y.; Zhang, C.; Jiang, H.; Wang, J.; Wang, Y.; Zheng, M.; et al. A glutathione S-transferase GhTT19 determines flower petal pigmentation via regulating anthocyanin accumulation in cotton. Plant Biotechnol. J. 2022, 21, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhou, N.; Wang, Y.; Sun, S.; Zhang, Y.; Ju, Z.; Yi, Y. Characterization and functional analysis of RdDFR1 regulation on flower color formation in Rhododendron delavayi. Plant Physiol. Biochem. 2021, 169, 203–210. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, X.; Wang, J.; Ran, J.; Xue, Z.; Zhang, X.; Yan, G.; Wu, C.; Zhou, Y.; Zhang, K. Combination of metabolome and transcriptome reveals flower color change candidate genes of Prunus humilis. Sci. Hortic. 2024, 336, 113364. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.-J.; Wang, Y.; Liu, S.; Geng, Z.; Song, A.; Jiang, J.; Chen, S.; Chen, F. Functional identification of a flavone synthase and a flavonol synthase genes affecting flower color formation in Chrysanthemum morifolium. Plant Physiol. Biochem. 2021, 166, 1109–1120. [Google Scholar] [CrossRef]
- Yao, P.; Deng, R.; Huang, Y.; Stael, S.; Shi, J.; Shi, G.; Lv, B.; Li, Q.; Dong, Q.; Wu, Q.; et al. Diverse biological effects of glycosyltransferase genes from Tartary buckwheat. BMC Plant Biol. 2019, 19, 339. [Google Scholar] [CrossRef]
- Rao, M.J.; Xu, Y.; Huang, Y.; Tang, X.; Deng, X.; Xu, Q. Ectopic expression of citrus UDP-GLUCOSYL TRANSFERASE gene enhances anthocyanin and proanthocyanidins contents and confers high light tolerance in Arabidopsis. BMC Plant Biol. 2019, 19, 603. [Google Scholar] [CrossRef]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Rev. Int. 2022, 39, 4581–4609. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Zhang, D.; Su, D.; Li, W.; Wang, X.; Chen, Q.; Cai, W.; Xu, L.; Cao, F.; et al. Comprehensive analysis of metabolome and transcriptome reveals the mechanism of color formation in different leave of Loropetalum Chinense var. Rubrum. BMC Plant Biol. 2023, 23, 133. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhou, H.; Ren, T.; Yu, E.-R.; Feng, B.; Wang, J.; Zhang, C.; Zhou, C.; Li, Y. Integrated transcriptome and metabolome analysis revealed that HaMYB1 modulates anthocyanin accumulation to deepen sunflower flower color. Plant Cell Rep. 2024, 43, 74. [Google Scholar] [CrossRef]
- Muhammad, N.; Luo, Z.; Yang, M.; Li, X.; Liu, Z.; Liu, M. The joint role of the late anthocyanin biosynthetic UFGT-encoding genes in the flowers and fruits coloration of horticultural plants. Sci. Hortic. 2022, 301, 111110. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Bartoszek, A. Relationship between Chemical Structure and Biological Activity Evaluated In Vitro for Six Anthocyanidins Most Commonly Occurring in Edible Plants. Molecules 2023, 28, 6156. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Mikami, R.; Akita, Y. Characterization of 5-O-glucosyltransferase involved in anthocyanin biosynthesis in Cyclamen purpurascens. Plant Biotechnol. 2021, 38, 263–268. [Google Scholar] [CrossRef]
- Tasaki, K.; Yoshida, M.; Nakajima, M.; Higuchi, A.; Watanabe, A.; Nishihara, M. Molecular characterization of an anthocyanin-related glutathione S-transferase gene in Japanese gentian with the CRISPR/Cas9 system. BMC Plant Biol. 2020, 20, 370. [Google Scholar] [CrossRef]
- Feng, Z.; Admas, T.; Cheng, B.; Meng, Y.; Pan, R.; Zhang, W. UGT gene family identification and functional analysis of HvUGT1 under drought stress in wild barley. Physiol. Mol. Biol. Plants 2024, 30, 1225–1238. [Google Scholar] [CrossRef]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Y.; Qu, X.; Wu, F.; Li, X.; Ren, M.; Tong, Y.; Wu, X.; Yang, A.; Chen, Y.; et al. Genome-wide analysis of UDP-glycosyltransferases family and identification of UGT genes involved in abiotic stress and flavonol biosynthesis in Nicotiana tabacum. BMC Plant Biol. 2023, 23, 204. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, X.; Li, B.; Zhao, X.; Shen, Y.; Yuan, Z. Genome-wide identification and characterization of bZIP gene family and cloning of candidate genes for anthocyanin biosynthesis in pomegranate (Punica granatum). BMC Plant Biol. 2022, 22, 170. [Google Scholar] [CrossRef]
- Song, Y.; Shang, W.; Ma, D.; Wang, Z.; He, S.; Shi, L.; Shen, Y.; He, D.; Wang, E.; Wang, X. Effect on the Growth and Photosynthetic Characteristics of Anthurium andreanum (‘Pink Champion’, ‘Alabama’) under Hydroponic Culture by Different LED Light Spectra. Horticulturae 2022, 8, 389. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Fu, Y.; Gao, Y.; Lu, H.; Xu, L. Transcriptome profiling in the spathe of Anthurium andraeanum ‘Albama’ and its anthocyanin-loss mutant ‘Xueyu’. Sci. Data 2018, 5, 180247. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Gao, Y.; Jing, Y.; Li, J.; Xu, L. The AnUFGT1 Is Involved in the Anthurium ‘Alabama’ Anthocyanidin Deficiency. Horticulturae 2024, 10, 369. [Google Scholar] [CrossRef]
- Fukuchi-Mizutani, M.; Okuhara, H.; Fukui, Y.; Nakao, M.; Katsumoto, Y.; Yonekura-Sakakibara, K.; Kusumi, T.; Hase, T.; Tanaka, Y. Biochemical and Molecular Characterization of a Novel UDP-Glucose:Anthocyanin 3′-O-Glucosyltransferase, a Key Enzyme for Blue Anthocyanin Biosynthesis, from Gentian. Plant Physiol. 2003, 132, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, X.; Yu, X.; Yuan, T.; Zhang, G.; Liu, C.; Li, X.; Wei, P.; Li, X.; Liu, X. Comparative Analysis of Chloroplast Genome of Meconopsis (Papaveraceae) Provides Insights into Their Genomic Evolution and Adaptation to High Elevation. Int. J. Mol. Sci. 2024, 25, 2193. [Google Scholar] [CrossRef]
- Qu, Y.; Ou, Z.; Wang, S. Coloration differences in three Meconopsis species: M. punicea, M. integrifolia and M. wilsonii. S. Afr. J. Bot. 2022, 150, 171–177. [Google Scholar] [CrossRef]
- Ou, Z.; Luo, J.; Qu, Y. Exploring the molecular mechanism of coloration differences in two Meconopsis wilsonii subspecies: Australis and orientalis. Dev. Biol. 2024, 505, 1–10. [Google Scholar] [CrossRef]
- Ren, Z.; Qiu, F.; Wang, Y.; Yu, W.; Liu, C.; Sun, Y.; Wang, Y.; Zhang, X.; Xing, S.; Tao, S.; et al. Network Analysis of Transcriptome and LC-MS Reveals a Possible Biosynthesis Pathway of Anthocyanins in Dendrobium officinale. BioMed Res. Int. 2020, 2020, 6512895. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Guan, R.; Sun, X.; Zhang, C.; Shan, C.; Liu, M.; Cui, N.; Wang, P.; Lin, H. Integrated metabolome and transcriptome analyses of anthocyanin biosynthesis reveal key candidate genes involved in colour variation of Scutellaria baicalensis flowers. BMC Plant Biol. 2023, 23, 643. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Fan, W.; Yang, J.; Appelhagen, I.; Wu, Y.; Zhang, P. A novel glycosyltransferase catalyses the transfer of glucose to glucosylated anthocyanins in purple sweet potato. J. Exp. Bot. 2018, 69, 5444–5459. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.H.; Jeong, B.R.; Koo, K.B.; Choi, J.W.; Hirsch, A.M.; Hawes, M.C. Modifying expression of closely related UDP-glycosyltransferases from pea and Arabidopsis results in altered root development and function. Physiol. Plant. 2007, 130, 250–260. [Google Scholar] [CrossRef]
- Woo, H.-H.; Jeong, B.R.; Hirsch, A.M.; Hawes, M.C. Characterization of Arabidopsis AtUGT85A and AtGUS gene families and their expression in rapidly dividing tissues. Genomics 2007, 90, 143–153. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Guo, D.; Gao, T.; Liu, T.; Jin, J.; Zhao, M.; Yu, K.; Tong, W.; Ge, H.; et al. Evolution and functional divergence of glycosyltransferase genes shaped the quality and cold tolerance of tea plants. Plant Cell 2025, 37, koae268. [Google Scholar] [CrossRef]
- Ma, Y.; Song, J.; Sheng, S.; Wang, D.; Wang, T.; Wang, N.; Chen, A.; Wang, L.; Peng, Y.; Ma, Y.; et al. Genome-wide characterization of Solanum tuberosum UGT gene family and functional analysis of StUGT178 in salt tolerance. BMC Genom. 2024, 25, 1206. [Google Scholar] [CrossRef]
- Feng, K.; Xu, Z.-S.; Liu, J.-X.; Li, J.-W.; Wang, F.; Xiong, A.-S. Isolation, purification, and characterization of AgUCGalT1, a galactosyltransferase involved in anthocyanin galactosylation in purple celery (Apium graveolens L.). Planta 2018, 247, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Arai, Y.; Nagashima, S.; Yoshikawa, T. Alteration of sugar donor specificities of plant glycosyltransferases by a single point mutation. Arch. Biochem. Biophys. 2004, 429, 198–203. [Google Scholar] [CrossRef]
- Zhao, A.; Li, R.; Guo, W.; Lei, K.; Ji, L.; Li, P. Plant secondary metabolites: Flavonoids and their glycosylation modification. Biol. Plant. 2024, 68, 39–49. [Google Scholar] [CrossRef]
- Sun, W.; Sun, S.; Xu, H.; Wang, Y.; Chen, Y.; Xu, X.; Yi, Y.; Ju, Z. Characterization of Two Key Flavonoid 3-O-Glycosyltransferases Involved in the Formation of Flower Color in Rhododendron Delavayi. Front. Plant Sci. 2022, 13, 863482. [Google Scholar] [CrossRef]
- Deng, J.; Su, M.; Zhang, X.; Liu, X.; Damaris, R.N.; Lv, S.; Yang, P. Proteomic and metabolomic analyses showing the differentially accumulation of NnUFGT2 is involved in the petal red-white bicolor pigmentation in lotus (Nelumbo nucifera). Plant Physiol. Biochem. 2023, 198, 107675. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, F.; Liu, Z.a.; Peng, L.; Shu, Q. PhUGT78A22, a novel glycosyltransferase in Paeonia ‘He Xie’, can catalyze the transfer of glucose to glucosylated anthocyanins during petal blotch formation. BMC Plant Biol. 2022, 22, 405. [Google Scholar] [CrossRef]
- De Vetten, N.; ter Horst, J.; van Schaik, H.-P.; de Boer, A.; Mol, J.; Koes, R. A cytochrome b5 is required for full activity of flavonoid 3′,5′-hydroxylase, a cytochrome P450 involved in the formation of blue flower colors. Proc. Natl. Acad. Sci. USA 1999, 96, 778–783. [Google Scholar] [CrossRef]
- Kusano, M.; He, H.; Ke, H.; Keting, H.; Qiaoyan, X.; Silan, D. Flower Colour Modification of Chrysanthemum by Suppression of F3’H and Overexpression of the Exogenous Senecio cruentus F3’5’H Gene. PLoS ONE 2013, 8, e74395. [Google Scholar] [CrossRef]
- Whang, S.S.; Um, W.S.; Song, I.-J.; Lim, P.O.; Choi, K.; Park, K.-W.; Kang, K.-W.; Choi, M.S.; Koo, J.C. Molecular Analysis of Anthocyanin Biosynthetic Genes and Control of Flower Coloration by Flavonoid 3′,5′-Hydroxylase (F3′5′H) in Dendrobium moniliforme. J. Plant Biol. 2011, 54, 209–218. [Google Scholar] [CrossRef]
- Han, X.; Luo, Y.; Lin, J.; Wu, H.; Sun, H.; Zhou, L.; Chen, S.; Guan, Z.; Fang, W.; Zhang, F.; et al. Generation of purple-violet chrysanthemums via anthocyanin B-ring hydroxylation and glucosylation introduced from Osteospermum hybrid F3′5′H and Clitoria ternatea A3′5′GT. Ornam. Plant Res. 2021, 1, 4. [Google Scholar] [CrossRef]
- Liu, W.; Tang, R.; Zhang, Y.; Liu, X.; Gao, Y.; Dai, Z.; Li, S.; Wu, B.; Wang, L. Genome-wide identification of B-box proteins and VvBBX44 involved in light-induced anthocyanin biosynthesis in grape (Vitis vinifera L.). Planta 2021, 253, 114. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Mu, H.; Yuan, L.; Li, Y.; Li, Y.; Li, S.; Ren, C.; Duan, W.; Fan, P.; Dai, Z.; et al. VvBBX44 and VvMYBA1 form a regulatory feedback loop to balance anthocyanin biosynthesis in grape. Hortic. Res. 2023, 10, uhad176. [Google Scholar] [CrossRef]
- Zhen, Z.; Cui, C.; Hong, L.; Changyue, J.; Yuhui, Z.; Yinshan, G. The VvHY5-VvMYB24-VvMYBA1 transcription factor cascade regulates the biosynthesis of anthocyanin in grape. Hortic. Plant J. 2024, in press. [Google Scholar] [CrossRef]
- Sun, W.; Liang, L.; Meng, X.; Li, Y.; Gao, F.; Liu, X.; Wang, S.; Gao, X.; Wang, L. Biochemical and Molecular Characterization of a Flavonoid 3-O-glycosyltransferase Responsible for Anthocyanins and Flavonols Biosynthesis in Freesia hybrida. Front. Plant Sci. 2016, 7, 410. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Bai, X.; Tan, Y.; Xie, W.; Feng, Y.; Yang, G.-Y. Glycosyltransferases: Mining, engineering and applications in biosynthesis of glycosylated plant natural products. Synth. Syst. Biotechnol. 2022, 7, 602–620. [Google Scholar] [CrossRef]
- Yoshida, K.; Oniduka, T.; Oyama, K.-i.; Kondo, T. Blue flower coloration of Corydalis ambigua requires ferric ion and kaempferol glycoside. Biosci. Biotechnol. Biochem. 2021, 85, 61–68. [Google Scholar] [CrossRef]
- Jiao, F.; Zhao, L.; Wu, X.; Song, Z.; Li, Y. Metabolome and transcriptome analyses of the molecular mechanisms of flower color mutation in tobacco. BMC Genom. 2020, 21, 611. [Google Scholar] [CrossRef]
- Houghton, A.; Appelhagen, I.; Martin, C. Natural Blues: Structure Meets Function in Anthocyanins. Plants 2021, 10, 726. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Lu, C.; Wang, Z.; Deng, C.; Gao, K.; Li, J.; Fang, Z.; Liu, H.; Hong, Y.; et al. Flavonoid extracts from chrysanthemum with appropriate anthocyanins turn blue when exposed to iron ions. Hortic. Plant J. 2024, 10, 837–852. [Google Scholar] [CrossRef]
- Momonoi, K.; Yoshida, K.; Mano, S.; Takahashi, H.; Nakamori, C.; Shoji, K.; Nitta, A.; Nishimura, M. A vacuolar iron transporter in tulip, TgVit1, is responsible for blue coloration in petal cells through iron accumulation. Plant J. 2009, 59, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kitahara, S.; Ito, D.; Kondo, T. Ferric ions involved in the flower color development of the Himalayan blue poppy, Meconopsis grandis. Phytochemistry 2006, 67, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Ning, G.; Xiao, X.; Lv, H.; Li, X.; Zuo, Y.; Bao, M. Shortening tobacco life cycle accelerates functional gene identification in genomic research. Plant Biol. 2012, 14, 934–943. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Chen, X.; Su, W.; Ou, Z.; Qu, Y. Identification of the UGT Family and Functional Validation of MwUGT2 in Meconopsis wilsonii. Plants 2025, 14, 944. https://doi.org/10.3390/plants14060944
Zhou L, Chen X, Su W, Ou Z, Qu Y. Identification of the UGT Family and Functional Validation of MwUGT2 in Meconopsis wilsonii. Plants. 2025; 14(6):944. https://doi.org/10.3390/plants14060944
Chicago/Turabian StyleZhou, Lin, Xiaojuan Chen, Wenkun Su, Zhi Ou, and Yan Qu. 2025. "Identification of the UGT Family and Functional Validation of MwUGT2 in Meconopsis wilsonii" Plants 14, no. 6: 944. https://doi.org/10.3390/plants14060944
APA StyleZhou, L., Chen, X., Su, W., Ou, Z., & Qu, Y. (2025). Identification of the UGT Family and Functional Validation of MwUGT2 in Meconopsis wilsonii. Plants, 14(6), 944. https://doi.org/10.3390/plants14060944





