Abstract
Pigmented rice, particularly the black and red varieties, is popular due to its better nutritional value. Anthocyanins and proanthocyanidins are two major flavonoid subcategories with broad physiological functions and therapeutic significance. However, pigment deposition is a complex process, and the molecular mechanism involved remains unknown. This review explores the metabolites responsible for the pigmentation in various rice tissues. Moreover, the current challenges, feasible strategies, and potential future directions in pigmented rice research are reported.
1. Introduction
Rice (Oryza sativa L.) is a major cereal crop for almost half of the world’s population [1]. Its demand continues to rise as the world’s population is projected to reach 9.7 billion by 2050 [2,3]. Rice offers diverse dietary nutrients, including carbohydrates, vitamins, and micronutrients [4,5]. Unfortunately, dehulling and milling processes remove many nutrients, particularly micronutrients, fatty acids, antioxidants, and fiber [6]. As a result, developing countries where rice is a staple food are experiencing micronutrient deficiencies, along with a significant increase in lifestyle-related diseases, such as diabetes, hypertension, and obesity [7]. Thus, there is an urgent need to produce rice with better nutritional value. To date, several bioactive compounds and micronutrient modulation processes have been employed to develop rice with superior nutritional quality for frequent consumers.
Pigmented rice exhibits significant genetic diversity in tissue coloration, including leaf, culm, apiculus, stigma, caryopses, and hull, with color variations ranging from dark purple to maroon to green (Figure 1) [8]. Black and red rice caryopses are rich in amino acids, functional lipids, dietary fiber, vitamins, minerals, anthocyanins, and phenolic compounds and are marketed as health-promoting foods for rice consumers [9,10]. Moreover, pigmented rice is known to offer a variety of potential health benefits, such as anti-inflammatory, antioxidant, anticancer, and hypoglycemic activities [11]. They also serve as a widely used natural food colorant [12]. Multi-omics approaches have facilitated numerous genetic and biochemical discoveries in pigmented rice cultivars [13]. Despite the widening knowledge of pigmented rice formation and function, the molecular mechanisms underlying rice pigment deposition remain to be elucidated.
In this review, we summarize the available information on the metabolites and corresponding genes responsible for grain pigment composition and highlight the challenges and strategies for future research in this field.
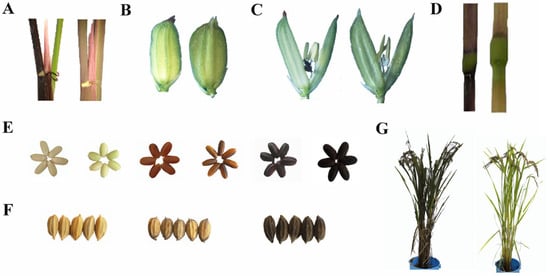
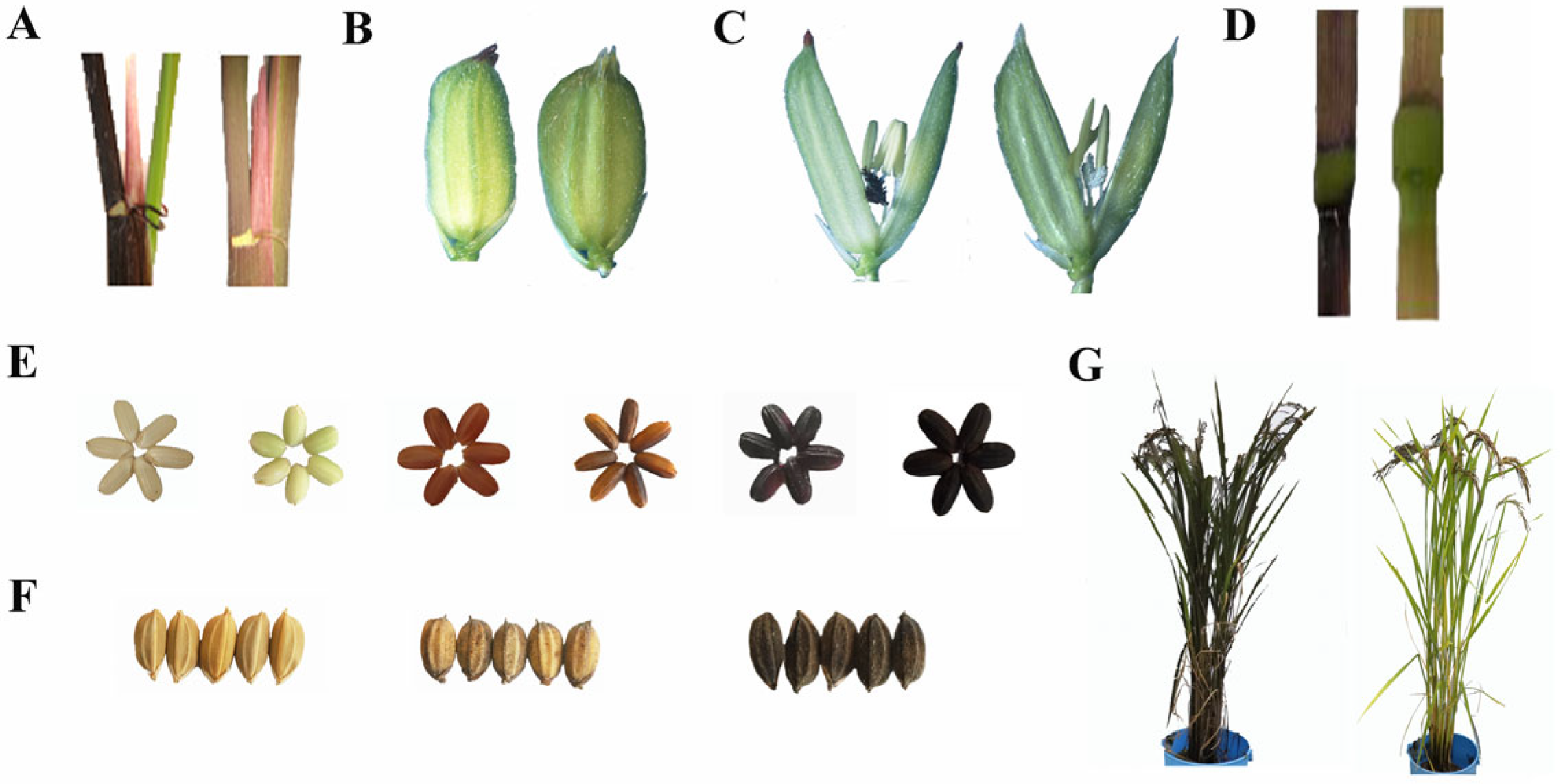
Figure 1.
Genetic diversity involving rice pigmentation. (A) Leaf sheath; (B) apiculi; (C) stigma; (D) culm; (E) seed pericarp; (F) seed hull; (G) whole plant.
Figure 1.
Genetic diversity involving rice pigmentation. (A) Leaf sheath; (B) apiculi; (C) stigma; (D) culm; (E) seed pericarp; (F) seed hull; (G) whole plant.
2. Identification of Metabolites in Black/Red Rice Grains
Colored grains are known for their superior nutritional value and are used as food colorants. Until recently, most studies on pigmented rice have focused on the complex relationship between bioactive compounds and antioxidants in black and red rice [14,15]. With the advancements in metabolomic technology, an increasing number of pigmented metabolites associated with pigmented rice grains have been identified [16,17]. Anthocyanins are responsible for the purple to black pigmentation in rice. Genetic diversity affects the anthocyanin content and composition in rice grains. Black rice contains approximately 18 distinct anthocyanins, with cyanidin 3-O-glucoside (C3G) and peonidin 3-O-glucoside (P3G) as the two primary anthocyanins, constituting 64–90% and 5–28% of the total anthocyanins, respectively [18,19,20,21]. Similarly, Mackon et al. (2023) employed high-performance liquid chromatography (HPLC) to examine anthocyanin content at different developmental stages of rice caryopsis [22]. They reported that anthocyanin deposition begins around 8 days post-flowering (DPF), continues from 10 to 20 DPF, and reaches a peak during the dough phase. Zhang et al. (2023) screened 12 anthocyanins, particularly cyanidin 3-O-galactoside, C3G, P3G, and cyanidin 3-O-rutinoside, all with relatively high content, using non-targeted metabolomics [23]. Furthermore, there is a complex diversity in the anthocyanin content among black rice cultivars. Proanthocyanidins, also known as condensed tannins, are responsible for red pigmentation through their oligomeric or polymerized flavan-3-ol units [24,25]. Procyanidin was identified as the major component in the bran layer of red-hulled rice [25]. Chen et al. (2016) analyzed 28 red rice varieties to systematically access the contents and proportions of proanthocyanidin oligomers and polymers in the bran layer [26,27]. The results showed significant differences in the phytochemical composition across genotypes, indicating variations in the proanthocyanidin biosynthesis among the varieties [26,27]. In addition, Sedeek et al. (2023) identified a total of 625 metabolites in 63 pigmented rice varieties, of which 375 metabolites showed significant differences in the abundance between red and black rice, indicating the genetic diversity of pigment deposition in grains [13]. Overall, there are limited reports on the anthocyanin or proanthocyanidin composition among different pigmented rice varieties, and further research is needed to elucidate these variations.
3. The Genetic Basis of Pigmentation
Flavonoids are common bioactive secondary metabolites found in higher plants, and they strictly regulate flowers, fruits, seeds, and other tissue pigmentation [28]. Anthocyanins and proanthocyanidins are the products of a specialized flavonoid axis (Figure 2) involving numerous structural (Table 1) and modulatory genes, whose combined action determines the pigmentation of various rice tissues (Table 2) [29]. Thus, rice pigmentation is managed by a CAP system, where “C” (chromogen) refers to structural genes, and “A” (activator) and “P” (tissue-specific modulator) refer to regulatory genes [30].
3.1. Anthocyanins and Proanthocyanidins Biosynthesis in Rice
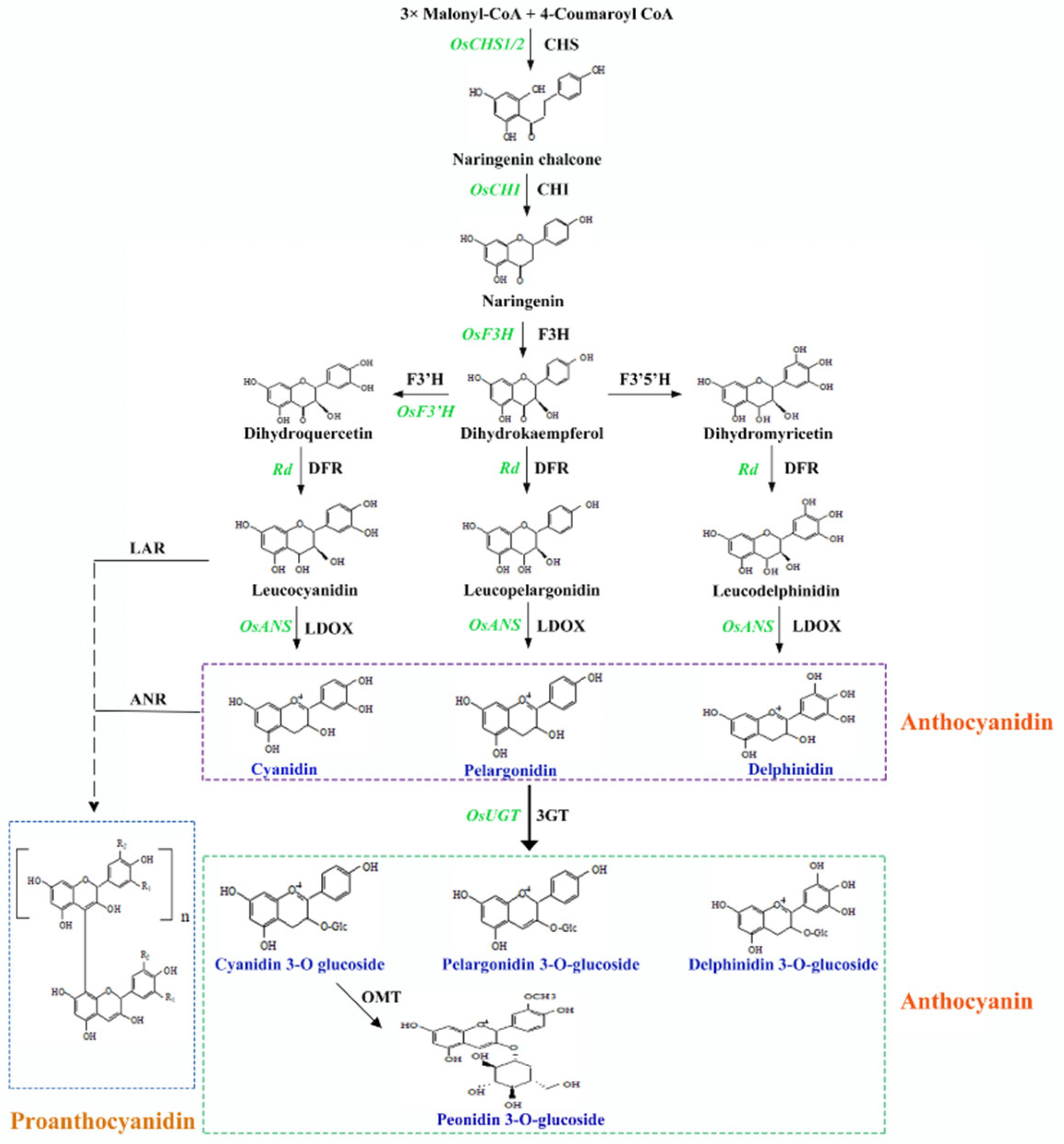
Anthocyanidin biosynthesis begins with malonyl-CoA and 4-coumaroyl-CoA, in the presence of chalcone synthase (CHS) and chalcone isomerase (CHI), to produce naringenin, the precursor of many flavonoids (Figure 2). Naringenin is then converted to dihydrokaempferol by flavanone 3-hydroxylase (F3H). Dihydrokaempferol is then hydroxylated to form dihydroquercetin and dihydromyricetin through the action of flavonoid 3′ hydroxylase (F3′H) and 3′5′ hydroxylase (F3′5′H), respectively. These three dihydroflavonols undergo reduction to leucoanthocyanidins under the action of dihydroflavonol reductase (DFR). Leucoanthocyanidins are then sequentially oxidized to form anthocyanidins under the action of leucoanthocyanidin oxidase (LDOX). The resulting anthocyanidins are then glycosylated, methylated, and acylated to form anthocyanins, which display different colors [31,32]. Proanthocyanidins belong to a separate flavonoid subgroup but share several biosynthetic genes with the anthocyanin pathway [33]. To synthesize proanthocyanins, leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) catalyze a reaction involving leucoanthocyanidin and cyanidin (Figure 2).
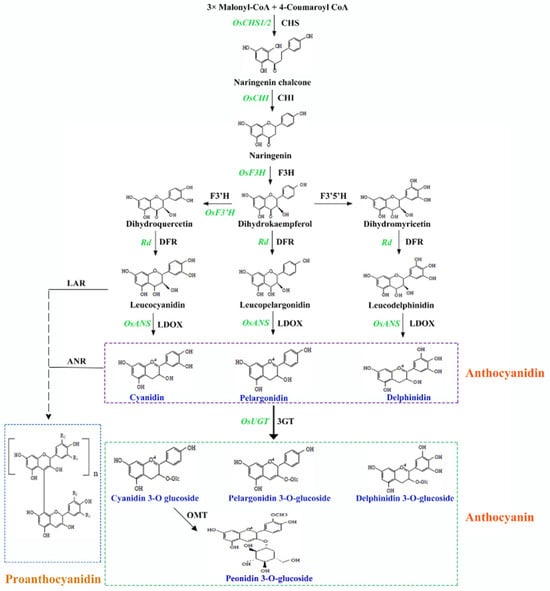
Figure 2.
A simple illustration of the anthocyanin and proanthocyanidin biosynthetic axes. Black text indicates synthesis enzymes, and green represents corresponding enzyme-encoding functional genes. CHS, chalcone synthesis; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; F3’H, flavonoid 3’ hydroxylase; F3’5’H, flavonoid 3’5’ hydroxylase (F3’5’H); DFR, dihydroflavonol 4-reductase; LDOX, leucoanthocyanidin oxidase; 3GT, 3-glucosyl transferase; OMT, O-methyltransferase; LAR, leucoanthocyanidin reductase; ANR, anthocyanidin reductase. The depicted biosynthetic axis supports evidence from Dixon et al. [30,34,35].
Figure 2.
A simple illustration of the anthocyanin and proanthocyanidin biosynthetic axes. Black text indicates synthesis enzymes, and green represents corresponding enzyme-encoding functional genes. CHS, chalcone synthesis; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; F3’H, flavonoid 3’ hydroxylase; F3’5’H, flavonoid 3’5’ hydroxylase (F3’5’H); DFR, dihydroflavonol 4-reductase; LDOX, leucoanthocyanidin oxidase; 3GT, 3-glucosyl transferase; OMT, O-methyltransferase; LAR, leucoanthocyanidin reductase; ANR, anthocyanidin reductase. The depicted biosynthetic axis supports evidence from Dixon et al. [30,34,35].
3.2. Identified Structural Genes in Rice
Multiple studies have identified genes that encode proteins involved in the anthocyanin and proanthocyanidin biosynthetic pathways. CHS catalyzes the conversion of 4-coumaroyl-CoA and malonyl-CoA to naringenin chalcone in anthocyanin biosynthesis. The frequency of CHS family genes varies significantly among the different plant species. In rice, four CHS gene copies have been identified (Table 1) [32,36,37,38]. CHI controls step 2 of the anthocyanin biosynthetic process, specifically the isomerization of p-coumaroyl-CoA to form naringenin. The OsCHI gene in rice is orthologous to ZmCHI1. Moreover, mutations in OsCHI in rice result in a golden hull and internode phenotype [39]. Lam et. al. (2022) revealed that the knockdown of CHS, CHI, and CHIL mutants significantly influenced the rice flavonoid pathway [37]. F3H plays a critical role in anthocyanin synthesis, and mutations in the F3H gene lead to variations in anthocyanin production [40]. So far, one OsF3H and three F3H homologs (OsF3H-1, OsF3H-2, and OsF3H-3) have been identified. OsF3H has been reported to have contrasting effects on rice resistance to brown planthopper and rice blast [41,42]. OsF3H2 also encodes F3H and is responsible for wide-ranging disease resistance in rice [43]. DFR accelerates the conversion of dihydroflavonol to leucoanthocyanidin. In rice, only the Rd gene has been reported to modulate proanthocyanidin synthesis [44]. Furthermore, several genes encoding F3’H, ANS, and UGT have been annotated in rice (Table 1).

Table 1.
A summary of structural genes that modulate anthocyanin and proanthocyanidin syntheses in rice.
Table 1.
A summary of structural genes that modulate anthocyanin and proanthocyanidin syntheses in rice.
| Gene Name | a MSU Locus | Proteins | Reference |
|---|---|---|---|
| OsCHS1; OsCHS24 | LOC_Os11g32650 | Chalcone synthase | [32,36,37,45] |
| OsCHS2; OsCHS8 | LOC_Os07g11440 | [37,45] | |
| OsCHS12 | LOC_Os07g31770 | [38] | |
| OsCHS28 | LOC_Os11g35930 | [38] | |
| OsCHI | LOC_Os03g60509 | Chalcone isomerase | [39] |
| OsCHIL1 | LOC_Os11g02440 | Chalcone isomerase-like | [37] |
| OsCHIL2 | LOC_Os12g02370 | ||
| OsF3H | LOC_Os03g03034 | Flavanone 3-hydroxylase | [42] |
| OsF3H-1 | LOC_Os04g56700 | Flavanone 3β-Hydroxylase | [38] |
| OsF3H-2 | LOC_Os10g39140 | ||
| OsF3H-3 | LOC_Os04g57160 | ||
| OsF3H2 | LOC_Os04g49194 | Flavanone 3-hydroxylase | [38,43] |
| OsF3’H | LOC_Os10g17260 | Flavanone 3’-hydroxylase | [32] |
| Rd/OsDFR | LOC_Os01g44260 | Dihydroflavonol reductase | [44] |
| OsANS1 | LOC_Os01g27490 | Anthocyanidin synthase | [32] |
| OsANS2 | LOC_Os06g42130 | ||
| OsUGT | LOC_Os06g09240 | Anthocyanidin 3-O-glucosyltransferase | [46] |
| OsANR | LOC_Os04g53850 | Anthocyanin reductase | [47] |
a MSU: rice genome annotation project.
3.3. Regulatory Systems for Anthocyanins and Proanthocyanidins in Rice
3.3.1. Purple-Black Pericarp
The purple-black pericarp color is highly popular among consumers, and its pigmentation is controlled by anthocyanins. Variations in pigmentation intensity among grains of this color suggest polygenic regulation. The ternary MBW complex, consisting of R2R3-MYB transcription factors (TFs), basic helix–loop–helix (bHLH) TFs, and WD-repeat (WDR) proteins, is proposed to bind promoter elements and activate the structural genes involved in the anthocyanin biosynthesis pathway [31,48,49].
OsMYB3, an R2R3-MYB gene, regulates anthocyanin-mediated pigmentation in black pericarp rice. The mutation of OsMYB3 strongly reduces 19 anthocyanin metabolites and several other flavonoids in grains (Table 2) [50,51]. Several bHLH TFs have been identified in rice, particularly Rc, OsB1/Ra1/Pb, and OsB2 (Table 2) [52,53]. Among these, the purple pericarp trait is primarily regulated by the PURPLE PERICARPA (Pp, Prp-a) and Ra/PURPLE PERICARPB (Pb, Prp-b) genes on chromosomes 4 and 1, respectively [53,54]. Plants that lack Pp but express Pb produce brown pericarp grains, whereas those that express Pp but lack Pb are white. On the other hand, plants that express both Pb and Pp develop a purple pericarp [2,53]. The Pb locus contains two genes, Ra and bHLH16. Ra corresponds to the OsB1 gene, which has an Lc homolog in maize that regulates anthocyanin biosynthesis. The bHLH16 gene is homologous to the TT8 gene in Arabidopsis thaliana, an MYC transcription factor that regulates pericarp pigmentation [53,55,56]. Sakulsingharoj et al. (2016) reported that a 2 bp (GT) insertion in exon 7 of OSB1 resulted in white rice [57]. Another investigation identified key activator loci for anthocyanin biosynthesis, referred to as KALA, which includes Kala1, Kala3, and Kala4, was responsible for the black pericarp phenotype [58]. Genetic and molecular analyses have shown that Kala1 and Kala3 correspond to Pp and OsMYB3, respectively [2]. On the other hand, Kala4 mimics OSB2, which regulates multiple structural genes encoding enzymes in the anthocyanin biosynthetic pathway, including F3H, DFR, and ANS [59,60,61]. Moreover, two newly identified transcription factors, OsBBX14 and OsHY5, reside in the nucleus, whereby they activate the transcription of genes involved in anthocyanin biosynthesis. OsBBX14 (AtBBX22) in Arabidopsis thaliana directly activates OsC1 or OsB2 in synergy with OsHY5 to regulate anthocyanin synthesis in black rice pericarp [62]. Despite these findings, only one gene, OsTTG1, encodes a WD40 protein that plays a key role in pericarp pigmentation (Table 2) [63].

Table 2.
A summary of regulatory genes associated with anthocyanin and proanthocyanidin syntheses in rice tissues.
Table 2.
A summary of regulatory genes associated with anthocyanin and proanthocyanidin syntheses in rice tissues.
| Locus | Allelic Locus | a MSU Locus | Gene Name | b CHRX | Tissues | Reference |
|---|---|---|---|---|---|---|
| Kala1 | Pp | 1 | Purple pericarp | [53,54,56] | ||
| Kala3 | LOC_Os03g29614 | OsMYB3 | 3 | Black pericarp | [50,51,58] | |
| Kala4 | Plw | LOC_Os04g47080 | OsB1; Ra1; Pb | 4 | Purple leaf, sheath, internode, caryopsis | [53,54,55,56,59] |
| LOC_Os04g47059 | OsB2; OsKala4 | 4 | Black pericarp; Purple leaf; sheath; apiculus; stigma | [59,60,64,65] | ||
| LOC_Os05g11510 | OsBBX14 | 5 | Black pericarp | [62] | ||
| Rc | Rc-s | LOC_Os07g11020 | bHLH | 7 | Light Red pericarp | [33,44,52,66] |
| Rc | Red pericarp | |||||
| rc | White pericarp | |||||
| Rc-g | Red pericarp | [67] | ||||
| Rcr | Red pericarp | [68] | ||||
| Rc-gl | white pericarp | [69] | ||||
| Rc-H2 | white pericarp | [70] | ||||
| Chromogen | LOC_Os06g10350 | OsC1; OsCPL1 | 6 | Purple leaf sheath; apiculus; stigma; hull | [71,72,73,74] | |
| OsPa | apiculi | [75] | ||||
| OsPs | stigmas | [75] | ||||
| LOC_Os02g45810 | OsTTG1 | 2 | Stigma; leaf; pericarp; culm; panicle; root; | [63] | ||
| LOC_Os04g52606 | SHR5-receptor-like kinase | 4 | Purple leaf | [76] | ||
| LOC_Os04g48840 | 4 | Purple leaf | [76] | |||
| PSH1 | Rb1 | LOC_Os01g39430 | anthocyanin regulatory protein | 1 | purple leaf sheath | [77] |
| PSH1 | Rb2 | LOC_Os01g39560 | anthocyanin regulatory Lc protein | 1 | purple leaf sheath | [77] |
a MSU: rice genome annotation project; b CHRX: chromosome.
3.3.2. Red Pericarp
The red pericarp is predominantly observed in the wild rice species (Oryza rufipogon L.), the ancestor of cultivated rice. Two complementary genes, namely Rc, which forms the basic bHLH TF, and Rd, which encodes the DFR protein, contribute to the red coloration in rice grains (Table 2) [7,33,44]. The Rc-Rd genotypes result in the red pericarp phenotype commonly observed in O. rufipogon. On the other hand, the Rc-rd genotypes produce brown pericarp grain [2]. Rc mainly determines pericarp pigmentation, with its expression masking the white pericarp phenotype. Moreover, the mutations in Rc alles include Rc (wild type), Rc-s, which introduces a premature stop codon leading to light red pericarp pigmentation, and rc, which contains a 14 bp deletion relative to the wild-type gene, resulting in a white pericarp phenotype [33,66]. Several variants have been identified that restore the wild-type red pericarp phenotype. For example, the Rc-g allele contains a 1 bp deletion located 20 bp upstream of the 14 bp rc deletion [67], whereas Rcr exhibits a 44 bp deletion upstream of the same region [68]. Most African domesticated rice (Oryza glaberrima) varieties exhibit a red pericarp, whereas white pericarp variants contain a loss-of-function Rc mutation. The O. glaberrima-specific mutation rc-gl introduces a premature stop codon 146 bp upstream of the Rc-s point mutation site [69]. Furthermore, Singh et al. (2017) identified a distinct haplotype, Rc-H2, which is strongly associated with the white pericarp phenotype in the Aus group of rice cultivars [70].
3.3.3. Leaf
Leaf color monitoring is simple and serves as a key morphological marker in rice breeding. Aberrations in any chlorophyll biosynthetic genes, namely OsCAO1, IspF, YGL1, CSP41b, YGL8, OsCOP1, and BC12/GDD1, can result in leaves that are primarily green but readily change to yellow or pale green [78,79,80,81,82,83]. So far, only a few genes have been identified as modulators of anthocyanin accumulation. The R2R3-MYB gene OsC1 was first identified in cultivated rice through comparative mapping between rice and maize or nucleotide sequence homology with known maize orthologs (Table 2) [71,84]. OsC1 is a functional chromogen gene that regulates anthocyanin biosynthesis in the leaf sheath, apiculus, stigma, and hull in rice. OsC1 null mutations result in a non-anthocyanin-pigmented phenotype [31,72]. Similarly, Qiao et al. (2021) reported that OrC1 in wild rice enhances the expression of OsCHI, OsF3H, and OsANS, therefore increasing anthocyanin accumulation in leaves [73]. Overall, OrC1 plays a significant role in anthocyanin accumulation in the purple apiculus, leaf sheath, and stigma in indica rice, while in japonica rice, it is responsible for the purple apiculus phenotype. These findings suggest that artificial selection and C1 gene domestication are independent events in the two subspecies. Further studies have revealed that OsC1 allelic diversity, rather than gene expression levels, regulates anthocyanin deposition [71,72,74,85]. The Pl locus consists of the Pb and Pl genes, which regulate purple pericarp and leaf pigmentation, respectively [59]. Three Pl alleles, Plw, Pli, and Plj, have been reported to have varying degrees of control over purple leaf pigmentation [64,65]. Another study suggested Os04g0577800 and Os04g0616400 as candidate genes for regulating the purple leaf phenotype [76]. Lastly, two strongly associated bHLH genes, Rb1 and Rb2, were identified through map-based cloning and found to play a significant role in leaf sheath pigmentation. Moreover, the overexpression of these genes considerably increased C3G and P3G accumulation in the leaf blade, leaf sheath, and pericarp [77].
3.3.4. Other Tissues
Some rice varieties show pigmentation in the apiculus, stigma, hulls, and other organs, with the genes regulating these traits being expressed in a tissue-specific manner [77]. Meng et al. (2021) identified two such genes, OsPa, which regulates apiculi pigmentation, and OsPs, which controls stigma pigmentation (Table 2) [75,86]. OsPa and OsPs are strongly associated with OsC1 and regulate the expressions and activity of OsDFR and other anthocyanin biosynthetic genes. Together, these genes act synergistically to produce purple pigmentation in the apiculi and stigmas, respectively. On the other hand, IBF1 and BBH/Lsi1 regulate rice hull pigmentation [87,88,89]. Sun et al. (2018) reported that C1 (OsC1), S1 (i.e., OsB2), and A1 (i.e., OsDFR) regulate anthocyanin deposition in the rice hull [85]. Recent studies have employed map-based cloning, genome-wide association study (GWAS), and multi-omics technology to identify genomic regions and genes that regulate anthocyanin synthesis [90,91].
In summary, several studies have identified R2R3-MYB and bHLH regulators involved in tissue-specific pigmentation; however, further research is needed to fully elucidate the regulatory mechanisms controlling pigmentation in different rice tissues. A comprehensive investigation of the genetic and signaling pathways involved in pigmentation across different rice tissues can improve the management of both beneficial and undesirable traits in pigmented rice.
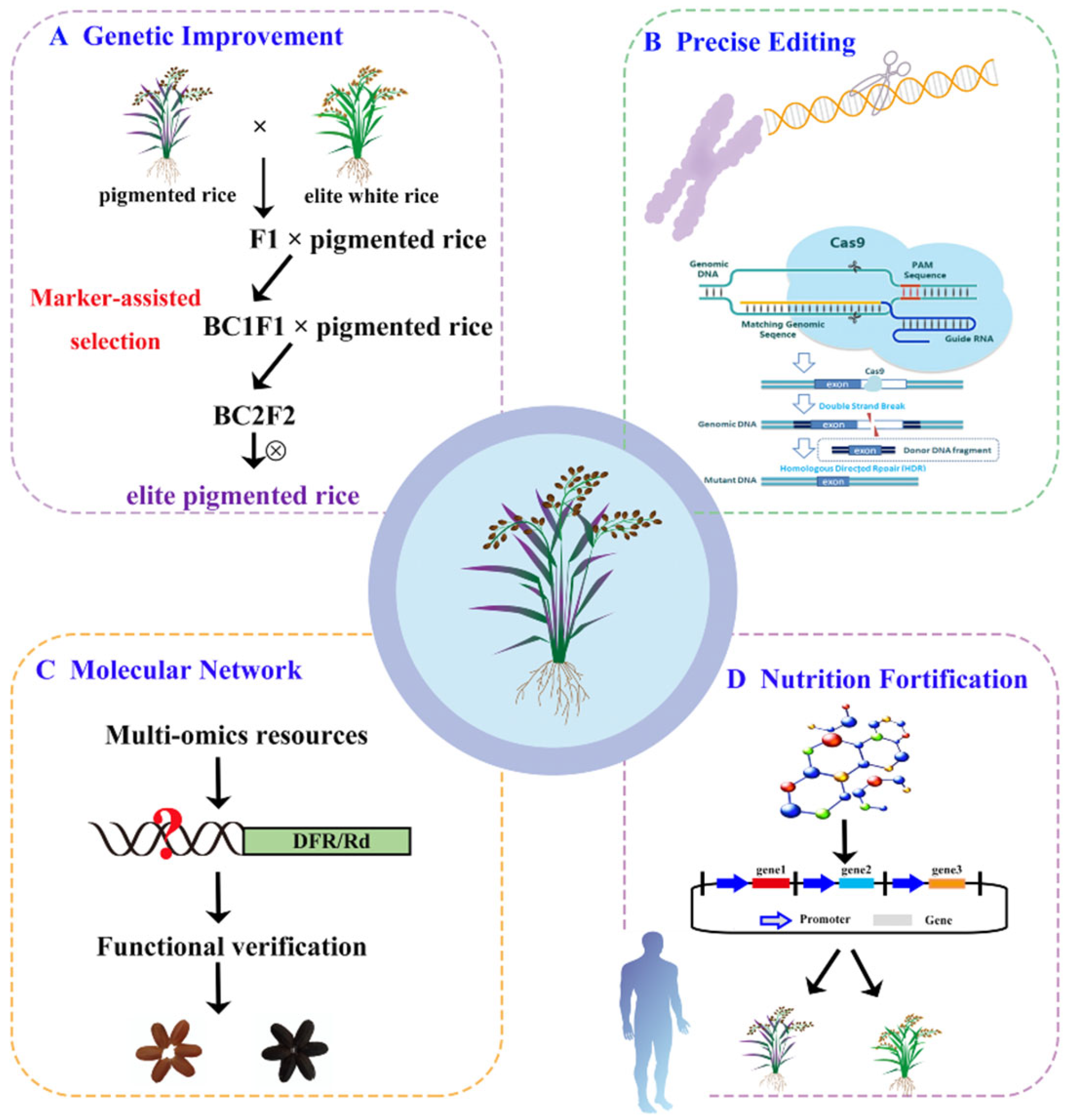
4. Concluding Remarks and Future Perspectives
4.1. Genetic Improvement of Pigmented Rice
There is growing interest in the study and production of pigmented rice due to its relatively high nutritional value and associated health benefits. Most pigmented rice varieties are landraces, which often exhibit suboptimal agronomic traits and lower yields compared to white rice, as they have undergone less artificial selection [92]. Moreover, pigmented rice presents other challenges, such as longer cooking times and poor eating quality (e.g., hard texture and insufficient viscoelasticity), which, in turn, limits its production. Both agronomic traits (e.g., plant architecture, life cycle, and yield) and quality traits (e.g., cooking and eating properties) must be improved to increase the nutritional and sensory appeal of pigmented rice (Figure 3). Although it is more feasible to introduce pigmentation into modern cultivars than to improve the quality and yield of pigmented rice landraces, no studies to date have examined the effect of introducing partial pigmented rice chromosomal segments into white rice on nutrient composition. As high-coverage molecular markers have been developed for white rice, it can serve as a donor parent, and molecular marker-assisted selection can accelerate the genetic improvement in pigmented rice. Moreover, the large-scale aggregation of phenotype and genotype data, combined with genomic selection based on multiple superior alleles, can further enhance efforts to improve pigmented rice.
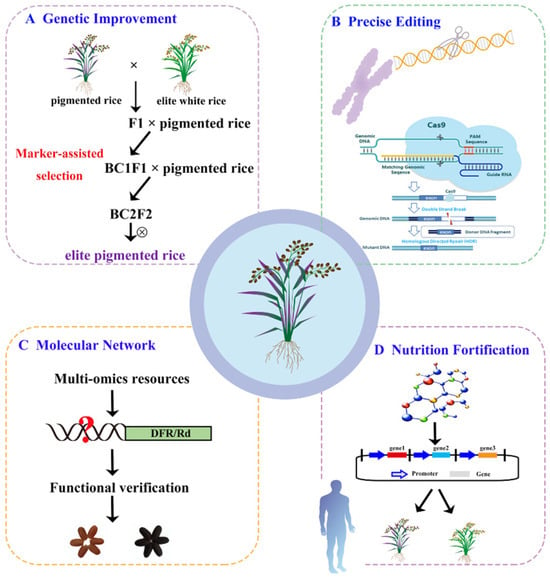
Figure 3.
Current challenges, adaptable strategies, and potential future directions of pigmented rice research. (A) Improvement strategies for agronomic traits of pigmented rice. (B) Precise editing of linked genes through the CRISPR/Cas9 system. (C) Molecular mechanism analysis of pigment deposition. (D) Engineering of nutritional fortification for pigmented rice.
4.2. Precise Editing Through the CRISPR/Cas9 System
Genes with significant physiological roles are often pleiotropic, having multiple functions. Thus, modifying these genes may adversely affect crop production. For instance, the genes that contribute to red pericarp color are often linked with the genes that control seed shattering and dormancy [93]. Moreover, mutations in regulatory genes that increase grain yield have been shown to significantly reduce 1000-grain weight [93,94]. Genome editing technologies, particularly CRISPR/Cas9, are promising tools for improving crop traits (Figure 3). Using CRISPR/Cas9, Sedeek et al. (2023) developed a regeneration and transformation system for the efficient production of an early maturing black rice germplasm resource [13]. CRISPR/Cas9 is a powerful tool for improving agronomic traits and enhancing yields in pigmented rice cultivation.
4.3. Elucidating the Molecular Network Regulating Grain Pigmentation
Although various structural and regulatory genes related to anthocyanins and proanthocyanidins have been cloned, the mechanisms responsible for grain pigmentation remain unclear. Therefore, it is essential to explore the upstream and downstream regulatory elements of known genes and identify genes involved in grain pigmentation. Publicly available genomic data on pigmented rice have facilitated the screening and characterization of genes associated with grain pigmentation. Furthermore, integrated analyses involving molecular genetics, transcriptomics, proteomics, and metabolomics have revealed complex details of grain pigmentation in rice. The characterization of molecular networks is facilitated by yeast one-hybrid and two-hybrid assays, as well as chromatin immunoprecipitation (ChIP) techniques. Rice grain color intensity is affected by both environmental factors and fertilizers [8]. Further research is required to explore the relationship between grain pigmentation and environmental factors to refine the regulatory policies governing pigmented rice breeding (Figure 3).
4.4. Engineering of Nutritional Fortification
Rice-consuming countries are currently facing widespread micronutrient deficiencies. To address this issue, the nutritional value, quality, and yield of pigmented rice must be improved to produce a healthier variety. The genetic fortification of rice grains with functional nutrients is a primary objective of breeding programs worldwide. In recent years, the emergence of “golden rice” and “purple endosperm rice” has gained significant attention [95,96]. Tian et al. (2021) employed synthetic biology approaches to improve the riboflavin content in the rice endosperm to address riboflavin deficiency. Pigmented rice varieties are rich in flavonoids, phenols, and other bioactive compounds, making them a nutritionally superior alternative to traditional white rice [97]. Further research to improve the nutritional quality and yield of rice is important for rice-consuming populations globally, particularly in countries where rice is a staple dietary component (Figure 3).
Author Contributions
Funding Acquisition, Investigation, Writing—Original Draft, H.L.; Supervision, Visualization, X.J.; Project Administration, Writing—Review and Editing, B.H.; Supervision, Visualization, Writing—Review and Editing, X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Jilin Province Science and Technology Development Program (grant number 20240303015NC).
Data Availability Statement
All the data used in this review paper are available online.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, W.; Chen, L.; Zhao, J.; Wang, J.; Li, W.; Yang, T.; Dong, J.; Ma, Y.; Zhou, L.; Chen, J.; et al. Genome-Wide association study of pericarp color in rice using different germplasm and phenotyping methods reveals different genetic architectures. Front. Plant Sci. 2022, 13, 841191. [Google Scholar] [CrossRef] [PubMed]
- Mbanjo, E.G.N.; Kretzschmar, T.; Jones, H.; Ereful, N.; Blanchard, C.; Boyd, L.A.; Sreenivasulu, N. The Genetic basis and nutritional benefits of pigmented rice grain. Front. Genet. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Mackon, E.; Ma, Y.; Mackon, G.C.J.D.E.; Usman, B.; Zhao, Y.; Li, Q.; Liu, P. Computational and transcriptomic analysis unraveled OsMATE34 as a putative anthocyanin transporter in black rice (Oryza sativa L.) caryopsis. Genes 2021, 12, 583. [Google Scholar] [CrossRef]
- Civáň, P.; Craig, H.; Cox, C.J.; Brown, T.A. Three geographically separate domestications of Asian rice. Nat. Plants 2015, 2, 15164. [Google Scholar] [CrossRef]
- Gondal, T.A.; Keast, R.S.J.; Shellie, R.A.; Jadhav, S.R.; Gamlath, S.; Mohebbi, M.; Liem, D.G. Consumer acceptance of brown and white rice varieties. Foods 2021, 10, 1950. [Google Scholar] [CrossRef]
- Mbanjo, E.G.N.; Pasion, E.A.; Jones, H.; Carandang, S.; Misra, G.; Ignacio, J.C.; Kretzschmar, T.; Sreenivasulu, N.; Boyd, L.A. Unravelling marker trait associations linking nutritional value with pigmentation in rice seed. Plant Genome 2023, 16, 20360. [Google Scholar] [CrossRef]
- Shao, Y.; Jin, L.; Zhang, G.; Jin, L.; Zhang, G.; Lu, Y.; Shen, Y.; Bao, J. Association mapping of grain color, phenolic content, flavonoid content and antioxidant capacity in dehulled rice. Theor. Appl. Genet. 2011, 122, 1005–1016. [Google Scholar] [CrossRef]
- Brunet-Loredo, A.; López-Belchí, M.D.; Cordero-Lara, K.; Noriega, F.; Cabeza, R.A.; Fischer, S.; Careaga, P.; Garriga, M. Assessing grain quality changes in white and black rice under water deficit. Plants 2023, 12, 4091. [Google Scholar] [CrossRef]
- Tiozon, R.J.N.; Sreenivasulu, N.; Alseekh, S.; Sartagoda, K.J.D.; Usadel, B.; Fernie, A.R. Metabolomics and machine learning technique revealed that germination enhances the multi-nutritional properties of pigmented rice. Commun. Biol. 2023, 6, 1000. [Google Scholar] [CrossRef]
- Gu, W.; Peng, Y.; Wang, R.; Wang, R.; Wu, H.; Zhu, J.; Ni, X.; Xiong, Q. Comparison of metabolites and main nutritional components between uncooked and cooked purple rice. Metabolites 2023, 13, 1018. [Google Scholar] [CrossRef]
- Zhu, F. Anthocyanins in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, K.; Zuccolo, A.; Fornasiero, A.; Weber, A.M.; Sanikommu, K.; Sampathkumar, S.; Rivera, L.F.; Butt, H.; Mussurova, S.; Alhabsi, A.; et al. Multi-omics resources for targeted agronomic improvement of pigmented rice. Nat. Food 2023, 4, 366–371. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, J.; Sun, C.; Wang, R.; Wei, H.; He, H.; Zhou, D.; Zhang, H.; Zhu, J. Metabolomics revealed metabolite biomarkers of antioxidant properties and flavonoid metabolite accumulation in purple rice after grain filling. Food Chem. X 2023, 18, 100720. [Google Scholar] [CrossRef]
- Tai, L.; Huang, S.; Zhao, Z.; Huang, G. Chemical composition analysis and antioxidant activity of black rice pigment. Chem. Biol. Drug Des. 2020, 97, 711–720. [Google Scholar] [CrossRef]
- Chen, W.; Gao, Y.; Xie, W.; Gong, L.; Lu, K.; Wang, W.; Li, Y.; Liu, X.; Zhang, H.; Dong, H.; et al. Genome-wide association analyses provide genetic and biochemical insights into natural variation in rice metabolism. Nat. Genet. 2014, 46, 714–721. [Google Scholar] [CrossRef]
- Tansawat, R.; Jindawatt, S.; Ekkaphan, P.; Ruengphayak, S.; Vanavichit, A.; Suttipanta, N.; Vimolmangkang, S.; De-Eknamkul, W. Metabolomics approach to identify key volatile aromas in Thai colored rice cultivars. Front. Plant Sci. 2023, 14, 973217. [Google Scholar] [CrossRef]
- Nakagawa, K.; Yooin, W.; Saenjum, C. Investigation of pigments in Thai purple rice using electron paramagnetic resonance imaging and HPLC. J. Nutr. Sci. Vitaminol. 2019, 65, 217–221. [Google Scholar] [CrossRef]
- Mackon, E.; Mackon, G.C.J.D.E.; Ma, Y.; Haneef Kashif, M.; Ali, N.; Usman, B.; Liu, P. Recent insights into anthocyanin pigmentation, synthesis, trafficking, and regulatory mechanisms in rice (Oryza sativa L.) caryopsis. Biomolecules 2021, 11, 394. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Identification and quantification of phenolic acids and anthocyanins as antioxidants in bran, embryo and endosperm of white, red and black rice kernels (Oryza sativa L.). J. Cereal Sci. 2014, 59, 211–218. [Google Scholar] [CrossRef]
- Ziegler, V.; Ferreira, C.D.; Hoffmann, J.F.; Chaves, F.C.; Vanier, N.L.; de Oliveira, M.; Elias, M.C. Cooking quality properties and free and bound phenolics content of brown, black, and red rice grains stored at different temperatures for six months. Food Chem. 2018, 242, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Mackon, E.; Mackon, G.C.J.D.E.; Yao, Y.; Guo, Y.; Ma, Y.; Dai, X.; Jandan, T.H.; Liu, P. Integrative HPLC profiling and transcriptome analysis revealed insights into anthocyanin accumulation and key genes at three developmental stages of black rice (Oryza sativa L.) caryopsis. Front. Plant Sci. 2023, 14, 1211326. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, D.; Ma, X.; Han, B.; Han, L. Comparative analysis of rice reveals insights into the mechanism of colored rice via widely targeted metabolomics. Food Chem. 2023, 399, 133926. [Google Scholar] [CrossRef]
- Gunaratne, A.; Wu, K.; Li, D.; Bentota, A.; Corke, H.; Cai, Y. Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem. 2013, 138, 1153–1161. [Google Scholar] [CrossRef]
- Oki, T.; Masuda, M.; Kobayashi, M.; Nishiba, Y.; Furuta, S.; Sato, T. Polymeric procyanidins as radical-scavenging components in red-hulled rice. J. Agric. Food Chem. 2002, 50, 7524–7529. [Google Scholar] [CrossRef]
- Chen, M.H.; McClung, A.M.; Bergman, C.J. Bran data of total flavonoid and total phenolic contents, oxygen radical absorbance capacity, and profiles of proanthocyanidins and whole grain physical traits of 32 red and purple rice varieties. Data Brief 2016, 8, 6–13. [Google Scholar] [CrossRef]
- Chen, M.H.; McClung, A.M.; Bergman, C.J. Concentrations of oligomers and polymers of proanthocyanidins in red and purple rice bran and their relationships to total phenolics, flavonoids, antioxidant capacity and whole grain color. Food Chem. 2016, 208, 279–287. [Google Scholar] [CrossRef]
- Tohge, T.; de Souza, L.P.; Fernie, A.R. Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J. Exp. Bot. 2017, 68, 4013–4028. [Google Scholar] [CrossRef]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef]
- Xia, D.; Zhou, H.; Wang, Y.; Li, P.; Fu, P.; Wu, B.; He, Y. How rice organs are colored: The genetic basis of anthocyanin biosynthesis in rice. Crop J. 2021, 9, 598–608. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, H.; Zhu, H.; Huang, C.; Liu, C.; Chang, Y.; Kong, Z.; Zhou, Z.; Wang, G.; Lin, Y.; et al. Determining factors, regulation system, and domestication of anthocyanin biosynthesis in rice leaves. New Phytol. 2019, 223, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.H.; Chu, H.; Tang, L.K.; Sakamoto, W.; Maekawa, M.; Chu, I.K.; Wang, M.; Lo, C. Functional characterization of key structural genes in rice flavonoid biosynthesis. Planta 2008, 228, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.T.; Thomson, M.J.; Pfeil, B.E.; McCouch, S. Caught red-handed: Rc encodes a basic helix-loop-helix protein conditioning red pericarp in rice. Plant Cell 2006, 18, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Liu, C.; Jun, J.H. Metabolic engineering of anthocyanins and condensed tannins in plants. Curr. Opin. Biotechnol. 2013, 24, 329–335. [Google Scholar] [CrossRef]
- Li, P.; Dong, Q.; Ge, S.; He, X.; Verdier, J.; Li, D.; Zhao, J. Metabolic engineering of proanthocyanidin production by repressing the isoflavone pathways and redirecting anthocyanidin precursor flux in legume. Plant Biotechnol. J. 2016, 14, 1604–1618. [Google Scholar] [CrossRef]
- Wu, H.; Xie, D.; Jia, P.; Tang, Z.; Shi, D.; Shui, G.; Wang, G.; Yang, W. Homeostasis of flavonoids and triterpenoids most likely modulates starch metabolism for pollen tube penetration in rice. Plant Biotechnol. J. 2023, 21, 1757–1772. [Google Scholar] [CrossRef]
- Lam, P.Y.; Wang, L.; Lui, A.C.W.; Liu, H.; Takeda-Kimura, Y.; Chen, M.X.; Zhu, F.Y.; Zhang, J.; Umezawa, T.; Tobimatsu, Y. Deficiency in flavonoid biosynthesis genes CHS, CHI, and CHIL alters rice flavonoid and lignin profiles. Plant Physiol. 2022, 188, 1993–2001. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Li, Y. Genome-wide identification and expression profiles of 13 key structural gene families involved in the biosynthesis of rice flavonoid scaffolds. Genes 2022, 13, 410. [Google Scholar] [CrossRef]
- Hong, L.; Qian, Q.; Tang, D.; Wang, K.; Li, M.; Cheng, Z. A mutation in the rice chalcone isomerase gene causes the golden hull and internode 1 phenotype. Planta 2012, 236, 141–151. [Google Scholar] [CrossRef]
- Dai, M.; Kang, X.; Wang, Y.; Huang, S.; Guo, Y.; Wang, R.; Chao, N.; Liu, L. Functional characterization of flavanone 3-hydroxylase (F3H) and its role in anthocyanin and flavonoid biosynthesis in mulberry. Molecules 2022, 27, 3341. [Google Scholar] [CrossRef]
- Dai, Z.; Tan, J.; Zhou, C.; Yang, X.; Yang, F.; Zhang, S.; Sun, S.; Miao, X.; Shi, Z. The OsmiR396-OsGRF8-OsF3H-flavonoid pathway mediates resistance to the brown planthopper in rice (Oryza sativa). Plant Biotechnol. J. 2019, 17, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, B.; Shi, Z.; Miao, X.; Li, H. Identification of the rice genes and metabolites involved in dual resistance against brown planthopper and rice blast fungus. Plant Cell Environ. 2022, 45, 1914–1929. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Wang, H.; Yang, C.; Wang, L.; Qi, L.; Guo, Z.; Chen, X. Salicylic acid is required for broad-spectrum disease resistance in rice. Int. J. Mol. Sci. 2022, 23, 1354. [Google Scholar] [CrossRef]
- Furukawa, T.; Maekawa, M.; Oki, T.; Suda, I.; Iida, S.; Shimada, H.; Shimada, H.; Takamure, I.; Kadowaki, K. The Rc and Rd genes are involved in proanthocyanidin synthesis in rice pericarp. Plant J. 2007, 49, 91–102. [Google Scholar] [CrossRef]
- Park, H.L.; Yoo, Y.; Bhoo, S.H.; Lee, T.H.; Lee, S.W.; Cho, M.H. Two chalcone synthase isozymes participate redundantly in UV-induced sakuranetin synthesis in rice. Int. J. Mol. Sci. 2020, 21, 3777. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Zaima, N.; Moriyama, T.; Kawamura, Y. Different localization patterns of anthocyanin species in the pericarp of black rice revealed by imaging mass spectrometry. PLoS ONE 2012, 7, e31285. [Google Scholar] [CrossRef]
- Kim, C.K.; Seol, Y.J.; Shin, Y.; Lim, H.M.; Lee, G.S.; Kim, A.R.; Lee, T.H.; Lee, J.H.; Park, D.S.; Yoo, S.; et al. Whole-genome resequencing and transcriptomic analysis to identify genes involved in leaf-color diversity in ornamental rice plants. PLoS ONE 2015, 10, e0124071. [Google Scholar] [CrossRef]
- Han, Z.; Li, F.; Qiao, W.; Nong, B.; Cheng, Y.; Zhang, L.; Huang, J.; Wang, Y.; Lou, D.; Ge, J.; et al. Identification of candidate genes and clarification of the maintenance of the green pericarp of weedy rice grains. Front. Plant Sci. 2022, 13, 930062. [Google Scholar] [CrossRef]
- Song, Y.E.; Wang, X.; Shen, Z.W.; Xu, Y.; Li, J.Y. Expressing the maize anthocyanin regulatory gene Lc increased flavonoid content in the seed of white pericarp rice and purple pericarp rice. Russ. J. Genet. 2013, 49, 1127–1133. [Google Scholar] [CrossRef]
- Kim, D.H.; Yang, J.; Ha, S.H.; Kim, J.K.; Lee, J.Y.; Lim, S.H. An OsKala3, R2R3 MYB TF, is a common key player for black rice pericarp as main partner of an OsKala4, bHLH TF. Front. Plant Sci. 2021, 12, 765049. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, H.; Zhao, M.; Yang, Z.; Zhou, Z.; Guo, Y.; Lin, Y.; Chen, H. OsMYB3 is a R2R3-MYB gene responsible for anthocyanin biosynthesis in black rice. Mol. Breed. 2021, 41, 51. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qiu, X.; Wang, Z.; Xie, T.; Sun, W.; Xu, J.; Zhang, F.; Yu, S. Deciphering the genetic architecture of color variation in whole grain rice by genome-wide association. Plants 2023, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Lee, K.E.; Lee, E.S.; Matin, M.N.; Lee, D.S.; Yun, J.S.; Kim, J.B.; Kang, S.G. The genetic constitutions of complementary genes Pp and Pb determine the purple color variation in pericarps with cyanidin-3-O-glucoside depositions in black rice. J. Plant Biol. 2013, 56, 24–31. [Google Scholar] [CrossRef]
- Ham, T.H.; Kwon, S.W.; Ryu, S.N.; Koh, H.J. Correlation analysis between grain color and cyanidin-3-glucoside content of rice grain in segregate population. Plant Breed. Biotechnol. 2015, 3, 160–166. [Google Scholar] [CrossRef]
- Hu, J.; Anderson, B.; Wessler, S.R. Isolation and characterization of rice R genes evidence for distinct evolutionary paths in rice and maize. Genetics 1996, 142, 1021–1031. [Google Scholar] [CrossRef]
- Wang, C.; Shu, Q. Fine mapping and candidate gene analysis of purple pericarp gene Pb in rice (Oryza sativa L.). Chin. Sci. Bull. 2007, 52, 3097–3104. [Google Scholar] [CrossRef]
- Sakulsingharoj, C.; Inta, P.; Sukkasem, R.; Pongjaroenkit, S.; Chowpongpang, S.; Sangtong, V. Cloning and characterization of OSB1 gene controlling anthocyanin biosynthesis from Thai black rice. Genom. Genet. 2016, 9, 7–18. [Google Scholar] [CrossRef]
- Maeda, H.; Yamaguchi, T.; Omoteno, M.; Takarada, T.; Fujita, K.; Murata, K.; Iyama, Y.; Kojima, Y.; Morikawa, M.; Ozaki, H.; et al. Genetic dissection of black grain rice by the development of a near isogenic line. Breed. Sci. 2014, 64, 134–141. [Google Scholar] [CrossRef]
- Sakamoto, W.; Ohmori, T.; Kageyama, K.; Miyazaki, C.; Saito, A.; Murata, M.; Noda, K.; Maekawa, M. The Purple leaf (Pl) locus of rice: The Pl(w) allele has a complex organization and includes two genes encoding basic helix-loop-helix proteins involved in anthocyanin biosynthesis. Plant Cell Physiol. 2001, 42, 982–991. [Google Scholar] [CrossRef]
- Oikawa, T.; Maeda, H.; Oguchi, T.; Yamaguchi, T.; Tanabe, N.; Ebana, K.; Yano, M.; Ebitani, T.; Izawa, T. The birth of a black rice gene and its local spread by introgression. Plant Cell 2015, 27, 2401–2414. [Google Scholar] [CrossRef]
- Sakulsingharoj, C.; Inta, P.; Sukkasem, R.; Pongjaroenkit, S.; Chowpongpang, S.; Sangtong, V. Overexpression of OSB2 gene in transgenic rice up-regulated expression of structural genes in anthocyanin biosynthesis pathway. Thai J. Genet. 2014, 7, 173–182. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, S.; Lee, J.Y.; Ha, S.H.; Lee, J.G.; Lim, S.H. A rice B-Box protein, OsBBX14, finely regulates anthocyanin biosynthesis in rice. Int. J. Mol. Sci. 2018, 19, 2190. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, J.; Xia, X.; Zhang, Z.; He, J.; Nong, B.; Luo, T.; Feng, R.; Wu, Y.; Pan, Y.; et al. OsTTG1, a WD40 repeat gene, regulates anthocyanin biosynthesis in rice. Plant J. 2021, 107, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jalil, S.; Cao, H.; Tsago, Y.; Sunusi, M.; Chen, Z.; Shi, C.; Jin, X. The purple leaf (pl6) mutation regulates leaf color by altering the anthocyanin and chlorophyll contents in rice. Plants 2020, 9, 1477. [Google Scholar] [CrossRef]
- Kang, S.G.; Lee, K.E.; Cho, J.; Lee, J.W.; Do, G.S.; Matin, M.N. The Purple leaf (Pl) alleles, Plw and Pli, regulate leaf color development independently from the Pb gene of purple pericarp (Prp) in rice. Agronomy 2023, 13, 2845. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Y.; Chen, S.; Liu, H.; Chen, Z.; Fan, M.; Hu, T.; Mei, F.; Chen, J.; Chen, L.; et al. CRISPR/Cas9-mediated functional recovery of the recessive rc allele to develop red rice. Plant Biotechnol. J. 2019, 17, 2096–2105. [Google Scholar] [CrossRef]
- Brooks, S.A.; Yan, W.; Jackson, A.K.; Deren, C.W. A natural mutation in rc reverts white-rice-pericarp to red and results in a new, dominant, wild-type allele: Rc-g. Theor. Appl. Genet. 2008, 117, 575–580. [Google Scholar] [CrossRef]
- Ferrari, B.; Gianinetti, A.; Finocchiaro, F.; Terzi, V. Rc gene sequence and expression evaluation in a red-kernel rice genotype. Rice Res. 2015, 3, 3. [Google Scholar] [CrossRef]
- Gross, B.L.; Steffen, F.T.; Olsen, K.M. The molecular basis of white pericarps in African domesticated rice novel mutations at the Rc gene. J. Evol. Biol. 2010, 23, 2747–2753. [Google Scholar] [CrossRef]
- Singh, N.; Singh, B.; Rai, V.; Sidhu, S.; Singh, A.K.; Singh, N.K. Evolutionary insights based on SNP haplotypes of red pericarp, grain size and starch synthase genes in wild and cultivated rice. Front. Plant Sci. 2017, 8, 972. [Google Scholar] [CrossRef]
- Saitoh, K.; Onishi, K.; Mikami, I.; Thidar, K.; Sano, Y. Allelic Diversification at the C (OsC1) locus of wild and cultivated rice. Genetics 2004, 168, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Chin, H.; Wu, Y.; Hour, A.; Hong, C.; Lin, Y. Genetic and evolutionary analysis of purple leaf sheath in rice. Rice 2016, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Wang, Y.; Xu, R.; Yang, Z.; Sun, Y.; Su, L.; Zhang, L.; Wang, J.; Huang, J.; Zheng, X.; et al. A functional chromogen gene C from wild rice is involved in a different anthocyanin biosynthesis pathway in indica and japonica. Theor. Appl. Genet. 2021, 134, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Wang, X.; Sun, T.; Rong, H.; Wu, L.; Deng, J.; Guo, T.; Wang, H.; Wang, J.; Huang, M. MYB transcription factor OsC1PLSr involves the regulation of purple leaf sheath in rice. Int. J. Mol. Sci. 2023, 24, 6655. [Google Scholar] [CrossRef]
- Meng, L.; Qi, C.; Wang, C.; Wang, S.; Zhou, C.; Ren, Y.; Cheng, Z.; Zhang, X.; Guo, X.; Zhao, Z.; et al. Determinant factors and regulatory systems for anthocyanin biosynthesis in rice apiculi and stigmas. Rice 2021, 14, 37. [Google Scholar] [CrossRef]
- Gao, J.; Dai, G.; Zhou, W.; Liang, H.; Huang, J.; Qing, D.; Chen, W.; Wu, H.; Yang, X.; Li, D.; et al. Mapping and identifying a candidate gene Plr4, a recessive gene regulating purple leaf in rice, by using bulked segregant and transcriptome analysis with next-generation sequencing. Int. J. Mol. Sci. 2019, 20, 4335. [Google Scholar] [CrossRef]
- Hu, W.; Zhou, T.; Han, Z.; Tan, C.; Xing, Y. Dominant complementary interaction between OsC1 and two tightly linked genes, Rb1 and Rb2, controls the purple leaf sheath in rice. Theor. Appl. Genet. 2020, 133, 2555–2566. [Google Scholar] [CrossRef]
- Huang, R.; Wang, Y.; Wang, P.; Li, C.; Xiao, F.; Chen, N.; Li, N.; Li, C.; Sun, C.; Li, L.; et al. A single nucleotide mutation of IspF gene involved in the MEP pathway for isoprenoid biosynthesis causes yellow-green leaf phenotype in rice. Plant Mol. Biol. 2017, 96, 5–16. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; He, B.; Diao, L.; Sheng, S.; Wang, J.; Guo, X.; Su, N.; Wang, L.; Jiang, L.; et al. A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiol. 2007, 145, 29–40. [Google Scholar] [CrossRef]
- Mei, J.; Li, F.; Liu, X.; Hu, G.; Fu, Y.; Liu, W. Newly identified CSP41b gene localized in chloroplasts affects leaf color in rice. Plant Sci. 2017, 256, 39–45. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, S.; Wang, Z.; Du, Q.; Xing, Y.; Zhang, T.; Shen, W.; Sang, X.; Ling, Y.; He, G. Map-based cloning and functional analysis of YGL8, which controls leaf colour in rice (Oryza sativa). BMC Plant Biol. 2016, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Piao, R.; Lee, G.; Koh, E.; Lee, Y.; Woo, S.; Reflinur; Jiang, W.; Septiningsih, E.M.; Thomson, M.J.; et al. OsCOP1 regulates embryo development and flavonoid biosynthesis in rice (Oryza sativa L.). Theor. Appl. Genet. 2021, 134, 2587–2601. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Chen, W.; Guo, L.; Liu, Y.; Pu, Z.; Zhang, G.; Tu, B.; Yuan, H.; Wang, Y.; Ma, B.; et al. Characterization of a novel allele of bc12/gdd1 indicates a differential leaf color function for BC12/GDD1 in Indica and Japonica backgrounds. Plant Sci. 2020, 298, 110585. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, C.; Ma, J.; Wang, S.; Tian, P.; Wang, J.; Cheng, Z.; Zhang, X.; Guo, X.; Lei, C. Map-based cloning and functional analysis of the chromogen gene C in rice (Oryza sativa L.). J. Plant Biol. 2016, 59, 496–505. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Z.; Chen, C.; Wu, W.; Ren, N.; Jiang, C.; Yu, J.; Zhao, Y.; Zheng, X.; Yang, Q.; et al. The C-S-A gene system regulates hull pigmentation and reveals evolution of anthocyanin biosynthesis pathway in rice. J. Exp. Bot. 2018, 69, 1485–1498. [Google Scholar] [CrossRef]
- Tong, J.; Han, Z.; Han, A. Mapping of quantitative trait loci for purple stigma and purple apiculus in rice by using a Zhenshan 97B/Minghui 63 RIL population. Czech J. Genet. Plant Breed. 2021, 57, 113–118. [Google Scholar] [CrossRef]
- Shao, T.; Qian, Q.; Tang, D.; Chen, J.; Li, M.; Cheng, Z.; Luo, Q. A novel gene IBF1 is required for the inhibition of brown pigment deposition in rice hull furrows. Theor. Appl. Genet. 2012, 125, 381–390. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, L.; Huang, W.; Luo, X.; Xie, J.; Hu, B.; Chen, Y. Flavonoid metabolic profiles and gene mapping of rice (Oryza sativa L.) purple gradient grain hulls. Rice 2022, 15, 43. [Google Scholar] [CrossRef]
- Yang, C.; Zeng, D.; Qin, R.; Alamin, M.; Jin, X.; Shi, C. Rice gene, BBH/Lsi1, regulates the color of rice hull by reducing the absorption and deposition of silicon and accumulating excess flavonoid. Plant Growth Regul. 2018, 85, 133–142. [Google Scholar] [CrossRef]
- Haghi, R.; Ahmadikhah, A.; Fazeli, A.; Shariati, V. Candidate genes for anthocyanin pigmentation in rice stem revealed by GWAS and whole-genome resequencing. Plant Genome 2022, 15, e20224. [Google Scholar] [CrossRef]
- Oh, J.H.; Lee, Y.J.; Byeon, E.J.; Kang, B.C.; Kyeoung, D.S.; Kim, C.K. Whole-genome resequencing and transcriptomic analysis of genes regulating anthocyanin biosynthesis in black rice plants. 3 Biotech 2018, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zuo, Z.; Yang, Z. Toward breeding pigmented rice balancing nutrition and yield. Trends Plant Sci. 2023, 23, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.Y.; Foley, M.E.; Horvath, D.P.; Anderson, J.V.; Feng, J.; Zhang, L.; Mowry, C.R.; Ye, H.; Suttle, J.C.; Kadowaki, K.; et al. Association between seed dormancy and pericarp color is controlled by a pleiotropic gene that regulates abscisic acid and flavonoid synthesis in weedy red rice. Genetics 2011, 189, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Chung, C.L.; Chen, K.Y.; Chen, R.K. A novel variation in the FRIZZLE PANICLE (FZP) gene promoter improves grain number and yield in rice. Genetics 2020, 215, 243–252. [Google Scholar] [CrossRef]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchliffe, E.; Adams, J.L.; Silverstone, A.L.; et al. Improving the nutritional value of golden rice through increased pro-vitamin A content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef]
- Zhu, Q.; Yu, S.; Zeng, D.; Liu, H.; Wang, H.; Yang, Z.; Xie, X.; Shen, R.; Tan, J.; Li, H.; et al. Development of “Purple Endosperm Rice” by engineering anthocyanin biosynthesis in the endosperm with a high-efficiency transgene stacking system. Mol. Plant. 2017, 10, 918–929. [Google Scholar] [CrossRef]
- Tian, Y.S.; Xu, J.; Wang, B.; Fu, X.Y.; Gao, J.J.; Han, H.J.; Li, Z.J.; Wang, L.J.; Zhang, F.J.; Zhang, W.H.; et al. Riboflavin fortification of rice endosperm by metabolic engineering. Plant Biotechnol. J. 2021, 19, 1483–1485. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).