Integrating RNA Interference and Nanotechnology: A Transformative Approach in Plant Protection
Abstract
1. Introduction
2. The Role of RNAi in Advancing Crop Protection Strategies
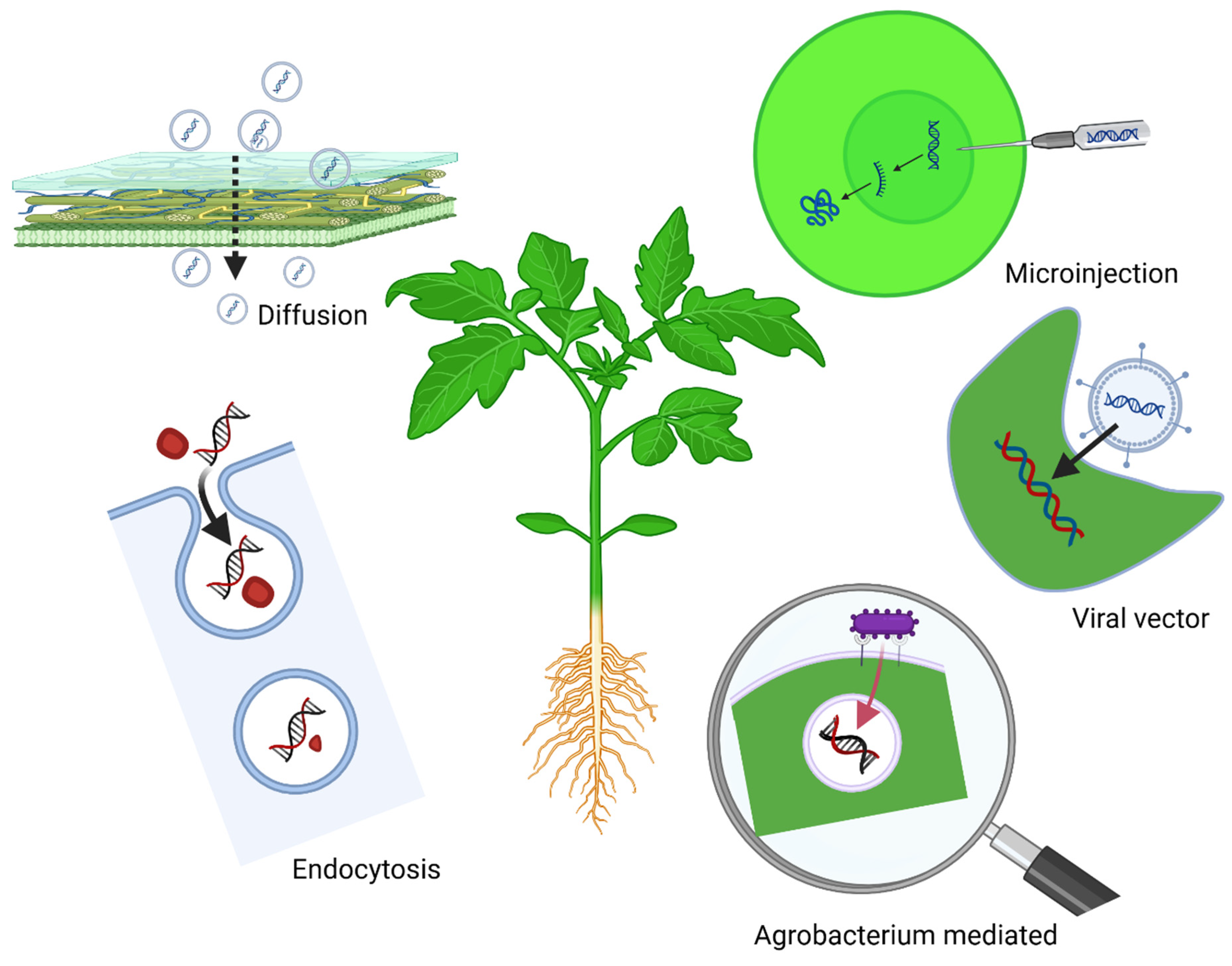
3. Mechanisms of Exogenous RNA Uptake by Crop Plants and Pathogens
4. Mechanism of RNAi-Based Crop Protection in Plants
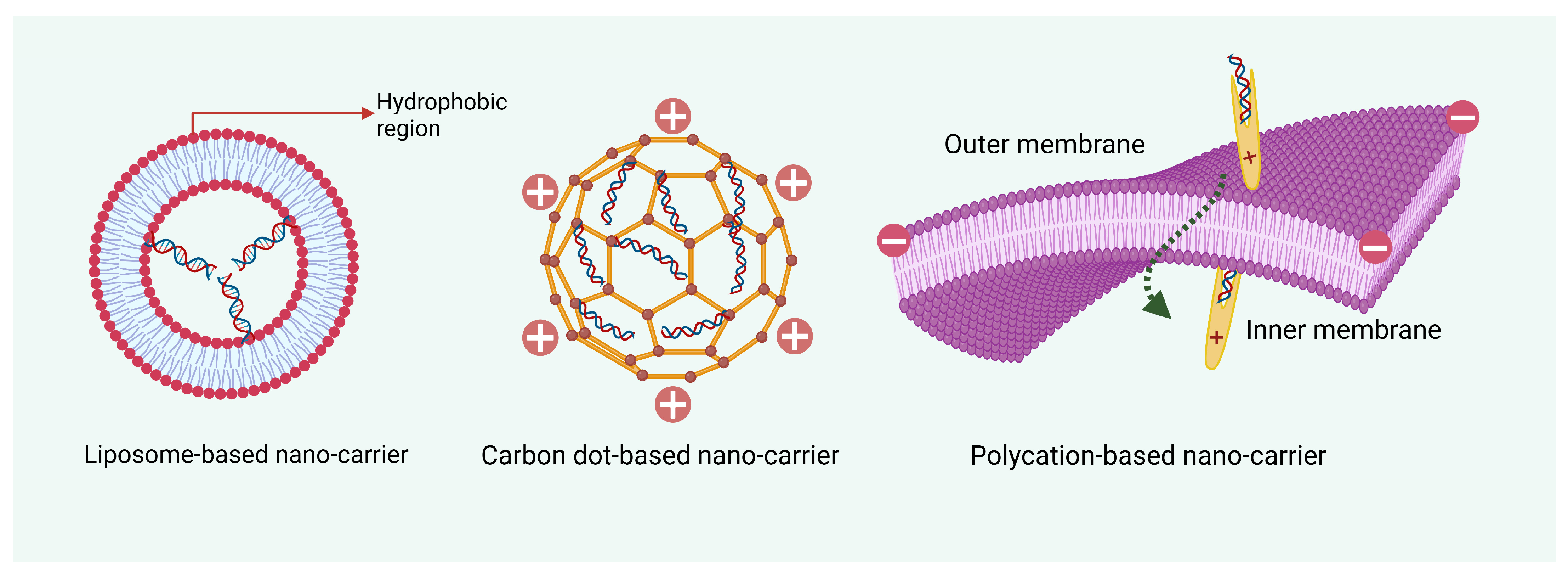
5. Nano-Enabled Delivery Systems for dsRNA/siRNA Stabilization
| Name | Composition | Function | References |
|---|---|---|---|
| Liposomes | The lipid bilayer is a vesicle that encapsulates and transports the RNA molecules. | Enhanced stability can be engineered to use nanoparticles in a controlled manner. | [50] |
| Metal nanoparticles, like gold or silica | Bind with RNA molecules and carry them into plant cells through endocytosis. | Stability enhancement | [34] |
| Polymeric biodegradable nanoparticles | Polylactic-co-glycolic acid (PLGA) and chitosan can form nanoparticles that encapsulate RNA molecules. | Provide stability to RNA and release RNA inside in a controlled manner. | [51] |
| Carbon nanotubes | Cylindrical nanostructures with colossal surface area. | Enormous surface area, it can be used to carry significant amounts of RNA. | [25] |
| Exosomes and nanovesicles | Naturally occurring nanoparticles derived from cells. | It can be loaded with RNA and used to deliver it to plant cells, having natural compatibility. | [52] |
| Nanospheres and nanorods | Spherical or rod-shaped nanoparticles made from various materials like metals, polymers, or silica. | Designed to deliver RNA molecules by attaching them to their surface or incorporating them into their structure. | [53] |
| Dendrimers | Dendrimers are highly branched, tree-like polymers. | Used to deliver RNA molecules by encapsulating them within their structure. | [54] |
| Micelles | Micelles are self-assembled nanoparticles formed from amphiphilic surfactants. | Can encapsulate RNA molecules in their core and facilitate their uptake into plant cells. | [55,56] |
| Nucleic acid nanostructures | RNA molecules can be assembled into nanostructures, like RNA nanorods or RNA nanoparticles. | These structures can improve the stability and cellular uptake of RNA molecules. | [57] |
| Magnetic nanoparticles | Magnetic nanoparticles produced from materials like iron oxide. | Employed to direct the delivery of RNA molecules to plant tissues through the application of an external magnetic field. | [58] |
| Pathogen | Host | Spray RNA | Target Gene | Main Effect | Reference |
|---|---|---|---|---|---|
| Magnaporthe oryzae/ Rice blast | Rice | dsRNA | MoDES11 | Systemic disease inhibition | [59] |
| Fusarium graminicum | Barley | dsRNA | CYP51-A, CYP51-B, CYP51-C | Restricts the growth of necrotrophic fungus | [60] |
| Fusarium graminicum | Barley | dsRNA | FgAG-01, FgAG-02 | Containment of infection areas | [61] |
| Fusarium graminicum | Barley | dsRNA | FgDCL1, FgDCL2 | Reduced fungal infection | [61] |
| Fusarium graminicum | Wheat | dsRNA | Myosin5 | Reduction in phenamacril resistance | [62] |
| Botrytis cinerea | Tomato, strawberry, grape, lettuce, onion, Arabidopsis, Grapevine | dsRNA | Bc-DCL1/2 | Inhibition of fungal growth reduced disease symptoms and suppressed fungal transcripts. BcCYP51, Bcchs1, BcEF2 | [32] |
| Phytophthora infestans/ Potato Late Blight | Potato | dsRNA | SDH, EF-1a, GPI, HAM344, PLD-3, HSP-90 | Enhanced disease resistance and less sporulation | [63] |
| Phytophthora infestans/ Potato Late Blight | Potato | dsRNA | PiGPB1, Pihmp1, PiCut3, PiEndo3 | Reduction in disease progression | [64] |
| Plasmopara viticola/ Grapevine downy mildew | Grapevine | dsRNA | PvDCL1/2 | Reduced disease progress rate | [65] |
| Phakopsara pachyrhizi/ Soybean rust | Soyabean | dsRNA | ATC, GCS-H, RP-S16 | Reduction in fungal biomass and a lower number of pustules on leaves | [66] |
| Rhizoctonia solani | Rice | dsRNA | DCTN1 + SAC1, PG | Transport of vesicles, pectin degradation | [67] |
| Delivery Vehicle | Delivery Mode | Target Site of Pathogen | Exposure | Durability | Efficacy | Reference |
|---|---|---|---|---|---|---|
| LDH | A. thaliana leaves or spray atomizer on V. unguiculata and N. tabacum | Silences the PMMoV replicase gene and the CMV target gene. | 200 µL samples of 15 µg CMV2b-dsRNA–LDH, sprayed on day 0 only. | After 2 min, uncoated (dsRNAs) showed minimal degradation, but dsRNA-loaded layered double hydroxides (dsRNA-LDHs) remained functionally intact. | Days 1–5 application: LDH-only treated plants developed more necrotic lesions than dsRNA-LDH-treated plants. LDH-dsRNA provided superior protection against the virus 20 days after application. Disease severity includes 10% for leaf spray, 15% for petioles adsorption, and 35% for sinking roots. | [68] |
| LDH | Spray the leaves, immerse the petioles, or drip the roots | The transcriptional activity of the fungus gene FoCYP51. | Spray leaves and petioles with 300 µg dsRNAs in 3 mL ddH2O per plant. | Degradation of naked dsRNA lasted from 1 to 10 min. | [69] | |
| CD (Carbon Dots) | Low-pressure spray | GFP transgene and endogenous gene silencing in N. benthamiana and S. lycopersicum. | SiRNA/CD is sprayed on plants at 12 ng/µL concentration on Days 1, 7, and 14. | SiRNA/CD is sprayed on plants at 12 ng/µL concentration on Days 1, 7, and 14. | Naked dsRNAs degrade entirely after 15 min. The dsRNA-CDs remain intact after 60 min of incubation. | [70] |
| Exogenous Spray | Fungus sporangia, CDQ complex. | Naked dsRNA does not show an effect, but dsRNA CQDs mixture 10 µL/mL shows a significant inhibitor. | [24] | |||
| CD bPEI-CD branched polyethylenimine | Petiole absorption and leaf spray | Virus. RNA polymerase and coat protein genes of grapevine leafroll associated virus-3 silenced. | The 0.00092 g/mL concentration was diluted 32 times. | Naked dsRNAs degrade after 2 h, while dsRNA-CDs-bPEI remains intact. | After a single dose, the virus titer dropped over three weeks, but several doses are necessary to enhance fruit quality. | [71] |
| CNT (Carbon nanotube) | Infiltration of N. benthamiana leaves using needleless syringes | The host plant silences mGFP5 transgenes in leaves. | 100 nM siRNA, 2 mg/L SWNT. | The degradation of naked dsRNAs was 94% after 6 h. 30% degradation of dsRNA-SNWT after 6 h. | Gene silencing effectiveness reached 95% within 1 day of invasion. | [25] |
| CPP (Cell-penetrating peptide) | Applying needleless syringe infiltration to A. thaliana leaves | For insects, silence GFP and firefly luciferase genes. | For up to 36 h, incubate 100 µL of dsRNA-peptide. | After 12 h, naked dsRNAs showed minor degradation, but dsRNA-peptides remained intact. | Naked dsRNAs did not show silencing effects, whereas dsRNA-peptides showed genetic down-regulation within 12 to 36 h. | [72] |
| Gold Nanoparticle | Various insect cell lines | Silences Spodopteria, frugiperda luciferase gene. | dsRNA (50 µg/mL) | Endosomal escape was improved by dsRNA-Au compared to dsRNA alone. | Luciferase activity for dsRNA-Au is reduced by up to 58% compared to dsRNA alone. | [73] |
| Needleless syringe infiltration N. benthamiana leaves | Inhibits mGFP5 transgenes in N. benthamiana leaves. | 100 ng siRNA | The degradation of naked dsRNAs was concluded after 30 min, while the dsRNA-Gold NP remained intact. | No NbrbohB overexpression indicates minimal stress to plant tissues. | [74] | |
| Chitosan Nanoparticle | Not tested | Silences tomato mosaic virus CP gene. | Use 200 µg/mL of dsRNA-chitosan. | Not reported | Low toxicity and no inhibition of root development are seen with dsRNA-chitosan. | [75] |
| SPc (star polycation) | To treat Myzus persicae-infested oilseed rape leaves, use a pneumatic water sprayer | Silences genes (ATP-A: 413 bp, LOC111039523; ATP-d: 383 bp, LOC111041166; ATP-G: 301 bp, LOC111040044) of M. persica. | Spray 0.2 µL dsRNA/SPc formulation on Day 0. | After 1.5 h, naked dsRNAs completely degraded in 12.5% of aphid hemolymph, though dsRNA-SPc remained intact. | Control effectiveness was 61% on Day 3 and 50% till Day 6 after SPc-dsRNA treatment. | [76] |
| Drenching roots | Silence genes associated with wing formation in M. persicae, such as vg and Ubx. | Applying 200 µL dsRNA/SPc formulation to radish seedlings on Day 0 before transplanting with M. persica. | Not reported | Approximately 40% of M. persica grew functional wings using both dsRNA genes. | [77] |
6. Nanotechnology-Based Enhanced Crop Protection Against Pathogens
7. Concluding Remarks and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Strange, R.N.; Scott, P.R. Plant Disease: A Threat to Global Food Security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Mason-D’Croz, D.; Robinson, S. Food System Consequences of a Fungal Disease Epidemic in a Major Crop. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2016, 371, 20150467. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef]
- Goswami, R.S.; Kistler, H.C. Heading for Disaster: Fusarium Graminearum on Cereal Crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef]
- Audenaert, K.; Vanheule, A.; Höfte, M.; Haesaert, G. Deoxynivalenol: A Major Player in the Multifaceted Response of Fusarium to Its Environment. Toxins 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, C.; Qi, H.; Wu, N.; Liu, Z.; Tian, Q.; Zhu, X.X.; Li, X.; Chen, Y.; Ma, Z. A Novel Highly Antifungal Compound ZJS—178 Targeting Myosin I Inhibits the Endocytosis and Mycotoxin Biosynthesis of Fusarium Graminearum. Crop Health 2024, 2, 14. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging Fungal Threats to Animal, Plant and Ecosystem Health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide Resistance Management: Maximizing the Effective Life of Plant Protection Products. Plant Pathol. 2022, 71, 150–169. [Google Scholar] [CrossRef]
- Islam, M.T.; Sherif, S.M. RNAi-Based Biofungicides as a Promising Next-Generation Strategy for Controlling Devastating Gray Mold Diseases. Int. J. Mol. Sci. 2020, 21, 2072. [Google Scholar] [CrossRef]
- Eamens, A.; Wang, M.-B.; Smith, N.A.; Waterhouse, P.M. RNA Silencing in Plants: Yesterday, Today, and Tomorrow. Plant Physiol. 2008, 147, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.C.; Conte, D. Revealing the World of RNA Interference. Nature 2004, 431, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Pandey, V.K.; Jha, A.K.; Srivastava, S.; Jakhar, S.; Vijay; Singh, G.; Rustagi, S.; Malik, S.; Choudhary, P. Intricacies of Plants’ Innate Immune Responses and Their Dynamic Relationship with Fungi: A Review. Microbiol. Res. 2024, 285, 127758. [Google Scholar] [CrossRef] [PubMed]
- Sang, H.; Kim, J.-I. Advanced Strategies to Control Plant Pathogenic Fungi by Host-Induced Gene Silencing (HIGS) and Spray-Induced Gene Silencing (SIGS). Plant Biotechnol. Rep. 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Šečić, E.; Kogel, K.-H. Requirements for Fungal Uptake of DsRNA and Gene Silencing in RNAi-Based Crop Protection Strategies. Curr. Opin. Biotechnol. 2021, 70, 136–142. [Google Scholar] [CrossRef]
- Wang, M.; Jin, H. Spray-Induced Gene Silencing: A Powerful Innovative Strategy for Crop Protection. Trends Microbiol. 2017, 25, 4–6. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Weiberg, A.; Buck, A.H.; Jin, H. Small RNAs and Extracellular Vesicles: New Mechanisms of Cross-Species Communication and Innovative Tools for Disease Control. PLoS Pathog. 2019, 15, e1008090. [Google Scholar] [CrossRef]
- Niu, D.; Hamby, R.; Sanchez, J.N.; Cai, Q.; Yan, Q.; Jin, H. RNAs—A New Frontier in Crop Protection. Curr. Opin. Biotechnol. 2021, 70, 204–212. [Google Scholar] [CrossRef]
- Niño, J.; Thanjavur Sambasivam, P.; Sawyer, A.; Hamby, R.; Chen, A.; Czislowski, E.; Li, P.; Manzie, N.; Gardiner, D.; Ford, R.; et al. BioClay TM Prolongs RNA Interference-mediated Crop Protection against Botrytis Cinerea. J. Integr. Plant Biol. 2022, 64, 2187–2198. [Google Scholar] [CrossRef]
- San Miguel, K.; Scott, J.G. The next Generation of Insecticides: DsRNA Is Stable as a Foliar-Applied Insecticide. Pest Manag. Sci. 2016, 72, 801–809. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay Nanosheets for Topical Delivery of RNAi for Sustained Protection against Plant Viruses. Nat. Plants 2017, 3, 1–10. [Google Scholar] [CrossRef][Green Version]
- Worrall, E.A.; Bravo-Cazar, A.; Nilon, A.T.; Fletcher, S.J.; Robinson, K.E.; Carr, J.P.; Mitter, N. Exogenous Application of RNAi-Inducing Double-Stranded RNA Inhibits Aphid-Mediated Transmission of a Plant Virus. Front. Plant Sci. 2019, 10, 265. [Google Scholar]
- Shang, W.; Xiong, Q.; Xie, Z.; Cheng, J.; Yu, B.; Zhang, H.; Su, Y.; Zhao, J. Functional, Eco-Friendly, and Starch-Based Nanocarriers with Sustained Release of Carvacrol for Persistent Control of Tomato Gray Mold. Crop Health 2023, 1, 1–12. [Google Scholar] [CrossRef]
- Kostov, K.; Andonova-Lilova, B.; Smagghe, G. Inhibitory Activity of Carbon Quantum Dots against Phytophthora Infestans and Fungal Plant Pathogens and Their Effect on DsRNA-Induced Gene Silencing. Biotechnol. Biotechnol. Equip. 2022, 36, 949–959. [Google Scholar] [CrossRef]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; Pinals, R.L.; Chang, R.; Landry, M.P. Carbon Nanocarriers Deliver SiRNA to Intact Plant Cells for Efficient Gene Knockdown. Sci. Adv. 2020, 6, eaaz0495. [Google Scholar] [CrossRef]
- Qiao, L.; Niño-Sánchez, J.; Hamby, R.; Capriotti, L.; Chen, A.; Mezzetti, B.; Jin, H. Artificial Nanovesicles for DsRNA Delivery in Spray-Induced Gene Silencing for Crop Protection. Plant Biotechnol. J. 2023, 21, 854–865. [Google Scholar] [CrossRef]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. SiRNA versus MiRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Li, C. Research Progress on MiRNAs and Artificial MiRNAs in Insect and Disease Resistance and Breeding in Plants. Genes 2024, 15, 1200. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P. Plant Hormone-Mediated Regulation of Stress Responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Katiyar-Agarwal, S.; Morgan, R.; Dahlbeck, D.; Borsani, O.; Villegas, A.; Zhu, J.-K.; Staskawicz, B.J.; Jin, H. A Pathogen-Inducible Endogenous SiRNA in Plant Immunity. Proc. Natl. Acad. Sci. USA 2006, 103, 18002–18007. [Google Scholar]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-Nucleotide RNAs Mediate RNA Interference in Cultured Mammalian Cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Wang, M.; Weiberg, A.; Lin, F.-M.; Thomma, B.P.H.J.; Huang, H.-D.; Jin, H. Bidirectional Cross-Kingdom RNAi and Fungal Uptake of External RNAs Confer Plant Protection. Nat. Plants 2016, 2, 1–10. [Google Scholar] [CrossRef]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-Based Control of Fusarium Graminearum Infections Through Spraying of Long DsRNAs Involves a Plant Passage and Is Controlled by the Fungal Silencing Machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef]
- Chen, A.; Halilovic, L.; Shay, J.-H.; Koch, A.; Mitter, N.; Jin, H. Improving RNA-Based Crop Protection through Nanotechnology and Insights from Cross-Kingdom RNA Trafficking. Curr. Opin. Plant Biol. 2023, 76, 102441. [Google Scholar] [CrossRef]
- Dalakouras, A.; Wassenegger, M.; McMillan, J.N.; Cardoza, V.; Maegele, I.; Dadami, E.; Runne, M.; Krczal, G.; Wassenegger, M. Induction of Silencing in Plants by High-Pressure Spraying of In Vitro-Synthesized Small RNAs. Front. Plant Sci. 2016, 7, 1327. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Numata, K. Advancing Biomolecule Delivery in Plants: Harnessing Synthetic Nanocarriers to Overcome Multiscale Barriers for Cutting-Edge Plant Bioengineering. Bull. Chem. Soc. Jpn. 2023, 96, 1026–1044. [Google Scholar] [CrossRef]
- Das, P.R.; Sherif, S.M. Application of Exogenous DsRNAs-Induced RNAi in Agriculture: Challenges and Triumphs. Front. Plant Sci. 2020, 11, 946. [Google Scholar] [CrossRef]
- Biedenkopf, D.; Will, T.; Knauer, T.; Jelonek, L.; Furch, A.C.U.; Busche, T.; Koch, A. Systemic Spreading of Exogenous Applied RNA Biopesticides in the Crop Plant Hordeum Vulgare. ExRNA 2020, 2, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Ou, Y.; Hou, L. Advances in RNA-Based Therapeutics: Challenges and Innovations in RNA Delivery Systems. Curr. Issues Mol. Biol. 2025, 47, 22. [Google Scholar] [CrossRef]
- Larsen, J.S.; Curtis, W.R. RNA Viral Vectors for Improved Agrobacterium-Mediated Transient Expression of Heterologous Proteins in Nicotiana Benthamiana Cell Suspensions and Hairy Roots. BMC Biotechnol. 2012, 12, 21. [Google Scholar] [CrossRef]
- Mahanty, B.; Mishra, R.; Joshi, R.K. Cross-Kingdom Small RNA Communication between Plants and Fungal Phytopathogens-Recent Updates and Prospects for Future Agriculture. RNA Biol. 2023, 20, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.; Palmquist, J.; Jin, H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar]
- Karimi, H.Z.; Baldrich, P.; Rutter, B.D.; Zajt, K.K.; Meyers, B.C.; Innes, R.W. Arabidopsis Apoplastic Fluid Contains SRNA- and Circular RNA—Protein Complexes That Are Located Outside Extracellular Vesicles. Plant Cell 2022, 34, 1863–1881. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Kogel, K.-H.; Jin, H. Cross-Kingdom RNA Trafficking and Environmental RNAi-Nature’s Blueprint for Modern Crop Protection Strategies. Curr. Opin. Microbiol. 2018, 46, 58–64. [Google Scholar] [CrossRef]
- Rego-Machado, C.M.; Nakasu, E.Y.T.; Silva, J.M.F.; Lucinda, N.; Nagata, T.; Inoue-Nagata, A.K. SiRNA Biogenesis and Advances in Topically Applied DsRNA for Controlling Virus Infections in Tomato Plants. Sci. Rep. 2020, 10, 22277. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, Y.; Mo, Q.; Huang, Y.; Tang, X. Spray-Induced Gene Silencing in Phytopathogen: Mechanisms, Applications, and Progress. Adv. Agrochem 2024, 3, 289–297. [Google Scholar] [CrossRef]
- Komarova, T.; Ilina, I.; Taliansky, M.; Ershova, N. Nanoplatforms for the Delivery of Nucleic Acids into Plant Cells. Int. J. Mol. Sci. 2023, 24, 16665. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.; Ahmed, T.; Wang, J.; Ijaz, M.; Shahid, M.; Islam, M.S.; Azizullah; Manzoor, I.; Li, D.; Song, F. Nano-Enabled Crop Resilience against Pathogens: Potential, Mechanisms and Strategies. Crop Health 2023, 1, 15. [Google Scholar] [CrossRef]
- Mat Jalaluddin, N.S.; Asem, M.; Harikrishna, J.A.; Ahmad Fuaad, A.A.H. Recent Progress on Nanocarriers for Topical-Mediated RNAi Strategies for Crop Protection—A Review. Molecules 2023, 28, 2700. [Google Scholar] [CrossRef]
- Wei, P.S.; Thota, N.; John, G.; Chang, E.; Lee, S.; Wang, Y.; Ma, Z.; Tsai, Y.H.; Mei, K.C. Enhancing RNA-Lipid Nanoparticle Delivery: Organ- and Cell-Specificity and Barcoding Strategies. J. Control. Release 2024, 375, 366–388. [Google Scholar] [CrossRef]
- Nafee, N.; Taetz, S.; Schneider, M.; Schaefer, U.F.; Lehr, C.-M. Chitosan-Coated PLGA Nanoparticles for DNA/RNA Delivery: Effect of the Formulation Parameters on Complexation and Transfection of Antisense Oligonucleotides. Nanomedicine 2007, 3, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Pandya, A.; Kumar, L.; Raval, N.; Vora, L.K.; Pulakkat, S.; Patravale, V.; Salwa; Duo, Y.; Tang, B.Z. Exosome Nanovesicles: A Potential Carrier for Therapeutic Delivery. Nano Today 2023, 49, 101771. [Google Scholar] [CrossRef]
- Byun, M.J.; Lim, J.; Kim, S.N.; Park, D.H.; Kim, T.H.; Park, W.; Park, C.G. Advances in Nanoparticles for Effective Delivery of RNA Therapeutics. Biochip J. 2022, 16, 128–145. [Google Scholar] [CrossRef]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.M.A.; Alfaidi, M.A.; Batiha, G.E.-S.; Akter, W.; Gautam, R.K.; Uddin, M.S.; Rahman, M.S. Dendrimers: A New Race of Pharmaceutical Nanocarriers. Biomed Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef]
- Sinani, G.; Durgun, M.E.; Cevher, E.; Özsoy, Y. Polymeric-Micelle-Based Delivery Systems for Nucleic Acids. Pharmaceutics 2023, 15, 2021. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Cao, Y.; Li, L.; Wang, X.; Lu, H.; Yang, J.; Wang, S. Self-Assembled Polymeric Micelle as a Novel MRNA Delivery Carrier. J. Control. release Off. J. Control. Release Soc. 2021, 338, 537–547. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, L.; Hao, Z.; Liu, D. DNA-Based Nanostructures for RNA Delivery. Med. Rev. 2024, 4, 207–224. [Google Scholar] [CrossRef]
- Mykhaylyk, O.; Sanchez-Antequera, Y.; Vlaskou, D.; Cerda, M.B.; Bokharaei, M.; Hammerschmid, E.; Anton, M.; Plank, C. Magnetic Nanoparticle and Magnetic Field Assisted SiRNA Delivery in Vitro. Methods Mol. Biol. 2015, 1218, 53–106. [Google Scholar] [CrossRef]
- Sarkar, A.; Roy-Barman, S. Spray-Induced Silencing of Pathogenicity Gene MoDES1 via Exogenous Double-Stranded RNA Can Confer Partial Resistance against Fungal Blast in Rice. Front. Plant Sci. 2021, 12, 733129. [Google Scholar] [CrossRef]
- Höfle, L.; Biedenkopf, D.; Werner, B.T.; Shrestha, A.; Jelonek, L.; Koch, A. Study on the Efficiency of DsRNAs with Increasing Length in RNA-Based Silencing of the Fusarium CYP51 Genes. RNA Biol. 2020, 17, 463–473. [Google Scholar] [CrossRef]
- Werner, B.T.; Gaffar, F.Y.; Schuemann, J.; Biedenkopf, D.; Koch, A.M. RNA-Spray-Mediated Silencing of Fusarium Graminearum AGO and DCL Genes Improve Barley Disease Resistance. Front. Plant Sci. 2020, 11, 476. [Google Scholar] [CrossRef]
- Song, X.S.; Gu, K.X.; Duan, X.X.; Xiao, X.M.; Hou, Y.P.; Duan, Y.B.; Wang, J.X.; Zhou, M.G. A Myosin5 DsRNA That Reduces the Fungicide Resistance and Pathogenicity of Fusarium Asiaticum. Pestic. Biochem. Physiol. 2018, 150, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bairwa, A.; Tomar, M.; Kumar, R.; Bhardwaj, V.; Jeevalatha, A.; Bakade, R.; Salaria, N.; Thakur, K.; Singh, B.P.; et al. Spraying of DsRNA Molecules Derived from Phytophthora Infestans, as a Plant Protection Strategy for the Management of Potato Late Blight. Pest Manag. Sci. 2022, 78, 3183. [Google Scholar]
- Kalyandurg, P.B.; Sundararajan, P.; Dubey, M.; Ghadamgahi, F.; Zahid, M.A.; Whisson, S.C.; Vetukuri, R.R. Spray-Induced Gene Silencing as a Potential Tool to Control Potato Late Blight Disease. Phytopathology 2021, 111, 2168–2175. [Google Scholar] [CrossRef]
- Haile, Z.M.; Gebremichael, D.E.; Capriotti, L.; Molesini, B.; Negrini, F.; Collina, M.; Sabbadini, S.; Mezzetti, B.; Baraldi, E. Double-Stranded RNA Targeting Dicer-like Genes Compromises the Pathogenicity of Plasmopara Viticola on Grapevine. Front. Plant Sci. 2021, 12, 667539. [Google Scholar] [CrossRef]
- Hu, D.; Chen, Z.Y.; Zhang, C.; Ganiger, M. Reduction of Phakopsora Pachyrhizi Infection on Soybean through Host-and Spray-induced Gene Silencing. Mol. Plant Pathol. 2020, 21, 794–807. [Google Scholar] [CrossRef]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-induced Gene Silencing for Disease Control Is Dependent on the Efficiency of Pathogen RNA Uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Xu, Z.P.; Carroll, B.J. Induction of Virus Resistance by Exogenous Application of Double-Stranded RNA. Curr. Opin. Virol. 2017, 26, 49–55. [Google Scholar] [CrossRef]
- Mosa, M.A.; Youssef, K. Topical Delivery of Host Induced RNAi Silencing by Layered Double Hydroxide Nanosheets: An Efficient Tool to Decipher Pathogenicity Gene Function of Fusarium Crown and Root Rot in Tomato. Physiol. Mol. Plant Pathol. 2021, 115, 101684. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. Carbon Dots for Efficient Small Interfering RNA Delivery and Gene Silencing in Plants. Plant Physiol. 2020, 184, 647–657. [Google Scholar] [CrossRef]
- Avital, A.; Muzika, N.S.; Persky, Z.; Bar, G.; Michaeli, Y.; Fridman, Y.; Karny, A.; Shklover, J.; Shainsky, J.; Savaldi-Goldstein, S.; et al. Foliar Delivery of SiRNA Particles for Treating Viral Infections in Agricultural Grapevines. Adv. Funct. Mater. 2021, 31, 2101003. [Google Scholar] [CrossRef]
- Numata, K.; Ohtani, M.; Yoshizumi, T.; Demura, T.; Kodama, Y. Local Gene Silencing in Plants via Synthetic Ds RNA and Carrier Peptide. Plant Biotechnol. J. 2014, 12, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Laisney, J.; Gurusamy, D.; Baddar, Z.E.; Palli, S.R.; Unrine, J.M. RNAi in Spodoptera Frugiperda Sf9 Cells via Nanomaterial Mediated Delivery of DsRNA: A Comparison of Poly-l-Arginine Polyplexes and Poly-l-Arginine-Functionalized Au Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 25645–25657. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Xu, D.; Goh, N.S.; Demirer, G.S.; Cestellos-Blanco, S.; Chen, Y.; Landry, M.P.; Yang, P. Gold-Nanocluster-Mediated Delivery of SiRNA to Intact Plant Cells for Efficient Gene Knockdown. Nano Lett. 2021, 21, 5859–5866. [Google Scholar] [CrossRef]
- Petrônio, M.S.; Barros-Alexandrino, T.T.; Lima, A.M.; de Assis, O.B.G.; Inoue-Nagata, A.K.; Nakasu, E.Y.; Tiera, M.J.; Pilon, L. Physicochemical and Toxicity Investigation of Chitosan-Based DsRNA Nanocarrier Formation. J. Name 2022, 12, 5266–5279. [Google Scholar]
- Ma, Z.; Zhang, Y.; Li, M.; Chao, Z.; Du, X.; Yan, S.; Shen, J. A First Greenhouse Application of Bacteria-Expressed and Nanocarrier-Delivered RNA Pesticide for Myzus Persicae Control. J. Pest Sci. 2023, 96, 181–193. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ma, Z.Z.; Zhou, H.; Chao, Z.J.; Yan, S.; Shen, J. Nanocarrier-delivered DsRNA Suppresses Wing Development of Green Peach Aphids. Insect Sci. 2022, 29, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Wang, D.; Carvalho, L.B.; Oliveira, J.L.; Do Espirito Santo Pereira, A.; Sharif, R.; Jogaiah, S.; Paidi, M.K.; Wang, L.; Ali, Q.; et al. Nanocarrier-Mediated Delivery of MiRNA, RNAi, and CRISPR-Cas for Plant Protection: Current Trends and Future Directions. ACS Agric. Sci. Technol. 2021, 1, 417–435. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Q.; Lan, C.; Tang, T.; Wang, K.; Shen, J.; Niu, D. Nanoparticle Carriers Enhance RNA Stability and Uptake Efficiency and Prolong the Protection against Rhizoctonia Solani. Phytopathol. Res. 2023, 5, 2. [Google Scholar] [CrossRef]
- Quilez-Molina, A.I.; Niño Sanchez, J.; Merino, D. The Role of Polymers in Enabling RNAi-Based Technology for Sustainable Pest Management. Nat. Commun. 2024, 15, 9158. [Google Scholar] [CrossRef]
- Mann, C.W.G.; Sawyer, A.; Gardiner, D.M.; Mitter, N.; Carroll, B.J.; Eamens, A.L. RNA-Based Control of Fungal Pathogens in Plants. Int. J. Mol. Sci. 2023, 24, 12391. [Google Scholar] [CrossRef] [PubMed]
- Ogunyemi, S.O.; Luo, J.; Abdallah, Y.; Yu, S.; Wang, X.; Alkhalifah, D.H.M.; Hozzein, W.N.; Wang, F.; Bi, J.; Yan, C.; et al. Copper Oxide Nanoparticles: An Effective Suppression Tool against Bacterial Leaf Blight of Rice and Its Impacts on Plants. Pest Manag. Sci. 2024, 80, 1279–1288. [Google Scholar] [CrossRef]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A Perspective on RNAi-Based Biopesticides. Front. Plant Sci. 2020, 11, 51. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Mishra, S.K.; Sridharan, K.; Barnes, E.R.; Alyokhin, A.; Tuttle, R.; Kokulapalan, W.; Garby, D.; Skizim, N.J.; Tang, Y.W.; et al. First Sprayable Double-Stranded RNA-Based Biopesticide Product Targets Proteasome Subunit Beta Type-5 in Colorado Potato Beetle (Leptinotarsa Decemlineata). Front. Plant Sci. 2021, 12, 728652. [Google Scholar] [CrossRef]
- Venu, E.; Ramya, A.; Babu, P.L.; Srinivas, B.; Kumar, S.; Reddy, N.K.; Babu, Y.M.; Majumdar, A.; Manik, S. Exogenous DsRNA-Mediated RNAi: Mechanisms, Applications, Delivery Methods and Challenges in the Induction of Viral Disease Resistance in Plants. Viruses 2025, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dai, Y.; Huang, Z.; Zhang, M.; Xiu, L.; Zhang, X.; Zhang, Y.; Huang, L. RNA-Based Biopesticides: Pioneering Precision Solutions for Sustainable Aquaculture in China. Anim. Res. One Health 2025, 1–12. [Google Scholar] [CrossRef]
- De Schutter, K.; Taning, C.N.T.; Van Daele, L.; Van Damme, E.J.M.; Dubruel, P.; Smagghe, G. RNAi-Based Biocontrol Products: Market Status, Regulatory Aspects, and Risk Assessment. Front. Insect Sci. 2021, 1, 818037. [Google Scholar] [CrossRef] [PubMed]
- Dietz-Pfeilstetter, A.; Mendelsohn, M.; Gathmann, A.; Klinkenbuß, D. Considerations and Regulatory Approaches in the USA and in the EU for DsRNA-Based Externally Applied Pesticides for Plant Protection. Front. Plant Sci. 2021, 12, 682387. [Google Scholar] [CrossRef]
- Yang, W.; Wang, B.; Lei, G.; Chen, G.; Liu, D. Advances in Nanocarriers to Improve the Stability of DsRNA in the Environment. Front. Bioeng. Biotechnol. 2022, 10, 974646. [Google Scholar] [CrossRef]
- Ahmed, T.; Luo, J.; Noman, M.; Ijaz, M.; Wang, X.; Masood, H.A.; Manzoor, N.; Wang, Y.; Li, B. Microbe-Mediated Nanoparticle Intervention for the Management of Plant Diseases. Crop Health 2023, 1, 3. [Google Scholar] [CrossRef]
- Qiao, H.; Chen, J.; Dong, M.; Shen, J.; Yan, S. Nanocarrier-Based Eco-Friendly RNA Pesticides for Sustainable Management of Plant Pathogens and Pests. Nanomaterials 2024, 14, 1874. [Google Scholar] [CrossRef]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically Modified Organism-Free RNA Interference: Exogenous Application of RNA Molecules in Plants. Plant Physiol. 2020, 182, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Bally, J.; Fishilevich, E.; Bowling, A.J.; Pence, H.E.; Narva, K.E.; Waterhouse, P.M. Improved Insect-Proofing: Expressing Double-Stranded RNA in Chloroplasts. Pest Manag. Sci. 2018, 74, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Li, N.; Guo, Y.; Chen, Y.; Ji, C.; Yin, M.; Shen, J.; Zhang, J. Chronic Exposure to the Star Polycation (SPc) Nanocarrier in the Larval Stage Adversely Impairs Life History Traits in Drosophila Melanogaster. J. Nanobiotechnol. 2022, 20, 515. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.S.; Ahmed, M.R.; Noman, M.; Zhang, Z.; Wang, J.; Lu, Z.; Cai, Y.; Ahmed, T.; Li, B.; Wang, Y.; et al. Integrating RNA Interference and Nanotechnology: A Transformative Approach in Plant Protection. Plants 2025, 14, 977. https://doi.org/10.3390/plants14060977
Islam MS, Ahmed MR, Noman M, Zhang Z, Wang J, Lu Z, Cai Y, Ahmed T, Li B, Wang Y, et al. Integrating RNA Interference and Nanotechnology: A Transformative Approach in Plant Protection. Plants. 2025; 14(6):977. https://doi.org/10.3390/plants14060977
Chicago/Turabian StyleIslam, Mohammad Shafiqul, Md Robel Ahmed, Muhammad Noman, Zhen Zhang, Jing Wang, Ziqi Lu, Yingying Cai, Temoor Ahmed, Bin Li, Yanli Wang, and et al. 2025. "Integrating RNA Interference and Nanotechnology: A Transformative Approach in Plant Protection" Plants 14, no. 6: 977. https://doi.org/10.3390/plants14060977
APA StyleIslam, M. S., Ahmed, M. R., Noman, M., Zhang, Z., Wang, J., Lu, Z., Cai, Y., Ahmed, T., Li, B., Wang, Y., Golam Sarwar, A. K. M., & Wang, J. (2025). Integrating RNA Interference and Nanotechnology: A Transformative Approach in Plant Protection. Plants, 14(6), 977. https://doi.org/10.3390/plants14060977







