Native Bacteria Are Effective Biocontrol Agents at a Wide Range of Temperatures of Neofusicoccum parvum, Associated with Botryosphaeria Dieback on Grapevine
Abstract
1. Introduction
2. Results
2.1. In Vitro Dual Antagonism Assays of Bacteria Against Grapevine Trunk Pathogens
2.2. In Vitro Biocontrol Agar Plug Diffusion Assays for Grapevine Trunk Pathogens
2.3. In Vitro Biocontrol Double Plate Assays for Grapevine Trunk Pathogens
2.4. In Vivo Test on Cuttings of Different Ages
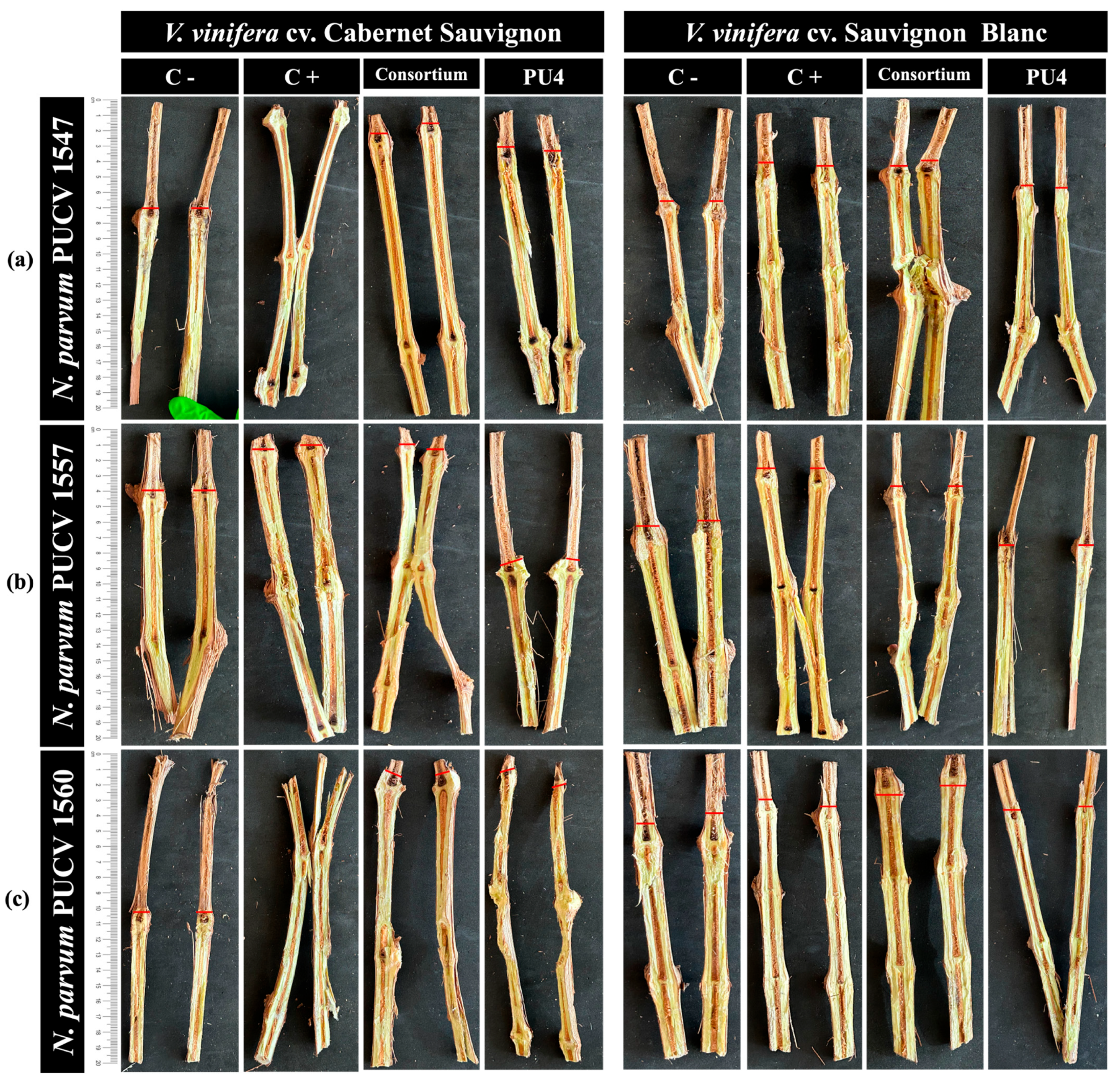
2.5. In Situ Biocontrol Test on Cuttings
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Culture Media
4.2. Microorganisms and Vegetable Materials of This Study
4.3. In Vitro Analysis of Biocontrol by Bacteria
4.3.1. Dual Antagonism Assays of Bacterial Strains Against Grapevine Trunk Pathogens
4.3.2. In Vitro Biocontrol Assays Using Agar Plug Diffusion for Grapevine Trunk Pathogens
4.3.3. In Vitro Biocontrol Assays Double Plate Method for Grapevine Trunk Pathogens
4.4. In Vivo Biocontrol by BCAs
4.4.1. Inoculations of Fungi and Bacteria
4.4.2. In Vivo Test on Cuttings
4.4.3. In Situ Biocontrol Test on Cuttings
4.4.4. Pathogen Damage Assessment and Recovery
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linnaeus, C. Species Plantarum; Laurentius Salvius: Stockholm, Sweden, 1753; 1200p. [Google Scholar]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 20 September 2023).
- Kenfaoui, J.; Radouane, N.; Mennani, M.; Tahiri, A.; El Ghadraoui, L.; Belabess, Z.; Fontaine, F.; El Hamss, H.; Amiri, S.; Lahlali, R.; et al. A panoramic view on Grapevine Trunk Diseases threats: Case of Eutypa Dieback, Botryosphaeria dieback, and Esca disease. J. Fungi 2022, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Mesguida, O.; Haidar, R.; Yacoub, A.; Dreux-Zigha, A.; Berthon, J.-Y.; Guyoneaud, R.; Attard, E.; Rey, P. Microbial biological control of fungi associated with Grapevine Trunk Diseases: A review of strain diversity, modes of action, and advantages and limits of current strategies. J. Fungi 2023, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Paredes, A.; Turrini, A.; Avio, L.; Stuardo, C.; Velásquez, A.; Becerra, J.; Giovannetti, M.; Seeger, M. Agricultural managements influence the diversity of arbuscular mycorrhizal fungi in vineyards from Chilean Mediterranean climate ecosystems. J. Soil Sci. Plant Nutr. 2024, 24, 6099–6112. [Google Scholar]
- Méndez, V.; Valenzuela, M.; Salvà-Serra, F.; Jaén-Luchoro, D.; Besoain, X.; Moore, E.R.B.; Seeger, M. Comparative genomics of pathogenic Clavibacter michiganensis subsp. michiganensis strains from Chile reveals potential virulence features for tomato plants. Microorganisms 2020, 8, 1679. [Google Scholar]
- Vásconez, I.-N.; Besoain, X.; Vega-Celedón, P.; Valenzuela, M.; Seeger, M. First report of bacterial wilt caused by Ralstonia solanacearum Phylotype IIB Sequevar 1 affecting tomato in different regions of Chile. Plant Dis. 2020, 104, 2023. [Google Scholar]
- Alfaro-Quezada, J.; Martínez, J.; Molinett, S.; Valenzuela, M.; Montenegro, I.; Ramírez, I.; Dorta, F.; Ávila-Valdés, A.; Gharbi, E.; Zhou, M.; et al. Rootstock increases the physiological defence of tomato plants to Pseudomonas syringae pv. tomato infection. J. Exp. Bot. 2023, 74, 2891–2911. [Google Scholar]
- Trouillas, F.P.; Úrbez-Torres, J.R.; Gubler, W.D. Diversity of Diatrypaceous fungi associated with grapevine canker diseases in California. Mycologia 2010, 102, 319–336. [Google Scholar]
- Gramaje, D.; Armengol, J. Fungal trunk pathogens in the grapevine propagation process: Potential inoculum sources, detection, identification and management strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar]
- Úrbez-Torres, J.R.; Peduto, F.; Striegler, K.; Rupe, J.C.; Cartwright, R.D.; Gubler, W.D. Characterization of fungal pathogens associated with grapevine trunk diseases in Arkansas and Missouri. Fungal Divers. 2011, 52, 169–189. [Google Scholar]
- Agustí-Brisach, C.; Armengol, J. Black-foot disease of grapevine: An update on taxonomy, epidemiology and management strategies. Phytopathol. Mediterr. 2013, 52, 245–261. [Google Scholar]
- Úrbez-Torres, J.R.; Peduto, F.; Smith, R.J.; Gubler, W.D. Phomopsis dieback: A grapevine trunk disease caused by Phomopsis viticola in California. Plant Dis. 2013, 97, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; Van Der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Lineages in Nectriaceae: Re-evaluating the generic status of Ilyonectria and allied genera. Phytopathol. Mediterr. 2014, 53, 515–532. [Google Scholar]
- Wilcox, W.F.; Gubler, W.D.; Uyemoto, J.K. Compendium of Grape Diseases, Disorders, and Pests, 2nd ed.; American Phytopathological Society Press: St. Paul, MN, USA, 2015. [Google Scholar]
- Carlucci, A.; Cibelli, F.; Lops, F.; Phillips, A.J.L.; Ciccarone, C.; Raimondo, M.L. Pleurostomophora richardsiae associated with trunk diseases of grapevines in Southern Italy. Phytopathol. Mediterr. 2015, 54, 109–123. [Google Scholar]
- Cloete, M.; Fischer, M.; Mostert, L.; Halleen, F. Hymenochaetales associated with Esca-related wood rots on grapevine with a special emphasis on the status of esca in South African vineyards. Phytopathol. Mediterr. 2015, 54, 299–312. [Google Scholar]
- Gramaje, D.; Mostert, L.; Groenewald, J.Z.; Crous, P.W. Phaeoacremonium: From Esca disease to phaeohyphomycosis. Fungal Biol. 2015, 119, 759–783. [Google Scholar] [CrossRef]
- Travadon, R.; Lawrence, D.P.; Rooney-Latham, S.; Gubler, W.D.; Wilcox, W.F.; Rolshausen, P.E.; Baumgartner, K. Cadophora species associated with wood-decay of grapevine in North America. Fungal Biol. 2015, 119, 53–66. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Travadon, R.; Pouzoulet, J.; Rolshausen, P.E.; Wilcox, W.F.; Baumgartner, K. Characterization of Cytospora isolates from wood cankers of declining grapevine in North America, with the descriptions of two new Cytospora species. Plant Pathol. 2016, 66, 713–725. [Google Scholar] [CrossRef]
- Araújo da Silva, M.; Correia, K.C.; Barbosa, M.A.G.; Cámara, M.P.S.; Gramaje, D.; Michereff, S.J. Characterization of Phaeoacremonium isolates associates with Petri disease of table grape in Northeastern Brazil, with description of Phaeoacremonium nordesticola sp. nov. Eur. J. Plant Pathol. 2017, 179, 695–709. [Google Scholar] [CrossRef]
- Gramaje, D. Avances en la investigación sobre el control de las enfermedades de la madera de la vid. Phytoma España 2017, 288, 50–52. [Google Scholar]
- Bertsch, C.; Ramirez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef]
- Makatini, G.; Mutawila, C.; Halleen, F.; Mostert, L. Grapevine sucker wounds as infection ports for trunk disease pathogens. Phytopathol. Mediterr. 2014, 53, 573. [Google Scholar]
- Mugnai, L.; Graniti, A.; Surico, G. Esca (black measles) and brown wood-streaking: Two old and elusive diseases of grapevines. Plant Dis. 1999, 83, 404–418. [Google Scholar] [PubMed]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [PubMed]
- Úrbez-Torres, J.R.; Gubler, W.D. Susceptibility of grapevine pruning wounds to infection by Lasiodiplodia theobromae and Neofusicoccum parvum. Plant Pathol. 2011, 60, 261–270. [Google Scholar] [CrossRef]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Millholland, R.D. Muscadine grapes: Some important diseases and their control. Plant Dis. 1991, 75, 113–117. [Google Scholar]
- Lorch, W. Fatal Wood Disease Affects 12 Percent of French Vineyards. 2014. Available online: https://www.wine-searcher.com/m/2014/10/fatal-wood-diseases-affect-12-percent-of-french-vineyards (accessed on 15 January 2025).
- Romanazzi, G.; Murolo, S.; Pizzichini, L.; Nardi, S. Esca in young and mature vineyards, and molecular diagnosis of the associated fungi. Eur. J. Plant Pathol. 2009, 125, 277–290. [Google Scholar]
- Martín, M.T.; Cobos, R. Identification of fungi associated with grapevine decline in Castilla y Léon (Spain). Phytopathol. Mediterr. 2007, 46, 18–25. [Google Scholar]
- Gramaje, D.; Armengol, J. Effects of hot-water treatment, posthot-water-treatment cooling and cold storage on the viability of dormant grafted grapevines under field conditions. Aust. J. Grape Wine Res. 2012, 18, 158–163. [Google Scholar]
- Hernandez, M.N.; Kc, A.N. A systematic survey on prevalence of grapevine trunk disease pathogens in Oregon vineyards. Plant Dis. 2023, 107, 1355–1364. [Google Scholar]
- Larach, A.; Torres, C.; Riquelme, N.; Valenzuela, M.; Salgado, E.; Seeger, M.; Besoain, X. Yield loss estimation and pathogen identification from Botryosphaeria dieback in vineyards of Central Chile over two growing seasons. Phytopathol. Mediterr 2020, 59, 537–548. [Google Scholar]
- Úrbez-Torres, J.R.; Gubler, W.D. Pathogenicity of Botryosphaeriaceae species isolated from grapevine cankers in California. Plant Dis. 2009, 93, 584–592. [Google Scholar] [PubMed]
- Pennycook, S.R.; Samuels, G.J. Botryosphaeria and Fusicoccum species associated with ripe fruit rot of Actinidia deliciosa (kiwifruit) in New Zealand. Mycotaxon 1995, 24, 445–458. [Google Scholar]
- Crous, P.W.; Slippers, B.; Wingfield, M.J.; Rheeder, J.; Marasas, W.F.; Philips, A.J.; Alves, A.; Burgess, T.; Barber, P.; Groenewald, J.Z. Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 2006, 55, 235–253. [Google Scholar]
- Latorre, B.A.; Besoain, X.; Flores, V. Botryosphaeria canker of table grapes. Phytopathology 1986, 76, 1112. [Google Scholar]
- Auger, J.; Esterio, M.; Ricke, G.; Pérez, I. Black dead arm and canker of Vitis vinifera cv. Red Globe caused by Botryosphaeria obtusa in Chile. Plant Dis. 2013, 88, 1286. [Google Scholar] [CrossRef]
- Díaz, G.A.; Prehn, D.; Besoain, X.; Chávez, E.R.; Latorre, B.A. Neofusicoccum parvum associated with grapevine trunk diseases in Chile. Plant Dis. 2011, 95, 1032. [Google Scholar]
- Morales, A.; Latorre, B.A.; Piontelli, E.; Besoain, X. Botryosphaeriaceae species affecting table grape vineyards in Chile and cultivar susceptibility. Cienc. Investig. Agrar. 2012, 39, 445–458. [Google Scholar]
- Besoain, X.; Torres, C.; Díaz, G.A.; Latorre, B.A. First report of Neofusicoccum australe associated with Botryosphaeria canker of grapevine in Chile. Plant Dis. 2013, 97, 143. [Google Scholar]
- Díaz, G.A.; Latorre, B.A. Efficacy of paste and liquid fungicide formulations to protect pruning wounds against pathogens associated with grapevine trunk diseases in Chile. Crop. Protect. 2013, 46, 106–112. [Google Scholar]
- Díaz, G.A.; Latorre, B.A. Infection caused by Phaeomoniella chlamydospora associated with Esca-like symptoms in grapevine in Chile. Plant Dis. 2014, 98, 351–360. [Google Scholar] [PubMed]
- Larach, A.; Riquelme, N.; Salinas, A.; Rolshausen, P.E.; Seeger, M.; Besoain, X. First Report of Diaporthe ambigua associated with dead arm disease on grapevine in Chile. Plant Dis. 2022, 106, 1988. [Google Scholar]
- Larignon, P.; Fulchic, R.; Cere, L.; Dubos, B. Observation on black dead arm in French vineyards. Phytopathol. Mediterr. 2001, 40, S336–S342. [Google Scholar]
- Úrbez-Torres, J.R.; Battany, M.; Bettiga, L.J.; Gispert, C.; McGourty, G.; Roncoroni, J.; Smith, R.J.; Verdegaal, P.; Gubler, W.D. Botryosphaeriaceae species spore-trapping studies in California vineyards. Plant Dis. 2010, 94, 717–724. [Google Scholar]
- Van Niekerk, J.M.; Crous, P.W.; Groenewald, J.Z.; Fourie, P.H.; Halleen, F. DNA phylogeny, morphology and pathogenicity of Botryosphaeria species on grapevines. Mycologia 2004, 96, 781–798. [Google Scholar]
- Sánchez, M.E.; Venegas, J.; Romero, M.A.; Phillips, A.J.L.; Trapero, A. Botryosphaeria and related taxa causing oak canker in southwestern Spain. Plant Dis. 2003, 87, 1515–1521. [Google Scholar]
- Bustamante, M.I.; Elfar, K.; Eskalen, A. Evaluation of the antifungal activity of endophytic and rhizospheric bacteria against grapevine trunk pathogens. Microorganisms 2022, 10, 2035. [Google Scholar] [CrossRef]
- Vega-Celedón, P.; Bravo, G.; Velásquez, A.; Cid, F.P.; Valenzuela, M.; Ramírez, I.; Vásconez, I.N.; Álvarez, I.; Jorquera, M.A.; Seeger, M. Microbial diversity of psychrotolerant bacteria isolated from wild flora of Andes Mountains and Patagonia of Chile towards the selection of plant growth-promoting bacterial consortia to alleviate cold stress in plants. Microorganisms 2021, 9, 538. [Google Scholar] [CrossRef]
- Larach, A.; Vega-Celedón, P.; Castillo-Novales, D.; Tapia, L.; Cuneo, I.; Cádiz, F.; Seeger, M.; Besoain, X. Diplodia seriata biocontrol is altered via temperature and the control of bacteria. Microorganisms 2024, 12, 350. [Google Scholar] [CrossRef]
- Montenegro, I.; Valenzuela, M.; Seeger, M.; Besoain, X.; Godoy, P.; Werner, E.; Caro, N.; Olguín, Y.; Valenzuela, M.; Silva, V.; et al. Natural bactericidal effects of Psoralea glandulosa essential oil for the control of bacterial canker and speck in tomato. Agronomy 2024, 14, 2615. [Google Scholar] [CrossRef]
- Orellana, R.; Macaya, C.; Bravo, G.; Dorochesi, F.; Cumsille, A.; Valencia, R.; Rojas, C.; Seeger, M. Living at the frontiers of life: Extremophiles in Chile and their potential for bioremediation. Front. Microbiol. 2018, 9, 2309. [Google Scholar]
- Niem, J.M.; Billones-Baaijens, R.; Stodart, B.; Savocchia, S. Diversity profiling of grapevine microbial endosphere and antagonistic potential of endophytic Pseudomonas against grapevine trunk diseases. Front. Microbiol. 2020, 11, 477. [Google Scholar]
- Velásquez, A.; Vega-Celedón, P.; Fiaschi, G.; Agnolucci, M.; Avio, L.; Giovannetti, M.; D’Onofrio, C.; Seeger, M. Responses of Vitis vinifera cv. Cabernet Sauvignon roots to the arbuscular mycorrhizal fungus Funneliformis mosseae and the plant growth-promoting rhizobacterium Ensifer meliloti include changes in volatile organic compounds. Mycorrhiza 2020, 30, 161–170. [Google Scholar] [PubMed]
- Velásquez, A.; Valenzuela, M.; Carvajal, M.; Fiaschi, G.; Avio, L.; Giovannetti, M.; D’Onofrio, C.; Seeger, M. The arbuscular mycorrhizal fungus Funneliformis mosseae induces changes and increases the concentration of volatile organic compounds in Vitis vinifera cv. Sangiovese leaf tissue. Plant Physiol. Biochem. 2020, 155, 437–443. [Google Scholar]
- Olivera, M.; Delgado, N.; Montenegro, I.; Besoain, X.; Seeger, M.; Bravo, G.; Barros-Parada, W.; Cadiz, F.; Pedreschi, R.; Riquelme, N. Diffusible compounds produced by Hanseniaspora osmophila and Gluconobacter cerinus help to control the causal agents of gray rot and summer bunch rot of table grapes. Antibiotics 2021, 10, 664. [Google Scholar] [CrossRef]
- Carvajal, M.; Olivares, M.; Lobaina, E.; Vergara, A.; Velásquez, A.; Jeldres, P.; Meza, D.; Dorta, F.; Jorquera, F.; Seeger, M. Addition of Trichoderma consortia to Chilean endemic flora compost teas strongly enhances in vitro and in vivo biocontrol of phytopathogenic fungi. J. Appl. Microbiol. 2023, 134, xad011. [Google Scholar]
- Álvarez-Hubert, I.; Durán, R.; Vega-Celedón, P.; Vásconez, I.-N.; Macaya, C.; Seeger, M. Draft genome sequences of halotolerant Halomonas spp. SpR1 and SpR8, potential plant growth-promoting bacteria associated to Salicornia rhizosphere in a hydrothermal lagoon ecosystem of the Altiplano, Northern Chile. Microbial. Resour. Announc. 2024, 13, e00822-23. [Google Scholar]
- Vega-Celedón, P.; Castillo-Novales, D.; Bravo, G.; Cárdenas, F.; Romero-Silva, M.; Seeger, M. Synthesis and degradation of the phytohormone indole-3-acetic acid by the versatile bacterium Paraburkholderia xenovorans LB400 and its growth promotion of Nicotiana tabacum plant. Plants 2024, 13, 3533. [Google Scholar] [CrossRef]
- Deyett, E.; Roper, C.M.; Ruegger, P.; Yang, J.; Borneman, J.; Rolshausen, P.E. Microbial landscape of the grapevine endosphere in the context of Pierce’s disease. Phytobiomes J. 2017, 1, 138–149. [Google Scholar]
- Leal, C.; Richet, N.; Guise, J.F.; Gramaje, D.; Armengol, J.; Fontaine, F.; Trotel-Aziz, P. Cultivar contributes to the beneficial effects of Bacillus subtilis PTA-271 and Trichoderma atroviride SC1 to protect grapevine against Neofusicoccum parvum. Front. Microbiol. 2021, 12, 726132. [Google Scholar]
- Langa-Lomba, N.; González-García, V.; Venturini-Crespo, M.E.; Casanova-Gascón, J.; Barriuso-Vargas, J.J.; Martín-Ramos, P. Comparison of the efficacy of Trichoderma and Bacillus strains and commercial biocontrol products against grapevine Botryosphaeria dieback pathogens. Agronomy 2023, 13, 533. [Google Scholar] [CrossRef]
- Brar, B.; Bala, K.; Saharan, B.S.; Sadh, P.K.; Duhan, J.S. Bio-boosting agriculture: Harnessing the potential of fungi-bacteria-plant synergies for crop improvement. Discov. Plants 2024, 1, 21. [Google Scholar]
- Bhimani, P.; Mahavar, P.; Rajguru, B.; Bhatt, V.D.; Nathani, N.; Shri, M. Unveiling the green dialogue: Advancements in omics technologies for deciphering plant–microbe interactions in soil. Discov. Plants 2024, 1, 4. [Google Scholar]
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar]
- Kotze, V. Evaluation of biocontrol agents for grapevine pruning wound protection against trunk pathogen infection. Phytopathol. Mediterr. 2011, 50, S247–S263. [Google Scholar]
- Trotel-Aziz, P.; Abou-Mansour, E.; Courteaux, B.; Rabenoelina, F.; Clément, C.; Fontaine, F.; Aziz, A. Bacillus subtilis PTA-271 counteracts Botryosphaeria dieback in grapevine, triggering immune responses and detoxification of fungal phytotoxins. Front. Plant Sci. 2019, 10, 25. [Google Scholar]
- Blundell, R.; Arreguin, M.; Eskalen, A. In vitro Evaluation of grapevine endophytes, epiphytes and sap microorganisms for potential use to control grapevine trunk disease pathogens. Phytopathol. Mediterr. 2021, 60, 535–548. [Google Scholar] [CrossRef]
- Haidar, R.; Yacoub, A.; Vallance, J.; Compant, S.; Antonielli, L.; Saad, A.; Habenstein, B.; Kauffmann, B.; Grélard, A.; Loquet, A.; et al. Bacteria associated with wood tissues of Esca-diseased grapevines: Functional diversity and synergy with Fomitiporia mediterranea to degrade wood components. Environ. Microbiol. 2021, 23, 6104–6121. [Google Scholar]
- Rangel-Montoya, E.A.; Paolinelli, M.; Rolshausen, P.; Hernandez-Martinez, R. The role of melanin in the grapevine trunk disease pathogen Lasiodiplodia gilanensis. Phytopathol. Mediterr. 2020, 59, 549–563. [Google Scholar]
- Eisenman, H.C.; Greer, E.M.; McGrail, C.W. The role of melanins in melanotic fungi for pathogenesis and environmental survival. Appl. Microbiol. Biotechnol. 2020, 104, 4247–4257. [Google Scholar]
- Valencia, D.; Torres, C.; Camps, R.; López, E.; Celis-Diez, J.; Beosain, X. Dissemination of Botryosphaeriaceae conidia in vineyards in the semiarid Mediterranean climate of the Valparaíso Region of Chile. Phytopathol. Mediterr. 2015, 54, 394–402. [Google Scholar]
- Kuntzmann, P.; Villaume, S.; Bertsch, C. Conidia dispersal of Diplodia species in a French vineyard. Phytopathol. Mediterr. 2009, 48, 150–154. [Google Scholar]
- Serra, S.; Mannoni, M.A.; Ligios, V. Studies on the susceptibility of pruning wounds to infections by fungi involved in grapevine wood disease in Italy. Phytopathol. Mediterr. 2008, 47, 234–246. [Google Scholar]
- Espinoza, J.G.; Briceńo, E.X.; Chávez, E.R.; Úrbez-Torres, J.R.; Latorre, B.A. Neofusicoccum spp. associated with stem canker and dieback of blueberry in Chile. Plant Dis. 2009, 93, 1187–1194. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Leavitt, G.M.; Voegel, T.; Gubler, W.D. Identification and distribution of Botryosphaeria species associated with grapevine cankers in California. Plant Dis. 2006, 90, 1490–1503. [Google Scholar] [CrossRef]
- Vega-Celedón, P.; Bravo, G.; Hernández, L.; Macaya, C.; Seeger, M. Psychrotolerant Bacterial Strains AMCR2b and AMTR8 of the Genus Pseudomonas with Plant Growth-Promoting Activities and Cold Stress Protection in Plants. Patent PCT/CL2022/050057, 26 May 2022. [Google Scholar]
- Fuentes, S.; Barra, B.; Caporaso, J.G.; Seeger, M. From rare to dominant: A fine-tunned soil bacterial bloom during petroleum hydrocarbon bioremediation. Appl. Environ. Microbiol. 2016, 82, 888–896. [Google Scholar] [CrossRef]
- Undabarrena, A.; Beltrametti, F.; Claverías, F.; González, M.; Moore, E.; Seeger, M.; Cámara, B. Exploring the diversity and antimicrobial potential of marine Actinobacteria from the Comau Fjord in Northern Patagonia, Chile. Front. Microbiol. 2016, 7, 1135. [Google Scholar] [CrossRef]
- Canchignia, H.; Altimira, F.; Montes, C.; Sánchez, E.; Tapia, E.; Miccono, M.; Espinoza, D.; Aguirre, C.; Seeger, M.; Prieto, H. Candidate nematicidal proteins in a new Pseudomonas veronii isolate identified by its antagonic properties against Xiphinema index. J. Gen. Appl. Microbiol. 2016, 63, 11–21. [Google Scholar] [CrossRef]
- Mandakovic, D.; Maldonado, J.; Pulgar, R.; Cabrera, P.; Gaete, A.; Urtuvia, V.; Seeger, M.; Cambiazo, V.; González, M. Microbiome analysis and bacterial isolation from Lejía Lake soil in Atacama Desert. Extremophiles 2018, 22, 665–673. [Google Scholar] [CrossRef]
- Durán, R.E.; Méndez, V.; Barra-Sanhueza, B.; Rodríguez-Castro, L.; Salvà-Serra, F.; Moore, E.R.B.; Castro-Nallar, E.; Seeger, M. Genomic and physiological traits of the marine bacterium Alcaligenes aquatilis QD168 isolated from Quintero Bay, Central Chile, reveal a robust adaptive response to environmental stressors. Front. Microbiol. 2019, 10, 528. [Google Scholar] [CrossRef]
- Morgante, V.; López-López, A.; Flores, C.; González, M.; González, B.; Vásquez, M.; Rosselló-Mora, R.; Seeger, M. Bioaugmentation with Pseudomonas sp. strain MHP41 promotes simazine attenuation and bacterial community changes in agricultural soils. FEMS Microbiol. Ecol. 2010, 71, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, A.; Li, X.; Chen, W. Control of Phytophthora nicotianae disease, induction of defense responses and genes expression of papaya fruits treated with Pseudomonas putida MGP1. J. Sci. Food Agric. 2013, 93, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Nieto, M.; Barret, M.; Morrissey, J.; Germaine, K.; Martínez-Granero, F.; Barahona, E.; Navazo, A.; Sánchez-Contreras, M.; Moynihan, J.; Muriel, C.; et al. Genome sequence reveals that Pseudomonas fluorescens F113 possesses a large and diverse array of systems for rhizosphere function and host interaction. BMC Genom. 2013, 14, 54. [Google Scholar]
- Van Der Voort, M.; Meijer, H.J.; Schmidt, Y.; Watrous, J.; Dekkers, E.; Mendes, R.; Dorrestein, P.; Gross, H.; Raaijmakers, J. Genome mining and metabolic profiling of the rhizosphere bacterium Pseudomonas sp. SH-C52 for antimicrobial compounds. Front. Microbiol. 2015, 6, 693. [Google Scholar] [CrossRef] [PubMed]
- Guyer, A.; De Vrieze, M.; Bönisch, D.; Gloor, R.; Musa, T.; Bodenhausen, N.; Bailly, A.; Weisskopf, L. The anti-phytophthora effect of selected potato-associated Pseudomonas strains: From the laboratory to the field. Front. Microbiol. 2015, 6, 1309. [Google Scholar]
- Ramette, A.; Moënne-Loccoz, Y.; Défago, G. Polymorphism of the polyketide synthase gene phID in biocontrol fluorescent pseudomonads producing 2,4-diacetylphloroglucinol and comparison of PhID with plant polyketide synthases. Mol Plant Microbe Interact. 2001, 14, 639–652. [Google Scholar]
- Ghadamgahi, F.; Tarighi, S.; Taheri, P.; Saripella, G.V.; Anzalone, A.; Kalyandurg, P.B.; Catara, V.; Ortiz, R.; Vetukuri, R.R. Plant Growth-Promoting activity of Pseudomonas aeruginosa FG106 and its ability to act as a biocontrol agent against potato, tomato and taro pathogens. Biology 2022, 11, 140. [Google Scholar] [CrossRef]
- Zachow, C.; Fatehi, J.; Cardinale, M.; Tilcher, R.; Berg, G. Strain-specific colonization pattern of Rhizoctonia antagonists in the root system of sugar beet. FEMS Microbiol. Ecol. 2010, 74, 124–135. [Google Scholar] [CrossRef]
- Abou-Mansour, E.; Débieux, J.L.; Ramírez-Suero, M.; Bénard-Gellon, M.; Magnin-Robert, M.; Spagnolo, A.; Chong, J.; Farine, S.; Bertsch, C.; L’Haridon, F.; et al. Phytotoxic metabolites from Neofusicoccum parvum, a pathogen of Botryosphaeria dieback of grapevine. Phytochemistry 2015, 115, 207–215. [Google Scholar]
- Lambert, C.; Bisson, J.; Waffo-Téguo, P.; Papastamoulis, Y.; Richard, T.; Corio-Costet, M.-F.; Mérillon, J.-M.; Cluzet, S. Phenolic and their antifungal role in grapevine wood decay: Focus on the Botryosphaeiriaceae family. J. Agric. Food Chem. 2012, 60, 11589–11868. [Google Scholar]
- Wagner, A.; Norris, S.; Chatterjee, P.; Morris, P.F.; Wildschutte, H. Aquatic Pseudomonads inhibit Oomycete plant pathogens of Glycine max. Front. Microbiol. 2018, 9, 1007. [Google Scholar]
- Zboralski, A.; Filion, M. Pseudomonas spp. can help plants face climate change. Front. Microbiol. 2023, 14, 1198131. [Google Scholar]
- Niem, J.M.; Billones-Baaijens, R.; Stodart, B.J.; Reveglia, P.; Savocchia, S. Biocontrol potential of an endophytic Pseudomonas poae strain against the grapevine trunk disease pathogen Neofusicoccum luteum and its mechanism of action. Plants 2023, 12, 2132. [Google Scholar] [CrossRef]
- Arseneault, T.; Goyer, C.; Filion, M. Biocontrol of potato common scab is associated with high Pseudomonas fluorescens LBUM223 populations and phenazine-1-carboxylic acid biosynthetic transcript accumulation in the potato geocaulosphere. Phytopathology 2016, 106, 963–970. [Google Scholar]
- Raaijmakers, J.M.; De Bruijn, I.; Nybro, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar]
- De Vrieze, M.; Pandey, P.; Bucheli, T.D.; Varadarajan, A.R.; Ahrens, C.H.; Weisskopf, L.; Bailly, A. Volatile organic compounds from native potato associated Pseudomonas as potential anti-oomycete agents. Front. Microbiol. 2015, 6, 1295. [Google Scholar]
- Hunziker, L.; Bonisch, D.; Groenhagen, U.; Bailly, A.; Schulz, S.; Weisskopf, L. Pseudomonas strains naturally associated with potato plants produce volatiles with high potential for inhibition of Phytophthora infestans. Appl. Environ. Microbiol. 2015, 81, 821–830. [Google Scholar]
- Matthijs, S.; Tehrani, K.A.; Laus, G.; Jackson, R.W.; Cooper, R.M.; Cornelis, P. Thioquinolobactin, a Pseudomonas siderophore with antifungal and anti-Pythium activity. Environ. Microbiol. 2007, 9, 425–434. [Google Scholar]
- Pierson, L.S., III; Pierson, E.A. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar]
- Rode, H.; Hanslo, D.; De Wet, P.M.; Millar, A.J.; Cywes, S. Efficacy of mupirocin in methicillin-resistant Staphylococcus aureus burn wound infection. Antimicrob. Agents Chemother. 1989, 33, 1358–1361. [Google Scholar] [CrossRef]
- Farrow, J.M., III; Pesci, E.C. Two distinct pathways supply anthranilate as a precursor of the Pseudomonas quinolone signal. J. Bacteriol. 2007, 189, 3425–3433. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, I.; De Kock, M.J.; Yang, M.; De Waard, P.; Van Beek, T.A.; Raaijmakers, J.M. Genome-based discovery, structure prediction and functional analysis of cyclic lipopeptide antibiotics in Pseudomonas species. Mol. Microbiol. 2007, 63, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Perneel, M.; D’hondt, L.; De Maeyer, K.; Adiobo, A.; Rabaey, K.; Hofte, M. Phenazines and biosurfactants interact in the biological control of soil-borne diseases caused by Pythium spp. Environ. Microbiol. 2008, 10, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Loper, J.E.; Hassan, K.A.; Mavrodi, D.V.; Davis, E.W.I.I.; Lim, C.K.; Shaffer, B.T.; Elbourne, L.D.H.; Stockwell, V.O.; Hartney, S.L.; Breakwell, K.; et al. Comparative genomics of plant-associated Pseudomonas spp.: Insights into diversity and inheritance of traits involved in multitrophic interactions. PLoS Genet. 2012, 8, e1002784. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 2012, 50, 403–424. [Google Scholar] [CrossRef]
- Begum, M.; Meon, S.; Abidin, M.; Puteh, A.; Rahman, M. Antagonistic potential of selected fungal and bacterial biocontrol agents against Colletotrichum truncatum of soybean seeds. Pertanika J. Trop. Agric. Sci. 2008, 31. [Google Scholar]
- Idris, H.A.; Labuschagne, N.; Korsten, L. Screening rhizobacteria for biological control of Fusarium root and crown rot of sorghum in Ethiopia. Biol. Control 2007, 40, 97–106. [Google Scholar] [CrossRef]
- Delgado, N.; Olivera, M.; Cádiz, F.; Bravo, G.; Montenegro, I.; Madrid, A.; Fuentealba, C.; Pedreschi, R.; Salgado, E.; Besoain, X. Volatile Organic Compounds (VOCs) produced by Gluconobacter cerinus and Hanseniaspora osmophila displaying control effect against table grape-rot pathogens. Antibiotics 2021, 10, 663. [Google Scholar] [CrossRef]
- Haidar, R.; Deschamps, A.; Roudet, J.; Calvo-Garrido, C.; Bruez, E.; Rey, P.; Fermaud, M. Multi-organ screening of efficient bacterial control agents against two major pathogens of grapevine. BioControl 2016, 92, 55–65. [Google Scholar] [CrossRef]
- Kaufmann, J.; Schering, A.G. Analysis of variance ANOVA. In Wiley StatsRef: Statistics Reference Online; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Vrbin, C.M. Parametric or nonparametric statistical tests: Considerations when choosing the most appropriate option for your data. Cytopathology 2022, 33, 663–667. [Google Scholar] [CrossRef]



| Strains | PUCV 1547 | PUCV 1557 | PUCV 1560 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 °C | 22 °C | 30 °C | 10 °C | 22 °C | 30 °C | 10 °C | 22 °C | 30 °C | |
| C− | 2.5 ± 0.2 abc | 7 ± 0.0 a | 7 ± 0.0 a | 2.1 ± 0.17 ab | 7 ± 0.0 a | 7 ± 0.0 a | 2.3 ± 0.15 ab | 7 ± 0.0 a | 7 ± 0.0 a |
| Pseudomonas sp. TmR1b | 2.25 ± 0.3 c | 3.2 ± 2.0 c | 5.9 ± 1.12 abc | 2 ± 0.14 ab | 5.22 ± 0.33 bc | 6.2 ± 0.6 a | 2.1 ± 0.15 ab | 2.87 ± 2.09 d | 2.77 ± 0.43 bcd |
| Pseudomonas sp. AMTR8 | 2.2 ± 0.05 c | 5.1 ± 1.3 ab | 3.5 ± 1.04 d | 2.1 ± 0.1 ab | 4.4 ± 0.28 cd | 2.8 ± 0.33 b | 2.3 ± 0.1 ab | 4.9 ± 0.21 bc | 4.45 ± 0.33 d |
| Pseudomonas sp. AMCR2b | 2.5 ± 0.14 abc | 4.5 ± 0.5 bc | 3.46 ± 0.24 d | 2 ± 0.05 ab | 3.95 ± 0.05 d | 3.5 ± 0.21 b | 2.2 ± 0.15 ab | 3.6 ± 0.20 cd | 2.8 ± 0.67 e |
| Pseudomonas sp. GcR15a | 2.27 ± 0.09 bc | 3.2 ± 0.99 c | 4.7 ± 0.56 bcd | 1.9 ± 0.05 ab | 4.5 ± 0.55 cd | 3.1 ± 0.7 b | 2.2 ± 0.24 ab | 4.7± 0.20 bc | 4.4 ± 0.86 d |
| Pseudomonas sp. TmR5a | 2.3 ± 0.28 bc | 6.7 ± 0.28 a | 7 ± 0.0 a | 1.8 ± 0.43 a | 5.9 ± 0.83 b | 6.6 ± 0.40 a | 1.9 ± 0.40 ab | 5.5 ± 0.95 ab | 7 ± 0.0 a |
| Brachybacterium sp. TmP30 | 2 ± 0.20 c | 7 ± 0.0 a | 6.0 ± 0.72 ab | 2.1 ± 0.17 ab | 7 ± 0.0 a | 6.5 ± 0.9 a | 2.2 ± 0.26 ab | 7 ± 0.0 a | 7 ± 0.0 a |
| Pseudomonas sp. TmR7 | 2.3 ± 0.14 bc | 6.36 ± 0.80 ab | 7 ± 0.0 a | 1.8 ± 0.09 ab | 7 ± 0.0 a | 5.8 ± 0.78 a | 1.7 ± 0.32 b | 7 ± 0.0 a | 5.9 ± 1.21 abc |
| Curtobacterium sp. BmP22c | 2.3 ± 0.14 bc | 7 ± 0.0 a | 7 ± 0.0 a | 2.1 ± 0.2 ab | 6.8 ± 0.4 a | 5.9 ± 1.18 a | 2.2 ± 0.22 ab | 7 ± 0.0 a | 6.0 ± 0.42 abc |
| Frondihabitans sp. GpP26d | 2.57 ± 0.15 abc | 7 ± 0.0 a | 6.9 ± 0.05 a | 2.1 ± 0.2 ab | 7 ± 0.0 a | 6.8 ± 0.4 a | 2.4 ± 0.24 a | 7 ± 0.0 a | 7 ± 0.0 a |
| Arthrobacter sp. BmP28 | 2.32 ± 0.26 bc | 7 ± 0.0 a | 6.5 ± 0.85 a | 2.3 ± 0.23 a | 7 ± 0.0 a | 6.3 ± 0.47 a | 2.4 ± 0.15 a | 7 ± 0.0 a | 6.75 ± 0.5 ab |
| Pseudomonas sp. NUR4a | 2.6 ± 0.14 abc | 7 ± 0.0 a | 6.57 ± 0.56 a | 1.7 ± 0.24 b | 7 ± 0.0 a | 6.9 ± 0.17 a | 2.2 ± 0.15 ab | 5.27 ± 1.6 abc | 6.5 ± 0.67 abc |
| Bacillus sp. PU3 | 3 ± 0.25 a | 7 ± 0.0 a | 7 ± 0.0 a | 1.9 ± 0.26 ab | 7 ± 0.0 a | 6.5 ± 0.86 a | 2 ± 0.25 ab | 7 ± 0.0 a | 6.7 ± 0.5 ab |
| Rhodococcus sp. PU4 | 3 ± 0.28 a | 6.7 ± 0.6 a | 6.22 ± 0.60 a | 1.9 ± 0.23 ab | 7 ± 0.0 a | 7 ± 0.0 a | 2 ± 0.28 ab | 7 ± 0.0 a | 6.7 ± 0.57 ab |
| Staphylococcus sp. PU18 | 2.8 ± 0.5 ab | 6.4 ± 0.35 a | 6.1 ± 0.98 ab | 1.9 ± 0.26 ab | 5.6 ± 0.23 b | 6.4 ± 0.4 a | 2 ± 0.25 ab | 6.5 ± 0.3 ab | 7 ± 0.0 a |
| Pseudomonas protegens CHA0 | 2.5 ± 0.15 ab | 6.36 ± 3.01 ab | 4.4 ± 0.45 cd | 2.2 ± 0.25 a | 7 ± 0.0 a | 6 ± 0.28 a | 2.2 ± 0.25 ab | 7 ± 0.0 a | 5.2 ± 0.5 cd |
| Essays | Variety | T °C | Grapevine Age | N. parvum Strain | Inhibition (%) by Biocontrol Agents | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C− | TmR1b | AMTR8 | AMCR2b | GcR15a | ||||||
| (a) | Agar plug diffusion method | - | 10 °C | - | PUCV 1547 | 0 e | 12 a | 16 a | 0 e | 13 a |
| PUCV 1557 | 0 e | 5 bc | 2 c | 4 cd | 7 b | |||||
| PUCV 1560 | 0 e | 7 b | 0 e | 1 e | 4 c | |||||
| 15 °C | - | PUCV 1547 | 0 h | 12 b | 16 a | 0 h | 13 b | |||
| PUCV 1557 | 0 h | 5 bc | 2 gh | 4 d | 7 de | |||||
| PUCV 1560 | 0 h | 7 fgh | 0 h | 1 de | 4 c | |||||
| 22 °C | - | PUCV 1547 | 0 f | 0 f | 31 ab | 34 a | 26 cd | |||
| PUCV 1557 | 0 f | 30 bc | 31 ab | 34 a | 26 de | |||||
| PUCV 1560 | 0 f | 28 d | 24 d | 33 ab | 20 e | |||||
| 30 °C | - | PUCV 1547 | 0 i | 37 ef | 39 de | 40 cde | 10 h | |||
| PUCV 1557 | 0 i | 46 bc | 44 bcd | 50 a | 31 f | |||||
| PUCV 1560 | 0 i | 34 f | 42 bcd | 45 b | 23 g | |||||
| (b) | Double plate method | - | 10 °C | - | PUCV 1547 | 0 g | 25 cd | 16 e | 22 d | 9 f |
| PUCV 1557 | 0 g | 36 a | 0 g | 15 e | 32 ab | |||||
| PUCV 1560 | 0 g | 27 cd | 30 bc | 22 d | 10 ef | |||||
| 15 °C | - | PUCV 1547 | 0 f | 34 b | 45 a | 30 bc | 46 a | |||
| PUCV 1557 | 0 f | 3 f | 0 f | 0 f | 5 e | |||||
| PUCV 1560 | 0 f | 27 cd | 30 bcd | 22 d | 10 e | |||||
| 22 °C | - | PUCV 1547 | 0 g | 35 bc | 5 g | 43 a | 35 bc | |||
| PUCV 1557 | 0 g | 36 bc | 39 b | 11 f | 31 cd | |||||
| PUCV 1560 | 0 g | 27 de | 30 de | 22 e | 10 f | |||||
| 30 °C | - | PUCV 1547 | 0 e | 0 e | 0 e | 26 a | 14 b | |||
| PUCV 1557 | 0 e | 0 e | 6 d | 8 cd | 13 bc | |||||
| PUCV 1560 | 0 e | 12 bc | 9 cd | 22 a | 21 a | |||||
| (c) | Cuttings | V. vinifera cv. Cabernet Sauvignon | 22 °C | C− | C+ | AMCR2b | GcR15a | |||
| 1 | PUCV 1547 | 0 c | 100 a | 90 a | 95 b | |||||
| 4 | 0 a | 100 a | 75 a | 55 a | ||||||
| 5 | 0 a | 100 a | 95 a | 6 a | ||||||
| 25 | 0 a | 100 a | 80 a | 90 a | ||||||
| 1 | PUCV 1557 | 0 c | 100 a | 95 a | 53 b | |||||
| 4 | 0 a | 100 a | 60 a | 70 a | ||||||
| 5 | 0 a | 100 a | 81 a | 88 a | ||||||
| 25 | 0 a | 100 a | 75 a | 84 a | ||||||
| 1 | PUCV 1560 | 0 c | 100 a | 80 ab | 50 b | |||||
| 4 | 0 a | 100 a | 90 a | 74 a | ||||||
| 5 | 0 a | 100 a | 83 a | 42 a | ||||||
| 25 | 0 a | 100 a | 84 a | 69 a | ||||||
| (d) | Shoots | V. vinifera cv. Cabernet Sauvignon | - | C− | C+ | PU4 | AMCR2b–GcR15a | |||
| 7 | PUCV 1547 | 0 d | 91 a | 68 b | 82 c | |||||
| PUCV 1557 | 0 d | 83 a | 26 b | 74 c | ||||||
| PUCV 1560 | 0 c | 94 a | 26 b | 89 b | ||||||
| V. vinifera cv. Sauvignon Blanc | - | 7 | PUCV 1547 | 0 c | 83 a | 74 a | 80 b | |||
| PUCV 1557 | 0 c | 97 a | 29 b | 30 b | ||||||
| PUCV 1560 | 0 d | 18 c | 39 a | 76 b | ||||||
| Native Bacteria | Locality, Region | Wild Plant | Closest Organism (Partial 16S rRNA Gene Sequence) | Identity (%) | Accession Number | Reference |
|---|---|---|---|---|---|---|
| Pseudomonas sp. TmR1b | Los Libertadores, Los Andes, Valparaíso | Thlaspi sp. (R) | Pseudomonas azotoformans strain 16d-S37 | 693/693 (100%) | MW548351 | [52] |
| Pseudomonas sp. AMTR8 | Los Libertadores, Los Andes, Valparaíso | Thlaspi sp. (R) | Pseudomonas brassicacearum strain DF41 | 657/657 (100%) | * | [80] |
| Pseudomonas sp. AMCR2b | Los Libertadores, Los Andes, Valparaíso | Calycera sp. (R) | Pseudomonas asgharzadehiana strain SWRI132 | 689/689 (100%) | * | [80] |
| Pseudomonas sp. GcR15a | Vicinity of El teniente Mine, Machalí, O’higgins | Gnaphallium sp. (R) | Pseudomonas orientalis strain R4-35-08 | 706/707 (99.86%) | MW548343 | [52] |
| Pseudomonas sp. TmR5a | Los Libertadores, Los Andes, Valparaíso | Thlaspi sp. (R) | Pseudomonas cedrina strain K19B | 722/722 (100%) | MW548356 | [52] |
| Brachybacterium sp. TmP30 | Los Libertadores, Los Andes, Valparaíso | Thlaspi sp. (P) | Brachybacterium tyrofermentans strain AFS097178 | 676/677 (99%) | MW548378 | [52] |
| Pseudomonas sp. TmR7 | Los Libertadores, Los Andes, Valparaíso | Thlaspi sp. (R) | Pseudomonas syringae pv. actinidiae strain 18YN-PSA-C2 | 693/695 (99%) | MW548359 | [52] |
| Curtobacterium sp. BmP22c | Chabunco Park, Punta Arena, Magallanes and Chilean Antartica | Berberis sp. (P) | Curtobacterium flaccumfaciens pv. flaccumfaciens strain Cff1037 | 657/657 (100%) | MW548393 | [52] |
| Frondihabitans sp. GpP26d | Shangri-La EcoPark, Las Trancas Valley, Ñuble | Gaultheria sp. (P) | Frondihabitans sucicola strain HP-S2 | 657/657 (100%) | MW548348 | [52] |
| Arthrobacter sp. BmP28 | Chabunco Park, Punta Arena, Magallanes and Chilean Antartica | Berberis sp. (P) | Arthrobacter citreus strain TTS-AB-A36 | 664/667 (99%) | MW548382 | [52] |
| Pseudomonas sp. NUR4a | Chabunco Park, Punta Arena, Magallanes and Chilean Antartica | Berberis sp. (P) | Pseudomonas baetica strain IHB B 4123 | 688/694 (99%) | MW548343 | [52] |
| Bacillus sp. PU3 | Peralillo, O’Higgins | Vitis vinifera (E) | Bacillus xiamenensis strain INV FIR70 | 725/725 (100%) | * | This study |
| Rhodococcus sp. PU4 | Peralillo, O’Higgins | Vitis vinifera (E) | Rhodococcus qingshengii strain H-cryo-48 | 682/685 (99.56%) | OQ244039 | [53] |
| Staphylococcus sp. PU18 | Peralillo, O’Higgins | Vitis vinifera (E) | Staphylococcus epidermidis strain VU-UCBMSH2 | 719/719 (100%) | * | This study |
| N. parvum Strains | Locality, Region | Access No. GenBank | Reference | |
|---|---|---|---|---|
| ITS | BT | |||
| PUCV 1547 | Peralillo, O’Higgins | KM870224 | KP762483 | [35] |
| PUCV 1557 | Palmilla, O’Higgins | KM870225 | KP762484 | [35] |
| PUCV 1560 | Talca, Maule | KM870226 | KP762485 | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Novales, D.; Vega-Celedón, P.; Larach, A.; Seeger, M.; Besoain, X. Native Bacteria Are Effective Biocontrol Agents at a Wide Range of Temperatures of Neofusicoccum parvum, Associated with Botryosphaeria Dieback on Grapevine. Plants 2025, 14, 1043. https://doi.org/10.3390/plants14071043
Castillo-Novales D, Vega-Celedón P, Larach A, Seeger M, Besoain X. Native Bacteria Are Effective Biocontrol Agents at a Wide Range of Temperatures of Neofusicoccum parvum, Associated with Botryosphaeria Dieback on Grapevine. Plants. 2025; 14(7):1043. https://doi.org/10.3390/plants14071043
Chicago/Turabian StyleCastillo-Novales, Diyanira, Paulina Vega-Celedón, Alejandra Larach, Michael Seeger, and Ximena Besoain. 2025. "Native Bacteria Are Effective Biocontrol Agents at a Wide Range of Temperatures of Neofusicoccum parvum, Associated with Botryosphaeria Dieback on Grapevine" Plants 14, no. 7: 1043. https://doi.org/10.3390/plants14071043
APA StyleCastillo-Novales, D., Vega-Celedón, P., Larach, A., Seeger, M., & Besoain, X. (2025). Native Bacteria Are Effective Biocontrol Agents at a Wide Range of Temperatures of Neofusicoccum parvum, Associated with Botryosphaeria Dieback on Grapevine. Plants, 14(7), 1043. https://doi.org/10.3390/plants14071043







