Over Time Changes in the Transcriptomic Profiles of Tomato Plants with or Without Mi-1 Gene During Their Incompatible or Compatible Interactions with the Whitefly Bemisia tabaci
Abstract
:1. Introduction
2. Results
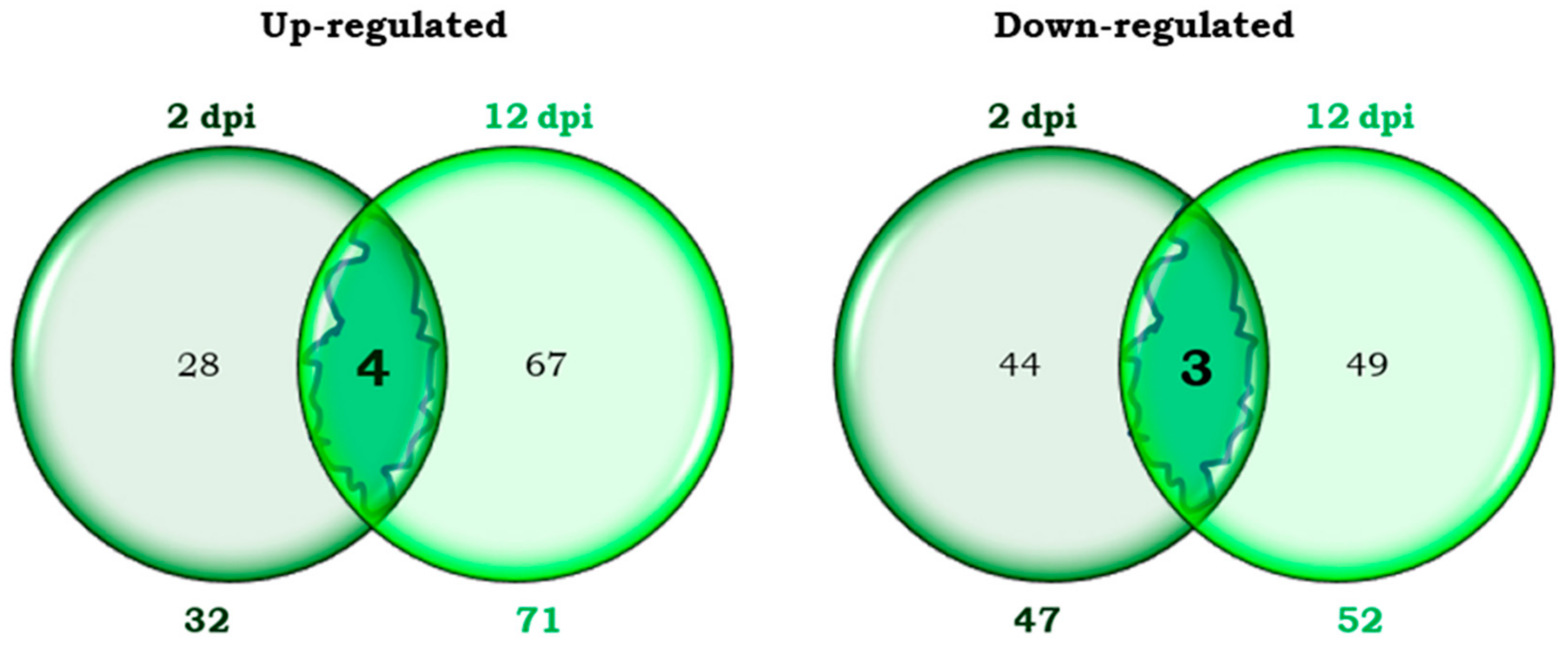
2.1. The Compatible Interaction Tomato/Whitefly: Transcriptional Reprogramming in Moneymaker in Two Phases of the Infestation by B. tabaci
2.1.1. Differential Transcripts of Moneymaker Tomato Common to Both Early and Late Phases of B. tabaci Infestation
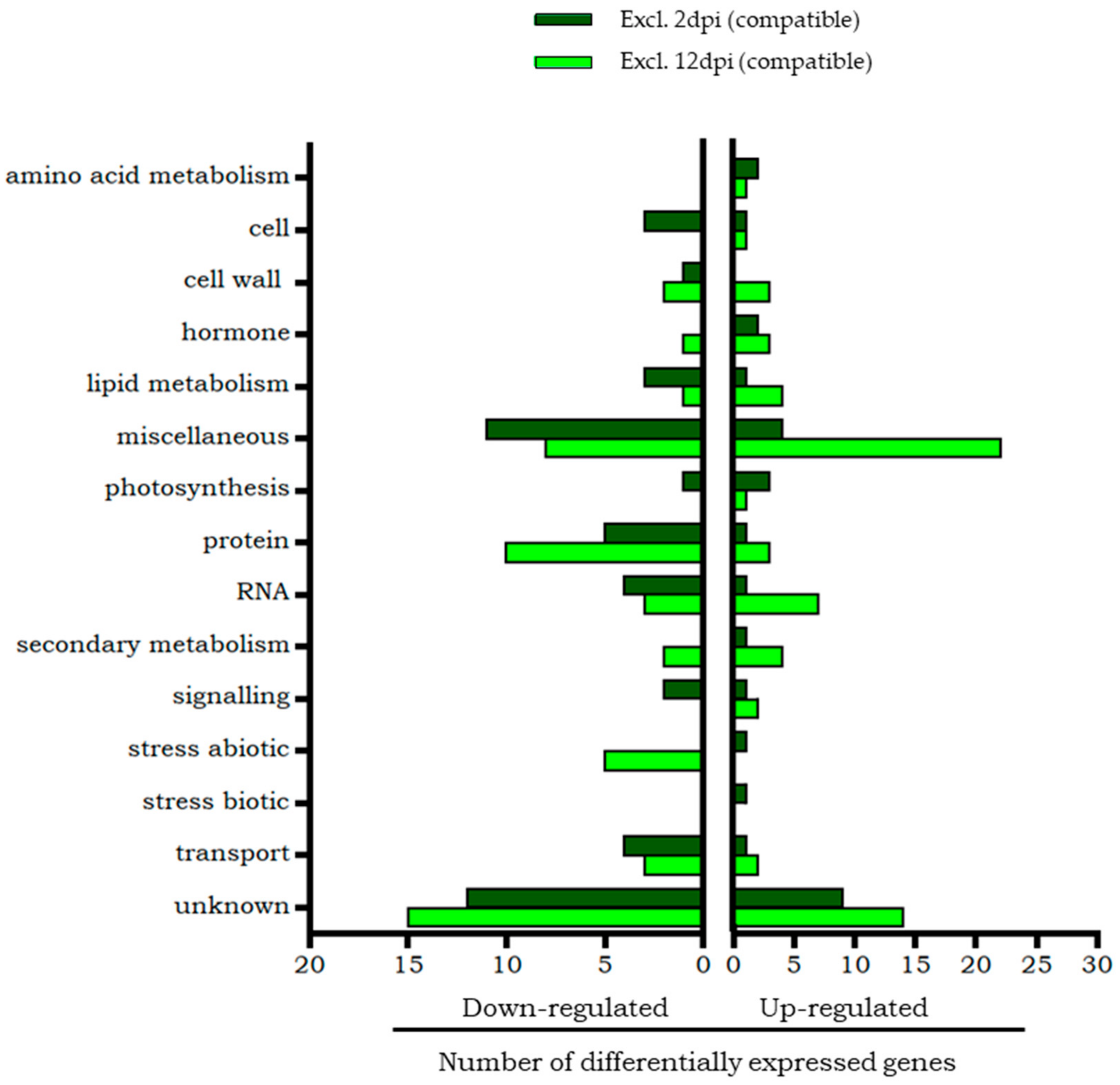
2.1.2. Differential Transcripts of Moneymaker Tomato Unique to the Early Phase of Infestation (2 dpi)
2.1.3. Differential Transcripts of Moneymaker Tomato Unique to the Late Phase of Infestation (12 dpi)
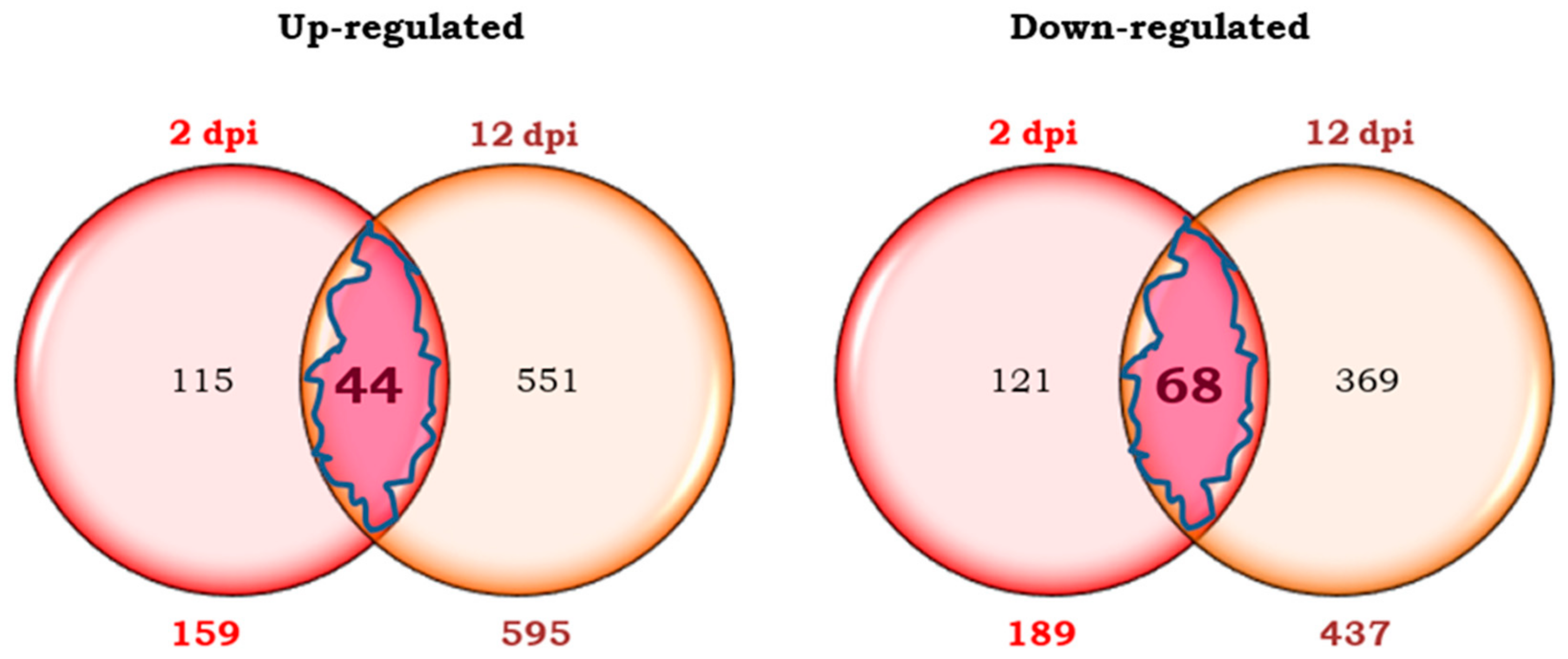
2.2. The Incompatible Interaction Tomato/Whitefly: Transcriptional Reprogramming in Motelle in Two Phases of the Infestation by B. tabaci
2.2.1. Differential Transcripts of Motelle Tomato Common to Both Early and Late Phases of B. tabaci Infestation
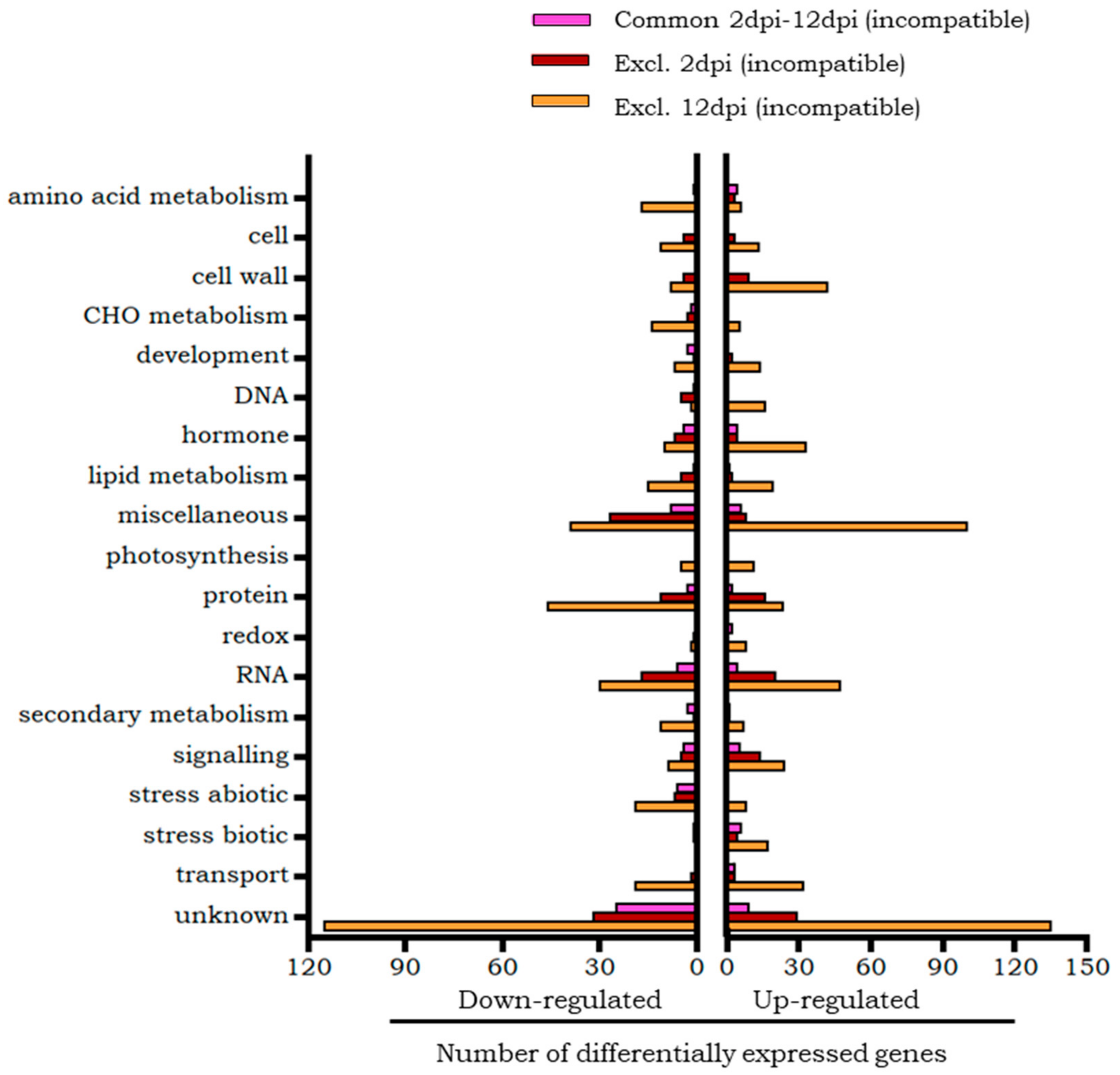
2.2.2. Differential Transcripts of Motelle Tomato Unique to the Early Phase of Infestation (2 dpi)
2.2.3. Differential Transcripts of Motelle Tomato Unique to the Late Phase of Infestation (12 dpi)
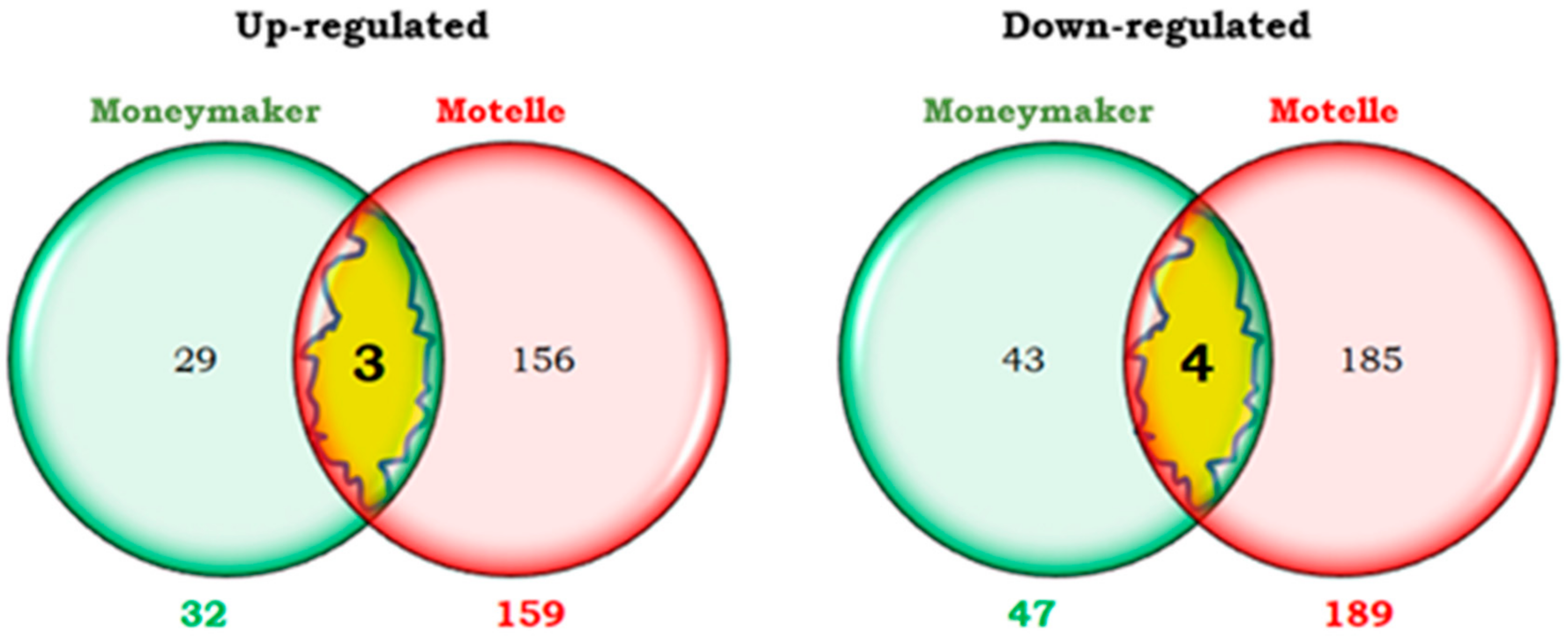
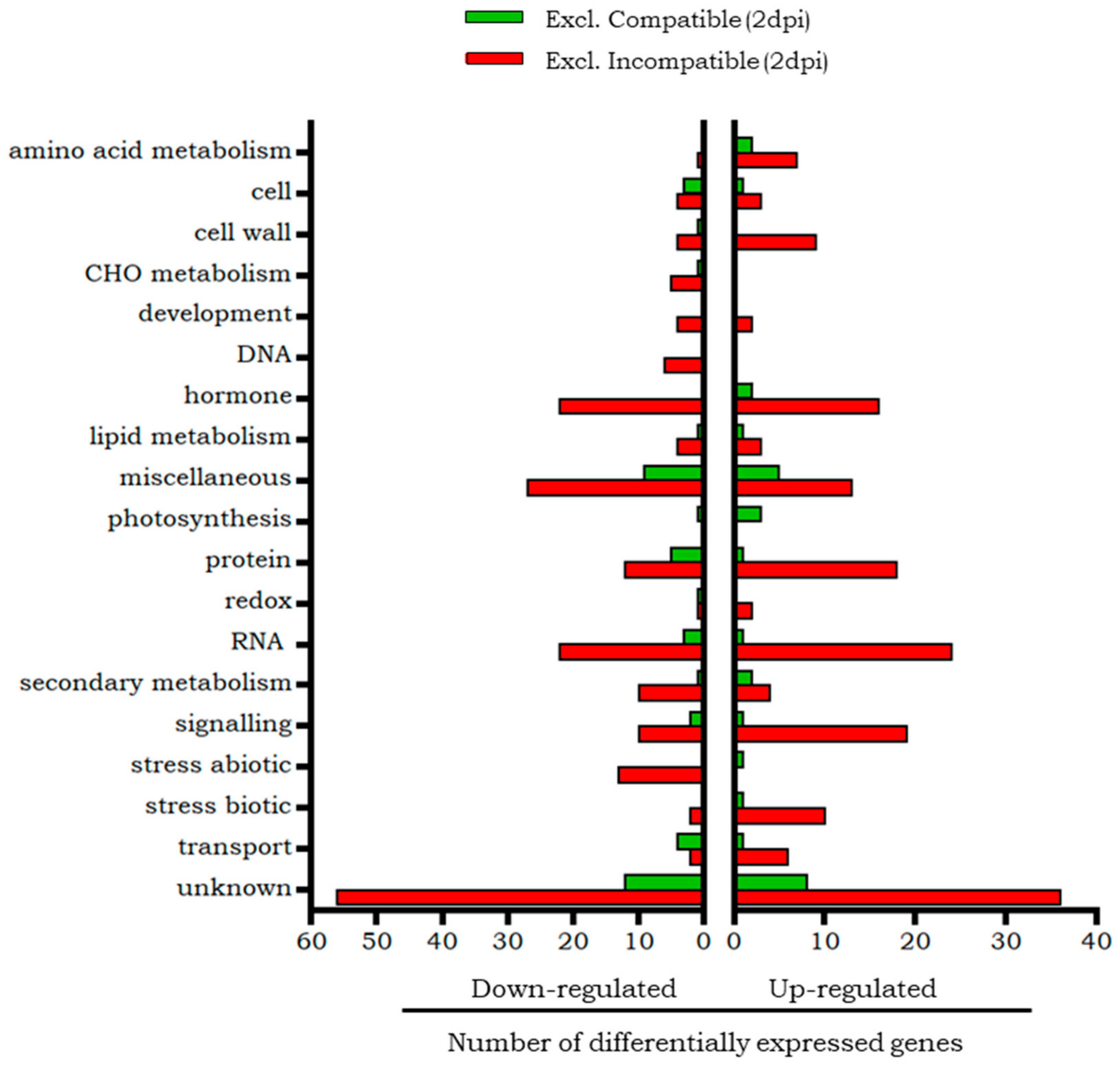
2.3. Similarities and Differences Between Compatible and Incompatible Interactions in the Early Phase of B. tabaci Infestation (2 dpi)
2.3.1. Transcripts Differentially Expressed at 2 dpi in Both Compatible and Incompatible Interactions
2.3.2. Transcripts Differentially Expressed at 2 dpi Only in the Compatible Interaction
2.3.3. Transcripts Differentially Expressed at 2 dpi Only in the Incompatible Interaction
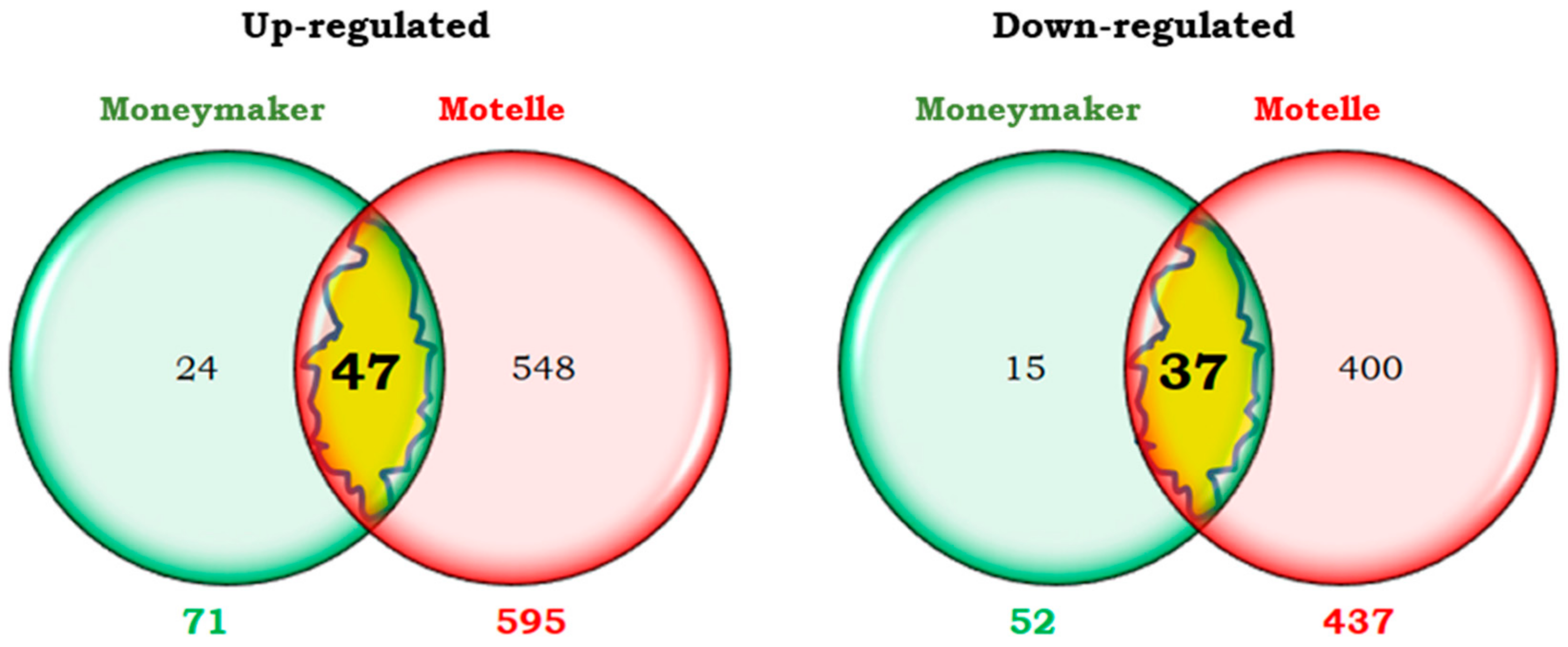
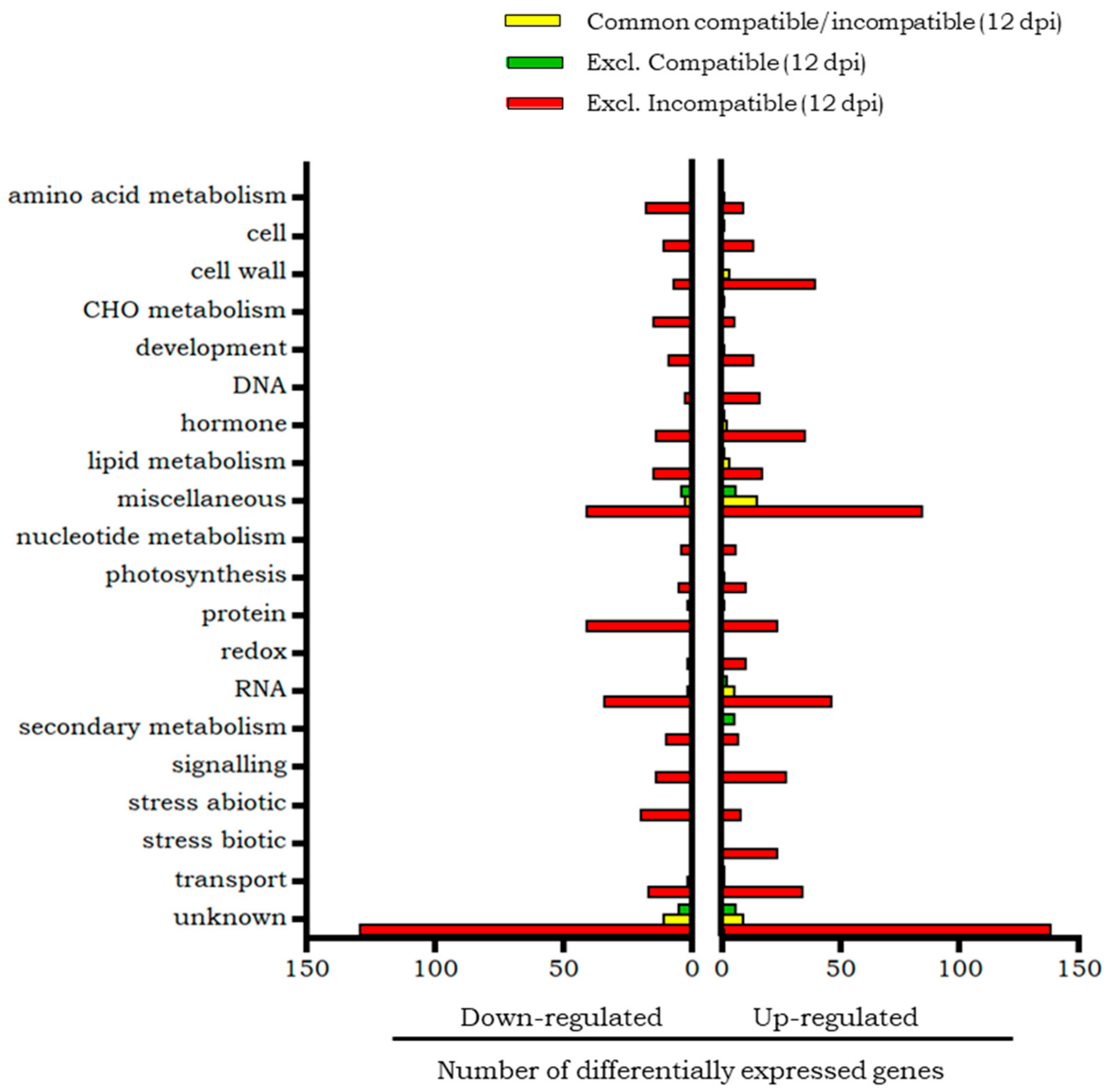
2.4. Similarities and Differences Between Compatible and Incompatible Interactions in the Late Phase of Whitefly Infestation (12 dpi)
2.4.1. Transcripts Differentially Expressed at 12 dpi in Both Compatible and Incompatible Interactions
2.4.2. Transcripts Differentially Expressed at 12 dpi Only in the Compatible Interaction
2.4.3. Transcripts Differentially Expressed at 12 dpi Only in the Incompatible Interaction
3. Discussion
3.1. Signalling
3.2. Respiratory Burst
3.3. Regulation of Transcription
3.4. Defence Genes
3.4.1. PR-Proteins
3.4.2. Heat Shock Proteins
3.4.3. Secondary Metabolites
3.5. Genes Putatively Involved in Biotic Stress
3.5.1. Cell Wall Genes
3.5.2. Hormone
3.5.3. Other Stress Genes
4. Materials and Methods
4.1. Plant Material
4.2. Whitefly Infestations
4.3. Sampling
- -
- 2 dpi (days post infestation): two days after the introduction of the females in the cages with the leaflets. At this time, the females have laid eggs and thus the effect of feeding and oviposition is assessed.
- -
- 12 dpi: twelve days after infestation. At this time, most of the individuals are N1 nymphs, which inject the stylet at different points into the leaves until the suitable spot is found to settle and continue development as sessile nymphs.
4.4. Sample Processing for Microarray Hybridization
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roberts, P.; Thomason, I. Variability in reproduction of isolates of Meloidogyne incognita and Meloidogyne javanica on resistant tomato genotypes. Plant Dis. 1986, 70, 547–551. [Google Scholar] [CrossRef]
- Rossi, M.; Goggin, F.; Milligan, S.; Kaloshian, I.; Ullman, D.; Williamson, V. The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc. Natl. Acad. Sci. USA 1998, 95, 9750–9754. [Google Scholar] [CrossRef] [PubMed]
- Casteel, C.L.; Walling, L.L.; Paine, T.D. Behavior and biology of the tomato psyllid, Bactericerca cockerelli, in response to the Mi-1.2 gene. Entomol. Exp. Appl. 2006, 121, 67–72. [Google Scholar] [CrossRef]
- Nombela, G.; Williamson, V.M.; Muñiz, M. The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol. Plant-Microbe Interact. 2003, 16, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olivé, E.; Navas-Castillo, J. Tomato chlorosis virus, an emergent plant virus still expanding its geographical and host ranges. Mol. Plant Pathol. 2019, 20, 1307–1320. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Simmons, A.M.; Harrison, H.F.; Ling, K.-S. Forty-nine new host plant species for Bemisia tabaci (Hemiptera: Aleyrodidae). Entomol. Sci. 2008, 11, 385–390. [Google Scholar] [CrossRef]
- Dropkin, V.H. The necrotic reaction of tomatoes and other hosts resistant to Meloidogyne. Reversal by temperature. Phytopathology 1969, 59, 1632–1637. [Google Scholar]
- Martínez de Ilarduya, O.; Xie, Q.; Kaloshian, I. Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol. Plant-Microbe Interact. 2003, 16, 699–708. [Google Scholar] [CrossRef]
- Rodriguez-Alvarez, C.I.; Lopez-Vidriero, I.; Franco-Zorrilla, J.M.; Nombela, G. Basal differences in the transcriptional profiles of tomato leaves associated with the presence/absence of the resistance gene Mi-1 and changes in these differences after infestation by the whitefly Bemisia tabaci. Bull. Entomol. Res. 2020, 110, 463–479. [Google Scholar] [CrossRef]
- Smith, G.P. Embryo culture of a tomato species hybrid. J. Am. Soc. Hortic. Sci. 1944, 44, 413–416. [Google Scholar]
- Martin, G.; Bogdanove, A.; Sessa, G. Understanding the functions of plant disease resistance proteins. Annu. Rev. Plant Biol. 2003, 54, 23–61. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Ilarduya, O.; Moore, A.; Kaloshian, I. The tomato Rme1 locus is required for Mi-1-mediated resistance to root-knot nematodes and the potato aphid. Plant J. 2001, 27, 417–425. [Google Scholar] [CrossRef]
- Martínez de Ilarduya, O.; Nombela, G.; Hwang, C.; Williamson, V.; Muniz, M.; Kaloshian, I. Rme1 is necessary for Mi-1-mediated resistance and acts early in the resistance pathway. Mol. Plant-Microbe Interact. 2004, 17, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xie, Q.-G.; Smith-Becker, J.; Navarre, D.A.; Kaloshian, I. Mi-1-mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. Mol. Plant-Microbe Interact. 2006, 19, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Mantelin, S.; Peng, H.-C.; Li, B.; Atamian, H.S.; Takken, F.L.W.; Kaloshian, I. The receptor-like kinase SlSERK1 is required for Mi-1-mediated resistance to potato aphids in tomato. Plant J. 2011, 67, 459–471. [Google Scholar] [CrossRef]
- Bhattarai, K.K.; Li, Q.; Liu, Y.; Dinesh-Kumar, S.P.; Kaloshian, I. The Mi-1-mediated pest resistance requires Hsp90 and Sgt1. Plant Physiol. 2007, 144, 312–323. [Google Scholar] [CrossRef]
- Pascual, S.; Rodríguez-Álvarez, C.I.; Kaloshian, I.; Nombela, G. Hsp90 gene is required for Mi-1-mediated resistance of tomato to the whitefly Bemisia tabaci. Plants 2023, 12, 641. [Google Scholar] [CrossRef]
- Barcala, M.; Garcia, A.; Cabrera, J.; Casson, S.; Lindsey, K.; Favery, B.; Garcia-Casado, G.; Solano, R.; Fenoll, C.; Escobar, C. Early transcriptomic events in microdissected Arabidopsis nematode-induced giant cells. Plant J. 2010, 61, 698–712. [Google Scholar] [CrossRef]
- Yokotani, N.; Hasegawa, Y.; Sato, M.; Hirakawa, H.; Kouzai, Y.; Nishizawa, Y.; Yamamoto, E.; Naito, Y.; Isobe, S. Transcriptome analysis of Clavibacter michiganensis subsp. michiganensis-infected tomatoes: A role of salicylic acid in the host response. BMC Plant Biol. 2021, 21, 476. [Google Scholar] [CrossRef]
- Coppola, V.; Coppola, M.; Rocco, M.; Digilio, M.C.; D’Ambrosio, C.; Renzone, G.; Martinelli, R.; Scaloni, A.; Pennacchio, F.; Rao, R.; et al. Transcriptomic and proteomic analysis of a compatible tomato-aphid interaction reveals a predominant salicylic acid-dependent plant response. BMC Genom. 2013, 14, 515. [Google Scholar] [CrossRef] [PubMed]
- Harrison, K.; Mendoza-Herrera, A.; Levy, J.G.; Tamborindeguy, C. Lasting consequences of psyllid (Bactericera cockerelli L.) infestation on tomato defense, gene expression, and growth. BMC Plant Biol. 2021, 21, 114. [Google Scholar] [CrossRef]
- Bonshtien, A.; Lev, A.; Gibly, A.; Debbie, P.; Avni, A.; Sessa, G. Molecular properties of the Xanthomonas AvrRxv effector and global transcriptional changes determined by its expression in resistant tomato plants. Mol. Plant-Microbe Interact. 2005, 18, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Gibly, A.; Bonshtien, A.; Balaji, V.; Debbie, P.; Martin, G.; Sessa, G. Identification and Expression profiling of tomato genes differentially regulated during a resistance response to Xanthomonas campestris pv. vesicatoria. Mol. Plant-Microbe Interact. 2004, 17, 1212–1222. [Google Scholar] [CrossRef]
- Bhattarai, K.K.; Xie, Q.-G.; Mantelin, S.; Bishnoi, U.; Girke, T.; Navarre, D.A.; Kaloshian, I. Tomato susceptibility to root-knot nematodes requires an intact jasmonic acid signaling pathway. Mol. Plant-Microbe Interact. 2008, 21, 1205–1214. [Google Scholar] [CrossRef]
- Portillo, M.; Cabrera, J.; Lindsey, K.; Topping, J.; Andres, M.; Emiliozzi, M.; Oliveros, J.; Garcia-Casado, G.; Solano, R.; Koltai, H.; et al. Distinct and conserved transcriptomic changes during nematode-induced giant cell development in tomato compared with Arabidopsis: A functional role for gene repression. New Phytol. 2013, 197, 1276–1290. [Google Scholar] [CrossRef]
- Chen, T.; Lv, Y.; Zhao, T.; Li, N.; Yang, Y.; Yu, W.; He, X.; Liu, T.; Zhang, B. Comparative transcriptome profiling of a resistant vs. susceptible tomato (Solanum lycopersicum) cultivar in response to infection by tomato yellow leaf curl virus. PLoS ONE 2013, 8, e80816. [Google Scholar] [CrossRef]
- D’Esposito, D.; Manzo, D.; Ricciardi, A.; Garonna, A.P.; De Natale, A.; Frusciante, L.; Pennacchio, F.; Ercolano, M.R. Tomato transcriptomic response to Tuta absoluta infestation. BMC Plant Biol. 2021, 21, 358. [Google Scholar] [CrossRef]
- Estrada-Hernandez, M.G.; Humberto Valenzuela-Soto, J.; Ibarra-Laclette, E.; Paul Delano-Frier, J. Differential gene expression in whitefly Bemisia tabaci-infested tomato (Solanum lycopersicum) plants at progressing developmental stages of the insect’s life cycle. Physiol. Plantarum 2009, 137, 44–60. [Google Scholar] [CrossRef]
- Kempema, L.A.; Cui, X.; Holzer, F.M.; Walling, L.L. Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. similarities and distinctions in responses to aphids. Plant Physiol. 2007, 143, 849–865. [Google Scholar] [CrossRef]
- Quintana-Camargo, M.; Mendez-Moran, L.; Ramirez-Romero, R.; Gurrola-Diaz, C.M.; Carapia-Ruiz, V.; Ibarra-Laclette, E.; Paul Delano-Frier, J.; Sanchez-Hernandez, C. Identification of genes differentially expressed in husk tomato (Physalis philadelphica) in response to whitefly (Trialeurodes vaporariorum) infestation. Acta Physiol. Plant. 2015, 37, 29. [Google Scholar] [CrossRef]
- Thompson, G.; Goggin, F. Transcriptomics and functional genomics of plant defence induction by phloem-feeding insects. J. Exp. Bot. 2006, 57, 755–766. [Google Scholar] [CrossRef]
- Li, Y.; Zou, J.; Li, M.; Bilgin, D.D.; Vodkin, L.O.; Hartman, G.L.; Clough, S.J. Soybean defense responses to the soybean aphid. New Phytol. 2008, 179, 185–195. [Google Scholar] [CrossRef]
- Bentur, J.S.; Rawat, N.; Divya, D.; Sinha, D.K.; Agarrwal, R.; Atray, I.; Nair, S. Rice-Gall midge interactions: Battle for survival. J. Insect Physiol. 2016, 84, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Duan, S.; Armstrong, M.R.; Xu, J.; Zheng, J.; Hu, J.; Chen, X.; Hein, I.; Li, G.; Jin, L. Comparative transcriptome profiling reveals compatible and incompatible patterns of potato toward Phytophthora infestans. G3 Genes Genom. Genet. 2020, 10, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Duhlian, L.; Koramutla, M.K.; Subramanian, S.; Chamola, R.; Bhattacharya, R. Comparative transcriptomics revealed differential regulation of defense related genes in brassica juncea leading to successful and unsuccessful infestation by aphid species. Sci. Rep. 2020, 10, 10583. [Google Scholar] [CrossRef]
- Ghaemi, R.; Pourjam, E.; Safaie, N.; Verstraeten, B.; Mahmoudi, S.B.; Mehrabi, R.; De Meyer, T.; Kyndt, T. Molecular insights into the compatible and incompatible interactions between sugar beet and the beet cyst nematode. BMC Plant Biol. 2020, 20, 483. [Google Scholar] [CrossRef]
- Schenk, P.M.; Kazan, K.; Manners, J.M.; Anderson, J.P.; Simpson, R.S.; Wilson, I.W.; Somerville, S.C.; Maclean, D.J. Systemic gene expression in Arabidopsis during an incompatible interaction with Alternaria brassicicola. Plant Physiol. 2003, 132, 2. [Google Scholar] [CrossRef]
- van Loon, L.C.; Geraats, B.P.J.; Linthorst, H.J.M. Ethylene as a modulator of disease resistance in plants. Trends Plant Sci. 2006, 11, 184–191. [Google Scholar] [CrossRef]
- Navarro, L.; Zipfel, C.; Rowland, O.; Keller, I.; Robatzek, S.; Boller, T.; Jones, J. The transcriptional innate immune response to Flg22. interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol. 2004, 135, 1113–1128. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, Z.; Chen, W.; Glazebrook, J.; Chang, H.; Han, B.; Zhu, T.; Zou, G.; Katagiri, F. Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell 2003, 15, 317–330. [Google Scholar] [CrossRef]
- Thilmony, R.; Underwood, W.; He, S. Genome-Wide Transcriptional analysis of the Arabidopsis thaliana Interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J. 2006, 46, 34–53. [Google Scholar] [CrossRef] [PubMed]
- van Esse, H.P.; Fradin, E.F.; de Groot, P.J.; de Wit, P.J.G.M.; Thomma, B.P.H.J. Tomato transcriptional responses to a foliar and a vascular fungal pathogen are distinct. Mol. Plant-Microbe Interact. 2009, 22, 245–258. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.-J.; Liu, B.-M.; Cui, H.-Y.; Zhang, Y.-Y.; Jiao, X.-G. Feeding behavior explains the different effects of cabbage on MEAM1 and MED cryptic species of Bemisia tabaci. Insect Sci. 2020, 27, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Muñiz, M.; Nombela, G. Differential variation in development of the B- and Q-Biotypes of Bemisia tabaci (Homoptera: Aleyrodidae) on sweet pepper at constant temperatures. Environ. Entomol. 2001, 30, 720–727. [Google Scholar] [CrossRef]
- Pascual, S.; Callejas, C. Intra- and Interspecific competition between Biotypes B and Q of Bemisia tabaci (Hemiptera: Aleyrodidae) from Spain. Bull. Entomol. Res. 2004, 94, 369–375. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, W.T.G.; LeVesque, C.S.; Perring, T.M.; Walling, L.L. Local and systemic changes in squash gene expression in response to silverleaf whitefly feeding. Plant Cell 2000, 12, 1409–1423. [Google Scholar] [CrossRef]
- Bouché, N.; Yellin, A.; Snedden, W.A.; Fromm, H. Plant-specific calmodulin-binding proteins. Annu. Rev. Plant Biol. 2005, 56, 435–466. [Google Scholar] [CrossRef]
- Bredow, M.; Monaghan, J. Regulation of plant immune signaling by calcium-dependent protein kinases. Mol. Plant-Microbe Interact. 2019, 32, 6–19. [Google Scholar] [CrossRef]
- Park, C.Y.; Lee, J.H.; Yoo, J.H.; Moon, B.C.; Choi, M.S.; Kang, Y.H.; Lee, S.M.; Kim, H.S.; Kang, K.Y.; Chung, W.S.; et al. WRKY group IId transcription factors interact with calmodulin. FEBS Lett. 2005, 579, 1545–1550. [Google Scholar] [CrossRef]
- Yokotani, N.; Hasegawa, Y.; Kouzai, Y.; Hirakawa, H.; Isobe, S. Transcriptome analysis of tomato plants following salicylic acid-induced immunity against Clavibacter michiganensis ssp. michiganensis. Plant Biotechnol. 2023, 40, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Alvarez, C.I.; Lopez-Climent, M.F.; Gomez-Cadenas, A.; Kaloshian, I.; Nombela, G. Salicylic acid is required for Mi-1-mediated resistance of tomato to whitefly Bemisia tabaci, but not for basal defense to this insect pest. Bull. Entomol. Res. 2015, 105, 574–582. [Google Scholar] [CrossRef]
- Branch, C.; Hwang, C.; Navarre, D.; Williamson, V. Salicylic acid is part of the Mi-1-mediated defense response to root-knot nematode in tomato. Mol. Plant-Microbe Interact. 2004, 17, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhu, Q.; Yuan, P.; Yan, Y.; Yi, K.; Du, L. Calmodulin and calmodulin-like protein-mediated plant responses to biotic stresses. Plant Cell Environ. 2023, 46, 3680–3703. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, D.; Ekengren, S.K.; Martin, G.B.; Dobney, S.L.; Snedden, W.A. Calmodulin-like proteins from arabidopsis and tomato are involved in host defense against Pseudomonas syringae pv. tomato. Plant Mol. Biol. 2005, 58, 887–897. [Google Scholar] [CrossRef]
- Jiao, C.; Li, K.; Zuo, Y.; Gong, J.; Guo, Z.; Shen, Y. CALMODULIN1 and WRKY53 function in plant defense by negatively regulating the jasmonic acid biosynthesis pathway in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 7718. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.-Y.; Chung, E.; Joung, Y.-H.; Pai, H.-S.; Hur, C.-G.; Choi, D. EST and microarray analyses of pathogen-responsive genes in hot pepper (Capsicum annuum L.) non-host resistance against soybean pustule pathogen (Xanthomonas axonopodis pv. glycines). Funct. Integr. Genom. 2004, 4, 196–205. [Google Scholar] [CrossRef]
- Zou, B.; Hong, X.; Ding, Y.; Wang, X.; Liu, H.; Hua, J. Identification and analysis of copine/BONZAI proteins among evolutionarily diverse plant species. Genome 2016, 59, 565–573. [Google Scholar] [CrossRef]
- Tomsig, J.L.; Snyder, S.L.; Creutz, C.E. Identification of targets for calcium signaling through the copine family of proteins: Characterization of a coiled-coil copine-binding motif. J. Biol. Chem. 2003, 278, 10048–10054. [Google Scholar] [CrossRef]
- Jin, H.; Liu, Y.; Yang, K.-Y.; Kim, C.Y.; Baker, B.; Zhang, S. Function of a mitogen-activated protein kinase pathway in n gene-mediated resistance in tobacco. Plant J. 2003, 33, 719–731. [Google Scholar] [CrossRef]
- Mayrose, M.; Bonshtien, A.; Sessa, G. LeMPK3 is a mitogen-activated protein kinase with dual specificity induced during tomato defense and wounding responses. J. Biol. Chem. 2004, 279, 14819–14827. [Google Scholar] [CrossRef]
- Zhang, G.; Jia, S.; Yan, Z.; Wang, Y.; Zhao, F.; Sun, Y. A Strawberry mitogen-activated protein kinase gene, FaMAPK19, is involved in disease resistance against Botrytis cinerea. Sci. Hortic. 2020, 265, 109259. [Google Scholar] [CrossRef]
- Zhang, M.; Su, J.; Zhang, Y.; Xu, J.; Zhang, S. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr. Opin. Plant Biol. 2018, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Klessig, D.F. MAPK Cascades in plant defense signaling. Trends Plant Sci. 2001, 6, 520–527. [Google Scholar] [CrossRef]
- Ekengren, S.; Liu, Y.; Schiff, M.; Dinesh-Kumar, S.; Martin, G. Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. Plant J. 2003, 36, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Nagata, T. The Possible involvement of a phosphate-induced transcription factor encoded by Phi-2 gene from tobacco in ABA-signaling pathways. Plant Cell Physiol. 2002, 43, 12–20. [Google Scholar] [CrossRef]
- Rivas, F.J.M.; Fernie, A.R.; Aarabi, F. Roles and regulation of the RBOHD enzyme in initiating ROS-mediated systemic signaling during biotic and abiotic stress. Plant Stress. 2024, 11, 100327. [Google Scholar] [CrossRef]
- Sagi, M.; Davydov, O.; Orazova, S.; Yesbergenova, Z.; Ophir, R.; Stratmann, J.; Fluhr, R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 2004, 16, 616–628. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, N.; Meng, J.; Yang, G.; Liu, W.; Zhou, X.; Ma, N.; Hou, X.; Luan, Y. LncRNA33732-respiratory burst oxidase module associated with WRKY1 in tomato- Phytophthora infestans interactions. Plant J. 2019, 97, 933–946. [Google Scholar] [CrossRef]
- Toum, L.; Torres, P.S.; Gallego, S.M.; Benavídes, M.P.; Vojnov, A.A.; Gudesblat, G.E. Coronatine inhibits stomatal closure through guard cell-specific inhibition of NADPH oxidase-dependent ROS production. Front. Plant Sci. 2016, 7, 1851. [Google Scholar] [CrossRef]
- Jabs, T.; Dietrich, R.A.; Dangl, J.L. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 1996, 273, 1853–1856. [Google Scholar] [CrossRef]
- Guan, T.; Shen, J.; Fa, Y.; Su, Y.; Wang, X.; Li, H. Resistance-breaking population of Meloidogyne incognita utilizes plant peroxidase to scavenge reactive oxygen species, thereby promoting parasitism on tomato carrying Mi-1 gene. Biochem. Biophys. Res. Commun. 2017, 482, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Achard, P.; Renou, J.-P.; Berthomé, R.; Harberd, N.P.; Genschik, P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef]
- Esposito, S.; Cappetta, E.; Tranchida-Lombardo, V.; Batelli, G.; Ruggiero, A.; Ruocco, M.; Sportelli, G.; Cillo, F.; De Palma, M. Genome-wide survey of glutaredoxin gene family in four solanaceae species and exploitation of duplicated cc-type following different environmental stimuli in tomato (Solanum lycopersicum). Sci. Hortic. 2023, 319, 112188. [Google Scholar] [CrossRef]
- Meyer, Y.; Siala, W.; Bashandy, T.; Riondet, C.; Vignols, F.; Reichheld, J.P. Glutaredoxins and thioredoxins in plants. BBA-Mol. Cell Res. 2008, 1783, 589–600. [Google Scholar] [CrossRef]
- Bolle, C. The role of GRAS proteins in plant signal transduction and development. Planta 2004, 218, 683–692. [Google Scholar] [CrossRef]
- Gambhir, P.; Raghuvanshi, U.; Kumar, R.; Sharma, A.K. Transcriptional regulation of tomato fruit ripening. Physiol. Mol. Biol. Plants 2024, 30, 289–303. [Google Scholar] [CrossRef]
- Huang, W.; Xian, Z.; Kang, X.; Tang, N.; Li, Z. Genome-wide identification, phylogeny and expression analysis of GRAS gene family in tomato. BMC Plant Biol. 2015, 15, 209. [Google Scholar] [CrossRef]
- Neves, C.; Ribeiro, B.; Amaro, R.; Expósito, J.; Grimplet, J.; Fortes, A.M. Network of GRAS transcription factors in plant development, fruit ripening and stress responses. Hortic. Res. 2023, 10, uhad220. [Google Scholar] [CrossRef]
- Reed, J.W. Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci. 2001, 6, 420–425. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Ratcliffe, O.J. A genomic perspective on plant transcription factors. Curr. Opin. Plant Biol. 2000, 3, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Amador, V.; Monte, E.; García-Martínez, J.-L.; Prat, S. Gibberellins signal nuclear import of PHOR1, a photoperiod-responsive protein with homology to Drosophila armadillo. Cell 2001, 106, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Andrasi, N.; Pettko-Szandtner, A.; Szabados, L. Diversity of plant heat shock factors: Regulation, interactions, and functions. J. Exp. Bot. 2021, 72, 1558–1575. [Google Scholar] [CrossRef]
- Chen, W.; Provart, N.J.; Glazebrook, J.; Katagiri, F.; Chang, H.-S.; Eulgem, T.; Mauch, F.; Luan, S.; Zou, G.; Whitham, S.A.; et al. Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 2002, 14, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Ngatia, J.N.; Wang, Y.; Khoso, M.A.; Farooq, U.; Chen, S. AP2/ERF, an important cold stress-related transcription factor family in plants: A Review. Physiol. Mol. Biol. Plants 2021, 27, 1953–1968. [Google Scholar] [CrossRef]
- Zhang, F.-B.; Ji, S.-X.; Yang, J.-G.; Wang, X.-W.; Han, W.-H. Genome-wide analysis of MYB family in Nicotiana benthamiana and the functional role of the key members in resistance to Bemisia tabaci. Int. J. Biol. Macromol. 2023, 235, 123759. [Google Scholar] [CrossRef]
- Li, S.; Li, K.; Ju, Z.; Cao, D.; Fu, D.; Zhu, H.; Zhu, B.; Luo, Y. Genome-wide analysis of tomato NF-Y factors and their role in fruit ripening. BMC Genom. 2016, 17, 36. [Google Scholar] [CrossRef]
- Song, Y.H.; Song, N.Y.; Shin, S.Y.; Kim, H.J.; Yun, D.-J.; Lim, C.O.; Lee, S.Y.; Kang, K.Y.; Hong, J.C. Isolation of CONSTANS as a TGA4/OBF4 interacting protein. Mol. Cells 2008, 25, 559–565. [Google Scholar]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Huang, Y.; Li, M.-Y.; Wu, P.; Xu, Z.-S.; Que, F.; Wang, F.; Xiong, A.-S. Members of WRKY Group III transcription factors are important in TYLCV defense signaling pathway in tomato (Solanum lycopersicum). BMC Genom. 2016, 17, 788. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Zhang, S.; Li, Y.; Wang, X.; Yao, L.; Sheng, J.; Shen, L. Over-Expression of SlWRKY46 in tomato plants increases susceptibility to Botrytis cinerea by modulating ROS homeostasis and SA and JA signaling pathways. Plant Physiol. Biochem. 2021, 166, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.-M.; Zou, C.; Shu, Y.-N.; Liu, S.-S. WRKY Transcription factors in nicotiana tabacum modulate plant immunity against whitefly via interacting with MAPK cascade pathways. Insects 2021, 12, 16. [Google Scholar] [CrossRef]
- Bhattarai, K.; Atamian, H.; Kaloshian, I.; Eulgem, T. WRKY72-type transcription factors contribute to basal immunity in tomato and Arabidopsis as well as gene-for-gene resistance mediated by the tomato R gene Mi-1. Plant J. 2010, 63, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.; Inbar, M.; McKenzie, C.; Shatters, R.; Borowicz, V.; Albrecht, U.; Powell, C.; Doostdar, H. Multitrophic interactions of the silverleaf whitefly, host plants, competing herbivores, and phytopathogens. Arch. Insect Biochem. 2002, 51, 151–169. [Google Scholar] [CrossRef]
- Jia, Y.; Martin, G. Rapid transcript accumulation of pathogenesis-related genes during an incompatible interaction in bacterial speck disease-resistant tomato plants. Plant Mol. Biol. 1999, 40, 455–465. [Google Scholar] [CrossRef]
- van der Westhuizen, A.; Qian, X.; Botha, A. β-1,3-Glucanases in wheat and resistance to the russian wheat aphid. Physiol. Plant. 1998, 103, 125–131. [Google Scholar] [CrossRef]
- Asea, A.; Calderwood, S.; Kaur, P. (Eds.) Heat Shock Proteins and Plants; Springer International Publishing AG: Cham, Switzerland, 2016; Volume 10, ISBN 1877-1246. [Google Scholar] [CrossRef]
- Gorovits, R.; Czosnek, H. The involvement of heat shock proteins in the establishment of tomato yellow leaf curl virus infection. Front. Plant Sci. 2017, 8, 355. [Google Scholar] [CrossRef]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef]
- Gorovits, R.; Akad, F.; Beery, H.; Vidavsky, F.; Mahadav, A.; Czosnek, H. Expression of stress-response proteins upon whitefly-mediated inoculation of tomato yellow leaf curl virus in susceptible and resistant tomato plants. Mol. Plant-Microbe Interact. 2007, 20, 1376–1383. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhu, L.; He, G. Differential gene expression in response to brown planthopper feeding in rice. J. Plant Physiol. 2004, 161, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Soto, M.; Restrepo, S.; Piégu, B.; Cooke, R.; Delseny, M.; Tohme, J.; Verdier, V. Gene expression profile in response to Xanthomonas axonopodis pv. manihotis infection in cassava using a cDNA microarray. Plant Mol. Biol. 2005, 57, 393–410. [Google Scholar] [CrossRef]
- Logemann, E.; Reinold, S.; Somssich, I.E.; Hahlbrock, K. A novel type of pathogen defense-related cinnamyl alcohol dehydrogenase. Biol. Chem. 1997, 378, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Nounurai, P.; Afifah, A.; Kittisenachai, S.; Roytrakul, S. Phosphorylation of CAD1, PLDdelta, NDT1, RPM1 proteins induce resistance in tomatoes infected by Ralstonia solanacearum. Plants 2022, 11, 726. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, G.; Li, S.; Li, J.; Jiang, J.; Zhang, H.; Kang, L.; Chen, X.; Xu, X. Differentially expressed gene transcripts related to the Cf-19-mediated resistance response to Cladosporium fulvum infection in tomato. Physiol. Mol. Plant Pathol. 2015, 89, 8–15. [Google Scholar] [CrossRef]
- Kaloshian, I.; Walling, L.L. Hemipteran and dipteran pests: Effectors and plant host immune regulators. J. Integr. Plant Biol. 2016, 58, 350–361. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Ezura, H. SlMBP3 Knockout/down in tomato: Normal-sized fruit with increased dry matter content through non-liquefied locular tissue by altered cell wall formation. Plant Cell Physiol. 2022, 63, 1485–1499. [Google Scholar] [CrossRef]
- Nicol, F.; His, I.; Jauneau, A.; Vernhettes, S.; Canut, H.; Höfte, H. A Plasma membrane-bound putative endo-1,4-β-d-glucanase is required for normal wall assembly and cell elongation in Arabidopsis. EMBO J. 1998, 17, 5563–5576. [Google Scholar] [CrossRef]
- Ellis, C.; Karafyllidis, L.; Turner, J. Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol. Plant-Microbe Interact. 2002, 15, 1025–1030. [Google Scholar] [CrossRef]
- Hernandez-Blanco, C.; Feng, D.X.; Hu, J.; Sanchez-Vallet, A.; Deslandes, L.; Llorente, F.; Berrocal-Lobo, M.; Keller, H.; Barlet, X.; Sanchez-Rodriguez, C.; et al. Impairment of cellulose synthases required for Arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell 2007, 19, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Jakob, K.; Kniskern, J.M.; Bergelson, J. The role of pectate lyase and the jasmonic acid defense response in Pseudomonas viridiflava virulence. Mol. Plant-Microbe Interact. 2007, 20, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Uluisik, S.; Seymour, G.B. Pectate lyases: Their role in plants and importance in fruit ripening. Food Chem. 2020, 309, 125559. [Google Scholar] [CrossRef]
- Palusa, S.G.; Golovkin, M.; Shin, S.-B.; Richardson, D.N.; Reddy, A.S.N. Organ-specific, developmental, hormonal and stress regulation of expression of putative pectate lyase genes in Arabidopsis. New Phytol. 2007, 174, 537–550. [Google Scholar] [CrossRef]
- De Lorenzo, G.; Ferrari, S. Polygalacturonase-inhibiting proteins in defense against phytopathogenic fungi. Curr. Opin. Plant Biol. 2002, 5, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Haeger, W.; Henning, J.; Heckel, D.G.; Pauchet, Y.; Kirsch, R. Direct evidence for a new mode of plant defense against insects via a novel polygalacturonase-inhibiting protein expression strategy. J. Biol. Chem. 2020, 295, 11833–11844. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, G.; Yu, Q.; Gao, T.; Zhu, Q.; Wang, R.; Huang, S.; Han, Z.; Cervone, F.; Yin, H.; et al. A plant mechanism of hijacking pathogen virulence factors to trigger innate immunity. Science 2024, 383, 732–739. [Google Scholar] [CrossRef]
- Tjallingii, W. Salivary secretions by aphids interacting with proteins of phloem wound responses. J. Exp. Bot. 2006, 57, 739–745. [Google Scholar] [CrossRef]
- Bargmann, B.O.; Munnik, T. The role of phospholipase D in plant stress responses. Curr. Opin. Plant Biol. 2006, 9, 515–522. [Google Scholar] [CrossRef]
- Yao, H.-Y.; Xue, H.-W. Phosphatidic acid plays key roles regulating plant development and stress responses. J. Integr. Plant Biol. 2018, 60, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Kant, M.; Ament, K.; Sabelis, M.; Haring, M.; Schuurink, R. Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol. 2004, 135, 483–495. [Google Scholar] [CrossRef]
- Cao, L.; Wang, W.; Zhang, W.; Staiger, C.J. Lipid signaling requires ROS production to elicit actin cytoskeleton remodeling during plant innate immunity. Int. J. Mol. Sci. 2022, 23, 2447. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Deng, Q.; Zhang, J.; Jia, C.; Gao, M.; Wang, Y.; Zhang, L.; Zhang, N.; Guo, Y.-D. Transcription factor SlSTOP1 regulates small auxin-up RNA genes for tomato root elongation under aluminum stress. Plant Physiol. 2024, 196, 2654–2668. [Google Scholar] [CrossRef]
- Mantelin, S.; Bhattarai, K.K.; Kaloshian, I. Ethylene contributes to potato aphid susceptibility in a compatible tomato host. New Phytol. 2009, 183, 444–456. [Google Scholar] [CrossRef]
- Yang, S.; Hoffman, N.E. Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Biol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Lin, Z.; Zhong, S.; Grierson, D. Recent advances in ethylene research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef]
- Solano, R.; Stepanova, A.; Chao, Q.; Ecker, J. Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 1998, 12, 3703–3714. [Google Scholar] [CrossRef]
- Hu, C.; Wu, S.; Li, J.; Dong, H.; Zhu, C.; Sun, T.; Hu, Z.; Foyer, C.H.; Yu, J. Herbivore-induced Ca2+ signals trigger a jasmonate burst by activating ERF16-mediated expression in tomato. New Phytol. 2022, 236, 1796–1808. [Google Scholar] [CrossRef]
- Sarwar, R.; Zhu, K.-M.; Jiang, T.; Ding, P.; Gao, Y.; Tan, X.-L. DELLAs directed gibberellins responses orchestrate crop development: A brief review. Crop Sci. 2023, 63, 1–28. [Google Scholar] [CrossRef]
- Mayrose, M.; Ekengren, S.K.; Melech-Bonfil, S.; Martin, G.B.; Sessa, G. A novel link between tomato GRAS genes, plant disease resistance and mechanical stress response. Mol. Plant Pathol. 2006, 7, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Ho-Plagaro, T.; Molinero-Rosales, N.; Farina Flores, D.; Villena Diaz, M.; Manuel Garcia-Garrido, J. Identification and expression analysis of GRAS transcription factor genes involved in the control of arbuscular mycorrhizal development in tomato. Front. Plant Sci. 2019, 10, 268. [Google Scholar] [CrossRef]
- Nir, I.; Shohat, H.; Panizel, I.; Olszewski, N.; Aharoni, A.; Weiss, D. The tomato DELLA protein PROCERA acts in guard cells to promote stomatal closure. Plant Cell 2017, 29, 3186–3197. [Google Scholar] [CrossRef]
- Habib, S.; Lwin, Y.Y.; Li, N. Down-regulation of SlGRAS10 in tomato confers abiotic stress tolerance. Genes 2021, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.; Hu, Y.; Liu, H.; He, M.; Yang, Z.; Kong, F.; Liu, X.; Hou, X. DELLA and EDS1 form a feedback regulatory module to fine-tune plant growth-defense tradeoff in Arabidopsis. Mol. Plant 2019, 12, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.-P.; Yu, T.-F.; Zheng, W.-J.; Chen, M.; Zhou, Y.-B.; Chen, J.; Ma, Y.-Z.; Xi, Y.-J.; Xu, Z.-S. The wheat bax Inhibitor-1 Protein interacts with an aquaporin TaPIP1 and enhances disease resistance in Arabidopsis. Front. Plant Sci. 2018, 9, 20. [Google Scholar] [CrossRef]
- Bhattarai, K.K.; Xie, Q.-G.; Pourshalimi, D.; Younglove, T.; Kaloshian, I. Coi1-Dependent signaling pathway is not required for Mi-1-mediated potato aphid resistance. Mol. Plant-Microbe Interact. 2007, 20, 276–282. [Google Scholar] [CrossRef]
- Shi, X.; Pan, H.; Xie, W.; Jiao, X.; Fang, Y.; Chen, G.; Yang, X.; Wu, Q.; Wang, S.; Zhang, Y. Three-way interactions between the tomato plant, tomato yellow leaf curl virus, and Bemisia tabaci (Hemiptera: Aleyrodidae) Facilitate Virus Spread. J. Econ. Entomol. 2014, 107, 920–926. [Google Scholar] [CrossRef]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef]
- Shen, G.; Sun, W.; Chen, Z.; Shi, L.; Hong, J.; Shi, J. Plant GDSL Esterases/Lipases: Evolutionary, physiological and molecular functions in plant development. Plants 2022, 11, 468. [Google Scholar] [CrossRef]
- Williams, C.E.; Nemacheck, J.A.; Shukle, J.T.; Subramanyam, S.; Saltzmann, K.D.; Shukle, R.H. Induced epidermal permeability modulates resistance and susceptibility of wheat seedlings to herbivory by hessian fly larvae. J. Exp. Bot. 2011, 62, 4521–4531. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Lam, E. Bax Inhibitor-1, a conserved cell death suppressor, is a key molecular switch downstream from a variety of biotic and abiotic stress signals in plants. Int. J. Mol. Sci. 2009, 10, 3149–3167. [Google Scholar] [CrossRef]
- Sanchez, P.; Zabala, M.; Grant, M. AtBI-1, a plant homologue of Bax Inhibitor-1, suppresses bax-induced cell death in yeast and is rapidly upregulated during wounding and pathogen challenge. Plant J. 2000, 21, 393–399. [Google Scholar] [CrossRef]
- Babaeizad, V.; Imani, J.; Kogel, K.-H.; Eichmann, R.; Huckelhoven, R. Over-expression of the cell death regulator BAX Inhibitor-1 in barley confers reduced or enhanced susceptibility to distinct fungal pathogens. Theor. Appl. Genet. 2009, 118, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Nirasawa, S.; Kiba, A.; Urasaki, N.; Saitoh, H.; Ito, M.; Kawai-Yamada, M.; Uchimiya, H.; Terauchi, R. Overexpression of Bax inhibitor suppresses the fungal elicitor-induced cell death in rice (Oryza sativa L.) Cells. Plant J. 2003, 33, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, R.; Bischof, M.; Weis, C.; Shaw, J.; Lacomme, C.; Schweizer, P.; Duchkov, D.; Hensel, G.; Kumlehn, J.; Hückelhoven, R. BAX INHIBITOR-1 is required for full susceptibility of barley to powdery mildew. Mol. Plant-Microbe Interact. 2010, 23, 1217–1227. [Google Scholar] [CrossRef]
- Ihara-Ohori, Y.; Nagano, M.; Muto, S.; Uchimiya, H.; Kawai-Yamada, M. Cell death suppressor Arabidopsis Bax Inhibitor-1 is associated with calmodulin binding and ion homeostasis. Plant Physiol. 2007, 143, 650–660. [Google Scholar] [CrossRef]
- Choi, H.W.; Kim, N.H.; Lee, Y.K.; Hwang, B.K. The pepper extracellular xyloglucan-specific endo-β-1,4-glucanase inhibitor protein gene, CaXEGIP1, is required for plant cell death and defense responses. Plant Physiol. 2013, 161, 384–396. [Google Scholar] [CrossRef]
- Aamir, M.; Shanmugam, V.; Dubey, M.K.; Husain, F.M.; Adil, M.; Ansari, W.A.; Rai, A.; Sah, P. Transcriptomic characterization of Trichoderma harzianum t34 primed tomato plants: Assessment of biocontrol agent induced host specific gene expression and plant growth promotion. BMC Plant Biol. 2023, 23, 552. [Google Scholar] [CrossRef]
- Qin, Q.; Bergmann, C.; Rose, J.; Saladie, M.; Kolli, V.; Albersheim, P.; Darvill, A.; York, W. Characterization of a tomato protein that inhibits a xyloglucan-specific endoglucanase. Plant J. 2003, 34, 327–338. [Google Scholar] [CrossRef]
- Medina-Filho, H.P.; Tanksley, S.D. Breeding for nematode resistance. In Handbook of Plant Cell Culture; Collier Macmillan Publishers: New York, NY, USA, London, UK, 1983; Volume 1, pp. 904–923. [Google Scholar]
- Fradin, E.F.; Zhang, Z.; Ayala, J.C.J.; Castroverde, C.D.M.; Nazar, R.N.; Robb, J.; Liu, C.-M.; Thomma, B.P.H.J. Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol. 2009, 150, 320–332. [Google Scholar] [CrossRef]
- Kawchuk, L.; Hachey, J.; Lynch, D.; Kulcsar, F.; van Rooijen, G.; Waterer, D.; Robertson, A.; Kokko, E.; Byers, R.; Howard, R.; et al. Tomato Ve disease resistance genes encode cell surface-like receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 6511–6515. [Google Scholar] [CrossRef]
- Hörmann, F.; Küchler, M.; Sveshnikov, D.; Oppermann, U.; Li, Y.; Soll, J. Tic32, an essential component in chloroplast biogenesis. J. Biol. Chem. 2004, 279, 34756–34762. [Google Scholar] [CrossRef]
- Kovács-Bogdán, E.; Soll, J.; Bölter, B. Protein import into chloroplasts: The tic complex and its regulation. BBA Mol. Cell Res. 2010, 1803, 740–747. [Google Scholar] [CrossRef]
- Dutta, S.; Mohanty, S.; Tripathy, B.C. Role of temperature stress on chloroplast biogenesis and protein import in pea. Plant Physiol. 2009, 150, 1050–1061. [Google Scholar] [CrossRef]
- Kim, S.A.; Ahn, S.Y.; Yun, H.K. Transcriptomic changes in dormant buds of two grapevine cultivars following exposure to freezing temperature. Hortic. Environ. Biotechnol. 2017, 58, 152–161. [Google Scholar] [CrossRef]
- Hao, X.; Wang, B.; Wang, L.; Zeng, J.; Yang, Y.; Wang, X. Comprehensive transcriptome analysis reveals common and specific genes and pathways involved in cold acclimation and cold stress in tea plant leaves. Sci. Hortic. 2018, 240, 354–368. [Google Scholar] [CrossRef]
- Wu, Q.; Park, S.; Kirkham, M.B.; Williams, K.A. Transcriptome analysis reveals potential mechanisms for inhibition of intumescence development by UV radiation in tomato. Environ. Exp. Bot. 2017, 134, 130–140. [Google Scholar] [CrossRef]
- Adhikary, D.; Mehta, D.; Uhrig, R.G.; Rahman, H.; Kav, N.N.V. A proteome-level investigation into Plasmodiophora brassicae resistance in Brassica napus canola. Front. Plant Sci. 2022, 13, 860393. [Google Scholar] [CrossRef]
- Lakmes, A.; Jhar, A.; Brennan, A.; Kahriman, A. Inheritance of early and late ascochyta blight resistance in wide crosses of chickpea. Genes 2023, 14, 316. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Xia, Q.; Xia, S. Genome-wide identification and multi-stress response analysis of the DABB-type protein-encoding genes in Brassica napus. Int. J. Mol. Sci. 2024, 25, 5721. [Google Scholar] [CrossRef]
- Lee, J.R.; Lee, S.S.; Park, S.-C.; Kang, J.S.; Kim, S.Y.; Lee, K.O.; Lee, S.Y. Functional characterization of pathogen-responsive protein AtDabb1 with an antifungal activity from Arabidopsis thaliana. Biochim. Biophys. Acta—Proteins Proteom. 2008, 1784, 1918–1923. [Google Scholar] [CrossRef]
- Didelon, M.; Khafif, M.; Godiard, L.; Barbacci, A.; Raffaele, S. Patterns of sequence and expression diversification associate members of the PADRE gene family with response to fungal pathogens. Front. Genet. 2020, 11, 491. [Google Scholar] [CrossRef]
- Ho, J.; Weide, R.; Ma, H.; Vanwoedragen, M.; Lambert, K.; Koornneef, M.; Zabel, P.; Williamson, V. The Root-knot nematode resistance gene (Mi) in tomato—Construction of a molecular linkage map and identification of dominant cDNA markers in resistant genotypes. Plant J. 1992, 2, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Laterrot, H. Near isogenic tomato lines in moneymaker type with different genes for disease resistances. Rep. Tomato Genet. Coop. 1987, 37, 91. [Google Scholar]
- Bonato, O.; Lurette, A.; Vidal, C.; Fargues, J. Modelling temperature-dependent bionomics of Bemisia tabaci (Q-biotype). Physiol. Entomol. 2007, 32, 50–55. [Google Scholar] [CrossRef]
- Medina, I.; Carbonell, J.; Pulido, L.; Madeira, S.C.; Goetz, S.; Conesa, A.; T�rraga, J.; Pascual-Montano, A.; Nogales-Cadenas, R.; Santoyo, J.; et al. Babelomics: An integrative platform for the analysis of transcriptomics, proteomics and genomic data with advanced functional profiling. Nucleic Acids Res. 2010, 38, W210–W213. [Google Scholar] [CrossRef]
- Irizarry, R.A.; Bolstad, B.M.; Collin, F.; Cope, L.M.; Hobbs, B.; Speed, T.P. Summaries of affymetrix genechip probe level data. Nucleic Acids Res. 2003, 31, e15. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Oliveros, J.C.; Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. 2007–2015. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 23 March 2025).
- Fernandez-Pozo, N.; Menda, N.; Edwards, J.D.; Saha, S.; Tecle, I.Y.; Strickler, S.R.; Bombarely, A.; Fisher-York, T.; Pujar, A.; Foerster, H.; et al. The Sol Genomics Network (SGN)—From genotype to phenotype to breeding. Nucleic Acids Res. 2015, 43, D1036–D1041. [Google Scholar] [CrossRef]
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. agriGO v2.0: A GO Analysis Toolkit for the Agricultural Community, 2017 Update. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis (3e), 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; p. 276. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual, S.; Rodríguez-Álvarez, C.I.; López-Vidriero, I.; Franco-Zorrilla, J.M.; Nombela, G. Over Time Changes in the Transcriptomic Profiles of Tomato Plants with or Without Mi-1 Gene During Their Incompatible or Compatible Interactions with the Whitefly Bemisia tabaci. Plants 2025, 14, 1054. https://doi.org/10.3390/plants14071054
Pascual S, Rodríguez-Álvarez CI, López-Vidriero I, Franco-Zorrilla JM, Nombela G. Over Time Changes in the Transcriptomic Profiles of Tomato Plants with or Without Mi-1 Gene During Their Incompatible or Compatible Interactions with the Whitefly Bemisia tabaci. Plants. 2025; 14(7):1054. https://doi.org/10.3390/plants14071054
Chicago/Turabian StylePascual, Susana, Clara I. Rodríguez-Álvarez, Irene López-Vidriero, José M. Franco-Zorrilla, and Gloria Nombela. 2025. "Over Time Changes in the Transcriptomic Profiles of Tomato Plants with or Without Mi-1 Gene During Their Incompatible or Compatible Interactions with the Whitefly Bemisia tabaci" Plants 14, no. 7: 1054. https://doi.org/10.3390/plants14071054
APA StylePascual, S., Rodríguez-Álvarez, C. I., López-Vidriero, I., Franco-Zorrilla, J. M., & Nombela, G. (2025). Over Time Changes in the Transcriptomic Profiles of Tomato Plants with or Without Mi-1 Gene During Their Incompatible or Compatible Interactions with the Whitefly Bemisia tabaci. Plants, 14(7), 1054. https://doi.org/10.3390/plants14071054







